Peer-review started: September 13, 2022
First decision: September 29, 2022
Revised: October 14, 2022
Accepted: January 3, 2023
Article in press: January 3, 2023
Published online: March 25, 2023
Processing time: 189 Days and 1 Hours
The coronavirus disease 2019 (COVID-19) disease was first detected in December 2019 in Wuhan, China. This disease is currently one of the most important global health problems. The novel coronavirus COVID-19 is a respiratory illness, that has caused a deadly pandemic that is spreading rapidly around the world. It is not only a respiratory system virus that causes severe lung disease, but also a systemic disease agent that can affect all systems. People with COVID-19 disease usually have respiratory signs, however, the liver disorder is not an uncommon presentation. In addition, many studies around the world have revealed that the liver is injured to various degrees in patients with severe acute respiratory syndrome coronavirus 2 disease. This review mainly focuses on the impact of COVID-19 on Liver Injury at various ages.
Core Tip: Studies have shown that neonates have rare evidence of liver damage, and in terms of age, they show the least amount of liver damage in the face of coronavirus disease 2019 (COVID-19) among affected people. Also, many studies reported different patterns of liver damage among children with COVID-19 much less than in adults, which is probably related to differences in their innate immune system and adaptation. The highest rate of liver damage is in adult patients and aspartate aminotransferase levels had the highest relevance with mortality compared to other indices reflecting liver injury.
- Citation: Sadeghi Dousari A, Hosseininasab SS, Sadeghi Dousari F, Fuladvandi M, Satarzadeh N. The impact of COVID-19 on liver injury in various age. World J Virol 2023; 12(2): 91-99
- URL: https://www.wjgnet.com/2220-3249/full/v12/i2/91.htm
- DOI: https://dx.doi.org/10.5501/wjv.v12.i2.91
Coronaviruses are a big family of viruses belonging to the realm Riboviria, order Nidovirales, family Coronaviridae and subfamily Coronavirinae. This virus contains an RNA genome and belongs to the Coronaviridae family[1,2]. This virus is spread in a wide spectrum of humans, other mammals, and avian species, also inducing acute respiratory infections[3]. Types of coronaviruses including HCoV-NL63, HCoV-HKU1, HCoV-229E, and HCoV-OC43 have been presented as mild virulent human viruses worldwide[4]. These viruses cause mild to severe acute respiratory illnesses in humans[3]. Coronavirus disease 2019 (COVID-19) was identified for the first time in December 2019, in Wuhan, located in the capital of Hubei Province in the People's Republic of China[1]. Coronavirus disease 2019 is an infectious illness that has caused a lethal pandemic that rapidly extends worldwide[5,6]. The signs of COVID-19 appear approximately 5.2 d after the disease and last for a minimum of 41 d and a maximum of 14 d until the end of life[4,7].
In the early stages of COVID-19, it has been found that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is not only a respiratory system virus that generates severe lung disease but a systemic disease factor that can involve all systems[8,9]. Some extrapulmonary involvement of SARS-CoV-2 disease is in organs like the liver, heart, or kidneys[10]. Many studies throughout the world have demonstrated that the liver is injured to differing degrees in patients affected by SARS-CoV-2 disease[8,9].
The liver is a vital member that is mostly responsible for the storage of glycogen and regulation of blood glucose levels, protein synthesis, metabolism of toxic substances, and very other physiological processes[8,9]. Liver dysfunction has been reported in 54% of hospitalized patients affected by COVID-19 disease, most of which are more severe in COVID-19[11]. Liver injuries have been documented in patients affected by COVID-19, and commonly have mild increasing liver enzymes range from 14% to 53%[12]. Patients with severe disease, especially those hospitalized in ICU, have shown a higher increase in transaminase enzymes than patients with mild to moderate severity[13]. Furthermore, few studies investigated the dynamic change of liver function during the COVID-19 pandemic. Also, no study to date has documented the incidence of a simultaneous increase in liver transaminases and total bilirubin levels in COVID-19 patients[14].
The purpose of this review is to evaluate the effect of COVID-19 on liver injury in various ages.
Patients who make severe acute liver injury in the absence of preexisting chronic liver disease, usually indicate noteworthy liver dysfunction marked with coagulopathy, which is described as an international normalized ratio ≥ 1.5 and is classically defined as acute liver failure (ALF) when any degree of hepatic encephalopathy (HE) is existing[15]. The ALF types include: (1) Hyperacute: < 7 d; (2) Acute: 7–28 d, and (3) Subacute: 28 d to 6 mo, depending on latency between the beginning of signs and development of encephalopathy and coagulopathy[16,17].
