Published online Sep 25, 2025. doi: 10.5527/wjn.v14.i3.108534
Revised: May 29, 2025
Accepted: July 15, 2025
Published online: September 25, 2025
Processing time: 153 Days and 22.7 Hours
Acute kidney injury is characterized by a sudden decline in renal function, often due to ischemia or nephrotoxins, leading to increased oxidative stress and inflammation.
To investigate the protective effects of adipose-derived mesenchymal stromal cell (ADMSC) secretome on renal tubular epithelial cells (NRK-52E) as an in vitro model of oxidative stress-associated kidney injury.
ADMSCs were isolated from human adipose tissue and characterized for mesenchymal markers and differentiation potential. Conditioned media (CM) was collected after 48-hour serum-free culture and applied to serum-deprived NRK-52E cells for 48 hours. Cell viability was assessed using the MTT assay, apoptosis was assessed by Annexin V-FITC/PI staining and flow cytometry, reactive oxygen species (ROS) levels via H2DCFDA staining, and mitochondrial membrane potential by the tetramethylrhodamine ethyl ester assay. The expression of heme oxygenase-1 (HO-1), nuclear factor erythroid 2-related factor 2 (Nrf2), and NAD(P)H quinone dehydrogenase 1 (Nqo1) genes was quantified by quantitative polymerase chain reaction. Comparative transcriptomic analysis was performed on ADMSCs and bone marrow-derived MSCs (BM-MSCs) using publicly available microarray data (GSE108511).
ADMSC secretome significantly reduced ROS production and enhanced mitochondrial membrane potential in NRK cells. Gene expression analysis revealed a significant upregulation of HO-1 mRNA levels in ADMSC-CM treated cells. However, no significant changes were observed in Nrf2 and Nqo1 mRNA levels. Transcriptome analysis of ADMSCs against BM-MSCs revealed significant differences in the expression of genes related to oxidative stress response, antioxidant activity, and mitochondrial function.
The results of this study suggest that the ADMSC secretome exerts multifaceted protective effects on NRK cells by reducing oxidative stress and enhancing mitochondrial function. The study demonstrates the potential beneficial applications of the ADMSC secretome in treating oxidative stress-related kidney injuries.
Core Tip: This study highlights the therapeutic potential of secretome derived from adipose-derived mesenchymal stromal cells (ADMSCs) in protecting renal tubular epithelial cells under oxidative stress conditions. The ADMSC secretome reduced reactive oxygen species levels, enhanced mitochondrial membrane potential, and upregulated heme oxygenase 1 gene expression, indicating activation of cytoprotective pathways. Comparative transcriptome analysis with bone marrow-derived mesenchymal stromal cells revealed distinct gene expression profiles related to mitochondrial and antioxidant functions. These findings support the use of ADMSC secretome as a promising cell-free approach for mitigating oxidative damage in acute kidney injury.
- Citation: Jafar H, Ababneh NA, Alhattab D, Alatoom RM, Zalloum S, Salah B, Alhawari H, Awidi A. Adipose-derived mesenchymal stromal cell secretome protects against kidney injury through induction of heme oxygenase 1 upregulation in vitro. World J Nephrol 2025; 14(3): 108534
- URL: https://www.wjgnet.com/2220-6124/full/v14/i3/108534.htm
- DOI: https://dx.doi.org/10.5527/wjn.v14.i3.108534
Acute kidney injury (AKI) comprises a syndrome of sudden renal function deterioration over a short period of time[1]. AKI is most often seen in hospitalized patients upon admission, with varying pathogenesis progressions depending on the underlying causes[2]. A common feature is the need for prolonged hospital stay leading to patient morbidity, possible hospital-acquired infections, increased healthcare costs and high mortality, especially in critical patients[3]. Currently, AKI treatment is tailored to the etiology, and can include diuretics, hyperkalemia control, dialysis, and even transplant when injury is persistent and the insult has not been corrected early. However, there is no definite drug therapy to protect against or reverse renal injury.
At a cellular level, the injury is generally secondary to ischemia or nephrotoxins resulting in tubular necrosis[4]. The most commonly accepted theory is the overactivated immune system causing a heightened inflammatory response in the glomeruli[5]. This includes both innate and adaptive immunity responses which can, directly and indirectly, lead to tubular cell apoptosis.
More recently, there is growing evidence that the molecular basis underlying AKI involves an increase in the levels of reactive oxygen species (ROS) in renal cells, with the resultant induction of oxidative stress[6-8]. Free oxygen radicals are physiologically derived as byproducts of biologically normal oxygen metabolism in the mitochondria. However, excess oxidative stress can activate the apoptotic pathway, trigger inflammation, and lead to inhibition of tubular function[7]. Novel therapeutic approaches targeting oxidative stress in AKI, with the purpose of protecting against insult and promoting an anti-inflammatory response, are needed[9].
Mesenchymal stromal cells (MSCs) are multipotent cells demonstrating special characteristics such immunomodulatory, regenerative, mitogenic, and anti-apoptotic abilities[10,11]; which renders them a unique source of advanced therapies. These cells are described to exert their effects via cellular interactions as well as cellular secretions[12]. The MSC secretome has been recently placed under the spotlight of research due to its relative ease of production, promising safety profile in pre-clinical and early clinical studies, in addition to its anticipated stability and as a result availability as a potential off-the-shelf therapy[13,14]. Previous studies have shown the positive effects of the MSC secretome on AKI in experimental models[15,16]. Responses seem to be heterogeneous and dependent on the cell source. Adipose-derived MSCs (ADMSCs) are a population of adult fibroblast-like stem cells isolated from subcutaneous fat tissue[17]. Their relative ease of isolation, and expansion and characterization make these cells and their products (i.e. secretome) an attractive source of advanced therapeutic medicinal products. Of special note, transcriptome analysis revealed an upregulation of oxidation-reduction processes genes in ADMSCs compared to their counterparts from bone marrow (BM), umbilical cord and placenta[18].
In this work, we explore the potential protective effects of the ADMSC secretome on renal tubular epithelial cells (NRK-52E cell line) as an in vitro model of oxidative stress-associated kidney injury. We also explore the underlying mechanism related to these effects in an effort to open future avenues for potential use of cell-free advanced therapeutics in renal injury.
