Published online Sep 25, 2024. doi: 10.5501/wjv.v13.i3.92647
Revised: June 4, 2024
Accepted: June 28, 2024
Published online: September 25, 2024
Processing time: 210 Days and 6.9 Hours
Chronic hepatitis C virus (HCV) has been associated with hepatic and extrahe
To study the prevalence of colorectal adenomas in patients with HCV compared to the general population and to evaluate if it is an independent risk factor for colorectal adenomas.
Patients were divided into HCV and non-HCV based on their HCV RNA titers. Patients with alcoholic liver disease, hepatitis B infection, and inflammatory bowel disease were excluded. Continuous variables were analyzed using the Mann-Whitney U test, and categorical variables using χ2 with P < 0.05 were considered statistically significant. The significant covariates (independent variables) were matched in both groups by propensity score matching, followed by multivariate regression analysis.
Of the 415 patients screened, 109 HCV patients and 97 non-HCV patients with colonoscopy results were included in the study. HCV patients were older, had a smoking history, had less frequent aspirin use, and had a lower body mass index (BMI) (P < 0.05). The HCV cohort had a significantly increased number of patients with adenomas (adenoma detection rate of 53.2% vs 34%. P = 0.006). We performed a propensity-matched multivariate analysis where HCV infection was significantly associated with colorectal adenoma (OR: 2.070, P = 0.019).
Our study shows a significantly higher rate of adenomas in HCV patients compared to the general population. Prospective studies would help determine if the increase in adenoma detection lowers the risk for colorectal cancer.
Core Tip: There is a paucity of data to suggest the role of chronic hepatitis C virus (HCV) infection and its role with ex
- Citation: Gogtay M, Yadukumar L, Singh Y, Suresh MG, Soni A, Yekula A, Bullappa A, Abraham GM. Retrospective study evaluating association of colorectal tumors and hepatitis C virus. World J Virol 2024; 13(3): 92647
- URL: https://www.wjgnet.com/2220-3249/full/v13/i3/92647.htm
- DOI: https://dx.doi.org/10.5501/wjv.v13.i3.92647
Approximately 4. 6 million people are infected by the hepatitis C virus (HCV) in the United States with approximately least 3. 5 million (range 2. 5 million-4. 7 million) currently infected; additional sources suggest that the actual prevalence could be much higher[1-4]. HCV is a multisystemic disease linked to pathological derangements in the body's immunological, cardiovascular, endocrine, respiratory, renal, and neurological systems[5]. In addition to raising the risk of hepatocellular carcinoma (HCC), HCV has been shown to significantly increase the risk of many non-hepatic cancers[6]. Although the HCV genome does not directly combine with the host genome, the proteins generated in response to chronic inflammation have cancer-causing effects, such as inactivating tumor suppressor genes and activating the pro-oncogenes[7,8]. The role of the hepatitis C virus in developing colorectal cancer (CRC) is still unclear due to the limited number of studies examining the association and possible carcinogenic mechanisms.
CRC is the third most common malignancy diagnosed globally, with 1. 8 million new cases reported annually[3,9]. The risk factors for CRC include obesity, a sedentary lifestyle, consumption of red meat and lack of fiber in their diet, excess alcohol consumption, and smoking[10]. More than 15% of cancers worldwide are linked to infective causes; one such is HCV[11]. A retrospective cohort study by Hurtado-Cordovi et al[12] discovered that HCV patients had more colorectal polyps than non-HCV patients. However, the results were not statistically significant. Another retrospective study by Su et al[13] found that the tumorigenesis within the malignant colonic cells exhibited antibodies to proteins akin to that of hepatocellular carcinoma in patients with chronic hepatitis C increased the risk of CRC on screening colonoscopies in the distal colon. Furthermore, it surprisingly showed that the risk ratio decreased with increasing age, with the highest odds ratio for CRC in HCV patients less than 45 years of age.
In 2020, the United States Preventive Services Task Force (USPSTF), American College of Gastroenterology (ACG), and American Cancer Society (ACS) updated their screening recommendations for CRC from the age of 50 to start at the age of 45 years[14,15]. Since timely intervention in the form of screening colonoscopy can help detect pre-neoplastic or early stages of CRC, it is imperative to understand the relationship between chronic hepatitis C and CRC[16]. Given the lack of literature in this area, we aimed to study the association of chronic hepatitis C and pre-cancerous lesions - colonic adenomatous polyps in patients undergoing screening colonoscopy.