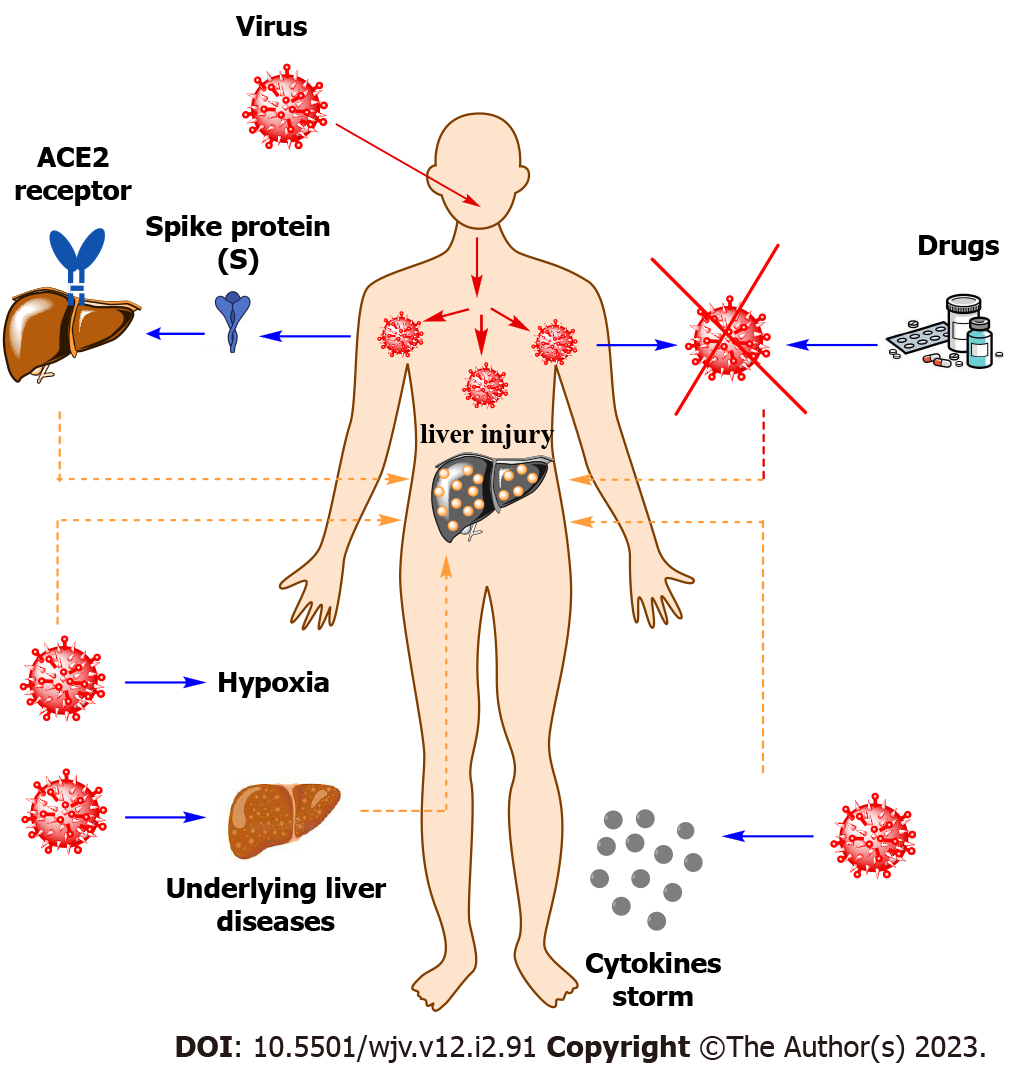
Liver injury is seen in patients with COVID-19, and its harshness is altered depending on the patient's age, geographical area, and disease severity[18]. Viral direct damage[19], immune damage, systemic inflammatory response, drug-induced, ischemia-reperfusion injury, mechanical ventilation, and underlying diseases may donate to liver injury[20] (Figure 1).