Adipose tissue samples were collected from patients undergoing routine liposuction at Jordan University Hospital (Amman, Jordan). All patients provided written informed consent before participation in the study, which was approved by the institutional review board (Approval No. IRB-CTC-1-2022-08) at the cell therapy center adhering to the ethical guidelines of the Declaration of Helsinki. ADMSCs were isolated and expanded as described in our previous study[19]. Briefly, three biological replicates of ADMSCs were maintained in cell culture medium (CCM) consisting of alpha-Minimum Essential Media (MEM; Gibco, Waltham, MA, United States) supplemented with 10% fetal bovine serum (FBS; Gibco), 200 mmol/L GlutaMax (Gibco) and Antibiotic-Antimycotic (100X; Gibco) and incubated at 37 °C and 5% CO2. Cells were sub-cultured and expanded when they reached subconfluence using 1X TrpleE (Gibco), and media was exchanged every other day.
The NRK-52E cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, United States). Cells were cultured and maintained in cell culture media composed of Advanced Dulbecco’s Modified Eagle Medium (DMEM; Gibco) supplemented with 1X GlutaMax, 1X, Antibiotic-Antimycotic, MEM Non-Essential Amino Acids, and 10% FBS. Cells were maintained at 37 °C and 5% CO2, with media exchange every other day and regular splitting upon reaching 75%–80% confluence.
Osteogenic and adipogenic differentiation: Samples were tested for their ability to differentiate into osteogenic and adipogenic lineages. For osteogenic differentiation, cells were harvested and seeded in triplicates in 6-well tissue culture plates at a seeding density of 2 × 105 cells/well. The next day, the media was replaced with an osteogenic differentiation medium (Gibco) and maintained for 21-28 days until calcium depositions were observed. Once minerals deposition was observed under the microscope, cells were stained with Alizarin Red stain and imaged using the EVOS XL Core Imaging System (Thermo Fisher Scientific, Waltham, MA, United States).
For adipogenic differentiation, cells seeded in 6-well tissue culture plates were maintained in adipogenic differentiation medium (Gibco) for 14-21 days. Media was exchanged every 2-3 days. When differentiation signs were observed under the microscope. Samples were stained with Oil Red O stain. The red-stained fat vacuoles were examined and imaged using the EVOS XL Core Imaging System.
Human MSC marker staining: The human (hMSC) markers were assessed using the BD Stem Flow hMSC Analysis Kit (BD Biosciences, Franklin Lakes, NJ, United States) using fluorescently conjugated antibodies against MSC-positive markers (cluster of differentiation 90 [CD90], CD105, CD73, and CD44) and their isotype controls as recommended by the manufacturer. Briefly, cultured ADMSCs from three different donors were harvested at early passage (passage < 4) and washed with phosphate-buffered saline (PBS). Cells were then resuspended in 800 µL BSA staining buffer, and each sample was aliquoted in four test tubes; each containing 200 µL of the cell suspension. Tubes were then centrifuged at
Quality control testing: Quality control testing was done to check sterility, mycoplasma contamination, and endotoxin levels as previously described by our group[20]. Briefly, cells along with their conditioned media (CM) were injected as a 5 mL suspension in aerobic and anaerobic bottles and incubated for 14 days on the BactecAlert Dual-T System (Biomeuriex, Craponne, France) to detect bacterial and fungal growth. For mycoplasma contamination detection, nucleic acid testing was performed using quantitative polymerase chain reaction (qPCR, MycoSEQ™ Mycoplasma Detection Kit; Thermo Fisher Scientific), as recommended by the manufacturer. Endotoxin level was checked using the Limulus Amebocyte Lysate method (Endosafe; Charles River Laboratories, Wilmington, MA, United States), as recommended by the manufacturer.
To prepare CM, three biological samples of ADMSCs at passage 4 were seeded in T-175 cm2 flasks (Nunc; Thermo Fisher Scientific) at a seeding density of 4000/cm2 in the same conditions described above. The next day, media was aspirated, and cells were washed with IX PBS and maintained in DMEM with 10% FBS, 1% GlutaMax, and 1% Antibiotic-Antimycotic and incubated for 48 hours. Then CM were collected, pooled, filtered using 0.22 μmol/L vacuum filtration (TPP), aliquoted, and stored at -80 °C.
For all experiments, and before treating NRK cells with CM, NRK cells were harvested using 1X Tryple E, centrifuged, seeded for the desired experiments into culture plates, and maintained in CCM at 37 °C and 5% CO2. After 24 hours of cell seeding, the cells were cultured in serum-free CCM to induce injury through serum deprivation. The next day, the media was replaced with either experimental CM or the control (CCM) and incubated for 48 hours at 37 °C and 5% CO2.
Cell viability was measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; ATCC) assay in 96-well plates. Briefly, after 24 hours and 48 hours, the MTT assay was performed by adding 10 µL MTT reagent into each well. Plates were incubated for 3 hours at 37 °C and 5% CO2. At the end of incubation, 100 µL stop solution was added to dissolve formazan crystals. Absorbance was measured at 560 nm via a microplate reader (GloMaXTM; Promega, Madison, WI, United States).
Flow cytometry using the Annexin V-FITC Cell Apoptosis Kit (Invitrogen, Carlsbad, CA, United States) was performed to measure the apoptosis level. After treatment of NRK cells for 48 hours with CM and CCM as described above, cells were harvested and centrifuged at 300 × g for 5 minutes and suspended in 200 µL binding buffer. A total of 5 μL Annexin V-FITC and 5 μL propidium iodide (PI) were mixed with the cells and kept in the dark for 15 minutes at room tem
ROS were assessed using the cell-permeant reagent 2,7–dichlorofluorescin diacetate (H2DCFDA), which is a fluorogenic dye that measures the activity of (ROS) within the cell (Abcam, Cambridge, MA, United States). Briefly, NRK cells were seeded in six T25 cm2 flasks, and the following day, cells were treated as described in the cell culture section. After 48 hours of treatment with the described CM or CCM, cells were trypsinized, washed with 1X PBS, and centrifuged. Then, 20 μmol/L DCFDA was prepared in 200 µL of 1X dilution buffer, added to the cells, incubated at 37 °C for 30 minutes, and washed with PBS three times to remove the residual probes. Fluorescence signs were detected using flow cytometry (FACSDiva software; BD Biosciences) at an excitation wavelength of 488 nm and an emission wavelength of 525 nm. Data were analyzed using Canto II FACS Diva software.