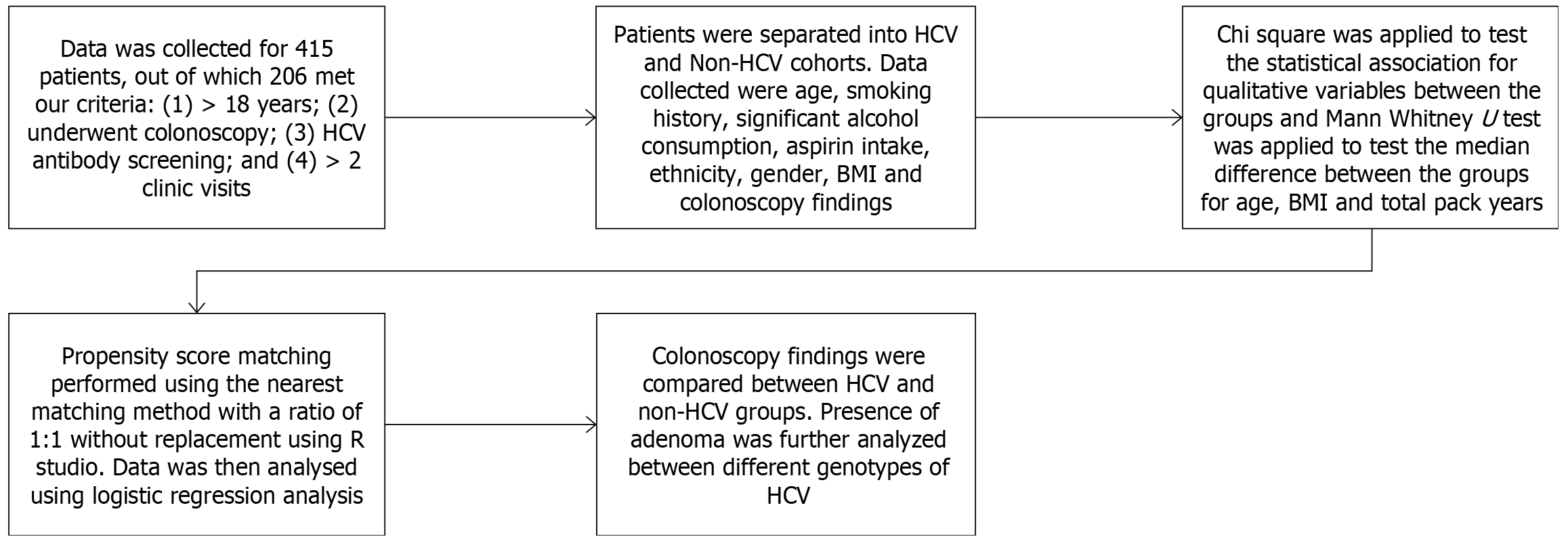
This is a retrospective cohort study that included patients who underwent screening colonoscopy in our single-centered community hospital from January 01, 2001, to December 31, 2021. The inclusion criteria were (1) patients aged 18 years and older; (2) patients who underwent screening colonoscopy during the study period as per USPSTF guidelines; (3) they had at least a one-time screening anti-HCV antibody assay done prior to the screening colonoscopy; and (4) the patients had at least 2 or more visits to our primary care clinic in our hospital. The exclusion criteria were (1) patients younger than 18 years of age; (2) patients with incomplete medical records; (3) patients with no biopsy reports when a polyp was found on colonoscopy; (4) patients with concurrent HIV infection, hepatitis B infection, alcoholic liver disease, or high-risk patients like those with inflammatory bowel disease or family history of CRC; (5) patients with documented cirrhosis; and (6) patients with history of colorectal carcinoma.
Colonoscopy findings were stratified into cohorts based on their results, i.e., hyperplastic, adenomatous, CRC, or normal colonic mucosa. The patients were divided into the HCV group and the non-HCV group based on their HCV RNA viral assay. The HCV group had a positive anti-HCV antibody assay followed by HCV RNA viral titer studies. The non-HCV group were defined as those who had a negative anti-HCV antibody assay. The primary outcome was the detection of adenomatous polyps on colonoscopy. Data collected included age, gender, ethnicity, body mass index (BMI) at the time of the initial clinic visit, family history of CRC, past medical history including diabetes mellitus (DM), medication use which included aspirin use and history of smoking. In patients who tested positive for HCV, their genotype, IL28 gene polymorphism, and HCV viral RNA titer were recorded. Colonoscopy findings included results of the procedure, num
Institutional review board statement: The study was reviewed and approved by our local Medical Center Institutional Review Board [(Approval No. 2021-035)].