There is much evidence that COVID-19 causes abnormal liver function experiment outcomes with increased levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in people with liver damage[21,22]. Studies performed in Wuhan, China, recorded mildly elevated ALT and AST levels in 14%–53% of cases, with higher rates of both enzymes in patients with intense infection, mostly in patients requiring admission to the intensive care unit[23]. In COVID-19 patients with injured biliary tract were increased serum bilirubin, alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT) levels[24]. Also, in cases where the virus causes notable liver injury and intense clinical symptoms, varying levels of ALP and GGT along with high levels of ALT and total bilirubin have been reported in 58%-78% of patients[25].
The pathophysiology of liver damage may include the cytopathic result, in which spike (S) protein of coronaviruses 2019 attaches to the angiotensin-converting enzyme 2 (ACE2) receptor, leading to reduced liver function and hepatobiliary disease[26]. S protein viral entry into the liver cells (hepatocytes and cholangiocytes), a process that involves binding to the surface of the host cell through binding of the surface unit (S1) to a receptor[27,28]. The virus attains access to the host via the ACE2 receptor (a type I integral membrane protein containing zinc, which indicates enzymatic action through cleaving the vasoconstrictor peptide angiotensin II to angiotensin I, a strong vasodilator peptide, therefore decreasing blood pressure). ACE2 receptor was abundantly demonstrated in epithelial cells that line a three-dimensional network of bile ducts named cholangiocytes (60%), hepatocytes (3%) in the liver, alveolar cells of the lungs, and in various organs such as the pancreas, kidney, and heart[29,30].
Drugs: There are several drugs that prescribed to manage the treatment of patients with COVID-19 and associated symptoms, including therapeutic agents such as antivirals, antibiotics, acetaminophen, immunomodulators, corticosteroids, steroids, and antipyretics, that are metabolized through the liver and their use may lead to hepatotoxicity[31,32]. It has been reported that liver damage caused by these drugs is reason of anomalies in liver experiments and histological variation like micro-vesicular steatosis and liver inflammation in COVID-19 patients. Drugs like oseltamivir, arbidol, hydroxychloroquine, as well as ritonavir, and lopinavir in the treatment of patients may induce variable degrees of hepatotoxicity[33].
Hypoxia: Hypoxia in patients with COVID-19 is known as a major factor that causes a decrease in oxygen saturation values and finally reduction in systemic blood pressure[34]. This will ultimately cause a reduction in liver arterial perfusion via liver ischemia and hypoxia reperfusion injury via liver cell hypoxia[35].
Cytokines storm: Another factor related to COVID-19 that causes liver damage is the occurrence of a cytokine storm. In cases of the moderate and severe phase of the disease, which includes endothelial damage, it is related via a strong immune response to the SARS-CoV-2 virus[36]. This step is accompanied by the stimulation of inflammasomes (cytosolic multiprotein oligomers) that are responsible for the activation of caspase-1 and the release of pro-inflammatory cytokines [Interleukin (IL)-1β, IL-6, and IL-18][37]. In the next step, these cytokines stimulate the expression of genes relevant to the immune response, and through intracellular signaling, especially using IL-6, other pro-inflammatory cytokine biomarkers like tumor necrosis factor-alpha, IL-2, IL-8, IL-10, IL-17, granulocyte colony-stimulating factor monocyte chemoattractant protein, and interferon-inducible protein[38]. In addition, IL-6 activates numerous downstream signal pathways using creating complexes with its receptor[39], and also the reason for raised ferritin and C-reactive protein levels, reduced lymphocytes and enhanced neutrophils[40].
Underlying liver diseases: Underlying liver diseases can aggravate liver damage in the face of COVID-19. The prevalence of underlying liver diseases in patients with COVID-19 has been reported to be between 3% -11% in large observational studies[27,41,42]. From cases of these underlying diseases can be mentioned chronic liver disease and cirrhosis, non-alcoholic fatty liver disease, and liver transplantation[27].