A mitochondrial membrane potential (MMP) assay kit (Abcam) was used as recommended by the manufacturer. Briefly, treated NRK cells were incubated with 400 nM tetramethylrhodamine ethyl ester (TMRE) in CM or CCM at 37 °C for 30 minutes. Positive controls were also prepared by adding 20 μmol/L FCCP 10 minutes before measurement. After incubation, cells were washed twice with PBS, and then trypsinized and centrifuged at 300 × g for 5 minutes. The pellets were resuspended in PBS. Flow cytometry detected TMRE staining at Ex/Em = 549/575, and data were analyzed using Canto II FACS Diva software.
RNA extraction was performed using the RNeasy mini kit (Qiagen, Germantown, MD, United States). RNA was quantified using the Nanodrop Spectrophotometer 2000 (Thermo Fisher Scientific), and cDNA synthesis was performed using the 5X Prime Script RT Master mix (Takara Bio Inc., Shiga, Japan), 1 µg RNA, and up to 20 µL nuclease-free water and incubated in a thermal cycler at 37 °C for 15 minutes then 85 °C for 5 seconds. cDNA samples were diluted at 1:10 before use in qPCR. qPCR was performed using the SYBR Green qPCR Ready Mix (Takara) and specific forward and reverse primers for the following genes: Nuclear factor erythroid 2-related factor 2 (Nrf2) (F-5′CACATCCAGACAGA CACCAGT-3′, R-5′CTACAAATGGGAATGTCTCTGC-3′), NAD(P)H quinone dehydrogenase 1 (Nqo1) (F-5′CAGCGGC TCCATGTACT-3′, R-5′GACCTGGAAGCCACAGAA-3’), and heme oxygenase 1 (HO-1) (F-5’CACCAAGTTCAAA CAGCTCT-3′,R-5′CAGGAAACTGAGTGTGAGGA-3′). The final reaction volume was 10 μL containing 5 μL 2 × qPCR master mix, 200 nM of each forward and reverse primers. The cycling conditions were as follows: 95 °C for 30 seconds, then 40X of 95 °C for 5 seconds and 60 °C for 1 minute. The CFX-96 Thermal Cycler (Bio-Rad, Hercules, CA, United States) was used for qPCR amplification and detection. Relative mRNA levels of the target genes were normalized to the expression of β-actin amplified using the following primers: Β-actin Rat-F-5’GGAGATTACTGCCCTGGCTCCTA-3’, β-actin Rat-R-5’ACTCATCGTACTCCTGCTTGCTG-3’.
To better understand the molecular basis for the observed differences in antioxidant capacity between ADMSCs and BM-derived MSCs (BM-MSCs), we performed comparative transcriptome analysis on gene expression data from both cell types. For this comparison, we utilized our previously published data set with Gene Expression Omnibus (Accession No. GSE108511)[18]. Raw microarray data were extracted and analyzed using R (version 4.2.0) with the Bioconductor package (version 3.15.0). Differential expression analysis was conducted using the limma package with Benjamini-Hochberg correction for multiple testing. Genes were considered significantly differentially expressed at false discovery rate (FDR) < 0.05 and absolute fold change > 1.5. Gene Ontology (GO) enrichment analysis for biological processes was performed using the clusterProfiler package. We specifically focused on genes related to oxidative stress response, antioxidant activity, and mitochondrial function based on GO annotations and keyword searches. Principal component analysis was used to visualize sample grouping and identify outliers. Heatmaps were generated using the pheatmap package to visualize expression patterns of key antioxidant and oxidative stress-related genes.
