Published online Nov 12, 2016. doi: 10.5501/wjv.v5.i4.170
Peer-review started: April 1, 2016
First decision: May 17, 2016
Revised: June 5, 2016
Accepted: July 11, 2016
Article in press: July 13, 2016
Published online: November 12, 2016
Processing time: 222 Days and 2.3 Hours
To assess hepatitis C virus (HCV) geographic integration, evaluate the spatial and temporal evolution of HCV worldwide and propose how to diminish its burden.
A literature search of published articles was performed using PubMed, MEDLINE and other related databases up to December 2015. A critical data assessment and analysis regarding the epidemiological integration of HCV was carried out using the meta-analysis method.
The data indicated that HCV has been integrated immensely over time and through various geographical regions worldwide. The history of HCV goes back to 1535 but between 1935 and 1965 it exhibited a rapid, exponential spread. This integration is clearly seen in the geo-epidemiology and phylogeography of HCV. HCV integration can be mirrored either as intra-continental or trans-continental. Migration, drug trafficking and HCV co-infection, together with other potential risk factors, have acted as a vehicle for this integration. Evidence shows that the geographic integration of HCV has been important in the global and regional distribution of HCV.
HCV geographic integration is clearly evident and this should be reflected in the prevention and treatment of this ongoing pandemic.
Core tip: Geographic integration of hepatitis C virus (HCV) is a newly described epidemiological phenomenon that is illustrated for the first time in this review article. The global burden of HCV infection has surpassed expectations and HCV genotypes are no longer restricted to certain countries or regions. All countries and their citizens are at a higher risk of HCV infection. HCV integration can be either intra-continental or trans-continental. Globalization, immigration and drug trafficking, in addition to the traditional HCV transmission factors, have acted as vectors for the geographical integration of HCV. International efforts and new strategies that go beyond borders should be combined to tackle this global threat.
- Citation: Daw MA, El-Bouzedi AA, Ahmed MO, Dau AA, Agnan MM, Drah AM. Geographic integration of hepatitis C virus: A global threat. World J Virol 2016; 5(4): 170-182
- URL: https://www.wjgnet.com/2220-3249/full/v5/i4/170.htm
- DOI: https://dx.doi.org/10.5501/wjv.v5.i4.170
Infection with hepatitis C virus (HCV) is a global public health threat that affects millions of individuals worldwide. In recent years, HCV has become one of the most important viruses. The global epidemic of HCV is distributed unevenly, with a high disease burden in low income regions and more than one-third of the estimated worldwide burden in the Western Pacific region. Approximately 32.2 million people have chronic HCV infection in Southeast Asia alone, Sub-Saharan Africa accounts for almost one-fifth of worldwide infections and over six million people are infected in Latin America[1].
Globally, about 27% of cirrhosis cases and 25% of hepatic cellular carcinoma cases are attributable to HCV[2]. Based on death certificate analyses, it has been estimated that there were about 3500 HCV-related deaths in France in 2001 and 15000 in the United States in 2007. In Egypt, there were an estimated 7379 HCV-related deaths in 1999 and the number is expected to more than double by 2020[3]. HCV-infected individuals have a 2.4 times higher risk of all-cause mortality compared to the non-infected population, 26.5 times the risk of liver-related mortality, and 1.8 times the risk of non-liver-related mortality[4].
Studies have reported an upsurge in the prevalence of HCV infection, particularly in developing countries and in some European regions. Southern provinces in Greece, Italy, France and Spain have reported higher levels of HCV infection (2%-7%) than in the North African nation of Libya[5]. Certain geographical spots in the Netherlands and Germany have a higher rate of HCV infection (7%) than other regions in the same countries[6].
The predisposing risk factors and modes of transmission of HCV have evolved in various ways in different parts of the world and this could have major implications for prevention programs[7]. However, many questions concerning the roles of risk factors and lifestyles that might be associated with the spread of HCV in different regions remain unanswered. Like some other important infectious diseases, HCV infection has been correlated with geographical, historical, social, economic and even political factors. Globalization and worldwide integration have added new epidemiological concepts that are clearly reflected in the prevalence of HCV worldwide. Neither HCV genotypes nor risk of exposure can be easily confined to certain regions or countries. Immigration, massive population displacement, unsettled conflicts and drug trafficking have aggravated the status of HCV infection and made it difficult to obtain a clear picture of HCV spread over the world. The objective of this review was to assess the worldwide geographical integration of HCV and its global evolution and to use the assessment to propose strategies to intervene in its spread.
This study was conducted in four stages: (1) identification of the literature on HCV integration; (2) selection of relevant studies; (3) extraction of data; and (4) data sensitivity analysis. This review was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement[8].
Relevant studies were identified by searching PubMed, Scopus, Google Scholar and other databases using the following key terms: HCV, HCV integration, history, geography, epidemiology, phylogeography and evolutionary analysis. Some articles were also found by checking the lists of references in published papers. No language or time restrictions were applied.
All identified abstracts were reviewed by two co-authors independently (Daw MA and El-Bouzedi AA or Ahmed MO/Agnan MM and Drah AM). They were considered eligible for full-text review if they provided accurate information on the geo-epidemiology of HCV and the distribution of genotypes. Data on evolutionary analyses were also included to estimate the dates of HCV origin and the temporal rates of virus spread from West Africa and China to Europe and North America.
The data were independently abstracted by two co-authors (Daw MA with El-Bouzedi AA or Dau AA and Agnan MM or Drah AM). The studies were assessed using standardized data collection forms. The collected data include publication details, type of study and information on HCV transmission, the vectors involved, phylogenetic analysis, historical follow-up and evaluation criteria of the spread of HCV. If the evaluation results were controversial, a consensus among the authors was reached.
Data on the spatial spread of HCV was combined with the phylogenetic and epidemiological information to understand the dispersal of the virus worldwide. Furthermore, sensitivity analysis was conducted and the consistency of the data search results was evaluated.
A statistical review of the study was performed by a biomedical statistician.
The publications were identified primarily by online searches. Unrelated studies were excluded based on the title or the abstract. The search results were categorized according to the historical, geographical, epidemiological and clinical parameters. Each of these fields was analyzed in the context of HCV integration.
History of HCV integration: Historical estimates have speculated about divergence time, distribution patterns and epidemic behaviors of HCV. Evolutionary analyses of the HCV genome have shed light on its epidemic history and transmission. Studies combining demographics with phylogeographic and molecular clock analyses have demonstrated the global dissemination of HCV[9,10]. The origin and evolution of HCV may date back to centuries ago in ancient China. An et al[11] recently estimated that the common ancestor of Chinese HCV variants (6 g and 6 w subtypes) isolated in Hainan Island dates back to between the sixth and ninth century. The authors speculated that the ancestors of a particular group of Austronesian-descended aborigines might have carried the earliest HCV-6 strains when they sailed to and settled in the Indochina peninsula in Southeast Asia, where HCV-6 is now indigenous.
Viral phylodynamic analysis indicates that HCV was disseminated in Africa before the rise of global travel and modern medicine[12]. It has been estimated that the most recent common ancestor of the CAR HCV-4 strains existed in the sixteenth century[13]. CAR HCV-4 strains spread rapidly and exponentially from 1935 to 1965, at about the same time as in Cameroon and Gabon. There is also epidemiological evidence of a wave of infection in Western countries during 1945-1965[14].
It has been suggested that colonization by European countries played a major role in the spread of these HCV genotypes, predominantly to the Americas but also to former colonial territories in Asia and Africa[14]. Global dissemination of HCV-2 seems to have been facilitated by the slave trade across the Atlantic and by colonization. During the intense period of slave trade (1700-1850), HCV-2 was disseminated from what is now Ghana/Benin to the Caribbean. HCV-2 also found its way from the Dutch colonies of Indonesia and Surinam to the Netherlands with the migration of Javanese workers to the Netherlands[14].
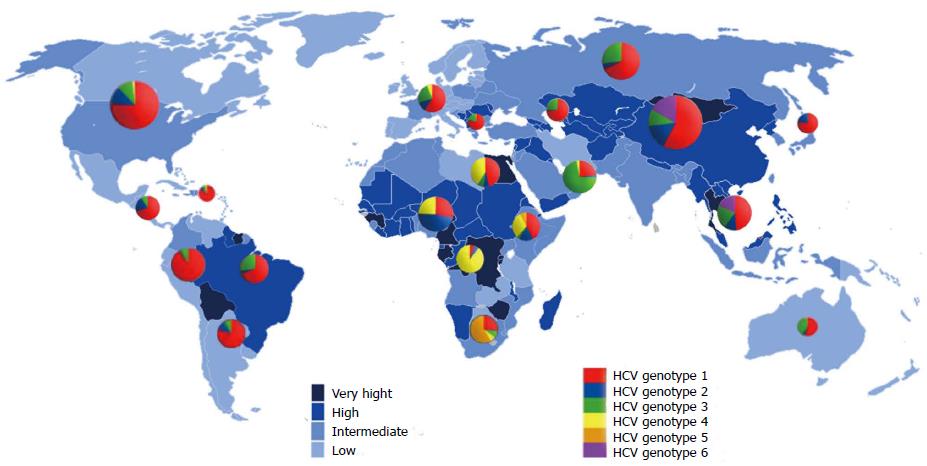
Epidemiological integration of HCV: The prevalence of HCV varies widely, from as low as 0.1% in certain Scandinavian countries to 23% in some African countries (Figure 1). The prevalence rate is classified as low (< 2.5%), intermediate (2.5%-10%) or high (> 10%). According to this classification, regions of low endemicity include North America, Europe, Australia and the Far East. Intermediate prevalence regions include some Mediterranean countries, the Middle East, Africa and South America. High prevalence countries are Egypt, Cameroon, Burundi, Rwanda, Gabon and Guinea in Africa, Bolivia in South America, and Mongolia in Asia[15]. However, 60% of all infected people are in Asia, particularly in its southern and eastern regions. China used to be considered a relatively high endemic area (average seroprevalence of HCV 3.2% in the general population)[16]. In Taiwan, the prevalence was estimated at 5.5%; it was 2.0%-14.2% in towns on the main island and higher (2.3%-26.4%) on the Penghu islands[17]. The overall prevalence in South Korea and Hong Kong is low (0.6%-1.1%) and is higher among females[18,19]. Japan has one of the highest endemic rates of HCV infection[20]. The prevalence rate of viral hepatitis in Southeast Asia is higher, where over 11 million people are estimated to have HCV. The prevalence of HCV infection among Malaysian adults has been estimated at 2.5%. Similar results have been reported from Indonesia, Cambodia, Thailand and the Philippines. However, it is less than 1% in Laos, Myanmar and Singapore, whereas the highest prevalence (> 6%) has been reported in Vietnam[21]. In India and Afghanistan, HCV prevalence ranged from 0.5%-1.5%. However, in Pakistan, about 6% of the population is suspected of having HCV infection and in some regions the estimated rate reached 31.9%[22-24].
In the Persian-Arab regions of West Asia, there are considerable regional differences in the prevalence of HCV. In Iran, the prevalence of anti-HCV antibodies in the general population ranges from 0.2% to 6.25% but the overall average is < 1%, which classifies the country as having a low frequency[25,26]. HCV prevalence was higher in Iraq (2.3%), Jordan (3.5%) and the Gaza strip (2.2%) but moderate in Lebanon (1%) and Syria (1%). There are no national studies on the prevalence HCV in the Arabian Peninsula and Yemen. HCV is considered endemic among the less populated countries, such as Qatar (6.3%), UAE (2.3%), Saudi Arabia (2%), Kuwait (1.8%) and Yemen (2.5%).
In Africa, > 28 million people have chronic HCV infection and future trends are difficult to predict[27]. Egypt has the highest prevalence in the world, with the prevalence rate estimated at 14.7% among people aged 15 to 59 years[1,28]. HCV has been well studied in the other North African countries, particularly in Libya. These countries are considered areas of low endemicity, with prevalence rates of 1%-1.5%[1,27]. In a recent study including 38 countries, seroprevalence was highest (after Egypt) in Central African countries, such as Gabon, Cameroon and Angola, and in some West African countries, such as Burkina Faso and Benin. The largest numbers of infected adults were in Nigeria, the Democratic Republic of Congo and Ethiopia[27].
In Latin America, it is estimated that 6.8 to 8.9 million adults are infected with HCV, of whom over 4 million are in Mexico and Brazil, which are the only South American countries that have carried out national population-based studies. The overall prevalence of HCV in Latin America is 1.5%; it varies from 0.1%-0.9% in Suriname, Chile, Peru, Venezuela, Panama and some other Latin American countries to 1.0%-3.4% in Brazil, Mexico and Argentina[29].
In Scandinavian countries, the spread of HCV infection started in the 1960s and peaked in the 1970s. Nosocomial and sexual transmission were minor routes and the main route was intravenous drug injection. The overall prevalence of HCV was 0.4%-0.6%, with higher rates among intravenous drug users and immigrants[30]. In central European countries, the prevalence of HCV was slightly higher than in the northern region. It varied from 0.6% in England, Luxemburg, Austria and Belgium to 1.5% in certain parts of Germany and Ireland[31]. High prevalence rates of HCV have been found in the southern European countries, particularly the southern regions of Spain, Portugal, Italy and Greece, which have the highest rates in Europe at 2.6%[32,33]. In Turkey, which is the European bridge to the oriental states, reported estimates range from 0.6% to 2.1%[34].
The HCV epidemic began later in Eastern European countries, particularly in the Czech Republic, Albania and Croatia. This delay is attributable to geographical barriers, limited immigration from neighboring endemic countries, and a delay in the increase in intravenous drug use[35]. The Czech Republic, Albania, Croatia, Estonia and Hungary are low-endemicity countries for HCV infection, where the prevalence rates range from 0.2% to 1%, but it was 1.4% in Latvia, Poland and Bulgaria. The highest prevalence was reported in Romania (3.3%), followed by Lithuania and Ukraine (2.3%). The Russian Federation and Baltic states have a high prevalence of HCV, ranging from 1.26% in the Republic of Belarus to 4.1% in some Russian states[36]. In the United States, Canada and Australia, the prevalence ranged from 0.61% to 1.8% and the number of people living with HCV is expected to continue to rise[37,38].
However, some studies indicated that there could be high rates of false positive results in HCV serological assays, as has been reported in Africa and China. In studies in Sub-Saharan Africa, such as in Uganda, Nigeria and the Republic of South Africa, the distinct variations in results were attributed to the wide variety of assays in use and to the different sample storage conditions[27,39]. Hence, future studies on HCV should give sufficient attention to testing strategies, sample handling and storage and include these details in the study reports.
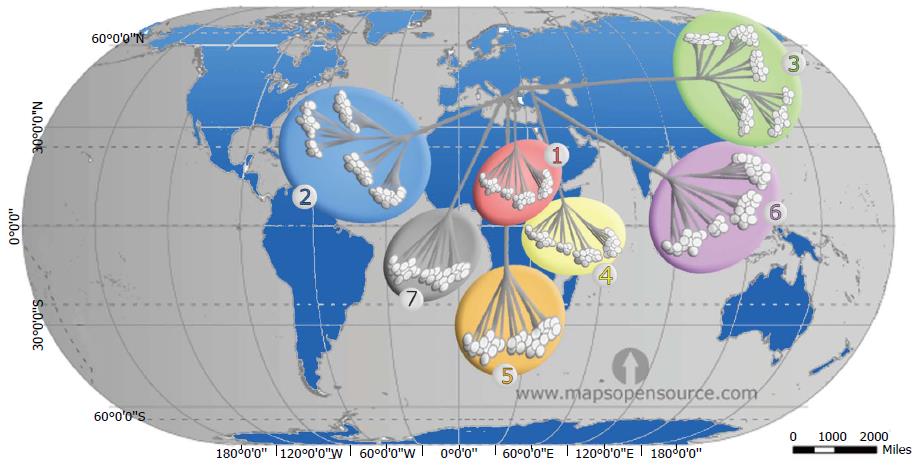
Phylogenetic analysis of HCV genomes led to the development of a nomenclature for distinct virus types and subtypes, comprising seven recognized genotypes[1,7]. The genotypes differ at 30%-35% of nucleotide sites. The 7 genotypes are sub-classified into 67 subtypes plus 20 provisional subtypes. For strains of the same subtype, nucleotide differences are less than 15%[40,41]. However, while some genotypes are ubiquitous, others are found only in specific regions, where they exhibit a high diversity of subtypes (Figure 2). This distribution pattern and the antigenic and biological differences between the HCV types point to long periods of endemic infection during which there was no significant exchange with types from other regions[42].
Globally, genotype 1 accounts for more HCV infections (46.2%) than any other single genotype. Subtypes 1a and 1b account for 90% of all genotype 1 strains, at a ratio of 1:2 respectively. Over one-third of infections with genotype 1 are in East Asia. The next most common genotype is 3, which accounts for 30.1% of cases and is found mainly in southern Asia and in regions of Scandinavia. Genotypes 2, 4 and 6 are responsible for most of the remaining cases of HCV worldwide (9.1%, 8.3% and 5.4% of cases, respectively)[43]. Genotype 2 is predominant in West Africa, genotype 4 in Central and North Africa, and genotype 6 in Southeast Asia. Genotype 5 is responsible for < 1% of all HCV cases worldwide and is found mainly in South Africa. The more recently identified genotype 7 was isolated from a Congolese immigrant in Canada[40,44].
In China, genotype 1 is predominant (69.6%) and type lb is more prevalent than 2a, whereas genotypes 3b and 6 are seen mainly in the southern provinces[45]. In India, the most prevalent genotypes are 3 and 1 (64% and 28% of HCV infections, respectively); genotype 1b is responsible for 16% of all infections, genotype 4a accounts for the remaining infections and the least prevalent is genotype 5 (< 1%)[46]. In Japan, 70% of infections are due to genotype 1b and 20% are caused by genotype 2a; the remaining infections are caused by genotype 2b. HCV genotype 6 is more geographically restricted than genotypes 1-3. It is found in parts of East Asia (South China, Hong Kong, Taiwan and Macao) and Southeast Asia (Singapore, Vietnam, Thailand, Indonesia and Burma). There is no information on HCV genotypes in the highly populated countries of Bangladesh, Malaysia and North Korea[47].
In most European countries, the most prevalent HCV subtype is 1b, although subtype 1a is more prevalent among patients co-infected with human immunodeficiency virus (HIV). In North America, parts of South America, United Kingdom, Scandinavia and Australia, 1a is the most prevalent subtype[42]. In Greece, Poland and the Netherlands, subtype 3 is responsible for 30% of all cases, and in Russia and the Baltic States, subtypes 1b and 3a share dominance[48]. It is noteworthy that the first HCV recombinant, RF2k/1b, was initially identified in Russia but since then has also been identified in Ireland, Estonia, Uzbekistan and Cyprus[49].
In Africa, genotypes 1, 2 and 4 appear to be endemic in regions of West and Central Africa and in the Middle East-North African region. Genotype 5 is more prevalent in southern and eastern Sub-Saharan Africa. Genotype 4 is the most frequent cause of chronic hepatitis C in the Middle East, North Africa and Sub-Saharan Africa[50]. In Egypt, 90% of all HCV infections are caused by type 4[51]. The emergent genotype 7, which originated from Central Africa, is phylogenetically very similar to genotype 2 variants. The greatest diversity of genotype 2 is observed in West Africa. It has been proposed that HCV genotype 2 originated from West Africa and then spread to the east[40]. Indeed, the finding of a new genotype further indicates the endemic nature of HCV in certain parts of Africa and again shows that HCV infection may have evolved from a common ancestor originating in this region of the world.
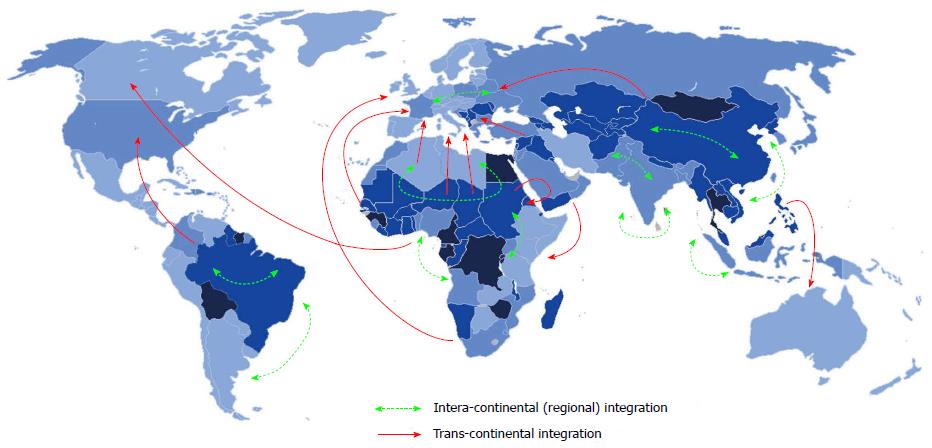
Geographic integration of HCV: The integration of HCV is influenced by host population size and density, the spatial distribution of the host, the frequency of contact between individuals, and other epidemiological factors. This is particularly evident in China and in many countries in Southeast Asia, as well as in Western Europe and Australia, possibly due to migration from Africa and/or Asia[45]. Endemic strains that are relatively rare have circulated for a long time in particular regions. Endemic strains of genotypes 1 and 2 are found mainly in West Africa, strains of genotype 3 in southern Asia, strains of genotype 4 in Central Africa and the Middle East, strains of genotypes 5 and 7 in southern Africa, and strains of genotype 6 in Southeast Asia. The global emergence of HCV has been either intra-continental or trans-continental (Figure 3). Nowadays, new strains of HCV have been reported in different regions and continents. They are due to surpass the commonly known strains resident in these places, particularly among high risk groups[52]. This evolving process of integration has played an important role in the spread of HCV and thus there is a need for developing specific strategies to combat the global spread of HCV infection.
Regional integration of HCV is seen in Africa and Asia. The endemic subtypes of HCV genotypes 1, 2 and 4, which are found mainly in geographically restricted areas of West Africa and Sub-Saharan Africa, are nowadays endemic in other parts of Africa. Egypt has the highest prevalence of HCV in the world (14.7%-32%) and has high rates of morbidity and mortality from chronic liver disease, cirrhosis and hepatocellular carcinoma. It is alarming that > 20% of Egyptian blood donors are seropositive for HCV. The dynamics of HCV in Egypt vary from one region to another. Desert areas have the lowest rates of anti-HCV positivity. The rates are higher in rural regions than in cities, and higher in the Nile Delta than in the Nile Valley[51]. Libya, neighboring Egypt, is considered an area of low endemicity for hepatitis C (1.2% average prevalence)[15]. The prevalence of HCV in Libya reaches its highest (1.6%) in the regions closer to Egypt and Sudan and lowest (0.2%) among other regions in the mid-coastal and western regions, where they resemble or are less than the rates in neighboring Tunisia[5]. The high prevalence rates in some parts of Libya could be due to its proximity to Sub-Saharan countries and the presence of large numbers of African immigrants. In the same way, the Albatnan region of Libya, which has a higher prevalence rate, borders Egypt, from where both legitimate and illegal workers come to Libya[5].