Many studies have demonstrated various patterns of disease and their outcomes between adults and children, possibly associated with the difference in their innate and adaptive immune systems. Children with or without chronic sickness are less likely to have a severe illness from COVID-19 confirmed in various studies[43]. However, children affected by COVID-19 have a milder infection than adults, possibly related to children having preserved effector and immunosuppressive components[44]. The differences in age, gender, and population are probably due to differences in immune responses and different variants of SARS-CoV-2[45]. Furthermore, children are less likely to have multiple chronic conditions than older people[44]. Children with a weakened immune system, such as liver illnesses considered at higher risk of coronavirus[43]. Some reports showed children to have higher ACE2 expression than older adults, that it conversion ang I (angiotensin I enzyme) into angiotensin 1-7 (ang 1-7) enzyme, thus ang 1-7 enzyme protecting against pulmonary capillary leak and inflammation. This issue can be the reason why children are more resistant to COVID-19 than adults. The mechanism of liver injury in cases by COVID- 19 is indistinct[46]. The liver damage associated with COVID-19 is described as any liver injury happening during the progression and treatment of this disease in cases with or without underlying liver illness[47]. The most common presentation of liver damage in patients is with COVID-19 shown by increasing liver enzymes and also decreasing Serum albumin in severe cases. However, reports of death in affected by COVID-19 patients due to severe liver injury rarely happen[48,49].
A clinical study of 10 neonates (including twins) to 9 born to mothers with COVID-19 showed that only two infants have thrombocytopenia accompanied using abnormal liver function[50]. Clinical Analysis of 48 Neonates Born to Mothers with COVID-19 (confirmed or clinically diagnosed) or without it accomplished by Liu et al[51] polymerase chain reaction (PCR) test of all neonates was negative. Evidence of vertical transmission and liver injury was not observed. Similarly, a clinical investigation of 19 neonates born to mothers with COVID-19 was investigated at Tongji Hospital, China. The COVID-19 real-time reverse-transcription–PCR Test of all neonates was negative. In this study also, vertical transfer of SARS-CoV-2 was not found[52]. Wang et al[53] investigated a case report of neonates with positive test results for coronavirus 36 h after birth. Nevertheless, whether this Newborn is vertical transfer from the mother to the neonate is yet to be verified. In this case, was observed a significant increase in AST and abnormalities in liver function tests. Stolfi et al[54] reported a neonate of vertical transmission of COVID-19 with liver injury, confirmed using an increase in serum transaminases in Italy. The positive PCR test of COVID-19 in a neonate less than 24 h after C-section probably indicates vertical transmission, therefore proposing a transplacental transfer of SARS-CoV-2. Liver damage in this neonate was created probably using a direct virus-mediated mechanism that correlated to ACE2 receptor expression, But the details are unknown. Out of 33 neonates born to mothers affected by COVID-19 in China, three cases have positive PCR tests for COVID-19. One neonate had observed increasing transaminases[55].
Guan et al[56] extracted information about 1099 patients with positive PCR tests for COVID-19 in 30 provinces in China (from 552 hospitals). Out of 1099 patients, 112 cases (with an average age of 47 years) had a slight increase of AST with mild illness, and 56 adults had a high increase of AST with severe illness. In 2020, in a national retrospective cohort study in France, Mallet et al[57] examined the danger of mortality after COVID-19 disease in adult with chronic liver disease. The study contained 259,110 of all adults with COVID-19 who were released from post-acute care and acute, public and private hospitals in France in 2020. From a total of 259,110 patients who were between 54 and 83 years old (average age 70 years) and 52% were men, including 10,006 (3.9%) and 15,746 (6.0%) patients with alcohol use disorders and chronic liver disease, respectively. The results of this study demonstrated that patients with uncompensated cirrhosis, primary liver cancer, and alcohol use disorders were at high risk for COVID-19 fatality, while patients with compensated cirrhosis, mild liver disease, organ, including liver transplant, or acquired depressive syndrome were not at risk of COVID-19 mortality. Overall, mortality was in 38,203 (15%) of the patients, including chronic liver disease 2,941 (19%) and 7,475 (28%) after mechanical ventilation.
In another study, Mantovani et al[42] evaluated the widespread outbreak of chronic liver disease among patients affected by COVID-19 with a meta-analysis of data in observational studies and investigating the association between the liver injury and COVID-19 disease. The number of 11 observational studies included 2034 adults aged between 45 and 54 years (average age of 49 years), and 57.2% were men. The results of this study revealed that the widespread outbreak of chronic liver disease was 3% and people with severe disease of COVID-19 had associated changes in liver enzymes and coagulation profiles, which were reported to be possibly due to an innate immune response to the virus. In addition, the findings of this study displayed that the gain in AST level in hospitalized severe patients was more frequent and significant than the gain in ALT, and AST levels had the highest relevance with mortality compared to other indices reflecting liver damage, and it was reported that common factors related with the increase in liver damage indicators were the enhance in the number of neutrophils, the decrease in the number of lymphocytes, and male gender. The association between liver damage and adverse events of Coronavirus disease is indistinct. In adult studies, a higher rate of liver enzymes was reported in adults with severe diseases than in milder diseases[43]. One of the limitations of this study is that it is a retrospective study, which may have inadvertently missed some studies with basic keyword searches. In addition, the mechanism of liver damage at COVID-19 patients with different ages in used studies has not been clarified. However, this study was summarized existing evidence on the effects of COVID-19 on the liver injury at various ages. Furthermore, this study might have helped in clinical diagnosis and treatment for COVID-19related liver disease.