Statistical analyses were conducted using GraphPad Prism software version 6.0 (GraphPad Software, San Diego, CA, United States). All data are presented as the mean ± SD from at least three independent biological replicates. For comparisons involving more than two groups or two independent variables (e.g., treatment and time), two-way analysis of variance (ANOVA) was employed. When the ANOVA indicated significant effects, post hoc analysis was conducted using Bonferroni’s multiple comparisons test to adjust for type I error due to multiple testing. For comparisons between two groups only, the unpaired two-tailed Student’s t-test was applied when assumptions of normality and equal variance were met; otherwise, the non-parametric Mann-Whitney U test was used. Flow cytometry data, including Annexin V/PI staining, ROS levels (H2DCFDA), and MMP (TMRE), were analyzed using geometric mean fluorescence intensity. For qPCR data, relative gene expression was calculated using the ΔΔCt method and normalized to the β-actin housekeeping gene. P < 0.05 was considered statistically significant.
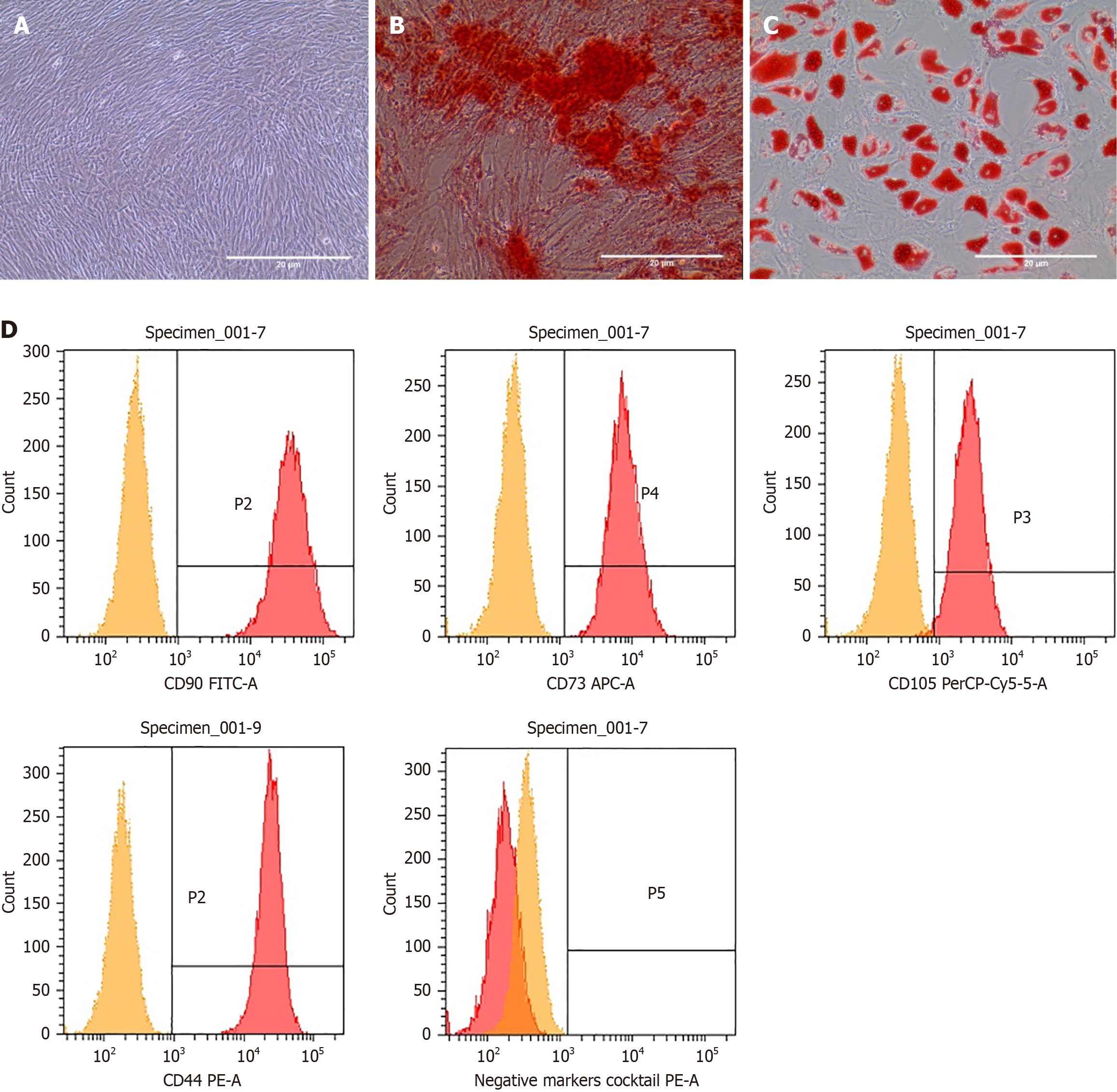
To ensure that ADMSCs possess typical MSC characteristics, cells were examined under an inverted phase-contrast microscope and showed a spindle-shaped plastic-adherent morphology (Figure 1A). Additionally, the cells were differentiated into osteogenic and adipogenic lineages. Alizarin Red staining showed the ability of ADMSCs to differentiate into osteogenic lines (Figure 1B). Oil Red O staining of fat vacuoles in adipocytes has also shown the positive differentiation of cells into adipocytes (Figure 1C). Undifferentiated cells were used as negative control.
Cells were also assessed for the positive expression of hMSC surface markers, including CD90, CD105, CD73, and CD44, and lack of expression of hematopoietic markers including CD45, CD34, CD11b, CD19 and human leukocyte antigen. Flow cytometry analysis showed that all of the analyzed samples successfully expressed hMSC surface markers (Figure 1D). The average percentages of hMSC surface markers among ADMSC samples were CD105 (94%), CD90 (99%), CD73 (94.3%), and CD44 (99%). Routine quality control tests showed accepted quality, and the results met the specifications previously set by our group[20]. The results included freedom of microbial contamination, absence of mycoplasma contamination, and endotoxin levels < 5 EU/mL.
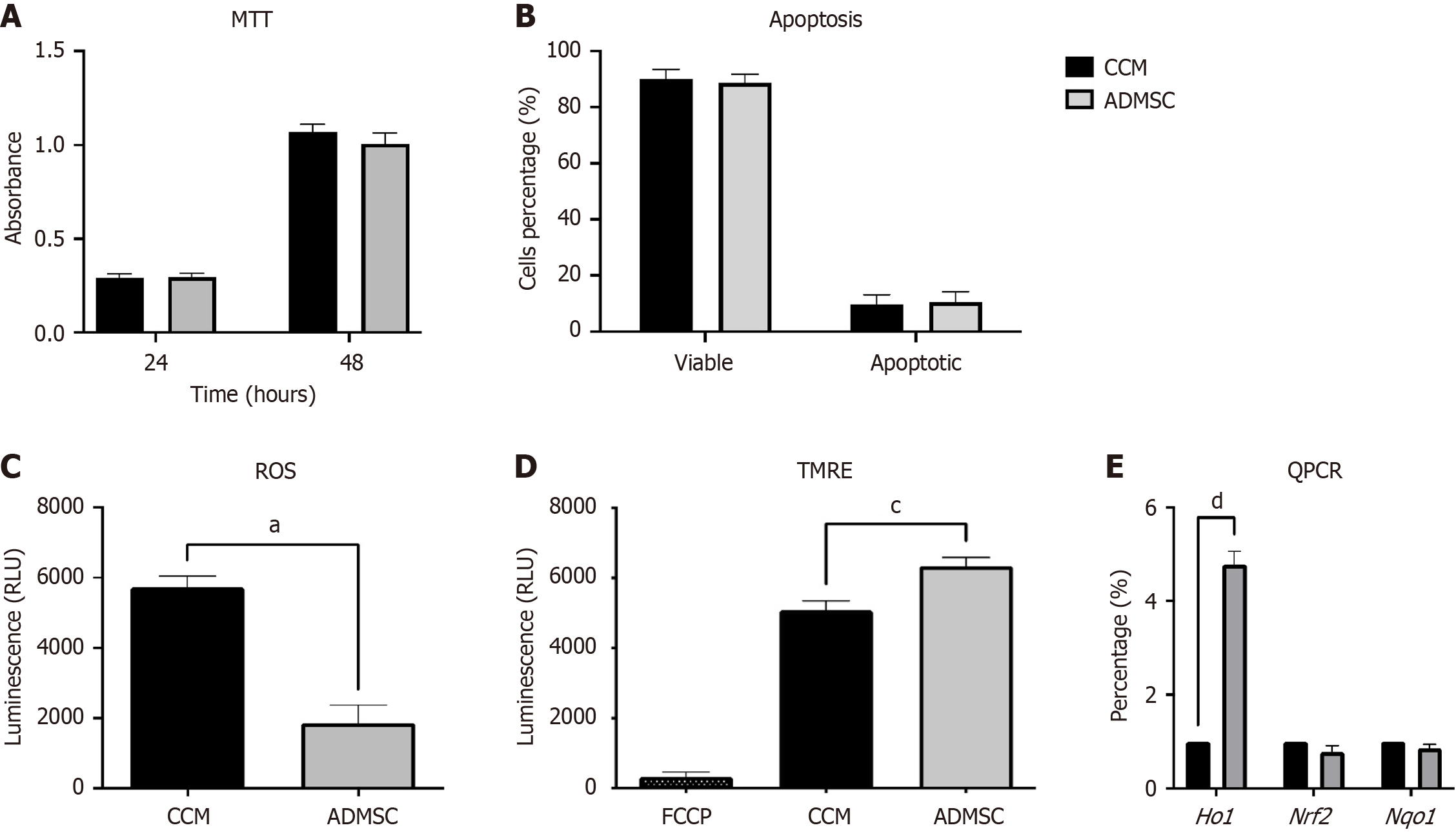
The MTT assay was performed after 24 hours and 48 hours of treating NRK cells with CM or CCM to explore the effect of MSC-CM treatment on cell viability. The reactive cell survival was measured by colorimetric detection of the insoluble formazan crystals produced after the reduction of tetrazolium MTT salt. Overall, no significant difference was observed in NRK cells treated with either CM or CCM (Figure 2A), indicating no cytotoxic effect on tubular cells by MSC-CM.