Informed consent statement: Waiver obtained as part of the IRB, owing to retrospective nature of study.
The data were subjected to the normalcy test (Shapiro-Wilk test) that showed non-normalcy distribution. Hence, non-parametric tests were employed. The baseline demographic characteristics between the two groups were assessed using
A total of 415 patients were screened, and 206 met our inclusion criteria. 109 HCV patients and 97 non-HCV patients that had colonoscopy results were included in the study. HCV patients were older, with a mean age of 62.73 years, and the non-HCV group with a mean age of 60.20 years (P = 0.026). 44% of HCV patients were females compared to 47.4% of patients in the non-HCV group (P = 0.626). Ethnic groups were equally distributed between both groups. 34.9% of HCV patients were currently smoking at the time of their clinic visit compared to 15.5% of non-HCV patients, and 18.3% of HCV were former smokers compared to 17.5% of non-HCV patients (P = 0.004). HCV patients had a lower BMI with a median BMI of 28 compared to non-HCV patients who had a BMI of 32 (P = 0.001). HCV patients had less frequent aspirin use, with 20.2% on aspirin compared to 36.1% of the non-HCV patients on aspirin (P = 0.011). The HCV group had 24.8% with DM vs 32% in the non-HCV group (P = 0.25). The HCV group had a significantly higher number of patients with colonic polyps, 53.2% vs 34% in the non-HCV group (P = 0. 006). Further demographics are included in Table 1.
| Variables | HCV (n = 109) | Non-HCV (n = 97) | OR | 95%CI | P value |
| Age (mean ± SD) | 62.73 ± 9.14 | 60.20 ± 7.06 | 0.0261 | ||
| Female gender | 48 (44) | 46 (47.4) | 0.872 | 0.504-1.511 | 0.6263 |
| Ethnicity | 0.4273 | ||||
| Caucasian | 81 (74.3) | 84 (86.6) | 0.447 | ||
| Hispanic | 18 (16.5) | 11 (11.3) | 1.546 | ||
| Asian | 4 (3.7) | 2 (2.1) | 1.809 | ||
| African American | 4 (3.7) | 0 | |||
| Others | 2 (1.8) | 0 | |||
| BMI > 25 | 28.0 (7) | 32.0 (7) | 0.0011,2 | ||
| Aspirin intake | 22 (20.2) | 35 (36.1) | 0.448 | 0.24-0.83 | 0.0111,3 |
| Smoking history | 2.31 | 1.31-4.07 | 0.0041,2 | ||
| Non-smoker | 51 (46.8) | 65 (67) | |||
| Current smoker | 38 (34.9) | 15 (15.5) | |||
| Former smoker | 20 (18.3) | 17 (17.5) | |||
| Total pack year, median (IQR) | 25 (7) | 25 (7) | 0.7572 | ||
| DM | 27 (24.8) | 31 (32) | 0.872 | 0.50-1.51 | 0.253 |
| Adenomatous polyps | 58 (53.2) | 33 (34) | 2.206 | 1.25-3.876 | 0.0061,3 |
| Bowel preparation | 0.1483 | ||||
| Good | 90 (82.6) | 88 (90.7) | 0.484 | ||
| Fair | 11 (10.1) | 7 (7.2) | 1.443 | ||
| Poor | 8 (7.3) | 2 (2.1) | 3.762 |
We performed bivariate logistic regression with adenoma as a dependent variable and other significant covariates as independent variables (Table 2). HCV patients had an OR of 2.070 for adenomas (P = 0.019). Aspirin use had an OR = 0.513 (P = 0.065).
| Patient characteristics | P value | OR | 95%CI for OR | |
| Lower | Upper | |||
| Age (> median) | 0.56 | 1.18 | 0.66 | 2.12 |
| Presence of HCV | 0.0191 | 2.07 | 1.12 | 3.81 |
| Aspirin intake | 0.06 | 0.51 | 0.25 | 1.04 |
| Smoking history | 0.49 | 1.22 | 0.67 | 2.23 |
| Female gender | 0.86 | 1.05 | 0.58 | 1.88 |
| Diabetes mellitus | 0.52 | 1.24 | 0.63 | 2.45 |
In terms of outcome, the analysis showed that patients with HCV showed a more significant number of polyps (88) as compared to patients with non-HCV (58). Other variables, such as location, size, and histopathology, were also analyzed in Table 3. Tubular and hyperplastic polyps showed more propensity for HCV (58 and 22, respectively) compared to non-Hep C, where the tubular adenoma was 38 and hyperplastic was 18. Our data consisted of patients who were predominantly HCV genotype 1a (63.7%, P = 0.8). Other genotypes included were 1b (10.3%), 2b (5.1%), 2 (13.7%), 4 (6.8%), and 6 (0%), as depicted in Table 4. Patients who were HCV-positive were tested for IL28 polymorphisms (interleukin 28). 56% had CT polymorphism, 33.9% had CC, and TT 10.1%, as shown in Table 5.
| Polyp characteristics | HCV | Non-HCV |
| Number of polyps | 88 (60.27) | 58 (39.72) |
| Polyp size < 5 mm | 59 (67.04) | 38 (65.51) |
| Range of polyp size (mm) | 1-30 | 1-18 |
| Histopathology of lesions: | ||
| Tubular Adenoma | 58 (74.6) | 38 (74.4) |
| Hyperplastic | 28 (23.7) | 18 (23.1) |
| Cancer | 2 (1.7) | 0 |
| Inflammatory | 0 | 2 (2.5) |
| Location: | ||
| Right colon | 29 (32.9) | 14 (24.1) |
| Left colon | 17 (19.3) | 9 (15.5) |
| Transverse colon | 14 (15.9) | 14 (24.1) |
| Sigmoid colon | 20 (22.7) | 8 (13.7) |
| Rectum | 8 (9.09) | 13 (22.4) |
| HCV genotype | With adenoma (n = 58) | Without adenoma (n = 51) |
| 1a | 37 (63.7) | 28 (54.9) |
| 1b | 6 (10.3) | 11 (21.5) |
| 2b | 3 (5.1) | 2 (3.9) |
| 3 | 8 (13.7) | 9 (17.6) |
| 4 | 4 (6.8) | 0 |
| 6 | 0 | 1 (1.9) |
| Il28 gene polymorphism | HCV patients |
| CC | 37 (33.9) |
| CT | 61 (56) |
| TT | 11 (10.1) |
We performed a 1:1 propensity score matching with HCV as the intervention and control for other covariates between the two groups. There were 97 matches between the cases and the control arm. Twelve were excluded as they were unmatched, and 0 were discarded. After matching, covariate adjustment was made, and we repeated the multivariate analysis where hepatitis C had an OR: 2.069, P = 0.019 for colonic adenoma. Age had an OR = 1.043, P = 0.0295, and aspirin use had an OR = 0.387, P = 0.0116 for colonic adenoma detection (Table 6).
Viruses are infective particles implicated in various disease processes, ranging from simple upper respiratory tract infections to malignancies. In the United States, hepatitis C is the most common chronic viral blood-borne infection[18]. Globally, an estimated 58 million people have chronic hepatitis C infection, with about 1.5 million new cases were detected yearly. HCV is associated with a variety of diseases, including autoimmune vasculitis, cryoglobulinemia, clotting disorders, lymphoproliferative neoplasm, and solid tumors[19,20]. The relationship between HCC and chronic HCV has been well established; however, there is limited literature about this infection's effects on the carcinogenesis of other common malignancies, namely CRC[7,21]. Most colon tumors arise from adenomatous polyps; it is not well known if HCV influences the growth and development of these precancerous lesions, thereby increasing the risk of CRC[22,23].
There have been a handful of retrospective studies that have investigated HCV and its association with CRC. In Allison et al[24], an increased incidence of rectal cancer was found in patients with chronic HCV infection (OR: 2.1, 95%CI: 1.3–2.8), but CRC incidence rate did not increase in chronic HCV-infected patients (OR: 0.4, 95%CI: 0.3–0.6). Another study showed that HCV infection is a separate risk factor for advanced neoplasia and hyperplastic polyps discovered during screening colonoscopies[25]. The total number of adenomas was higher in the HCV group as compared to the non-HCV group; however, not statistically significant (0.69 vs 0.58 per patient; P > 0.05). According to a 2018 systematic review article, young adults with hepatitis C have a higher risk of developing CRC, which was associated with worse outcomes[26].