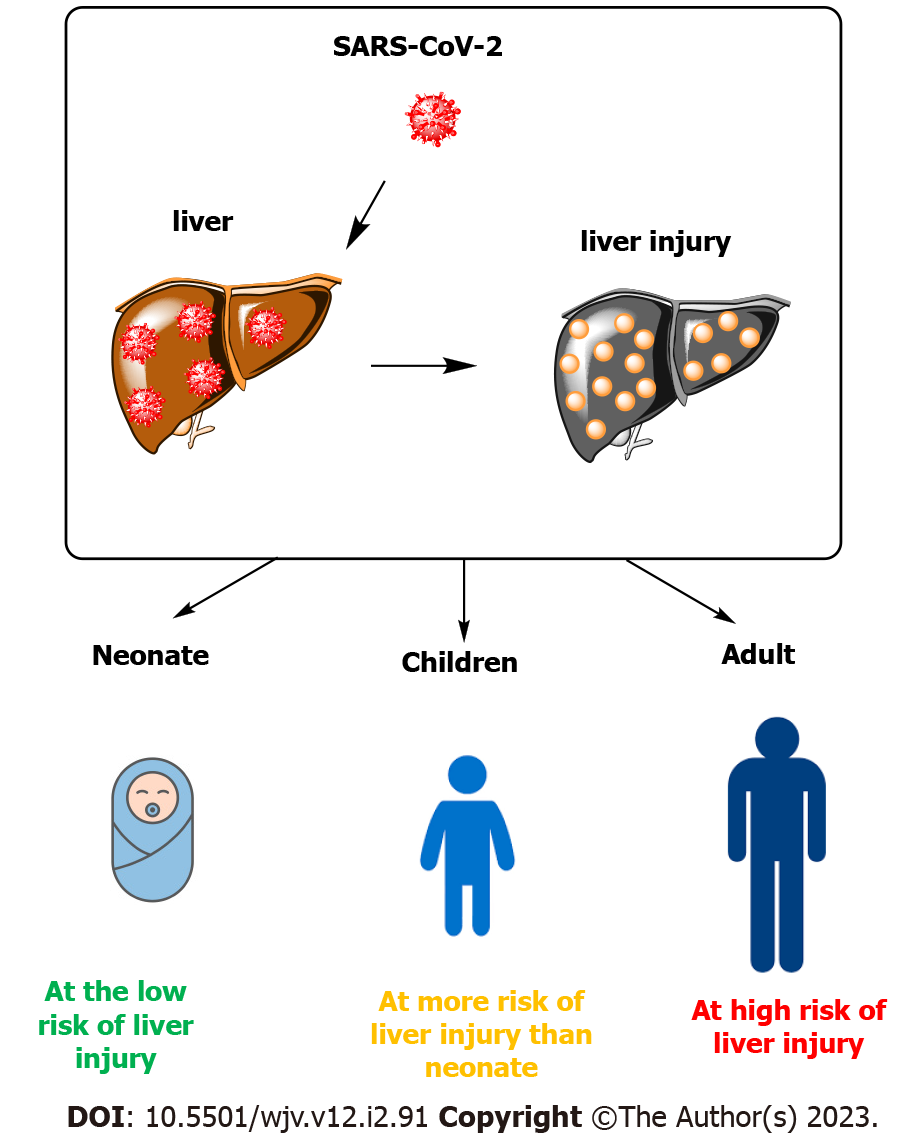
Figure 2 shows a summary of the effects of COVID-19 on Liver Injury at various ages.
The mechanisms of liver damage in either adults or children with COVID-19 are not fully unclear and the impact of liver injury caused by new variants of COVID-19 in patients is unexplained. Furthermore, further investigation is required to determine liver involvement and the consequence of COVID-19 on various ages with liver disease. Also, the pathogenetic mechanisms of COVID-19 on liver injury of patients in different age groups need to be investigated.