NRK-52E cells were exposed to CM for 72 hours, and apoptosis level was determined through Annexin V-FITC and PI double staining to investigate the apoptotic effect of using ADMSC-CM on NRK cells. Flow cytometry analysis revealed no significant increase in apoptosis level on NRK cells treated with either CM or CCM (Figure 2B), indicating MSC-CM does not induce tubular cell apoptosis.
Treated NRK cells were stained with 2,7–dichlorofluorescein diacetate (DCFDA), a fluorogenic permanent-cell dye oxidized in cells by ROS to a strongly fluorescent compound. Accordingly, the fluorescent intensity directly reflects the total ROS production. TBHP was used as a positive control to induce ROS production. Overall, as illustrated in Figure 2C, a significant reduction in total ROS production was detected in cells treated with ADMSCs-CM (aP ≤ 0.05).
MMP was detected after staining cultured cells with the cationic TMRE dye. FCCP was used as positive control to induce mitochondrial depolarization. Herein, alterations in the relative MMP of NRK-52E cells were investigated, and a highly significant increase in MMP was observed in ADMSC-CM treated cells (cP < 0.001) compared with CM (Figure 2D).
The mRNA levels of HO-1, Nrf2, and Nqo1 genes were tested to assess the protective effect of CM. The results showed a significant increase in HO-1 mRNA expression (dP < 0.0001) in ADMSC-CM-treated NRK cells (Figure 2E). By contrast, no significant increase in Nrf2 and Nqo1 mRNA expression levels was detected.
Comparative transcriptome analysis between ADMSCs and BM-MSCs revealed significant differences in the expression of genes related to oxidative stress response, antioxidant activity, and mitochondrial function. Among the 3220 genes analyzed, we identified 1749 significantly differentially expressed genes (FDR < 0.05) between the two cell types (Supplementary material).
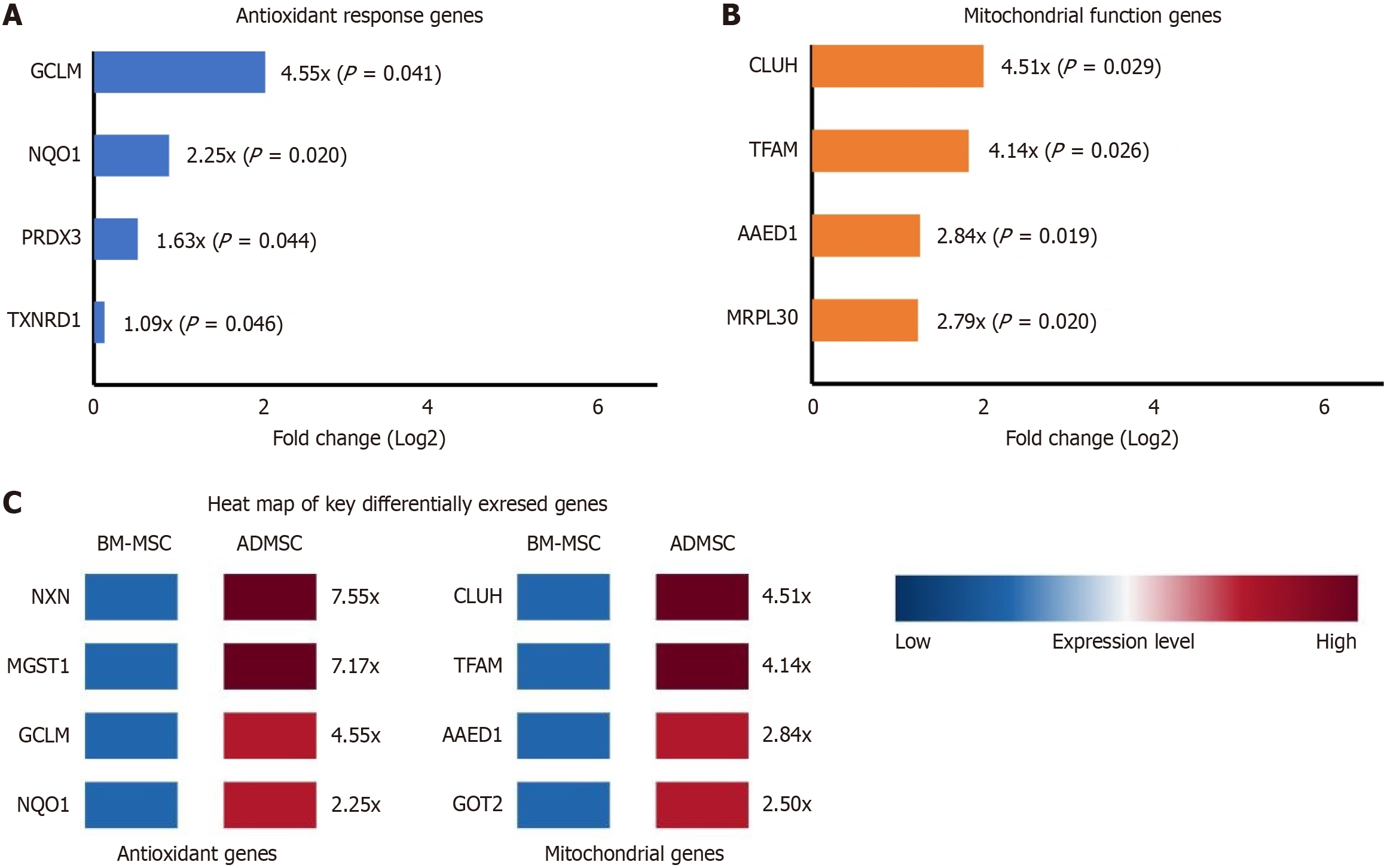
Multiple genes involved in glutathione metabolism and antioxidant defense showed significantly higher expression in ADMSCs compared to BM-MSCs (Figure 3). Notably, the glutamate-cysteine ligase modifier subunit, a rate-limiting enzyme in glutathione synthesis, was upregulated 4.55-fold in ADMSCs (FDR = 0.0414). Nqo1, a critical downstream target of the Nrf2 pathway and important antioxidant enzyme, showed 2.25-fold higher expression in ADMSCs (FDR = 0.0202). Other antioxidant genes upregulated in ADMSCs included peroxiredoxin 3 (fold change = 1.63, FDR = 0.0437) and thioredoxin reductase 1 (fold change = 1.09, FDR = 0.0459) (Figure 3A).