To understand this association better, we conducted a retrospective cohort study on 415 consecutive patients who underwent screening colonoscopy that were previously screened for hepatitis C. The patients were then divided into two groups, those with chronic hepatitis C (HCV group) and those without (non-HCV group). Patients in the HCV group were older, smoked more often, had a lower BMI, and used aspirin less frequently than the non-HCV group. Ethnicity groups, female gender and comorbidities like DM were equally distributed between both groups. HCV patients had a higher risk of polyps that were detected on their screening colonoscopy than non-HCV patients. We found that HCV patients had higher odds of colonic adenomatous polyps than non-HCV patients on logistic regression analysis.
Given that pre-cancerous lesions and cancer have multiple risk factors, many of which are unmodifiable, like age, gender, and ethnicity. There are also certain modifiable factors like smoking, aspirin intake, higher BMI, and chronic hepatitis C, which influence the risk for CRC. As these risk factors could impact our results and act as confounders, we applied propensity score matching to control for these potential confounders. The propensity score was estimated using a logistic regression model, in which treatment status was regressed on observed baseline demographics. HCV status was used as intervention arm and the propensity score was calculated. Following this, 1:1 propensity score matching was conducted. Whereby, matched sets of treated and untreated patients who share a similar value of the propensity score are reanalyzed. After covariate adjustment, we repeated the logistic regression analysis, which also showed that patients with HCV have 1. 89 higher odds of colonic adenoma than non-HCV patients.
In 2020, CDC and USPTF recommended a universal one-time HCV screening for the population aged 18 years and above, irrespective of their year of birth[26]. Given the updated screening recommendation, we expect the incidence of new acute and chronic hep C patients to increase steadily over the next few years. Furthermore, HCV affects multiple generations, the highest among two age groups: 20–39 and 55–70 years[20]. The USPSTF, ACG, and ACS recently re
One of the limitations of our study is the small sample size. Since the study is single-centered and retrospective, the generalizability of the study is limited. Furthermore, due consideration was given to possible confounding factors; we used special statistical analyses like propensity score, 1:1 matching, and covariate adjustment. Another limitation of our study is that we did not document if a patient underwent > 1 colonoscopy. As most of our patients were younger than the age for CRC screening, they mostly had only a single colonoscopy. We also did not document the temporal relationship of HCV treatment with the timing of their colonoscopy. Since our study period extended from 2001 to 2021, there have been varied treatment regimens for HCV. This could be an area of future studies to investigate if treatment with novel direct-acting antivirals reduces the risk of association between HCV and colonic adenomas by documenting serial colonoscopies.
We did not analyze data for possible correlation between HCV viral load, specific genotype, Il28 gene polymorphism of HCV, and risk of adenoma/ advanced neoplasia. We postulate that the cumulative effect of duration and HCV viral load increases the risk of adenoma production. HCV patients with chronically elevated viral loads for extended periods could be at highest risk of adenoma and colorectal carcinogenesis. Prospective studies could investigate if the treatment of hepatitis C reverses the risk of colorectal adenomas.
Adenoma detection rate is variable depending on the experience of the endoscopist, withdrawal time, first time endo
Our study shows a significantly higher rate of colonic adenomas in chronic hepatitis C patients. On multivariate analysis with and without propensity score matching, HCV infection was found to be an independent risk factor for colorectal adenoma. Current guidelines do not recommend earlier screening for CRC for patients with chronic hepatitis C. Pro
| 1. | Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, Holmberg SD. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 582] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 2. | Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62:1353-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 340] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 3. | Hofmeister MG, Rosenthal EM, Barker LK, Rosenberg ES, Barranco MA, Hall EW, Edlin BR, Mermin J, Ward JW, Ryerson AB. Estimating Prevalence of Hepatitis C Virus Infection in the United States, 2013-2016. Hepatology. 2019;69:1020-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 387] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 4. | Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2042] [Cited by in RCA: 2026] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 5. | Gill K, Ghazinian H, Manch R, Gish R. Hepatitis C virus as a systemic disease: Reaching beyond the liver. Hepatol Int. 2016;10:415-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Nyberg AH, Sadikova E, Cheetham C, Chiang KM, Shi JX, Caparosa S, Younossi ZM, Nyberg LM. Increased cancer rates in patients with chronic hepatitis C. Liver Int. 2020;40:685-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Levrero M. Viral hepatitis and liver cancer: The case of hepatitis C. Oncogene. 2006;25:3834-3847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 298] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 8. | Zhang DY, Goossens N, Guo J, Tsai MC, Chou HI, Altunkaynak C, Sangiovanni A, Iavarone M, Colombo M, Kobayashi M, Kumada H, Villanueva A, Llovet JM, Hoshida Y, Friedman SL. A hepatic stellate cell gene expression signature associated with outcomes in hepatitis C cirrhosis and hepatocellular carcinoma after curative resection. Gut. 2016;65:1754-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56702] [Article Influence: 7087.8] [Reference Citation Analysis (135)] |
| 10. | Keum N, Giovannucci E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 1783] [Article Influence: 254.7] [Reference Citation Analysis (3)] |
| 11. | Schottenfeld D, Beebe-Dimmer J. The cancer burden attributable to biologic agents. Ann Epidemiol. 2015;25:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Hurtado-Cordovi J, Davis-Yadley AH, Lipka S, Vardaros M, Shen H. Association between chronic hepatitis C and hepatitis C/HIV co-infection and the development of colorectal adenomas. J Gastrointest Oncol. 2016;7:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Su FH, Bai CH, Le TN, Muo CH, Chang SN, Te A, Sung FC, Yeh CC. Patients With Chronic Hepatitis C Virus Infection Are at an Increased Risk of Colorectal Cancer: A Nationwide Population-Based Case-Control Study in Taiwan. Front Oncol. 2020;10: 561420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Singh Y, Gogtay M, Yekula A, Soni A, Mishra AK, Tripathi K, Abraham GM. Detection of colorectal adenomas using artificial intelligence models in patients with chronic hepatitis C. World J Hepatol. 2023;15:107-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 15. | Knudsen AB, Rutter CM, Peterse EFP, Lietz AP, Seguin CL, Meester RGS, Perdue LA, Lin JS, Siegel RL, Doria-Rose VP, Feuer EJ, Zauber AG, Kuntz KM, Lansdorp-Vogelaar I. Colorectal Cancer Screening: An Updated Modeling Study for the US Preventive Services Task Force. JAMA. 2021;325:1998-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 255] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 16. | Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, Etzioni R, McKenna MT, Oeffinger KC, Shih YT, Walter LC, Andrews KS, Brawley OW, Brooks D, Fedewa SA, Manassaram-Baptiste D, Siegel RL, Wender RC, Smith RA. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 1363] [Article Influence: 170.4] [Reference Citation Analysis (1)] |
| 17. | Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6382] [Cited by in RCA: 8099] [Article Influence: 539.9] [Reference Citation Analysis (0)] |
| 18. | Alter MJ. Hepatitis C virus infection in the United States. J Hepatol. 1999;31 Suppl 1:88-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 162] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 19. | Gumber SC, Chopra S. Hepatitis C: A multifaceted disease. Review of extrahepatic manifestations. Ann Intern Med. 1995;123:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 299] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Singh Y, Gogtay M, Gurung S, Trivedi N, Abraham GM. Assessment of Predictive Factors of Hepatic Steatosis Diagnosed by Vibration Controlled Transient Elastography (VCTE) in Chronic Hepatitis C Virus-Infected Patients. J Community Hosp Intern Med Perspect. 2022;12:58-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (2)] |
| 21. | El-Serag HB, Hampel H, Yeh C, Rabeneck L. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 912] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 23. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8087] [Cited by in RCA: 8101] [Article Influence: 225.0] [Reference Citation Analysis (1)] |
| 24. | Allison RD, Tong X, Moorman AC, Ly KN, Rupp L, Xu F, Gordon SC, Holmberg SD; Chronic Hepatitis Cohort Study (CHeCS) Investigators. Increased incidence of cancer and cancer-related mortality among persons with chronic hepatitis C infection, 2006-2010. J Hepatol. 2015;63:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 25. | Rustagi T, Zarookian EI, Qasba O, Diez LF. Chronic hepatitis C as a risk factor for colorectal adenoma. Int J Colorectal Dis. 2014;29:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 26. | Schillie S, Wester C, Osborne M, Wesolowski L, Ryerson AB. CDC Recommendations for Hepatitis C Screening Among Adults - United States, 2020. MMWR Recomm Rep. 2020;69:1-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 389] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4. 0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/