Liver damage is seen in patients affected by COVID-19, and factors including viral direct damage, immune damage, systemic inflammatory response, drug-induced, ischemia-reperfusion injury, mechanical ventilation, and underlying diseases contribute to liver injury. The association between liver damage and adverse clinical outcomes in patients affected by COVID-19 and the mechanism of SARS-CoV-2 in creating this injury is also unclear. Studies have shown that neonates have rare evidence of liver damage, and in terms of age, they show the least amount of liver damage in the face of COVID-19 disease among affected people. Most patients with COVID-19 have maintained their normal liver function during the disease, but patients with more severe disease probably had an abnormal liver function. Also, many studies reported different patterns of liver damage among children with COVID-19 much less than in adults, which is probably related to differences in their innate immune system and adaptation. Most patients with COVID-19 have a mild increase in aspartate aminotransferase, alanine aminotransferase, or total bilirubin. The highest rate of liver damage is in adult patients and AST levels had the highest relevance with mortality compared to other indices reflecting liver injury.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Virology
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu Y, China; Naswhan AJ, Qatar; Skrypnik D, Poland S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Sadeghi Dousari A, Taati Moghadam M, Satarzadeh N. COVID-19 (Coronavirus Disease 2019): A New Coronavirus Disease. Infect Drug Resist. 2020;13:2819-2828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Meena P, Bhargava V, Rana DS, Bhalla AK, Gupta A. COVID-19 and the kidney: A matter of concern. Curr Med Res Pract. 2020;10:165-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Moghadam MT, Taati B, Paydar Ardakani SM, Suzuki K. Ramadan Fasting During the COVID-19 Pandemic; Observance of Health, Nutrition and Exercise Criteria for Improving the Immune System. Front Nutr. 2020;7:570235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Shakibnia P, Ahmadi RH, Fallah F, Ebrahimzadeh F, Dosari AS, Mojtahedi A. et al Iran as the Center of challenges in the Middle East for the Outbreak of COVID-19 Delta Variant. Iranian Red Crescent Medical Journal. 2021;23:1394. [DOI] [Full Text] |
| 5. | Taati B, Paydar Ardakani SM, Suzuki K, Sadat Modaresi M, Taati Moghadam M, Roozbeh B. Protective Roles of Exercise and Nutritional Factors for Immune System During Delta Variant-COVID-19 Outbreaks: Evidence Review and Practical Recommendations. Iranian Journal of Medical Microbiology. 2022;16:2345-4342. [DOI] [Full Text] |
| 6. | Moghadam MT, Babakhani S, Rajabi S, Baravati FB, Raeisi M, Dousari AS. Does stress and anxiety contribute to COVID-19? Iranian Journal of Psychiatry and Behavioral Sciences. 2021;15. [DOI] [Full Text] |
| 7. | Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11224] [Cited by in RCA: 9398] [Article Influence: 1566.3] [Reference Citation Analysis (0)] |
| 8. | Kayaaslan B, Guner R. COVID-19 and the liver: A brief and core review. World J Hepatol. 2021;13:2013-2023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. 2017;27:R1147-R1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 1017] [Article Influence: 127.1] [Reference Citation Analysis (0)] |
| 10. | Carvalho T. Extrapulmonary SARS-CoV-2 manifestations. Nat Med. 2020;26:1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Davidov-Derevynko Y, Ben Yakov G, Wieder A, Segal G, Naveh L, Orlova N, Gringauz I, Amit S, Mor O, Klempfner R, Rahav G, Ben Ari Z. The liver in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Eur J Gastroenterol Hepatol. 2021;33:e313-e319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14864] [Article Influence: 2477.3] [Reference Citation Analysis (1)] |
| 13. | Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2493] [Cited by in RCA: 2319] [Article Influence: 386.5] [Reference Citation Analysis (0)] |