Genes related to mitochondrial function also showed significantly different expression patterns between the two MSC sources (Figure 3B). Transcription factor A mitochondrial, a key regulator of mitochondrial DNA replication and tran
Interestingly, nucleoredoxin (NXN), a redox-sensitive regulator of Wnt signaling that participates in cellular redox homeostasis, showed 7.55-fold higher expression in ADMSCs (FDR = 0.0227) (Figure 4). Microsomal glutathione S-transferase 1, which protects against oxidative stress, was also significantly upregulated (fold change = 7.17, FDR = 0.0306) (Figure 3C).
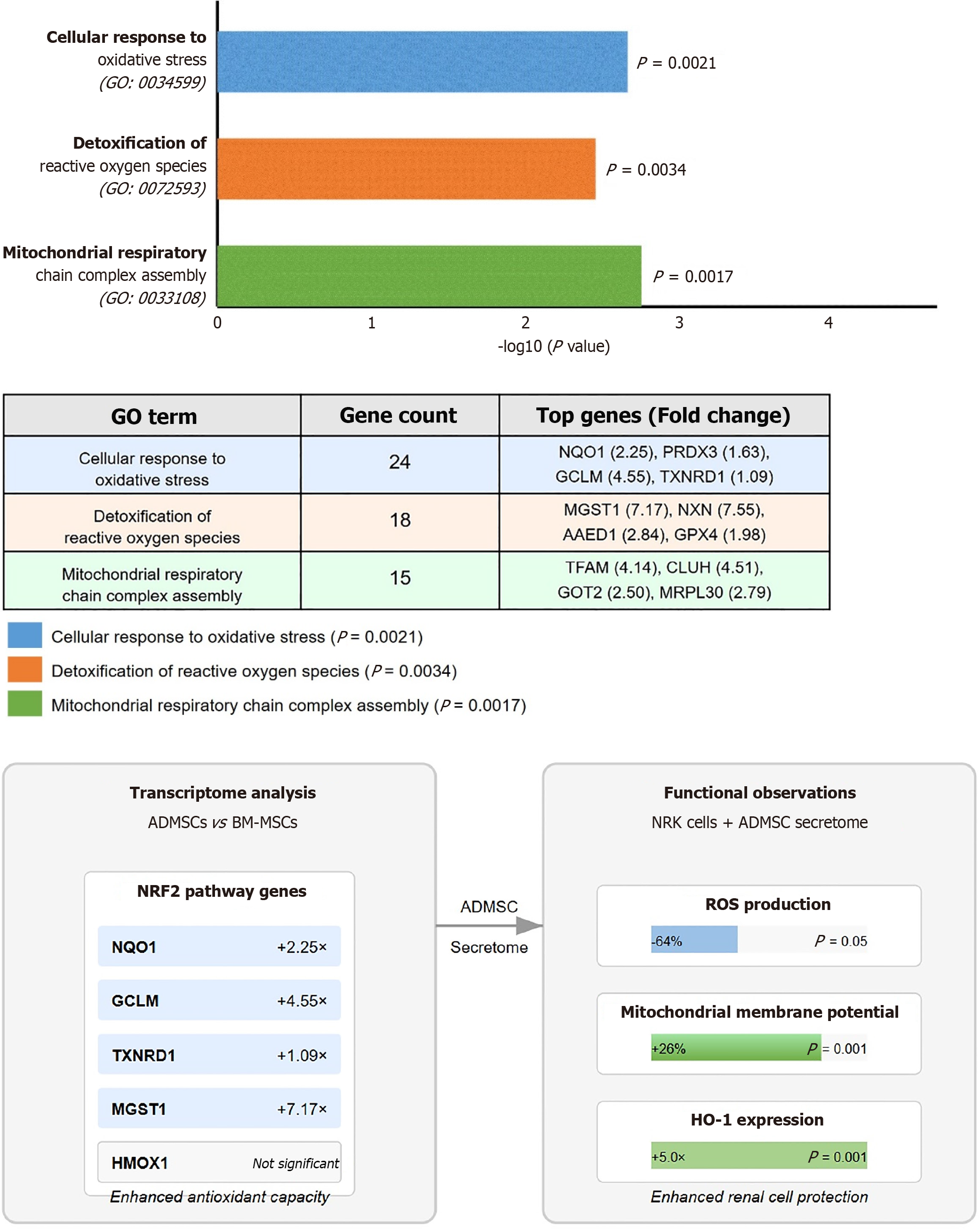
GO enrichment analysis of differentially expressed genes revealed significant enrichment of biological processes related to "cellular response to oxidative stress" (GO: 0034599; P = 0.0021), "detoxification of reactive oxygen species" (GO: 0072593; P = 0.0034), and "mitochondrial respiratory chain complex assembly" (GO: 0033108; P = 0.0017) in ADMSCs compared to BM-MSCs (Figure 4).
Our work showed that the MSC secretome has no adverse effects on kidney tubular cells, as it showed no decrease in cell viability, nor did it induce apoptosis. This is consistent with the work by Faria et al[21] on ischemic kidney injury. The authors showed that the MSC secretome can ameliorate proximal tubule damage. However, no mechanism of action or target of the secretome components were discussed. The potential of MSC secretome as a viable treatment option has been explored in several disease models including muscle injury[22], cerebral ischemia[23], kidney injury[21], and cancer[24]. This is due to their content of growth factors, cytokines, chemokines, and anti-inflammatory factors[11].
