Published online Sep 25, 2022. doi: 10.5501/wjv.v11.i5.283
Peer-review started: March 31, 2022
First decision: June 16, 2022
Revised: June 30, 2022
Accepted: August 22, 2022
Article in press: August 22, 2022
Published online: September 25, 2022
Processing time: 177 Days and 10.2 Hours
Acute kidney injury (AKI) and electrolyte disorders are important complications of hospitalized coronavirus disease 2019 (COVID-19) patients. AKI is thought to occur due to multiple pathophysiological mechanisms, such as multiple organ dysfunction (mainly cardiac and respiratory), direct viral entry in the renal tubules, and cytokine release syndrome. AKI is present in approximately one in every ten hospitalized COVID-19 patients. The incidence rates of AKI increase in patients who are admitted to the intensive care unit (ICU), with levels higher than 50%. Additionally, renal replacement therapy (RRT) is used in 7% of all AKI cases, but in nearly 20% of patients admitted to an ICU. COVID-19 patients with AKI are considered moderate-to-severe cases and are managed with multiple interdisciplinary conducts. AKI acts as a risk factor for mortality in severe acute respiratory syndrome coronavirus 2 infection, especially when RRT is needed. Electrolyte disorders are also common manifestations in hospitalized COVID-19 patients, mainly hyponatremia, hypokalemia, and hypocalcemia. Hyponatremia occurs due to a combination of syndrome of inappropriate secretion of antidiuretic hormone and gastrointestinal fluid loss from vomiting and diarrhea. When it comes to hypokalemia, its mechanism is not fully understood but may derive from hyperaldosteronism due to renin angiotensin aldosterone system overstimulation and gastrointestinal fluid loss as well. The clinical features of hypokalemia in COVID-19 are similar to those in other conditions. Hypocalcemia is the most common electrolyte disorder in COVID-19 and seems to occur because of vitamin D deficiency and parathyroid imbalance. It is also highly associated with longer hospital and ICU stay.
Core Tip: Acute kidney injury and electrolyte disorders are frequent clinical complications in hospitalized patients with coronavirus disease 2019, being directly related to the severity of the disease and increasing the mortality.
- Citation: Nogueira GM, Silva NLOR, Moura AF, Duarte Silveira MA, Moura-Neto JA. Acute kidney injury and electrolyte disorders in COVID-19. World J Virol 2022; 11(5): 283-292
- URL: https://www.wjgnet.com/2220-3249/full/v11/i5/283.htm
- DOI: https://dx.doi.org/10.5501/wjv.v11.i5.283
The coronavirus disease 2019 (COVID-19) outbreak initiated in the first months of 2020. It corresponds to an illness caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Although frequently asymptomatic, the malady is known for its wide variety of clinical signs and symptoms. These can range from pulmonary manifestations, such as dyspnea and cough, to extrapulmonary ones, which include fever, anosmia, ageusia, diarrhea, and myalgia[1-3]. This heterogeneity of clinical features is an indicative of the systemic character of COVID-19.
COVID-19 had an outstanding impact in the nephrology community. With over 4 million chronic kidney disease patients on maintenance dialysis at risk, the pandemic caused profound changes to the sector[4,5]. The kidney was also a commonly affected organ by COVID-19; one in every four patients presented abnormal renal function at hospital admission[6]. According to the classification proposed by the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, acute kidney injury is defined as any of the following situations: Increase in serum creatinine (SCr) by ≥ 0.3 mg/dL within 48 h; or increase in SCr ≥ 1.5 times baseline from 7 d prior; or urinary volume < 0.5 mL/kg/h for a period of 6 h. The KDIGO guidelines also propose a stratification of AKI in three stages, numbered from 1 to 3 in crescent order of injury severity[7].
In primary analyses of patients with COVID-19, it was also noticed that, among the various systemic complications caused by the SARS-CoV-2, changes in electrolyte concentrations are not only present, but also independently associated with a poor outcome[8,9].
In this review, we discuss the pathophysiology, epidemiology, clinical history, risk factors, mana-gement, and prognosis of COVID-19 associated acute kidney injury (AKI) and the most reported electrolyte disorders in COVID-19, which are hyponatremia, hypokalemia, and hypocalcemia.
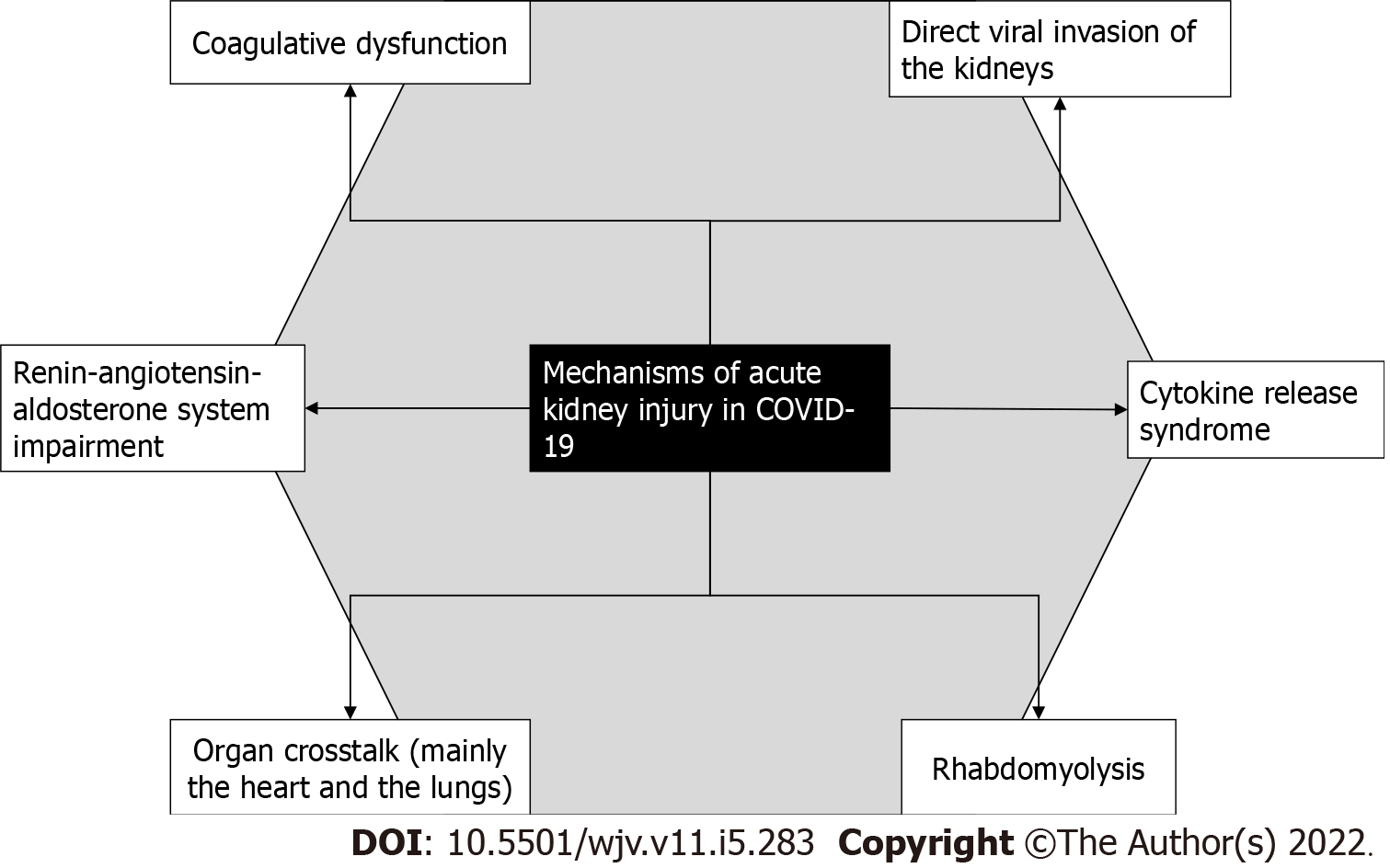
The mechanism of AKI in COVID-19 is most likely multifactorial. Some of the proposed alterations induced by the viral disease that could damage the kidneys can be seen in Figure 1[10].
Coronaviruses have high affinity for the angiotensin-converting enzyme 2 (ACE2), a metal-lopeptidase often bound to cell membranes that is responsible for catalyzing the conversion of angiotensin 2 to angiotensin 1-7[11-13]. The transmembrane protease serine 2 contributes to the entry of SARS-CoV-2 in the cell by cleaving and activating the spike (S) protein[14]. After entry, followed by endocytosis, coronavirus infection causes upregulation of PAK1, a kinase that mediates inflammation and is associated with risk factors for mortality. Increased PAK1 levels also suppress the adaptive immune response, facilitating viral replication[15]. It was previously shown that SARS-CoV could bind to ACE2 via the virus’ S protein[16]. Being structurally similar to SARS-CoV, SARS-CoV-2 also uses ACE2 in order to enter the host cell and replicate in its cytoplasm[17]. This enzyme is distributed across multiple tissues, such as the vascular endothelium, alveolar epithelium, proximal tubular cells of the kidney, and glomerular epithelium[18].
The fact that kidney cells express ACE2 explains how they also act as host cells of the novel coronavirus, a piece of information that was shown in autopsy studies. Histopathological examination found out varying degrees of tubular injury, such as diffuse proximal tubule injury with loss of the brush border, vacuolar degeneration, necrosis, hemosiderin granules, and pigment casts[19-21]. RNA in situ hybridization and electron microscopy also found evidence that SARS-CoV-2 directly infects the renal tubules[20,21]. A small number of patients with AKI may present virus in urine samples, which also supports a direct viral cytopathic effect hypothesis. These patients may have a greater predilection for proteinuria[22].
Due to the binding of SARS-CoV-2 to ACE2, the expression of this molecule is downregulated, which leads to increased activity of angiotensin 2 that is unopposed by angiotensin 1-7[23,24]. In normal conditions, angiotensin 1-7 has anti-thrombotic, anti-inflammatory, and vasodilator effects that counter the actions of angiotensin 2 through activation of Mas receptors[25-27]. It is suggested that the overactivation of angiotensin type 1 receptors may contribute to AKI onset mostly due to hemodynamic alterations, such as hypoxia, hypertension-induced proteinuria, and oxidative stress[27,28].
Furthermore, a hypercoagulative state induced by the lack of anti-thrombotic effects of angiotensin 1-7 could cause renal microangiopathy capable of causing AKI[29]. Rhabdomyolysis is also a frequent cause of COVID-19 associated AKI, being responsible for around 7% of the cases[30]. The occurrence of skeletal muscle injury is present in up to one in every five COVID-19 patients, which explains the occurrence of rhabdomyolysis nephropathy in this disease[29].
Cytokine release syndrome consists of an extreme rise of inflammatory cytokines, frequently called a “cytokine storm”, caused by a systemic response that can be triggered by a wide variety of conditions[31,32]. It has been implied that cytokine storm is a significant component of the disease course of severe cases of COVID-19[33]. The binding of SARS-CoV-2 to ACE2 promotes an inflammatory response with a prominent release of inflammatory cytokines, such as IL-6, IL-8, IL-22, and TNF-α, and chemokines, like CCL2, CCL3, and CCL5[29,34,35]. Lymphopenia, a common feature of SARS-CoV-2 infection, also contributes to the rise of inflammatory cytokines[36].
The crosstalk between the kidneys, lungs, and cardiovascular system seems to be significant for the development of AKI. Cases of acute respiratory distress syndrome (ARDS) are knowingly associated with a greater risk of AKI onset, including those related to SARS-CoV-2 infection[37-39]. This is likely a result of renal damage triggered by inflammatory mediators that cause tubular injury, which by itself culminates in IL-6 upregulation that harms the lungs[39,40].
The cardiovascular system is another important topic regarding AKI in COVID-19. Acute viral myocarditis and cytokine cardiomyopathy can induce a reduction of the estimated glomerular filtration rate (eGFR) through hemodynamic changes. Type 1 cardiorenal syndrome (CRS) can occur due to a cytokine storm or myocarditis and type 3 CRS can occur after the onset of AKI. Furthermore, upon the onset of sepsis, type 5 CRS can occur[41,42]. Right ventricular failure caused by pneumonia induced by SARS-CoV-2 and reduction of cardiac output due to left ventricle failure are also possible mechanisms of eGFR diminishment and AKI[43,44].