| 14. | Wang Q, Zhao H, Liu LG, Wang YB, Zhang T, Li MH, Xu YL, Gao GJ, Xiong HF, Fan Y, Cao Y, Ding R, Wang JJ, Cheng C, Xie W. Pattern of liver injury in adult patients with COVID-19: a retrospective analysis of 105 patients. Mil Med Res. 2020;7:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 15. | Koch DG, Speiser JL, Durkalski V, Fontana RJ, Davern T, McGuire B, Stravitz RT, Larson AM, Liou I, Fix O, Schilsky ML, McCashland T, Hay JE, Murray N, Shaikh OS, Ganger D, Zaman A, Han SB, Chung RT, Brown RS, Munoz S, Reddy KR, Rossaro L, Satyanarayana R, Hanje AJ, Olson J, Subramanian RM, Karvellas C, Hameed B, Sherker AH, Lee WM, Reuben A. The Natural History of Severe Acute Liver Injury. Am J Gastroenterol. 2017;112:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 16. | Lemmer P, Pospiech JC, Canbay A. Liver failure-future challenges and remaining questions. Ann Transl Med. 2021;9:734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Canbay A, Tacke F, Hadem J, Trautwein C, Gerken G, Manns MP. Acute liver failure: a life-threatening disease. Dtsch Arztebl Int. 2011;108:714-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Zhao JN, Fan Y, Wu SD. Liver injury in COVID-19: A minireview. World J Clin Cases. 2020;8:4303-4310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Satarzadeh N, Behzadi A, Khalilabadi RM. Donors with Positive Hepatitis B and C Infections and Their Demographic Characteristics, a Study in Jirof Blood Donation Center From 2011 to 2016. Int J Basic Sci Med. 2022;7:57-60.. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Shousha HI, Ramadan A, Lithy R, El-Kassas M. Patterns of liver profile disturbance in patients with COVID-19. World J Clin Cases. 2022;10:2063-2071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 21. | Kukla M, Skonieczna-Żydecka K, Kotfis K, Maciejewska D, Łoniewski I, Lara LF, Pazgan-Simon M, Stachowska E, Kaczmarczyk M, Koulaouzidis A, Marlicz W. COVID-19, MERS and SARS with Concomitant Liver Injury-Systematic Review of the Existing Literature. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 22. | Krishnan A, Prichett L, Tao X, Alqahtani SA, Hamilton JP, Mezey E, Strauss AT, Kim A, Potter JJ, Chen PH, Woreta TA. Abnormal liver chemistries as a predictor of COVID-19 severity and clinical outcomes in hospitalized patients. World J Gastroenterol. 2022;28:570-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 13055] [Article Influence: 2175.8] [Reference Citation Analysis (4)] |
| 24. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 666] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 25. | McGrowder DA, Miller F, Anderson Cross M, Anderson-Jackson L, Bryan S, Dilworth L. Abnormal Liver Biochemistry Tests and Acute Liver Injury in COVID-19 Patients: Current Evidence and Potential Pathogenesis. Diseases. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Lozano-Sepulveda SA, Galan-Huerta K, Martínez-Acuña N, Arellanos-Soto D, Rivas-Estilla AM. SARS-CoV-2 another kind of liver aggressor, how does it do that? Ann Hepatol. 2020;19:592-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | McGrowder DA, Miller F, Anderson Cross M, Anderson-Jackson L, Bryan S, Dilworth L. Abnormal Liver Biochemistry Tests and Acute Liver Injury in COVID-19 Patients: Current Evidence and Potential Pathogenesis. Diseases. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Sariani OK, Dousari AS, Moghadam MT. Possible psychological consequences in public of Omicron variant (B. 1.1. 529) of SARS-CoV-2 identification in Iran. Iran J Psychiatry Behav Sci. 2022;16:485-487. [DOI] [Full Text] |
| 29. | Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS-CoV-2 Infection. Clin Gastroenterol Hepatol. 2020;18:2128-2130.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 481] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 30. | Ozkurt Z, Çınar Tanrıverdi E. COVID-19: Gastrointestinal manifestations, liver injury and recommendations. World J Clin Cases. 2022;10:1140-1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (7)] |
| 31. | Boeckmans J, Rodrigues RM, Demuyser T, Piérard D, Vanhaecke T, Rogiers V. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch Toxicol. 2020;94:1367-1369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 32. | D'Ardes D, Boccatonda A, Cocco G, Fabiani S, Rossi I, Bucci M, Guagnano MT, Schiavone C, Cipollone F. Impaired coagulation, liver dysfunction and COVID-19: Discovering an intriguing relationship. World J Gastroenterol. 2022;28:1102-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 33. | Marjot T, Webb GJ, Barritt AS 4th, Moon AM, Stamataki Z, Wong VW, Barnes E. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 286] [Article Influence: 57.2] [Reference Citation Analysis (2)] |