ROS are implicated in the pathophysiology of AKI. As ROS levels rise excessively, they lead to oxidative stress, which damages vital cellular components such as lipids, proteins, and DNA[6-8]. This oxidative damage can lead to cell death and inflammation, further impairing kidney function. Studies have shown that ROS can trigger apoptosis and necrosis in kidney cells, exacerbating the injury[25]. The HO-1 gene encodes an enzyme that helps protect against oxidative stress[26]. HO-1 breaks down heme into biliverdin, carbon monoxide, and free iron, all of which have antioxidant, anti-inflammatory, and anti-apoptotic properties. Inducing HO-1 has been shown to reduce oxidative stress and inflammation in kidney tissues, which can help mitigate the severity of AKI. Preclinical models have demonstrated that HO-1 induction can prevent the progression of AKI to acute renal failure and improve renal outcomes[27]. The protective effects of HO-1 also include modulation of the immune response, promoting the polarization of macrophages towards an anti-inflammatory phenotype (M2 macrophages), and increasing the production of anti-inflammatory cytokines like interleukin 10[28]. This helps to suppress pro-inflammatory cytokines and reduce overall inflammation in the kidneys. As such, HO-1 might have therapeutic potential in various renal diseases, making it a promising target for new treatments[29].
Our study demonstrates the beneficial effects of the ADMSC-derived secretome on oxidative stress and mitochondrial function in NRK cells, as a model of kidney tubular cells. The significant reduction in ROS production, as evidenced by the decreased fluorescence intensity of DCFDA-stained cells, highlights the potent antioxidant properties of the ADMSC secretome. This finding is consistent with previous research showing that MSC-derived exosomes can mitigate oxidative stress and inflammation[30]. Moreover, the increase in MMP observed in ADMSC-CM treated cells suggests enhanced mitochondrial function. This improvement in MMP (P ≤ 0.001) compared to the control group aligns with studies demonstrating the protective effects of the MSC secretome on mitochondrial health[31].
Gene expression analysis further supports these observations. The significant upregulation of HO-1 mRNA levels (P < 0.001) in ADMSC-CM treated cells indicate activation of protective oxidative stress response pathways, likely contributing to the observed reduction in ROS. This aligns with previous studies emphasizing HO-1 as a key antioxidant and cytoprotective enzyme in renal cells[27,32]. Interestingly, we did not observe a concomitant significant increase in Nrf2 mRNA expression, despite the established regulatory relationship between Nrf2 and HO-1. Several plausible explanations may account for this discrepancy. First, Nrf2 is primarily regulated at the post-translational level, where its stability and nuclear translocation are tightly controlled by Kelch-like ECH-associated protein 1 (KEAP1)-mediated ubiquitination and degradation under homeostatic conditions[33]. Upon oxidative stress, Nrf2 escapes degradation and rapidly translocates to the nucleus, activating its downstream targets including HO-1. Therefore, the absence of detectable increases in Nrf2 transcript levels does not necessarily preclude its functional activation[34]. Moreover, the transient nature of Nrf2 activation, which often peaks within 1-2 hours of stimulation, may mean that its mRNA levels return to baseline by the time of sampling, while downstream targets like HO-1 remain elevated due to sustained promoter activation[35]. While we did not find HO-1 among the significantly differentially expressed genes in the basal state transcriptome comparison, the upregulation of multiple Nrf2 pathway genes and other antioxidant-related genes suggests that ADMSCs possess an inherently enhanced antioxidant capacity[36]. This supports the idea that Nrf2 may have been activated transiently or post-transcriptionally in response to ADMSC-CM, which in turn could explain the strong HO-1 induction observed (Figure 4). Taken together, although we did not observe a significant change in Nrf2 mRNA levels, the pronounced upregulation of Ho1, a canonical NRF2 target, strongly suggests engagement of the Nrf2/HO-1 axis at the protein and functional levels. Future studies involving time-course analyses of nuclear Nrf2 translocation and KEAP1-Nrf2 protein interactions, along with antioxidant response element reporter assays, are warranted to validate this mechanism.
These findings provide molecular evidence for the enhanced antioxidant and mitochondrial protective properties of ADMSCs compared to the gold standard of MSCs, supporting the potential superiority of ADMSCs as a source for cell-free therapeutics targeting oxidative stress-related conditions such as AKI. Further validation is needed using an appropriate animal model. Additionally, markers, such as 4-hydroxynonenal, should be quantified, to provide a more complete picture.
In conclusion, our study demonstrates the beneficial effects of the ADMSC secretome on oxidative stress and mito
The authors wish to thank Dr. Huda Qubbaj for her valuable review of the statistics implemented in this work.
| 1. | Turgut F, Awad AS, Abdel-Rahman EM. Acute Kidney Injury: Medical Causes and Pathogenesis. J Clin Med. 2023;12:375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 98] [Reference Citation Analysis (0)] |
| 2. | Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394:1949-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 1314] [Article Influence: 187.7] [Reference Citation Analysis (0)] |
| 3. | Pickkers P, Darmon M, Hoste E, Joannidis M, Legrand M, Ostermann M, Prowle JR, Schneider A, Schetz M. Acute kidney injury in the critically ill: an updated review on pathophysiology and management. Intensive Care Med. 2021;47:835-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 341] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 4. | Goyal A, Daneshpajouhnejad P, Hashmi MF, Bashir K. Acute Kidney Injury. 2023 Nov 25. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 5. | Kinsey GR, Okusa MD. Expanding role of T cells in acute kidney injury. Curr Opin Nephrol Hypertens. 2014;23:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Lee H, Jose PA. Coordinated Contribution of NADPH Oxidase- and Mitochondria-Derived Reactive Oxygen Species in Metabolic Syndrome and Its Implication in Renal Dysfunction. Front Pharmacol. 2021;12:670076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Rashid H, Jali A, Akhter MS, Abdi SAH. Molecular Mechanisms of Oxidative Stress in Acute Kidney Injury: Targeting the Loci by Resveratrol. Int J Mol Sci. 2023;25:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 8. | Zheng Y, Yi H, Zhan Z, Xue SS, Tang G, Yu X, Zhang DY. Reactive oxygen/nitrogen species scavenging and inflammatory regulation by renal-targeted bio-inspired rhodium nanozymes for acute kidney injury theranostics. J Colloid Interface Sci. 2024;662:413-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 9. | Kovacevic S, Mitovic N, Brkic P, Ivanov M, Zivotic M, Miloradovic Z, Nesovic Ostojic J. Hyperbaric Oxygenation: Can It Be a Novel Supportive Method in Acute Kidney Injury? Data Obtained from Experimental Studies. Cells. 2024;13:1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Merimi M, El-Majzoub R, Lagneaux L, Moussa Agha D, Bouhtit F, Meuleman N, Fahmi H, Lewalle P, Fayyad-Kazan M, Najar M. The Therapeutic Potential of Mesenchymal Stromal Cells for Regenerative Medicine: Current Knowledge and Future Understandings. Front Cell Dev Biol. 2021;9:661532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 11. | Eleuteri S, Fierabracci A. Insights into the Secretome of Mesenchymal Stem Cells and Its Potential Applications. Int J Mol Sci. 2019;20:4597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 221] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 12. | Alvites R, Branquinho M, Sousa AC, Lopes B, Sousa P, Maurício AC. Mesenchymal Stem/Stromal Cells and Their Paracrine Activity-Immunomodulation Mechanisms and How to Influence the Therapeutic Potential. Pharmaceutics. 2022;14:381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 13. | Dahbour S, Jamali F, Alhattab D, Al-Radaideh A, Ababneh O, Al-Ryalat N, Al-Bdour M, Hourani B, Msallam M, Rasheed M, Huneiti A, Bahou Y, Tarawneh E, Awidi A. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci Ther. 2017;23:866-874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 14. | Awidi A, Al Shudifat A, El Adwan N, Alqudah M, Jamali F, Nazer F, Sroji H, Ahmad H, Al-Quzaa N, Jafar H. Safety and potential efficacy of expanded mesenchymal stromal cells of bone marrow and umbilical cord origins in patients with chronic spinal cord injuries: a phase I/II study. Cytotherapy. 2024;26:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Tsuji K, Kitamura S, Wada J. Secretomes from Mesenchymal Stem Cells against Acute Kidney Injury: Possible Heterogeneity. Stem Cells Int. 2018;2018:8693137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Ullah MM, Collett JA, Monroe JC, Traktuev D, Coleman M, March KL, Basile DP. Subcutaneous injection of adipose stromal cell-secretome improves renal function and reduces inflammation in established acute kidney injury. Stem Cell Res Ther. 2024;15:119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Rautiainen S, Laaksonen T, Koivuniemi R. Angiogenic Effects and Crosstalk of Adipose-Derived Mesenchymal Stem/Stromal Cells and Their Extracellular Vesicles with Endothelial Cells. Int J Mol Sci. 2021;22:10890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | Alhattab D, Jamali F, Ali D, Hammad H, Adwan S, Rahmeh R, Samarah O, Salah B, Hamdan M, Awidi A. An insight into the whole transcriptome profile of four tissue-specific human mesenchymal stem cells. Regen Med. 2019;14:841-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Ababneh NA, Al-Kurdi B, Barham R, Ali D, Sharar N, Abuarqoub D, Jafar H, Salah B, Awidi A. Derivation of three human induced pluripotent stem cell lines (JUCTCi014-A, JUCTCi015-A, JUCTCi016-A) from mesenchymal stem cells (MSCs) derived from bone marrow, adipose tissue and Wharton's jelly samples. Stem Cell Res. 2020;49:102000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Jafar H, Alqudah D, Rahmeh R, Al-Hattab D, Ahmed K, Rayyan R, Abusneinah A, Rasheed M, Rayyan Y, Awidi A. Safety and Potential Efficacy of Expanded Umbilical Cord-Derived Mesenchymal Stromal Cells in Luminal Ulcerative Colitis Patients. Stem Cells Dev. 2024;33:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Faria J, Calcat-I-Cervera S, Skovronova R, Broeksma BC, Berends AJ, Zaal EA, Bussolati B, O'Brien T, Mihăilă SM, Masereeuw R. Mesenchymal stromal cells secretome restores bioenergetic and redox homeostasis in human proximal tubule cells after ischemic injury. Stem Cell Res Ther. 2023;14:353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 22. | Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, Kamei N, Akimoto T, Higashi Y, Ochi M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;589:1257-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 421] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 23. | Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl Med. 2015;4:1131-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 620] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 24. | Lee CS, Lee M, Na K, Hwang HS. Stem Cell-Derived Extracellular Vesicles for Cancer Therapy and Tissue Engineering Applications. Mol Pharm. 2023;20:5278-5311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 25. | Priante G, Gianesello L, Ceol M, Del Prete D, Anglani F. Cell Death in the Kidney. Int J Mol Sci. 2019;20:3598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 26. | Nath M, Agarwal A. New insights into the role of heme oxygenase-1 in acute kidney injury. Kidney Res Clin Pract. 2020;39:387-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Zhai H, Ni L, Wu X. The roles of heme oxygenase-1 in renal disease. Front Nephrol. 2023;3:1156346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 28. | Li Y, Ma K, Han Z, Chi M, Sai X, Zhu P, Ding Z, Song L, Liu C. Immunomodulatory Effects of Heme Oxygenase-1 in Kidney Disease. Front Med (Lausanne). 2021;8:708453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Uddin MJ, Kim EH, Hannan MA, Ha H. Pharmacotherapy against Oxidative Stress in Chronic Kidney Disease: Promising Small Molecule Natural Products Targeting Nrf2-HO-1 Signaling. Antioxidants (Basel). 2021;10:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 30. | Kao YH, Chang CY, Lin YC, Chen PH, Lee PH, Chang HR, Chang WY, Chang YC, Wun SF, Sun CK. Mesenchymal Stem Cell-Derived Exosomes Mitigate Acute Murine Liver Injury via Ets-1 and Heme Oxygenase-1 Up-regulation. Curr Stem Cell Res Ther. 2024;19:906-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Zhang Z, Sheng H, Liao L, Xu C, Zhang A, Yang Y, Zhao L, Duan L, Chen H, Zhang B. Mesenchymal Stem Cell-Conditioned Medium Improves Mitochondrial Dysfunction and Suppresses Apoptosis in Okadaic Acid-Treated SH-SY5Y Cells by Extracellular Vesicle Mitochondrial Transfer. J Alzheimers Dis. 2020;78:1161-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 32. | Chiang SK, Chen SE, Chang LC. The Role of HO-1 and Its Crosstalk with Oxidative Stress in Cancer Cell Survival. Cells. 2021;10:2401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 33. | Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73:3221-3247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1641] [Cited by in RCA: 2052] [Article Influence: 205.2] [Reference Citation Analysis (0)] |
| 34. | Min KJ, Lee JT, Joe EH, Kwon TK. An IκBα phosphorylation inhibitor induces heme oxygenase-1(HO-1) expression through the activation of reactive oxygen species (ROS)-Nrf2-ARE signaling and ROS-PI3K/Akt signaling in an NF-κB-independent mechanism. Cell Signal. 2011;23:1505-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Biswas C, Shah N, Muthu M, La P, Fernando AP, Sengupta S, Yang G, Dennery PA. Nuclear heme oxygenase-1 (HO-1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and anti-oxidant defenses. J Biol Chem. 2014;289:26882-26894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 216] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 36. | Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells. 2019;8:467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 348] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/