Most cases of HCV in Egypt are of genotype 4a. This strong homogeneity indicates epidemic spread of HCV. On the other hand, different genotypes were isolated from the Libyan population: Genotypes 1, 2, 3 and 4, as well as the newly emerged genotype 5. The prevalence of these genotypes in Libya varies from one region to another and is influenced by demographics and risk factors[53]. The dynamics of integration are applicable in Central Africa and East Africa, notably in Cameroon and Angola which have a high prevalence of HCV comparable to that in the Democratic Republic of Congo[54]. HCV seroprevalence was intermediate in the Horn of Africa: 2.7% in Ethiopia, 2.6% in Somalia and 0.3% in Djibouti[27]. In Southeast Asia, HCV genotype 6 integrated within northern countries, such as Myanmar, Laos and Vietnam, while genotype 3 integrated in Thailand and Malaysia. In the island nations of Singapore, Indonesia and the Philippines, genotype 1 was the most prevalent. Similar integration dynamics have been observed in the Caribbean, India and the Baltic region[55].
The transcontinental integration of HCV is clearly mirrored between African and European countries, particularly around the Mediterranean regions of north African and southern European countries. The relatively high rates of HCV genotype 4 in southern Europe could be attributed to different factors: (1) the historic link between regions in southern Italy and Spain on the one hand and North Africa and the Middle East on the other hand; (2) the employment of multiple-use needles and glass syringes; and (3) the use of blood products that have not been tested for HCV. HCV genotype 4 seems to have recently spread from its endemic reservoir in Africa to southern Europe by immigrants. The prevalence rates of HCV type 4 have been rising in France, Italy, Greece and Spain[56]. In France, the prevalence of genotype 4 increased from 4% in 1990 to > 11% within one decade. In Europe, most patients infected with HCV genotype 4 are intravenous drug users or patients co-infected with HCV and HIV[57]. Recently, genotype 4 was shown to be the second most frequently detected genotype. One study identified genotype 4 in 23% of a large cohort of HIV-positive homosexual men from England, the Netherlands, France, Germany and Australia[58].
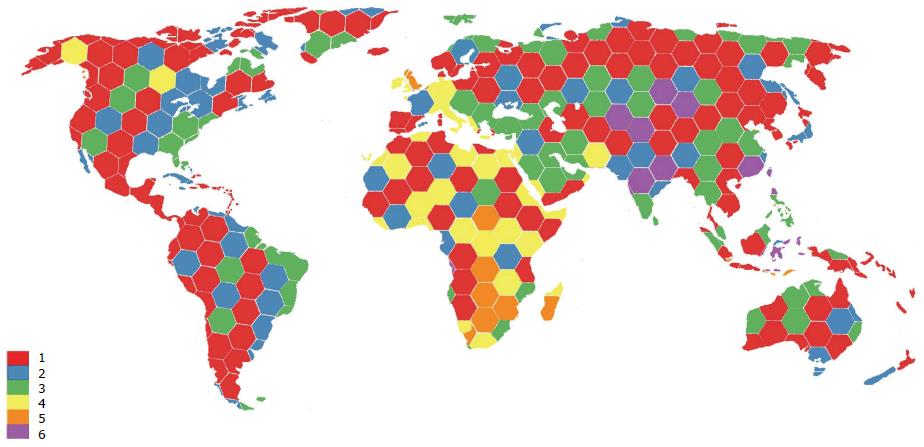
Another example of trans-continental integration is the existence of multiple types of “migrant clusters” of people who moved from West Africa to other regions of the world. These HCV types include the transfer of HCV-2e and 2f to Indonesia, HCV-2i to Morocco, France, Vietnam and Quebec, HCV-2j to Venezuela, HCV-2k to Martinique and France, HCV-2m to Vietnam, HCV-2r to Haiti and the Dominican Republic, and many unclassified type 2 lineages to Suriname[59]. The fact that genotype 2 is the most prevalent in West Africa, Europe, North America and parts of South America could reflect population dynamics resulting from the trans-Atlantic slave trade in the past and/or immigration, as illustrated by the identification of the new genotype 7 in Canada in an immigrant from Central Africa[40]. Figure 4 illustrates the integration dynamics of HCV genotypes all over the world. These trends clearly mirror the regional and global integration of HCV. Such a profile of interaction is associated with transmission and population dynamics of HCV and thus may reflect differences in when HCV infection occurred, which could influence the time of the peak burden of complications of HCV infection, such as cirrhosis and hepatocellular carcinoma. In view of these trends, regional differences in the prevalence of HCV genotypes and HCV epidemiology might have to be taken into consideration by tailoring prevention and treatment strategies to local needs.
Factors associated with HCV integration: HCV integration is a continuous dynamic phenomenon clearly influenced by population movements, demographic factors, clinical practice and personal behaviors in addition to the genetic entity of HCV. These global integration vectors play an important role in the spread of HCV worldwide. The outcomes of this integration coincide with the epidemiological evidence associated with immigration, trafficking and massive use of iatrogenic procedures and IDU, which escalated as early as the mid-twentieth century. The global prevalence of HCV is determined more by these social, behavioral and demographic factors than by genetic variation of the virus.
In 2013, 231 million people (3.2% of the world’s population) migrated to new host nations. Migrants come mainly from developing countries in the south and migrate to the developed nations in North America and Western Europe. Migrants can be classified as immigrants, migrants and seasonal workers, refugees, asylum seekers, international students and others[60]. These newly emerged populations may suffer from infectious diseases usually more exotic or more prevalent in their own environment. These individuals come from regions in which HCV is endemic and thus pose a unique challenge to controlling the global prevalence of viral hepatitis[61]. A Canadian study from 2000 to 2007 compared immigrants with Canadian-born individuals and demonstrated high rates of HCV infection among immigrants. Compared to Canadian-born patients, immigrant patients were more likely to be female, non-white, older and to be infected with genotypes 4, 5 or 6[62]. United States studies on refugee populations found that the rates of HCV infection were up to 8% and only 1.8% among nationals[63].
One study estimated that 50% of HCV infections in the Netherlands are among immigrants, in whom the prevalence (2%) was tenfold higher than in the native population (0.2%)[61]. Likewise, the prevalence of chronic HCV in the United Kingdom among South Asians, and especially among migrants from Pakistan, may be as high as 2.7%, which is over fivefold higher than in the general population (0.5%)[64]. In Italy, the prevalence of HCV among Sub-Saharan refugees varied between 2.7% and 7.1%, while in Spain it was 12.5%, considerably higher than in the autochthonous population[65]. A recent study from Switzerland showed that the molecular epidemiology of HCV infection in low prevalence countries such as Switzerland is driven mainly by migration rather than by the distribution of virus genotypes in the native population[66].
The predominant HCV genotype in Middle Eastern and African countries is genotype 4. It is noteworthy that the prevalence of this genotype has been increasing in southern Europe, with region-specific increases in particular subtypes: 4a in Greece, 4d in Italy, 4c and 4d in Spain, and 4d in the Netherlands. The prevalence of genotype 4 among high-risk individuals in France increased from 15% of infections in 2003 to 22% in 2012. These changes in the trends of genotypes in these countries corresponds well with the increasing numbers of migrants from Africa[64]. As the foreign-born populations expand, the integration dynamics of HCV will become globally imminent[67].
HCV transmission is closely associated with drug trafficking routes worldwide and HCV positivity is found among 15.6-98.7% of injection drug users. About 0.5% of the world’s population injects drugs and of these about 6.8% are infected with HCV. Drug injection is the most common HCV transmission route and the main risk factor for acute and chronic hepatitis C (33.3% and 83.7%, respectively)[68].
The largest populations of people who inject drugs are found in China, Russia, the United States and Brazil, followed by Mexico, Pakistan and Thailand. People who inject drugs have a high seroprevalence of HCV in almost all European countries. This pattern is seen in Austria, Bulgaria, Cyprus, Greece and Romania and the rates are even higher in Latvia, Portugal, Turkey and Cyprus. The prevalence rate is < 30% only in the Czech Republic, Hungary and Slovenia. It is noteworthy that the rates of HCV seroprevalence have declined in Germany, France, the United Kingdom and Italy[69]. In most developing countries, transmission via drug injection is becoming more prevalent and replacing the iatrogenic and habitual transmission methods that have been reported for decades.
Intravenous drug use has become a predominant factor in the integration and transmission dynamics of HCV. This is clearly mirrored by the variations of the genotypes among these at-risk populations. The most commonly isolated genotypes worldwide from people who inject drugs are genotypes 1a and 3a. HCV subtype 3a is endemic in Southeast Asia and is spreading among intravenous drug users in the United States and Europe. An increase in the prevalence of genotypes 1a and 3a has been observed in Germany, France, Italy and Portugal. Mixed infections have been identified in some European countries: Italy (1b/3a), Germany (2a/3b) and Sweden (1a/1b)[70]. Eastern Europe (Russia and Estonia) and Central Asia have the largest drug epidemics globally, where the rapidly expanding HCV epidemic is associated with the injection of heroin and new synthetic or homemade drugs[71]. The frequency of genotype 3a is also rising in Eastern and Central European countries, as reported in Romania, Bulgaria, Poland and Serbia and Montenegro. It has been reported that people who use drugs in England are more likely to have genotype 3a in comparison to other risk groups, in whom genotype 1a is the most prevalent[71]. Thus, people who use drugs are important contributors to the spread of infections to the general population and drug trafficking is a key vector in the integration and diversification of HCV. This factor is boosted by other risk factors, such poverty, incarceration and HIV co-infection.
Worldwide, HCV and HIV are among the leading causes of death from infectious diseases[71]. Rates of co-infection with HCV and HIV range from 1.2% to 98.5% and co-infections are endemic, particularly in Asia and Africa[72]. In Europe and the United States, about one-fourth of HIV-infected individuals are co-infected with HCV. HCV infection outbreaks have been reported in HIV-positive men who have sex with men in North America, Europe and Asia. HCV co-infection reached up to 26.9% among heterosexuals compared with the HCV infection rate alone, which is reported to be 2.5%[1]. A cross-sectional study conducted between 2008 and 2010 in the Mazandaran province of Iran demonstrated that 33.8% of HIV-positive patients were co-infected with HCV and 25% were co-infected with both HBV and HCV[72]. The co-infection rates among intravenous drug users ranged between 58.2% and 91.6% and among commercial blood donors between 15.8% and 71.6%. These rates are higher than among those who become co-infected via sex (5.3%-20.0%). In Vietnam, between 89.8% and 98.5% of HIV-positive intravenous drug users are infected with HCV[73]. In China, 62.4% of HIV-infected individuals are seropositive for HCV. The co-infection rate is much lower in India (8.3%) and central, southeast and west African regions (4.9% to 8.5%) and much lower in North Africa (1.3%)[74].
Although HCV integration is clearly driven by migration, drug trafficking and HIV infection, there are many other additional factors: Health practices, hemodialysis, poverty, imprisonment and HBV/tuberculosis co-infections. A deeper understanding of HCV epidemiology should take into consideration all these factors. In developing countries, the routes of transmission of HCV are also found within medical care, such as the use of unsafe injections or improperly sterilized medical equipment, which are responsible for 40% of worldwide HCV infections, as well as the use of blood and blood products that have not been screened properly[1]. The prevalence of HCV infection in patients on maintenance hemodialysis reached 63% in the Arabian Peninsula (Kuwait, Saudi Arabia, Qatar and Yemen) and in China it was 41%. Moreover, hemodialysis patients who had blood transfusions were 5.65 times more likely to be infected with HCV than their counterparts who had no transfusions[1]. HCV co-infection among HBV-infected individuals ranged from 3% in Thailand to 22% in Japan, 23% in the United Kingdom and 30% in Spain[75]. HBV/HCV co-infection is more prevalent among the homeless, sexual assault victims and victims of intimate partner violence. Tuberculosis infection is also a major public health issue associated with HCV; it is responsible for more than a third of the opportunistic infections among drug users who are co-infected with HCV and HIV[75].
HCV infection can be acquired during travel for tourism or for medical treatment, particularly if the distant area is to the Indian subcontinent or Africa. Patients traveling abroad in general, and particularly if they are planning to have hemodialysis abroad, should be made aware of the risks and possibility of bringing new HCV genotypes to their homeland. Homosexuality is another prevailing factor for HCV transmission and outbreaks have been increasingly reported among men who have sex with men in Europe, Australia, Asia and the United States[76]. This is also emerging in developing countries, such as in the Arabian Peninsula, where such behavior is stigmatized. The spread of HCV may be influenced by habitual and social vulnerabilities between and within countries, urban and rural settings, and according to the burden of risk groups and economic status. This is clearly seen along the river Nile of Egypt and in China and Southeast Asian islands. Economic crises can influence HCV seroprevalence and the distribution of circulating genotypes. This was shown by outbreaks of HCV/HIV co-infections among intravenous drug users in Athens and Bucharest between 2011 and 2013, as both Greece and Bucharest have the highest unemployment rates among young people.
HCV is widely integrated, resulting in great heterogeneity both in the prevalence of infection and in the distribution of viral genotypes. This imposes a tremendous health burden globally in terms of morbidity and mortality. Hence, effective intervention requires a clear understanding of the dynamics of viral epidemics. Despite all scientific advances and the mounting knowledge of HCV, it remains “a hidden pandemic”[77]. HCV is considered to be endemic in developing countries, which have inadequate genotype data, and the largest populations of HCV-infected individuals are in Asia (accounting for 3.6% of the global population), followed by Africa (3.2% of the global population) and Latin America (1.4% of the global population)[15]. Most of these regions face many structural, cultural, societal and political obstacles in responding to this epidemic.
While preventive screening programs should be mandatory, 99 countries do not perform routine screening of blood donors for infectious agents that can be transmitted by transfusion. In other countries, the testing of blood donors for HIV, HBV and HCV is not consistent and attention is given mostly to HIV. Even where testing is done, there is preference for rapid assays with poor quality control and the result is lack of sensitivity. Testing based on nucleic acid is rare in countries with low or middle income due to lack of financial resources and skills. Even in Egypt, only 20% of the blood supply is tested by nucleic acid methods. Health authorities worldwide should give priority to this issue because any efforts to prevent or limit HCV transmission will not be effective unless the safety of blood and blood products is guaranteed. Moreover, most HCV infections are asymptomatic and in the absence of comprehensive, coordinated surveillance systems, the result is fragmented reporting and underestimation of the disease burden[1]. Improved surveillance is important not only for gaining a better understanding of the epidemiology of HCV but is also needed to identify population groups that should be targeted in prevention, testing and treatment programs.
Public healthcare systems, particularly in developing countries, should simplify service delivery to HCV-infected patients and specifically track progress to guarantee a high quality of services for both prevention and treatment[78]. People who inject drugs should have priority because prevalence estimates of HCV among this group is lacking in most countries. This is especially the case in countries with low or middle income, where data are scanty even for the general population[79]. Effective data collection and accurate reporting at the national level is the key in any program targeting HCV because it enables healthcare providers to implement policies targeting the populations that require the greatest attention.
Social and educational programs have to be promoted, particularly in countries that still ignore and stigmatize certain behaviors leading to HCV infection. All groups prone to HCV and associated co-infections should be included, whether they involve intravenous drug use, heroin sniffing or sexual promiscuity. Disseminating awareness of HCV infection in the general population can be beneficial in two ways. First, people who become aware exert pressure for provision of treatment. Second, promoting appropriate behavior helps to limit the risk of disease progression. Peer support has been proposed as one way to overcome these barriers. This is usually accompanied by screening as recommended by key United Nations agencies, such as the World Health Organization, United Nations Office on Drugs and Crime and Joint United Nations Program on HIV/AIDS. The high rates of HIV/HCV/TB co-infection in some settings indicate the need for another approach to increase access to HCV care, namely, to move towards an integrated policy resembling those used for HIV and tuberculosis[1,79].
A national action plan accompanied with national guidelines for the treatment of HCV infection should be advocated. Targeted populations should be provided with equal access to medical care, reliable supplies of medications and medical follow-up. Nowadays, it is possible to treat almost every person with HCV regardless of liver disease stage, viral genotype, past therapies and comorbidities. However, this approach imposes a heavy burden on the health system, particularly in developing countries where it is most needed. Research and operational projects supported by international funds should be established particularly in low-income countries[80].
Sequential omission was performed for sensitivity analysis and we consider our data reliable. We observed no publication bias. The main limitation we noticed is the lack of specific data on certain aspects contributing to HCV integration, such as spatial and social factors associated with the global spread of HCV. Hence, further studies are needed to overcome such limitations.
From all that is described and discussed above, we conclude the following: HCV is an integrating dynamic threat and no country can be considered safe enough. Despite awareness of the spread of HCV infections, epidemiological data remain scarce. This study highlights the need for integrated cooperative actions at the local, regional and global levels if the spread and burden of hepatitis C virus are to be contained. Governments, the scientific community, industry and non-governmental organizations should develop a cooperation framework for combating HCV infection. Priority should be directed to help low or middle income countries to gain access to effective screening and medical care for HCV and associated infectious diseases.
We are deeply grateful to the Libyan Study Group of Hepatitis and HIV and to the Department of Medical Microbiology and Immunology, Faculty of Medicine, Tripoli, Libya for their assistance and particularly those who helped to analyze and critically review the data published here. Special thanks go to Dr. Amin Bredan, http://www.theeditor.be, for his professional editing of the manuscript.
Hepatitis C virus (HCV) infection is a major public health threat and its geo-epidemiology varies widely worldwide and over time. HCV genotypes and the risk of infection can be easily transmitted to any country and cannot be restricted to certain regions. Therefore, new evaluations of the epidemiology of HCV should be considered.
Geographical integration is an epidemiological phenomenon that highlights the spread of HCV globally. Globalization, immigration and drug trafficking are important driving forces in this integration.
Worldwide HCV integration is a newly described phenomenon that can be either intra-continental or trans-continental.
Geographical integration should be taken into consideration in both prevention and treatment policies for HCV. National and international strategies should be designed on the basis of accurate analysis of HCV epidemiology.
Intra-continental (regional) integration: The dynamic spread and dissemination of HCV genotypes within a certain region or continent is clearly evident in Africa and Asia; Trans-continental integration: The dynamic spread of HCV from one continent to another. This is clearly evident in the Mediterranean basin (Africa-Europe), Africa-America.
This is a very well-conceived, lucid, informative mini review that should be shared with the scientific community. The readers will gain valuable insight into the evolution of HCV at the global level.
| 1. | Daw MA, Dau AA. Hepatitis C virus in Arab world: a state of concern. ScientificWorldJournal. 2012;2012:719494. [PubMed] |
| 2. | Tanaka K, Hirohata T, Koga S, Sugimachi K, Kanematsu T, Ohryohji F, Nawata H, Ishibashi H, Maeda Y, Kiyokawa H. Hepatitis C and hepatitis B in the etiology of hepatocellular carcinoma in the Japanese population. Cancer Res. 1991;51:2842-2847. [PubMed] |
| 3. | Deuffic-Burban S, Mohamed MK, Larouze B, Carrat F, Valleron AJ. Expected increase in hepatitis C-related mortality in Egypt due to pre-2000 infections. J Hepatol. 2006;44:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Daw MA, Dau AA, Agnan MM. Influence of healthcare-associated factors on the efficacy of hepatitis C therapy. ScientificWorldJournal. 2012;2012:580216. [PubMed] |
| 5. | Daw MA, El-Bouzedi A. Prevalence of hepatitis B and hepatitis C infection in Libya: results from a national population based survey. BMC Infect Dis. 2014;14:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Kauhl B, Heil J, Hoebe CJ, Schweikart J, Krafft T, Dukers-Muijrers NH. The Spatial Distribution of Hepatitis C Virus Infections and Associated Determinants--An Application of a Geographically Weighted Poisson Regression for Evidence-Based Screening Interventions in Hotspots. PLoS One. 2015;10:e0135656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Wedemeyer H, Duberg AS, Buti M, Rosenberg WM, Frankova S, Esmat G, Örmeci N, Van Vlierberghe H, Gschwantler M, Akarca U. Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepat. 2014;21 Suppl 1:60-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9247] [Cited by in RCA: 9006] [Article Influence: 529.8] [Reference Citation Analysis (0)] |
| 9. | Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4996] [Cited by in RCA: 4669] [Article Influence: 126.2] [Reference Citation Analysis (1)] |
| 10. | Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 753] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 11. | An Y, Wu T, Wang M, Lu L, Li C, Zhou Y, Fu Y, Chen G. Conservation in China of a novel group of HCV variants dating to six centuries ago. Virology. 2014;464-465:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Volz EM, Koelle K, Bedford T. Viral phylodynamics. PLoS Comput Biol. 2013;9:e1002947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 13. | Njouom R, Nerrienet E, Dubois M, Lachenal G, Rousset D, Vessière A, Ayouba A, Pasquier C, Pouillot R. The hepatitis C virus epidemic in Cameroon: genetic evidence for rapid transmission between 1920 and 1960. Infect Genet Evol. 2007;7:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Markov PV, van de Laar TJ, Thomas XV, Aronson SJ, Weegink CJ, van den Berk GE, Prins M, Pybus OG, Schinkel J. Colonial history and contemporary transmission shape the genetic diversity of hepatitis C virus genotype 2 in Amsterdam. J Virol. 2012;86:7677-7687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Transmission of hepatitis C virus. Ann Intern Med. 1990;113:411-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Xia GL, Liu CB, Cao HL, Bi SL, Zhan MY, Su CA, Nan JH, Qi XQ. Prevalence of hepatitis B and C virus infections in the general Chinese population. Results from a nationwide cross-sectional seroepidemiologic study of hepatitis A, B, C, D, and E virus infections in China, 1992. International Hepatology Communications. 1996;5:62-73. [RCA] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 187] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Sun CA, Chen HC, Lu CF, You SL, Mau YC, Ho MS, Lin SH, Chen CJ. Transmission of hepatitis C virus in Taiwan: prevalence and risk factors based on a nationwide survey. J Med Virol. 1999;59:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Kim YS, Pai CH, Chi HS, Kim DW, Min YI, Ahn YO. Prevalence of hepatitis C virus antibody among Korean adults. J Korean Med Sci. 1992;7:333-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Leung N, Chu C, Tam JS. Viral hepatitis C in Hong Kong. Intervirology. 2006;49:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Nishioka K, Watanabe J, Furuta S, Tanaka E, Iino S, Suzuki H, Tsuji T, Yano M, Kuo G, Choo QL. A high prevalence of antibody to the hepatitis C virus in patients with hepatocellular carcinoma in Japan. Cancer. 1991;67:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Nguyen VT, McLaws ML, Dore GJ. Prevalence and risk factors for hepatitis C infection in rural north Vietnam. Hepatol Int. 2007;1:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Chowdhury A, Santra A, Chaudhuri S, Dhali GK, Chaudhuri S, Maity SG, Naik TN, Bhattacharya SK, Mazumder DN. Hepatitis C virus infection in the general population: a community-based study in West Bengal, India. Hepatology. 2003;37:802-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 126] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Raja NS, Janjua KA. Epidemiology of hepatitis C virus infection in Pakistan. J Microbiol Immunol Infect. 2008;41:4-8. [PubMed] |
| 24. | Rajabali A, Moin O, Ansari AS, Khanani MR, Ali SH. Communicable disease among displaced Afghans: refuge without shelter. Nat Rev Microbiol. 2009;7:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Alavian SM, Adibi P, Zali MR. Hepatitis C virus in Iran: Epidemiology of an emerging infection. Arch Iranian Med. 2005;8:84-90. |
| 26. | Merat S, Rezvan H, Nouraie M, Jafari E, Abolghasemi H, Radmard AR, Zaer-rezaii H, Amini-Kafiabad S, Maghsudlu M, Pourshams A. Seroprevalence of hepatitis C virus: the first population-based study from Iran. Int J Infect Dis. 2010;14 Suppl 3:e113-e116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Riou J, Aït Ahmed M, Blake A, Vozlinsky S, Brichler S, Eholié S, Boëlle PY, Fontanet A. Hepatitis C virus seroprevalence in adults in Africa: a systematic review and meta-analysis. J Viral Hepat. 2016;23:244-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Daw MA, Elkaber MA, Drah AM, Werfalli MM, Mihat AA, Siala IM. Prevalence of hepatitis C virus antibodies among different populations of relative and attributable risk. Saudi Med J. 2002;23:1356-1360. [PubMed] |
| 29. | Kershenobich D, Razavi HA, Sánchez-Avila JF, Bessone F, Coelho HS, Dagher L, Gonçales FL, Quiroz JF, Rodriguez-Perez F, Rosado B. Trends and projections of hepatitis C virus epidemiology in Latin America. Liver Int. 2011;31 Suppl 2:18-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Fitzsimons D, François G, Alpers K, Radun D, Hallauer J, Jilg W, Gerlich W, Rombo L, Blystad H, Nøkleby H. Prevention of viral hepatitis in the Nordic countries and Germany. Scand J Infect Dis. 2005;37:549-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Bosetti C, Levi F, Boffetta P, Lucchini F, Negri E, La Vecchia C. Trends in mortality from hepatocellular carcinoma in Europe, 1980-2004. Hepatology. 2008;48:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 32. | Guadagnino V, Stroffolini T, Rapicetta M, Costantino A, Kondili LA, Menniti-Ippolito F, Caroleo B, Costa C, Griffo G, Loiacono L. Prevalence, risk factors, and genotype distribution of hepatitis C virus infection in the general population: a community-based survey in southern Italy. Hepatology. 1997;26:1006-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 265] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 33. | Sánchez-Quijano A, Abad MA, Torronteras R, Rey C, Pineda JA, Leal M, Macias J, Lissen E. Unexpected high prevalence of hepatitis C virus genotype 4 in Southern Spain. J Hepatol. 1997;27:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Dursun M, Ozekinci T, Ertem M, Saka G, Yilmaz S, Canoruc F, Celenk S, Celik M, Paşa S, Aydin K. Prevalence of Hepatitis C in adults in the south-eastern region of Anatolia: a community-based study. Hepatol Res. 2004;29:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Naoumov NV. Hepatitis C virus infection in Eastern Europe. J Hepatol. 1999;31 Suppl 1:84-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Maksyutov RA, Gavrilova EV, Maksyutov AZ, Kanev AN. Genotyping of hepatitis B and C virus Russian isolates for reference serum panel construction. J Med Virol. 2015;87:1192-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62:1353-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 339] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 38. | Zou S, Tepper M, Giulivi A. Current status of hepatitis C in Canada. Can J Public Health. 2000;91 Suppl 1:S10-S15, S10-S15. [PubMed] |
| 39. | Cui Y, Jia J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol. 2013;28 Suppl 1:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 40. | Murphy DG, Sablon E, Chamberland J, Fournier E, Dandavino R, Tremblay CL. Hepatitis C virus genotype 7, a new genotype originating from central Africa. J Clin Microbiol. 2015;53:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 41. | Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, Simmonds P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 965] [Cited by in RCA: 991] [Article Influence: 82.6] [Reference Citation Analysis (2)] |
| 42. | Dusheiko G, Schmilovitz-Weiss H, Brown D, McOmish F, Yap PL, Sherlock S, McIntyre N, Simmonds P. Hepatitis C virus genotypes: an investigation of type-specific differences in geographic origin and disease. Hepatology. 1994;19:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 274] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 43. | Pybus OG, Barnes E, Taggart R, Lemey P, Markov PV, Rasachak B, Syhavong B, Phetsouvanah R, Sheridan I, Humphreys IS. Genetic history of hepatitis C virus in East Asia. J Virol. 2009;83:1071-1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 44. | Jeannel D, Fretz C, Traore Y, Kohdjo N, Bigot A, Pê Gamy E, Jourdan G, Kourouma K, Maertens G, Fumoux F. Evidence for high genetic diversity and long-term endemicity of hepatitis C virus genotypes 1 and 2 in West Africa. J Med Virol. 1998;55:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Zhou Y, Wang X, Mao Q, Fan Y, Zhu Y, Zhang X, Lan L, Jiang L, Tan W. Changes in modes of hepatitis C infection acquisition and genotypes in southwest China. J Clin Virol. 2009;46:230-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Narahari S, Juwle A, Basak S, Saranath D. Prevalence and geographic distribution of Hepatitis C Virus genotypes in Indian patient cohort. Infect Genet Evol. 2009;9:643-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Bennett H, Waser N, Johnston K, Kao JH, Lim YS, Duan ZP, Lee YJ, Wei L, Chen CJ, Sievert W. A review of the burden of hepatitis C virus infection in China, Japan, South Korea and Taiwan. Hepatol Int. 2015;9:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 48. | Olinger CM, Lazouskaya NV, Eremin VF, Muller CP. Multiple genotypes and subtypes of hepatitis B and C viruses in Belarus: similarities with Russia and western European influences. Clin Microbiol Infect. 2008;14:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Karchava M, Waldenström J, Parker M, Hallack R, Sharvadze L, Gatserelia L, Chkhartishvili N, Dvali N, Dzigua L, Dolmazashvili E. High incidence of the hepatitis C virus recombinant 2k/1b in Georgia: Recommendations for testing and treatment. Hepatol Res. 2015;45:1292-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Iles JC, Raghwani J, Harrison GL, Pepin J, Djoko CF, Tamoufe U, LeBreton M, Schneider BS, Fair JN, Tshala FM. Phylogeography and epidemic history of hepatitis C virus genotype 4 in Africa. Virology. 2014;464-465:233-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 51. | Medhat A, Shehata M, Magder LS, Mikhail N, Abdel-Baki L, Nafeh M, Abdel-Hamid M, Strickland GT, Fix AD. Hepatitis c in a community in Upper Egypt: risk factors for infection. Am J Trop Med Hyg. 2002;66:633-638. [PubMed] |
| 52. | Ciccozzi M, Lo Presti A, Ciccaglione AR, Zehender G, Ciotti M. Phylogeny and phylodinamic of Hepatitis C in Italy. BMC Infect Dis. 2012;12 Suppl 2:S5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Daw MA, El-Bouzedi A, Dau AA. Geographic distribution of HCV genotypes in Libya and analysis of risk factors involved in their transmission. BMC Res Notes. 2015;8:367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Njouom R, Caron M, Besson G, Ndong-Atome GR, Makuwa M, Pouillot R, Nkoghé D, Leroy E, Kazanji M. Phylogeography, risk factors and genetic history of hepatitis C virus in Gabon, central Africa. PLoS One. 2012;7:e42002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Chao DT, Abe K, Nguyen MH. Systematic review: epidemiology of hepatitis C genotype 6 and its management. Aliment Pharmacol Ther. 2011;34:286-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 56. | Chiaramonte M, Pupo A, Menegon T, Baldo V, Malatesta R, Trivello R. HBV and HCV infection among non-European Union immigrants in North-East Italy. Epidemiol Infect. 1998;121:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Peters L, Klein MB. Epidemiology of hepatitis C virus in HIV-infected patients. Curr Opin HIV AIDS. 2015;10:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Roman F, Hawotte K, Struck D, Ternes AM, Servais JY, Arendt V, Hoffman P, Hemmer R, Staub T, Seguin-Devaux C. Hepatitis C virus genotypes distribution and transmission risk factors in Luxembourg from 1991 to 2006. World J Gastroenterol. 2008;14:1237-1243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Tokita H, Okamoto H, Iizuka H, Kishimoto J, Tsuda F, Lesmana LA, Miyakawa Y, Mayumi M. Hepatitis C virus variants from Jakarta, Indonesia classifiable into novel genotypes in the second (2e and 2f), tenth (10a) and eleventh (11a) genetic groups. J Gen Virol. 1996;77:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 99] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Greenaway C, Thu Ma A, Kloda LA, Klein M, Cnossen S, Schwarzer G, Shrier I. The Seroprevalence of Hepatitis C Antibodies in Immigrants and Refugees from Intermediate and High Endemic Countries: A Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0141715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 61. | Manzardo C, Treviño B, Gómez i Prat J, Cabezos J, Monguí E, Clavería I, Luis Del Val J, Zabaleta E, Zarzuela F, Navarro R. Communicable diseases in the immigrant population attended to in a tropical medicine unit: epidemiological aspects and public health issues. Travel Med Infect Dis. 2008;6:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 62. | Gushulak BD, MacPherson DW. Globalization of infectious diseases: the impact of migration. Clin Infect Dis. 2004;38:1742-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 63. | Redditt VJ, Janakiram P, Graziano D, Rashid M. Health status of newly arrived refugees in Toronto, Ont: Part 1: infectious diseases. Can Fam Physician. 2015;61:e303-e309. [PubMed] |
| 64. | Owiti JA, Greenhalgh T, Sweeney L, Foster GR, Bhui KS. Illness perceptions and explanatory models of viral hepatitis B & amp; C among immigrants and refugees: a narrative systematic review. BMC Public Health. 2015;15:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 65. | García Comas L, Ordobás Gavín M, Sanz Moreno JC, Ramos Blázquez B, Gutiérrez Rodríguez A, Astray Mochales J, Moreno Guillén S. Prevalence of hepatitis C antibodies in the population aged 16-80 years in the Community of Madrid 2008-2009. J Med Virol. 2015;87:1697-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Hirzel C, Wandeler G, Owczarek M, Gorgievski-Hrisoho M, Dufour JF, Semmo N, Zürcher S. Molecular epidemiology of hepatitis B virus infection in Switzerland: a retrospective cohort study. BMC Infect Dis. 2015;15:483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Wandeler G, Dufour JF, Bruggmann P, Rauch A. Hepatitis C: a changing epidemic. Swiss Med Wkly. 2015;145:w14093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Matheï C, Buntinx F, van Damme P. Seroprevalence of hepatitis C markers among intravenous drug users in western European countries: a systematic review. J Viral Hepat. 2002;9:157-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Pierce RD, Hegle J, Sabin K, Agustian E, Johnston LG, Mills S, Todd CS. Strategic information is everyone’s business: perspectives from an international stakeholder meeting to enhance strategic information data along the HIV Cascade for people who inject drugs. Harm Reduct J. 2015;12:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 70. | Ruta S, Cernescu C. Injecting drug use: A vector for the introduction of new hepatitis C virus genotypes. World J Gastroenterol. 2015;21:10811-10823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 71. | Platt L, Bobrova N, Rhodes T, Uusküla A, Parry JV, Rüütel K, Talu A, Abel K, Rajaleid K, Judd A. High HIV prevalence among injecting drug users in Estonia: implications for understanding the risk environment. AIDS. 2006;20:2120-2123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 72. | Babamahmoodi F, Heidari Gorji MA, Mahdi Nasehi M, Delavarian L. The prevalence rate of hepatitis B and hepatitis C co-infection in HIV positive patients in Mazandaran province, Iran. Med Glas (Zenica). 2012;9:299-303. [PubMed] |
| 73. | Zhang L, Celentano DD, Le Minh N, Latkin CA, Mehta SH, Frangakis C, Ha TV, Mo TT, Sripaipan T, Davis WW. Prevalence and correlates of HCV monoinfection and HIV and HCV coinfection among persons who inject drugs in Vietnam. Eur J Gastroenterol Hepatol. 2015;27:550-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 74. | Solomon SS, Srikrishnan AK, Mehta SH, Vasudevan CK, Murugavel KG, Thamburaj E, Anand S, Kumar MS, Latkin C, Solomon S. High prevalence of HIV, HIV/hepatitis C virus coinfection, and risk behaviors among injection drug users in Chennai, India: a cause for concern. J Acquir Immune Defic Syndr. 2008;49:327-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 75. | Gao X, Cui Q, Shi X, Su J, Peng Z, Chen X, Lei N, Ding K, Wang L, Yu R. Prevalence and trend of hepatitis C virus infection among blood donors in Chinese mainland: a systematic review and meta-analysis. BMC Infect Dis. 2011;11:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 76. | Götz HM, van Doornum G, Niesters HG, den Hollander JG, Thio HB, de Zwart O. A cluster of acute hepatitis C virus infection among men who have sex with men--results from contact tracing and public health implications. AIDS. 2005;19:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 77. | Dalgard O, Mauss S. No strategy to meet the HCV epidemic. BMC Infect Dis. 2014;14 Suppl 6:S2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 78. | Daw MA, Shabash A, El-Bouzedi A, Dau AA, Habas M. Modelling the prevalence of hepatitis C virus amongst blood donors in Libya: An investigation of providing a preventive strategy. World J Virol. 2016;5:14-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 79. | Harris M, Albers E, Swan T. The promise of treatment as prevention for hepatitis C: Meeting the needs of people who inject drugs? Int J Drug Policy. 2015;26:963-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 80. | Daw MA, El-Bouzedi A, Ahmed MO, Dau AA, Agnan MM. Epidemiology of Hepatitis C Virus and Genotype Distribution in Immigrants Crossing to Europe from North and Sub-Saharan Africa. Travel Med Infect Dis. 2016;14:517-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (2)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Virology
Country of origin: Libya
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Ghiringhelli PD, Krishnan T, Pandey KK, Rezaee-Zavareh MS S- Editor: Ji FF L- Editor: Roemmele A E- Editor: Wu HL