| 34. | Sahu T, Pande B, Pl M, Verma HK. Liver dysfunction during COVID-19 pandemic: Contributing role of associated factors in disease progression and severity. World J Hepatol. 2022;14:1099-1110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 35. | Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P, Hosein Z, Padda I, Mangat J, Altaf M. Comorbidity and its Impact on Patients with COVID-19. SN Compr Clin Med. 2020;2:1069-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 973] [Cited by in RCA: 795] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 36. | García LF. Immune Response, Inflammation, and the Clinical Spectrum of COVID-19. Front Immunol. 2020;11:1441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 482] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 37. | Shah A. Novel Coronavirus-Induced NLRP3 Inflammasome Activation: A Potential Drug Target in the Treatment of COVID-19. Front Immunol. 2020;11:1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 38. | Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 552] [Cited by in RCA: 794] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 39. | Gonçalves Júnior J. COVID-19, liver dysfunction and pathophysiology: A conceptual discussion. World J Gastroenterol. 2022;28:683-688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 40. | Samprathi M, Jayashree M. Biomarkers in COVID-19: An Up-To-Date Review. Front Pediatr. 2020;8:607647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 41. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 558] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 42. | Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2020;40:1316-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 43. | Di Giorgio A, Hartleif S, Warner S, Kelly D. COVID-19 in Children With Liver Disease. Front Pediatr. 2021;9:616381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Dhochak N, Singhal T, Kabra SK, Lodha R. Pathophysiology of COVID-19: Why Children Fare Better than Adults? Indian J Pediatr. 2020;87:537-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 45. | Forster P, Forster L, Renfrew C, Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci U S A. 2020;117:9241-9243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 662] [Cited by in RCA: 620] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 46. | Wu J, Shi C, Sheng X, Xu Y, Zhang J, Zhao X, Yu J, Shi X, Li G, Cao H, Li L. Prognostic Nomogram for Patients with Hepatitis E Virus-related Acute Liver Failure: A Multicenter Study in China. J Clin Transl Hepatol. 2021;9:828-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 48. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Implication of non-alcoholic fatty liver diseases (NAFLD) in patients with COVID-19: a preliminary analysis. J Hepatol. 2020;73. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 413] [Article Influence: 68.8] [Reference Citation Analysis (2)] |
| 49. | Mao R, Doyon G, Gordon IO, Li J, Lin S, Wang J, Le THN, Elias M, Kurada S, Southern B, Olman M, Chen M, Zhao S, Dejanovic D, Chandra J, Mukherjee PK, West G, Van Wagoner DR, Fiocchi C, Rieder F. Activated intestinal muscle cells promote preadipocyte migration: a novel mechanism for creeping fat formation in Crohn's disease. Gut. 2022;71:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 50. | Word Health Organization. Opening remarks at the media briefing on COVID-19. [Internet] [accessed March 11, 2020]. Available from:https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. |
| 51. | Liu W, Cheng H, Wang J, Ding L, Zhou Z, Liu S, Chang L, Rong Z. Clinical Analysis of Neonates Born to Mothers with or without COVID-19: A Retrospective Analysis of 48 Cases from Two Neonatal Intensive Care Units in Hubei Province. Am J Perinatol. 2020;37:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Liu W, Wang J, Li W, Zhou Z, Liu S, Rong Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front Med. 2020;14:193-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 53. | Wang S, Guo L, Chen L, Liu W, Cao Y, Zhang J, Feng L. A Case Report of Neonatal 2019 Coronavirus Disease in China. Clin Infect Dis. 2020;71:853-857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 304] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 54. | Stolfi I, Conti MG, Marciano A, Dito L, Natale F, Bartolucci M, Cellitti R, Regoli D, Ticchiarelli A, Pangallo I, Pagano F, Ajassa C, Brunelli R, Terrin G. Liver Involvement in SARS-CoV-2 Vertically Infected Newborn: A Case Report. Front Pediatr. 2021;9:701722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 55. | Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, Benachi A, De Luca D. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11:3572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 595] [Cited by in RCA: 742] [Article Influence: 123.7] [Reference Citation Analysis (1)] |
| 56. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 19015] [Article Influence: 3169.2] [Reference Citation Analysis (9)] |
| 57. | Mallet V, Beeker N, Bouam S, Sogni P, Pol S; Demosthenes research group. Prognosis of French COVID-19 patients with chronic liver disease: A national retrospective cohort study for 2020. J Hepatol. 2021;75:848-855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |