Published online Jun 25, 2025. doi: 10.5501/wjv.v14.i2.99663
Revised: November 7, 2024
Accepted: November 26, 2024
Published online: June 25, 2025
Processing time: 331 Days and 1.4 Hours
In the initial stages of the coronavirus disease 2019 (COVID-19) pandemic, healthcare workers (HCWs) who were immunologically naive to COVID-19, were exposed to a highly transmissible virus.
To compare infection risk among HCWs in high-risk (HR) and low-risk (LR) areas.
Data on reverse transcriptase-polymerase chain reaction confirmed clinical infection and samples for nucleocapsid, and spike protein antibodies were collected at five time-points (T1 to T5) from HCWs in the emergency department and intensive care unit (HR group) and pre-clinical and para-clinical areas (LR). For the sero-study, only participants who provided at least one baseline sample and one during the second wave (T4 or T5) were analysed. Since CovishieldTM elicits only spike protein antibodies, subclinical infection was diagnosed if asymptomatic unvaccinated and CovishieldTM vaccinated individuals tested positive for nucleocapsid antibody.
Overall, by T5, clinical infection rate was similar in the HR (120/366, 32.8%) and LR (22/82, 26.8%) groups (P = 0.17). However, before vaccination (T3), more HCWs in the HR group developed COVID-19 infection (21.9% vs 8.8%, P = 0.046). In the sero-study group, clinical infection occurred in 31.5% (45/143) and 23.7% (14/59) in the HR and LR groups respectively (P = 0.23). Spike antibody was detected in 140/143 (97.9%) and 56/59 (94.9%) and nucleocapsid antibody was positive in 95/143 (66.4%) and 35/59 (59.3%) in the HR and LR groups respectively (P = 0.34). Subclinical infection rate (HR 34.9%, LR 35.6%, P = 0.37) and hospitalization rate were similar. There was no mortality.
Before vaccination, HCWs in HR areas had a higher risk of infection. Seroprevalence studies suggest that sub-clinical infection was not uncommon.
Core Tip: This study tracked clinical infection and seroprevalence to severe acute respiratory syndrome-coronavirus 2 virus during the two waves of the pandemic in India and compared infection risk between health care workers (HCWs) in high-risk (HR) areas (emergency department, critical care) and low-risk (LR) non-clinical areas. The seroprevalence rate of 1.1% at the start of the pandemic increased to 34.1% and 60.1% during the first and second waves respectively. Prior to vaccination, more HCWs in HR areas developed clinical infection. Following vaccination, clinical infection rates and seropositivity were similar in HR and LR groups. About 1/3rd had evidence of subclinical infection.
- Citation: Abhilash KPP, Nellimootil MV, Chacko B, Hazra D, Coelho V, Jesudasan JE, Gunasekaran K, Thomas L, Ramchandra MA, Melchizedek J, Gunaraj HM, Moorthy M, Peter JV. Risk of COVID-19 infection among frontline healthcare workers during the COVID-19 pandemic. World J Virol 2025; 14(2): 99663
- URL: https://www.wjgnet.com/2220-3249/full/v14/i2/99663.htm
- DOI: https://dx.doi.org/10.5501/wjv.v14.i2.99663
The rapid spread of the coronavirus disease 2019 (COVID-19) pandemic constrained medical services worldwide. As of May 2023, there were over 700 million confirmed cases of COVID-19 and 6.5 million deaths[1]. The pandemic raised concerns regarding the safety of healthcare workers (HCWs) who were in close contact with infected patients[2-4]. This led to anxiety, mental stress, and decreased morale that resulted in absenteeism[5,6]. Governments resorted to nationwide lockdowns and infection control measures were strictly enforced.
Infection control measures were put in place during the pandemic which included surveillance, personal protection equipment (PPE), and environmental measures[7]. Vaccination against COVID-19 was considered to be the panacea and was rapidly developed[8-10]. Frontline workers such as HCWs were preferentially vaccinated in order to protect them against serious illness including intensive care unit (ICU) admission and death[11,12].
Studies during the H1N1 pandemic showed that HCWs were at a higher risk of contracting influenza when compared to the general population[13]. Seroprevalence studies showed that sub-clinical infection was common[14]. However, positivity rates were similar among HCWs working in high-risk (HR) and low-risk (LR) areas[15].
In the context of the COVID-19 pandemic, it was important to evaluate the risk of COVID-19 infection among HCWs, who were immunologically naive to COVID-19 and exposed to a highly transmissible virus at the workplace. It was also essential to track infection and seroconversion rates as the pandemic evolved and vaccination became available. This study was thus undertaken to evaluate clinical and sub-clinical infection among HCWs during different time-points in the pandemic and to assess if the risk of COVID-19 infection was higher among HCWs working in HR areas such as the emergency department (ED) and the ICU where they are in direct contact with COVID-19-infected patients when compared with LR areas such as pre-clinical and para-clinical departments of a medical institution, where HCWs do not come in direct contact with patients.
This prospective single-center observational study was conducted in a large tertiary care referral hospital in India between June 1, 2020, to August 31, 2021 (15 months), and included HCWs from various departments. HCWs working in HR areas included those working in the ED, ICU, and general medical wards, and HCWs working in LR areas included the departments of anatomy, physiology, biochemistry, and community medicine. HCWs were excluded if they did not consent to participate in the study.
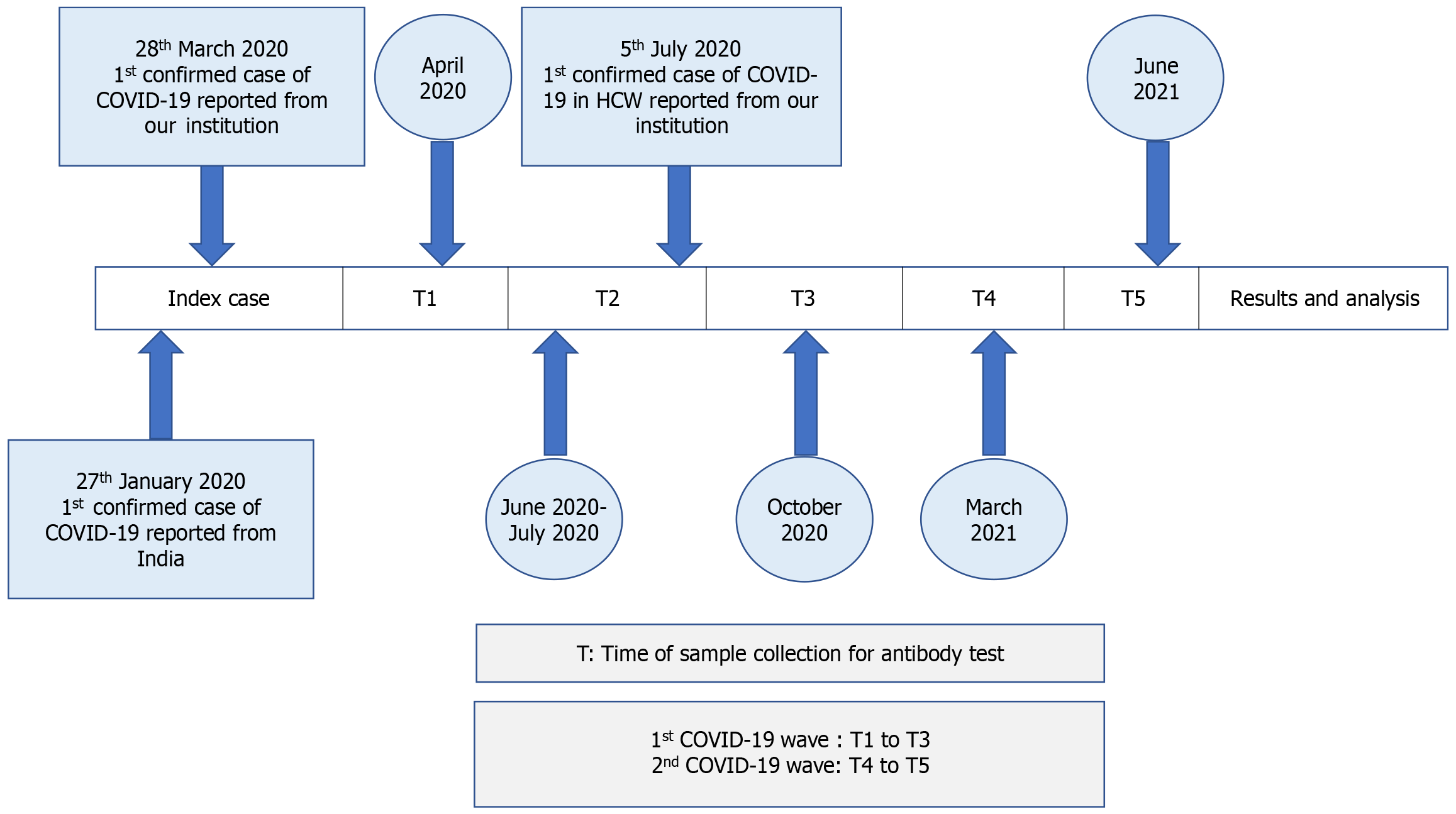
Demographic data including age, gender, and co-morbidities were recorded. Serum samples were collected at five time-points for the seroprevalence component of the study (Figure 1). The first wave of the COVID-19 pandemic in India was witnessed between June 2020 and September 2020 while the second wave started in March 2021 and started waning by June 2021. The first sample was taken in April 2020 (T1), after the lockdown was initiated and after the first COVID-19 positive patient was diagnosed in the institution on 28th March 2020. The second sample was collected in June 2020-July 2020 (T2) as the cases started increasing during the first wave of the pandemic. The first health care worker (HCW) in our institution was tested positive for COVID-19 on 5th July 2020. The third sample was collected in October 2020 (T3) after the first wave of the pandemic waned and before vaccination became available in the country. The fourth sample was collected in March 2021 (T4) about 2-months after vaccination was rolled out in the country and as the country witnessed the start of the second wave of the pandemic, while the final sample was collected in June 2021 (T5) as the second wave of the pandemic waned and when two doses of vaccination among HCWs was completed in a large proportion of HCWs. For the seroprevalence component of the study, only those who had given at least one sample in the initial period of the pandemic (T1 to T3) and at least one sample during the second wave of the pandemic (T4 or T5) were included.
At each time point, when samples were collected for the seroprevalence part of the study, participants were asked if they had COVID-19 infection in the preceding period. COVID-19 infection was diagnosed when severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) RNA was detected in respiratory samples of symptomatic individuals using reverse transcriptase-polymerase chain reaction assays (Altona RealStar® SARS-CoV-2) and/or Cartridge Based Nucleic Acid Amplification Test (CBNAAT) assay (Xpert® Xpress SARS-CoV-2). Vaccination status and the details of vaccination were recorded. In India, the Oxford-Astra Zeneca’s chimpanzee adenovirus vectored ChadOx1 vaccine, CovishieldTM, and the Bharat biotech’s whole virion inactivated BBV152, Covaxin® were available[16]. Since CovishieldTM was available much earlier than Covaxin®, more HCWs received CovishieldTM; Sputnik V (also known as Gam-COVID-Vac), an adenovirus viral vector vaccine, was available at a much later stage and only one HCW included in the study received it.
The duration of work in COVID-19 areas was documented for all the HCWs. Since the healthcare system was overwhelmed during the second wave of the pandemic, some of the HCWs working in LR areas were posted for a limited time to work in COVID-19 areas. Data on number of HCWs who developed COVID-19 infection, need for home isolation or hospitalization, requirement of oxygen therapy, ventilation and hospital outcome were recorded.
The primary outcome of the study was to determine if HCWs working in HR areas were at a higher risk of developing COVID-19 infection when compared with HCWs working in LR areas at different time-points of the pandemic. The study also looked at seroconversion of HCWs to COVID-19 at five time points to evaluate the proportion of HCWs who had subclinical infection. Since CovishieldTM elicits only spike protein antibodies, subclinical infection was diagnosed if asymptomatic HCWs who were either unvaccinated or were vaccinated with CovishieldTM tested positive for nucleocapsid antibody. Covaxin® elicits both spike protein and nucleocapsid antibodies. This would have made it difficult to differentiate antibody response to clinical or subclinical infection vs vaccination. Hence Covaxin®-vaccinated individuals were excluded for the determination of subclinical infection among HCWs. For the same reason, seroprevalence data among HCWs during the 5 time periods was also ascertained based on positivity to the nucleocapsid protein.
Blood samples were drawn for in vitro qualitative detection of antibodies against SARS-CoV-2 virus; both nucleocapsid (N) and spike (S) protein antibodies were estimated using Elecsys® Anti-SARS-CoV-2, Roche diagnostics. This employs the chemiluminescent immunosorbent assay method which is more sensitive and specific than other available serological methods[17]. The antibody levels were considered positive if either the N or S antibody levels were above the cut-off index (COI) reference limits. Nucleocapsid antibody was considered positive if COI was ≥ 1.0 U/mL, while for the spike antibody, a COI of ≥ 0.8 U/mL was considered positive.
The proportion of influenza-like illness in high and LR HCWs was assumed to be 10% and 1% respectively, with an anticipated odds ratio of 1.72. The study was powered to 80% with a type 1 error of 0.05. The calculated sample size worked out to 150 with 75 in each arm.
Data was analyzed using the Statistical Package for the Social Sciences for Windows software released in 2015, version 23.0, Armonk, New York, United States. Mean and SD were used for continuous variables and percentages for categorical and nominal variables. Seropositivity and clinical outcomes were compared between the HR and LR using student’s t-test or Fisher’s exact test as appropriate. For all tests, a 2-sided P-value less than 0.05 was considered statistically significant.
The study was approved by the Institutional Review Board And Ethics Committee (No. 12751, dated 1 May, 2020).
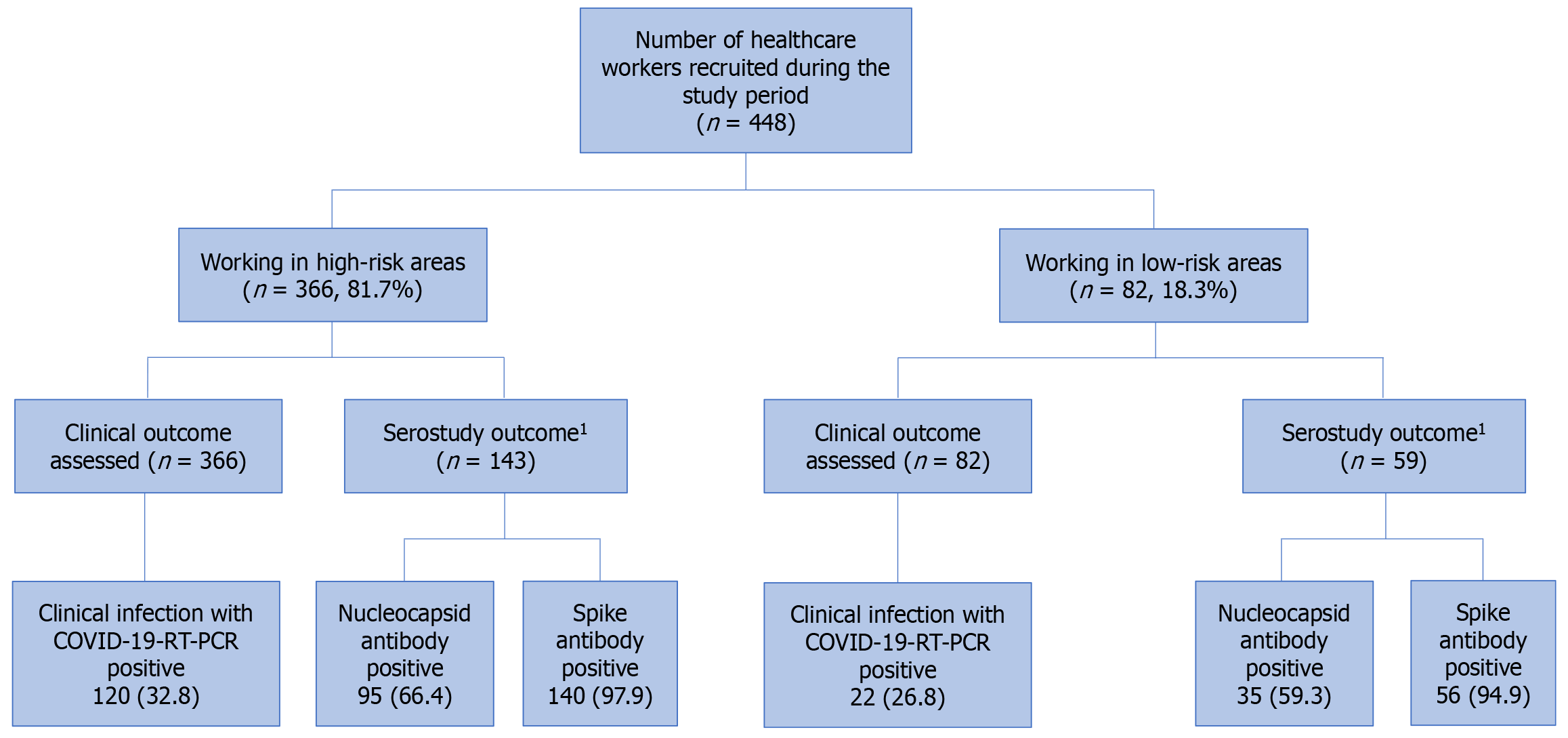
Between June 2020 and August 2021, 448 HCWs consented to participate. A majority (n = 366, 81.7%) worked in HR areas while the remaining worked in LR areas (n = 82, 18.3%). Clinical infection during the study period was assessed in all HCWs, while sero-study outcomes were available on 143 HCWs in the HR group and 59 HCWs in the LR group (Figure 2).
Demographic characteristics, duration of work in COVID areas and vaccination status are summarized in Table 1. The mean (SD) age was significantly lower in HCWs working in HR areas when compared with those working in LR areas [31.7 (8.3 years) years vs 39.9 (10.4 years) years, P < 0.001]. A higher proportion of males worked in HR areas (54.9% vs 39.1%, P = 0.01). The frequency of co-morbidities was low (13.4%) and similar in the two groups (P = 0.27). The duration of work in COVID-19 areas was significantly higher among HCWs working in HR areas when compared with LR areas [13 (4.6 months) months vs 1.8 (1.2 months) months, P < 0.001]. Four hundred and two HCWs (89.7%) had received at least one dose of COVID-19 vaccine by the end of the study period. A majority were vaccinated with CovishieldTM vaccine (83.7%) while a small proportion (5.8%) received Covaxin®; one HCW received Sputnik V.
| Characteristic | High-risk areas (n = 366) | Low-risk areas (n = 82) |
| Age, mean (SD) years | 31.7 (8.3) | 39.9 (10.4) |
| Gender, male sex | 201 (54.9) | 32 (39.1) |
| Comorbidities | ||
| Presence of at least one co-morbid illness | 49 (13.4) | 49 (13.4) |
| Diabetes mellitus | 17 (4.6) | 8 (9.7) |
| Hypertension | 14 (3.8) | 10 (12.2) |
| Obstructive airway disease | 31 (8.5) | 9 (10.9) |
| Duration of work in COVID-19 areas, mean (SD) months1 | 13 (4.6) | 1.8 (1.2) |
| Vaccination details2 | ||
| Vaccinated against (COVID-19)2 | 325 (88.8) | 77 (93.9) |
| Received two doses | 288 (78.7) | 65 (79.3) |
| Received one dose | 37 (10.1) | 12 (14.6) |
| Unvaccinated | 41 (11.2) | 5 (6.1) |
| Type of vaccine | ||
| COVISHIELD™ | 306 (94.2) | 69 (84.4) |
| COVAXIN™ | 18 (5.5) | 8 (10.4) |
| Sputnik V | 1 (0.3) | 0 (0) |
Overall, 144 (32.1%) HCWs developed COVID-19 infection during the study period (Table 2). There was no difference between the HR and LR groups in terms of clinical infection (32.8% vs 26.8%, P = 0.17), hospital admission (44.2% vs 54.5%, P = 0.58), home isolation (55.8% vs 45.5%, P = 0.51), and length of hospitalization [7.4 (3.6) vs 6.5 (4), P = 0.22]. Only one HCW required oxygen therapy. There was no mortality.
| Parameter | COVID-19-positive in high-risk areas (n = 120) | COVID-19-positive in low-risk areas (n = 22) | P value |
| COVID-19 positivity rate | 120/366 (32.8) | 22/82 (26.8) | 0.17 |
| Treated as home isolation | 67 (55.8) | 10 (45.5) | 0.51 |
| Hospital admission | 53 (44.2) | 12 (54.5) | 0.58 |
| Admission duration, mean (SD), days | 7.4 (3.6) | 6.5 (4) | 0.22 |
| Oxygen requirement | 1 (0.01) | 0 | Not applicable |
| Ventilation (invasive or non-invasive) | 0 | 0 | - |
| Mortality | 0 | 0 | - |
Table 3 summarizes the cross-sectional data of clinical infection and seropositivity for the nucleocapsid and spike proteins at five time points (T1-T5). The seroprevalence rate among HCWs, based on the nucleocapsid antibodies, was 1.1% (T1), 17.4% (T2), 34.1% (T3), 44.9% (T4) and 60.1% (T5) at the different time points.
| Time point | Clinical infection | Nucleocapsid (N) antibody | Spike (S) antibody | |||||||||
| Overall | HR | LR | P value | Overall | HR | LR | P value | Overall | HR | LR | P value | |
| Baseline | ||||||||||||
| T1 (n = 283) [HR | 0/283 (0) | 0/204 (0) | 0/79 (0) | - | 3/283 (1.1) | 3/204 (1.5) | 0/79 (0) | - | 0/283 (0) | 0/204 (0) | 0/79 (0) | - |
| First wave | ||||||||||||
| T2 (n = 168) [HR | 5/168 (3.0) | 3/121 (2.5) | 2/47 (4.3) | 0.56 | 30/168 (17.9) | 21/121 (17.4) | 9/47 (19.1) | 0.82 | 13/168 (7.7) | 10/121 (8.3) | 3/47 (6.4) | 0.70 |
| T3 (n = 214) [HR | 38/214 (17.8) | 32/146 (21.9) | 6/68 (8.8) | 0.046 | 73/214 (34.1) | 54/146 (37) | 19/68 (27.9) | 0.36 | 59/214 (27.6) | 46/146 (31.5) | 13/68 (19.1) | 0.15 |
| Second wave | ||||||||||||
| T4 (n = 156) [HR | 6/156 (3.9) | 5/110 (4.5) | 1/46 (2.2) | 0.50 | 70/156 (44.9) | 51/110 (46.4) | 19/46 (41.3) | 0.72 | 138/156 (88.5) | 98/110 (89.1) | 40/46 (86.9) | 0.92 |
| T5 (n = 175) [HR | 29/175 (16.6) | 21/122 (17.2) | 8/53 (15.1) | 0.77 | 106/175 (60.1) | 77/122 (63.1) | 29/53 (54.7) | 0.60 | 172/175 (98.3) | 121/122 (99.1) | 51/53 (96.2) | 0.11 |
| T51 (n = 166) [HR | 24/166 (14.5) | 17/118 (14.4) | 7/48 (14.6) | 0.78 | 100/166 (60.2) | 73/118 (61.9) | 27/48 (56.3) | 0.42 | 164/166 (98.8) | 117/118 (99.2) | 47/48 (97.9) | 0.52 |
The T1 to T3 period represented the first COVID-19 wave when vaccination was still unavailable in India, during which time seropositivity increased from 1.1% to 34.1%. The first case of COVID-19 infection was reported in India on 27th January 2020. At T1, which was in April 2020, no clinical infections were reported among HCWs in the institution; however, 3 HCWs in the HR area and none in the LR area tested positive for the nucleocapsid antibody but not for the spike protein. During the second sampling (T2) which was from June 2020 to July 2020, 5 HCWs reported clinical infection in the preceding period (HR 2.5% vs LR 4.3%, P = 0.56). COVID-19 antibodies for the nucleocapsid protein were positive in 17.4% and 19.1% in HR and LR groups respectively (P = 0.82) and the spike protein in 8.3% and 6.4% respectively (P = 0.70).
Between T2 and T3, when the first wave of COVID-19 peaked, HCWs in the HR area had a 2.48 times higher risk (21.9% in HR vs 8.8% in LR, P = 0.046) of developing COVID-19 infection. However, there was no demonstrable difference in seroconversion rates between the HR and LR groups by this time-point (Table 3).
During the second wave (T4 and T5), when the majority of the HCWs were vaccinated, the proportion of HCWs who developed clinical infection was similar in the HR and LR groups. Antibodies to spike protein were evident in 99.2% in the HR group and 97.9% in the LR group (P = 0.52), suggesting good response to vaccination, while 61.9% in the HR group and 56.3% in the LR group respectively had antibodies to nucleocapsid protein (P = 0.42), suggesting prior clinical or subclinical COVID-19 infection.
When the sero-study component was assessed (Table 4), out of the 202 patients who had given at least one sample in the T1 to T3 period and one sample in T4 or T5, clinical infection occurred in 31.5% (45/143) and 23.7% (14/59) in the HR and LR groups respectively (P = 0.23). Those vaccinated with Covaxin® (11/213, 5.1%) and Sputnik V were excluded from the final sero-study analysis. Given that nucleocapsid antibody was positive in 66.4% in the HR group and 59.3% in the LR group (Table 4), the subclinical infection rates worked out to 34.9% in the HR group and 35.6% in the LR group (P = 0.37).
| Antibody positive | High-risk areas (n = 143) | Low-risk areas (n = 59) | Inference1 | |
| Nucleocapsid + | Spike + | 95 (66.4) | 34 (57.6) | Infection with vaccination |
| Nucleocapsid - | Spike + | 45 (31.5) | 22 (37.3) | Vaccination |
| Nucleocapsid + | Spike - | 0 (0) | 1 (1.7) | Infection |
| Nucleocapsid - | Spike - | 3 (2.1) | 2 (3.4) | Not infected or vaccinated |
Our study, involving 448 HCWs, tracked seroprevalence to the SARS-CoV-2 virus during the first two waves of the pandemic at five time-points and provided valuable insights into the virus's impact and vaccination efficacy. The seroprevalence rate among HCWs was 1.1% at the start of the pandemic and increased to 34.1% during the first wave and to 60.1% during the second wave of the pandemic. Prior to vaccination, during the first wave of the pandemic, a significantly higher proportion of HCWs in HR areas developed clinical infection when compared with HCWs working in LR areas (21.9% vs 8.8%, P = 0.046); seropositivity was, however, similar in both groups (37% vs 27.9%, P = 0.36). Following vaccination, during the second wave of the pandemic, clinical infection rates (17.2% vs 15.1%, P = 0.77) and seropositivity (63.1% vs 54.7%, P = 0.60) were similar in HR and LR groups. Subclinical infection rates were 34.9% in the HR group and 35.6% in the LR group (P = 0.37).
Table 5 presents the seroprevalence data among HCWs from studies across the globe[18-33]. At the initial stages of the pandemic, when the population was immunologically naïve to the novel COVID-19 pathogen, as expected, only 1.1% of the HCWs were seropositive in the current study in April 2020. These results are comparable with the seropositivity of 0.3% from Japan in 2020[18] and the 0.5% in March 2020 from Italy[19]. The seroprevalence data from Brazil (5.5%, June 2020)[20], the United States (6%, April 2020 to June 2020)[21], Switzerland (9.6%, April 2020)[22], Netherlands (13.9%, June 2020 to July 2020)[23], Sweden (19.1%, April 2020 to May 2020)[24], and the United Kingdom (24.4%, April 2020)[25] probably correspond to the seroprevalence of 17.9% during the upslope of the first wave of the pandemic in our study (T2 sampling, June 2020 to July 2020).
| Ref. | Country | Period | Sample size | Sero-prevalence |
| Current study | India | April 2020 (T1) | 283 | 1.1% |
| June 2020 to July 2020 (T2) | 168 | 17.9% | ||
| October 2020 (T3) | 214 | 34.1% | ||
| March 2021(T4) | 156 | 44.9% | ||
| June 2021(T5) | 175 | 60.1% | ||
| Milazzo et al[19] | Italy | March 2020 | 697 | 0.5% |
| May 2020 | 5.4% | |||
| Piccoli et al[22] | Switzerland | April 16th to April 30th 2020 | 4726 | 9.6% |
| Shields et al[25] | United Kingdom | April 2020 | 545 | 24.4% |
| Rudberg et al[24] | Sweden | April 2020 to May 2020 | 2149 | 19.1% |
| Self et al[21] | United States | April 2020 to June 2020 | 3248 | 6% |
| Airoldi et al[27] | Italy | May 2020 to June 2020 | 2252 | 17.1% |
| Oliveira et al[20] | Brazil | June 2020 | 1996 | 5.5% |
| Recanatini et al[23] | Netherlands | June 2020 to July 2020 | 2328 | 13.9% |
| Kataria et al[28] | United States | July 2020 | 1743 | 5.5% |
| Prakash et al[26] | India | August 2020 | 1710 | 23.65% |
| Sonmezer et al[30] | Turkey | October 2020 | 1974 | 19% |
| Halili et al[29] | Kosovo | October 2020 to December 2020 | 647 | 17.5% |
| Wiggen et al[31] | United States | November 2020 to February 2021 December 2020 to February 2021 | 459 | 9.47% 17.7% |
| Ige et al[32] | Nigeria | December 2020 to July 2021 | 413 | 30.9% |
| Kanamori et al[18] | Japan | 2020 | 3788 | 0.3% |
| 2021 | 1.6% | |||
| 2022 | 17.7% | |||
| Taher et al[33] | Saudi Arabia | June 2022 to September 2022 | 404 | 94% |
As the first wave of the pandemic progressed, seroprevalence rates increased as more HCWs were exposed for a longer time to patients with infection. During the first wave of the pandemic in India, the seroprevalence was reported to be 23.65% in August 2020 in a cohort of 1710 HCWs[26]. The seroprevalence of 34.1% in the current study in October 2020 (T3) is consistent with the trajectory of seroconversion as the pandemic evolved in India. Other centers from across the world reported[27-30] much lower seroprevalence rates ranging from 5.5%[28] to 19%[30] during the period May to December 2020.
The seroprevalence rate in the current study in June 2021 of 60.1% (T5) was much higher than the seroprevalence rates for 2021 from the United States (17.7%, December 2020 to February 2021)[31], and Nigeria (30.9%, December 2020 to July 2021)[32]. Seroprevalence rates in 2022 were 17.7% in Japan (annual checkup of HCWs in 2022)[18] and very high rates of 94% in Saudi Arabia (June 2022 to September 2022)[33].
These results highlight significant regional and temporal disparities in seroprevalence. These variations in seroprevalence rates among HCWs reflect the pandemic's complexity and emphasize factors such as geographic location and vaccination timing. The prevalence of SARS-COV-2 antibodies among HCWs can differ significantly based on local epidemiological conditions and workplace settings.
Our study showed that HCWs working in HR areas were at a higher risk of developing clinical infection during the initial period of the pandemic when vaccination was not available. This can be attributed to the disease's novelty, lack of vaccines, and PPE shortages. Another possible contributing factor could be the significant differences in work duration between HR and LR areas, as illustrated in Table 1. However, clinical infection risks became comparable by the end of the second wave (T5), with over 96% of HCWs in both groups showing seroconversion. This shows the evolving nature of the pandemic and the protective effects of vaccination over time. In our institution, which had around 11000 workers including HCWs and support staff at the time of the pandemic, only 2 HCWs succumbed to COVID-19 infection. Vaccination has a positive impact on the severity of illness and mortality with full vaccination conferring a substantially higher protective effect over partial vaccination[16]. A study by Murhekar et al[34] showed that the seroprevalence status among HCWs and the general population who received two doses of COVID-19 vaccination was comparable (89.8% vs 88.6%) confirming the efficacy of infection control policies and vaccination strategies.
Our study shows that roughly half of the COVID-19 infections among HCWs were symptomatic, highlighting the need to address both symptomatic and asymptomatic HCWs from spreading the virus. These findings align with similar findings in other studies, reinforcing that many infections may present with milder symptoms or be asymptomatic, underscoring the need for vigilant surveillance and infection control measures[35,36]. Although routine screening of asymptomatic HCWs for COVID-19 infection was undertaken in several centers, its role in prevention of transmission of infection to patients is unclear[37]. Lastly, our study reveals that a notable percentage of HCWs were hospitalized, with some admissions being mandated by government directives, which highlights the seriousness of COVID-19 infections and their immense pressure on healthcare systems. Similar trends were seen in other research, underlining the need for prepared healthcare infrastructure and safeguarding frontline HCWs[38].
A few limitations merit mention. This study was conducted in a single center and hence this limits generalizability to other regions. Not all HCWs provided samples at all time points given that some HCWs, particularly postgraduate trainees, joined after the initial sampling or left the institution on completion of their course before study completion. However, it must be highlighted that the proportion of HCWs with clinical infection was similar in the entire cohort and the subgroup on whom seroprevalence data was analyzed. It is likely that the subset reflects the characteristics of the entire cohort of HCWs. Covaxin® elicits antibodies to nucleocapsid and spike proteins as a natural infection does. This would have made it difficult to differentiate the antibody response to vaccination versus infection (clinical and subclinical). Hence, Covaxin®-vaccinated individuals were excluded from the seroprevalence analysis.
This study demonstrates an increase in seroprevalence to COVID-19 infection among HCWs as the pandemic evolved, from 1.1% at the start of the pandemic to 60.2% by the end of the second wave of the pandemic, reflecting the progression of the pandemic among a population that was immunologically naïve to COVID-19. HCWs working in HR areas were more prone to develop clinical infection in the pre-vaccination period. About 1/3rd of HCWs had evidence of sub-clinical infection. By the end of the study period, 98.8% were positive for spike antibody suggesting good response to vaccination.
| 1. | World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int. |
| 2. | Nguyen LH, Drew DA, Graham MS, Joshi AD, Guo CG, Ma W, Mehta RS, Warner ET, Sikavi DR, Lo CH, Kwon S, Song M, Mucci LA, Stampfer MJ, Willett WC, Eliassen AH, Hart JE, Chavarro JE, Rich-Edwards JW, Davies R, Capdevila J, Lee KA, Lochlainn MN, Varsavsky T, Sudre CH, Cardoso MJ, Wolf J, Spector TD, Ourselin S, Steves CJ, Chan AT; COronavirus Pandemic Epidemiology Consortium. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475-e483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1446] [Cited by in RCA: 1407] [Article Influence: 234.5] [Reference Citation Analysis (1)] |
| 3. | CDC COVID-19 Response Team. Characteristics of Health Care Personnel with COVID-19 - United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:477-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 522] [Cited by in RCA: 584] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 4. | Sim MR. The COVID-19 pandemic: major risks to healthcare and other workers on the front line. Occup Environ Med. 2020;77:281-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 5. | Los Angeles Times. Doctors and nurses fighting coronavirus in China die of both infection and fatigue. 2020. Available from: https://www.latimes.com/world-nation/story/2020-02-25/doctors-fighting-coronavirus-in-china-die-of-both-infection-and-fatigue. |
| 6. | Wu P, Fang Y, Guan Z, Fan B, Kong J, Yao Z, Liu X, Fuller CJ, Susser E, Lu J, Hoven CW. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54:302-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1086] [Cited by in RCA: 1013] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 7. | Ingram C, Downey V, Roe M, Chen Y, Archibald M, Kallas KA, Kumar J, Naughton P, Uteh CO, Rojas-Chaves A, Shrestha S, Syed S, Cléirigh Büttner F, Buggy C, Perrotta C. COVID-19 Prevention and Control Measures in Workplace Settings: A Rapid Review and Meta-Analysis. Int J Environ Res Public Health. 2021;18:7847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 8. | Pfizer. Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study. 2020. Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against. |
| 9. | Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, Bailey R, Swanson KA, Xu X, Roychoudhury S, Koury K, Bouguermouh S, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Yang Q, Liberator P, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Gruber WC, Jansen KU; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N Engl J Med. 2021;385:1761-1773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 937] [Cited by in RCA: 1076] [Article Influence: 215.2] [Reference Citation Analysis (0)] |
| 10. | Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7073] [Cited by in RCA: 7923] [Article Influence: 1584.6] [Reference Citation Analysis (1)] |
| 11. | Woolf K, Gogoi M, Martin CA, Papineni P, Lagrata S, Nellums LB, McManus IC, Guyatt AL, Melbourne C, Bryant L, Gupta A, John C, Carr S, Tobin MD, Simpson S, Gregary B, Aujayeb A, Zingwe S, Reza R, Gray LJ, Khunti K, Pareek M; UK-REACH Study Collaborative Group. Healthcare workers' views on mandatory SARS-CoV-2 vaccination in the UK: A cross-sectional, mixed-methods analysis from the UK-REACH study. EClinicalMedicine. 2022;46:101346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Symons X, Matthews S, Tobin B. Why should HCWs receive priority access to vaccines in a pandemic? BMC Med Ethics. 2021;22:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Lietz J, Westermann C, Nienhaus A, Schablon A. The Occupational Risk of Influenza A (H1N1) Infection among Healthcare Personnel during the 2009 Pandemic: A Systematic Review and Meta-Analysis of Observational Studies. PLoS One. 2016;11:e0162061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Hudson B, Toop L, Mangin D, Brunton C, Jennings L, Fletcher L. Pandemic influenza A(H1N1)pdm09: risk of infection in primary healthcare workers. Br J Gen Pract. 2013;63:e416-e422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Moorthy M, Chacko B, Ramakrishna K, Samuel P, Karthik G, Kalki RC, Abraham AM, Akhuj A, Valsan A, Abraham OC, Peter JV. Risk of pandemic (H1N1) 2009 virus infection among healthcare workers caring for critically ill patients with pandemic (H1N1) 2009 virus infection. J Hosp Infect. 2011;77:365-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Abhilash KPP, Mathiyalagan P, Krishnaraj VRK, Selvan S, Kanagarajan R, Reddy NP, Rajendiran N, Hazra D, Gunasekaran K, Moorthy M, Jasmine S, Davis JP, George T, George K, Varghese GM, Rupali P, Barney Isaac TJ, Gupta R, Pichamuthu K, Joy M, Jayaseelan L, Mathews P, Peter JV. Impact of prior vaccination with Covishield(TM) and Covaxin® on mortality among symptomatic COVID-19 patients during the second wave of the pandemic in South India during April and May 2021: a cohort study. Vaccine. 2022;40:2107-2113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Gong F, Wei HX, Li Q, Liu L, Li B. Evaluation and Comparison of Serological Methods for COVID-19 Diagnosis. Front Mol Biosci. 2021;8:682405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 18. | Kanamori R, Yan Y, Ito K, Fukuda H, Hori S, Yamamoto T, Igawa G, Saito K, Horiuchi Y, Nojiri S, Nishizaki Y, Tabe Y, Takahashi K, Naito T. Increased SARS-CoV-2 seroprevalence and spread of infection without awareness among healthcare workers through 2020-2022 in a Japanese medical center. Sci Rep. 2023;13:4941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 19. | Milazzo L, Lai A, Pezzati L, Oreni L, Bergna A, Conti F, Meroni C, Minisci D, Galli M, Corbellino M, Antinori S, Ridolfo AL. Dynamics of the seroprevalence of SARS-CoV-2 antibodies among healthcare workers at a COVID-19 referral hospital in Milan, Italy. Occup Environ Med. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Oliveira MS, Lobo RD, Detta FP, Vieira-Junior JM, Castro TLS, Zambelli DB, Cardoso LF, Borges IC, Tozetto-Mendoza TR, Costa SF, Mendes-Correa MC. SARS-Cov-2 seroprevalence and risk factors among health care workers: Estimating the risk of COVID-19 dedicated units. Am J Infect Control. 2021;49:1197-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Self WH, Tenforde MW, Stubblefield WB, Feldstein LR, Steingrub JS, Shapiro NI, Ginde AA, Prekker ME, Brown SM, Peltan ID, Gong MN, Aboodi MS, Khan A, Exline MC, Files DC, Gibbs KW, Lindsell CJ, Rice TW, Jones ID, Halasa N, Talbot HK, Grijalva CG, Casey JD, Hager DN, Qadir N, Henning DJ, Coughlin MM, Schiffer J, Semenova V, Li H, Thornburg NJ, Patel MM; CDC COVID-19 Response Team; IVY Network. Seroprevalence of SARS-CoV-2 Among Frontline Health Care Personnel in a Multistate Hospital Network-13 Academic Medical Centers, April-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1221-1226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 22. | Piccoli L, Ferrari P, Piumatti G, Jovic S, Rodriguez BF, Mele F, Giacchetto-Sasselli I, Terrot T, Silacci-Fregni C, Cameroni E, Jaconi S, Sprugasci N, Bartha I, Corti D, Uguccioni M, Lanzavecchia A, Garzoni C, Giannini O, Bernasconi E, Elzi L, Albanese E, Sallusto F, Ceschi A. Risk assessment and seroprevalence of SARS-CoV-2 infection in healthcare workers of COVID-19 and non-COVID-19 hospitals in Southern Switzerland. Lancet Reg Health Eur. 2021;1:100013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Recanatini C, GeurtsvanKessel CH, Pas SD, Broens EM, Maas M, van Mansfeld R, Mutsaers-van Oudheusden AJG, van Rijen M, Schippers EF, Stegeman A, Tami A, Veldkamp KE, Visser H, Voss A, Wegdam-Blans MCA, Wertheim HFL, Wever PC, Koopmans MPG, Kluytmans JAJW, Kluytmans-van den Bergh MFQ; COCON Study Group. Seroprevalence of SARS-CoV-2 antibodies among healthcare workers in Dutch hospitals after the 2020 first wave: a multicentre cross-sectional study with prospective follow-up. Antimicrob Resist Infect Control. 2023;12:137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Rudberg AS, Havervall S, Månberg A, Jernbom Falk A, Aguilera K, Ng H, Gabrielsson L, Salomonsson AC, Hanke L, Murrell B, McInerney G, Olofsson J, Andersson E, Hellström C, Bayati S, Bergström S, Pin E, Sjöberg R, Tegel H, Hedhammar M, Phillipson M, Nilsson P, Hober S, Thålin C. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11:5064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 25. | Shields A, Faustini SE, Perez-Toledo M, Jossi S, Aldera E, Allen JD, Al-Taei S, Backhouse C, Bosworth A, Dunbar LA, Ebanks D, Emmanuel B, Garvey M, Gray J, Kidd IM, McGinnell G, McLoughlin DE, Morley G, O'Neill J, Papakonstantinou D, Pickles O, Poxon C, Richter M, Walker EM, Wanigasooriya K, Watanabe Y, Whalley C, Zielinska AE, Crispin M, Wraith DC, Beggs AD, Cunningham AF, Drayson MT, Richter AG. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75:1089-1094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 191] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 26. | Prakash O, Solanki B, Sheth J, Makwana G, Kadam M, Vyas S, Shukla A, Pethani J, Tiwari H. SARS-CoV2 IgG antibody: Seroprevalence among health care workers. Clin Epidemiol Glob Health. 2021;11:100766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Airoldi C, Patrucco F, Milano F, Alessi D, Sarro A, Rossi MA, Cena T, Borrè S, Faggiano F. High Seroprevalence of SARS-CoV-2 among Healthcare Workers in a North Italy Hospital. Int J Environ Res Public Health. 2021;18:3343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Kataria Y, Cole M, Duffy E, de la Cena K, Schechter-Perkins EM, Bouton TC, Werler MM, Pierre C, Ragan EJ, Weber SE, Jacobson KR, Andry C. Seroprevalence of SARS-CoV-2 IgG antibodies and risk factors in health care workers at an academic medical center in Boston, Massachusetts. Sci Rep. 2021;11:9694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Halili R, Bunjaku J, Gashi B, Hoxha T, Kamberi A, Hoti N, Agahi R, Basha V, Berisha V, Hoxha I. Seroprevalence of anti-SARS-CoV-2 antibodies among staff at primary healthcare institutions in Prishtina. BMC Infect Dis. 2022;22:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Sonmezer MC, Erul E, Sahin TK, Rudvan Al I, Cosgun Y, Korukluoglu G, Zengin H, Telli Dizman G, Inkaya AC, Unal S. Seroprevalence of SARS-CoV-2 Antibodies and Associated Factors in Healthcare Workers before the Era of Vaccination at a Tertiary Care Hospital in Turkey. Vaccines (Basel). 2022;10:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Wiggen TD, Bohn B, Ulrich AK, Stovitz SD, Strickland AJ, Naumchik BM, Walsh S, Smith S, Baumgartner B, Kline S, Yendell S, Hedberg C, Beebe TJ, Demmer RT. SARS-CoV-2 seroprevalence among healthcare workers. PLoS One. 2022;17:e0266410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Ige FA, Ohihoin GA, Osuolale K, Dada A, Onyia N, Johnson A, Okwuraiwe AP, Odediran O, Liboro G, Aniedobe M, Mogaji S, Nwaiwu SO, Akande IR, Audu RA, Salako BL. Seroprevalence of SARS-CoV-2 IgG among healthcare workers in Lagos, Nigeria. PLoS One. 2023;18:e0292440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Taher WT, Bawazir AA, Sallam TA, Alsurimi K. Seroprevalence and factors associated with SARS-CoV-2 infection among healthcare workers: cross-sectional study. BMC Infect Dis. 2023;23:761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | Murhekar MV, Bhatnagar T, Thangaraj JWV, Saravanakumar V, Santhosh Kumar M, Selvaraju S, Rade K, Kumar CPG, Sabarinathan R, Asthana S, Balachandar R, Bangar SD, Bansal AK, Bhat J, Chakraborty D, Chopra V, Das D, Devi KR, Dwivedi GR, Jain A, Khan SMS, Kumar MS, Laxmaiah A, Madhukar M, Mahapatra A, Ramesh T, Rangaraju C, Turuk J, Yadav S, Bhargava B; ICMR serosurveillance group. Seroprevalence of IgG antibodies against SARS-CoV-2 among the general population and healthcare workers in India, June-July 2021: A population-based cross-sectional study. PLoS Med. 2021;18:e1003877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 35. | Olmos C, Campaña G, Monreal V, Pidal P, Sanchez N, Airola C, Sanhueza D, Tapia P, Muñoz AM, Corvalan F, Hurtado S, Meneses C, Orellana A, Montecino M, Arriagada G, Bustos FJ. SARS-CoV-2 infection in asymptomatic healthcare workers at a clinic in Chile. PLoS One. 2021;16:e0245913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Mehreen SF, Neelima A, Padmaja K, Teja V. Clinical Characteristics, Diagnosis & Outcome of Covid 19 Infections among Health Care Workers at a Tertiary Care Centre. Indian J Med Microbi. 2021;39:S72. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Jabs JM, Schwabe A, Wollkopf AD, Gebel B, Stadelmaier J, Erdmann S, Radicke F, Grundmann H, Kramer A, Monsef I, Rücker G, Rupp J, Scheithauer S, Schmucker C, Simon A, Mutters NT. The role of routine SARS-CoV-2 screening of healthcare-workers in acute care hospitals in 2020: a systematic review and meta-analysis. BMC Infect Dis. 2022;22:587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Ferland L, Carvalho C, Gomes Dias J, Lamb F, Adlhoch C, Suetens C, Beauté J, Kinross P, Plachouras D, Hannila-Handelberg T, Fabiani M, Riccardo F, van Gageldonk-Lafeber AB, Teirlinck AC, Mossong J, Vergison A, Melillo J, Melillo T, Mook P, Pebody R, Coutinho Rehse AP, Monnet DL. Risk of hospitalization and death for healthcare workers with COVID-19 in nine European countries, January 2020-January 2021. J Hosp Infect. 2022;119:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/