Data available refers to hospitalized patients, since AKI is a complication typical of moderate and severe cases of COVID-19 that require inpatient healthcare[45-50]. The incidence of AKI in COVID-19 is highly variable depending on the study analyzed. Our review found results between 5% and 75%, and the article with the largest sample size indicated a frequency of approximately 36%, whilst a systematic review of observational studies found an incidence of AKI of 11%[30,45-49,51-53].
A multicenter study showed that about 45% of the patients had no significant kidney injury caused by the viral illness; that 34% developed AKI without need for renal replacement therapy (RRT); and that 26% developed COVID-19 AKI with need of RRT (AKI-RRT)[44]. In contrast, a systematic review found that RRT was used in only 7% of COVID-19 cases with renal manifestations[51]. The modality of first choice is usually continuous renal replacement therapy, mostly because it is a suitable modality for hemodynamically unstable patients[54].
In a Chinese cohort study, three quarters of the patients had developed renal symptomatology, including proteinuria and/or hematuria, but only one in every ten patients had an AKI onset[55]. Out of all renal clinical findings, the most common ones were proteinuria, hematuria, elevated SCr and blood urea nitrogen, reduction of eGFR, and AKI[46,47,55-57]. One study found that three in every four patients that were at least moderately ill had renal involvement to some extent. These levels were as low as 62% in moderately (3.5% being AKI) ill patients and as high as 91% in critically ill patients (43% being AKI). It is suspected that most cases of AKI in COVID-19 occur due to intrinsic rather than prerenal mechanisms[55].
A multicenter American study found that patients who developed AKI because of SARS-CoV-2 infection were older and predominantly male individuals with higher levels of comorbidities associated with more severe cases of COVID-19, such as systemic arterial hypertension, diabetes mellitus, and heart failure. Additionally, the same article shows that patients who developed AKI were usually admitted to an intensive care unit (ICU) and were more likely to be on use of vasopressors (52.6% vs 3.4%) as well as mechanical ventilation (89.7% vs 21.7% in nonventilated patients), indicating that patients who developed AKI were critically ill. In that sample of AKI patients, about one third of patients died[46]. Independent risk factors for the development of COVID-19 associated AKI include pre-existing renal impairment (such as chronic kidney disease), hypertension, and inpatient diuretic use[45,51,52,57].
It is also known that there is an association between ARDS and AKI in general and it also applies to COVID-19 due to the release of inflammatory cytokines, especially IL-6[37-40]. This is clinically notable as well, since patients on mechanical ventilators are more likely to develop AKI with or without need of RRT[45-47]. Additionally, abnormal serum urea and serum creatinine values were associated in a bivariate Cox regression model with either ARDS development or progression from ARDS to death[58].
Laboratory examinations show that most AKI patients are admitted with abnormal kidney function, represented by high levels of SCr and low eGFR. Patients who do not develop AKI are admitted with higher levels of SCr and lower eGFR than at discharge, while AKI patients are discharged with worsened kidney function[45-47]. Patients who develop stage 3 AKI are usually discharged with a median SCr of 4.0 mg/dL and median eGFR of 14.0 mL/min/1.73 m², as opposed to a median SCr of 1.19 mg/dL and median eGFR of 62 mL/min/1.73m² at admission[46].
The management of COVID-19 associated AKI is a largely discussed theme among intensive care professionals. The 25th Acute Disease Quality Initiative Workgroup defined a few strategies for dealing with COVID-19 associated AKI[54]. The standard measures that have an evidence level of 1B or above include:
Measurement of kidney function through serum creatinine and urine output[56].
Use of dynamic assessment of cardiovascular status to mitigate the risk of AKI and ARDS, avoiding hemodynamic imbalance[56].
Volume expansion with balanced crystalloids to decrease the chances of developing AKI, unless there are indications for the use of other kinds of fluids[56].
Limit the patients’ exposure to nephrotoxins whenever possible and monitor their kidney functionality when the use of nephrotoxins is necessary[56].
When contrast media are required, optimize intravascular volume as a means to prevent AKI[56].
The prognosis of COVID-19 associated AKI and AKI-RRT is arguably poor. AKI was associated with a longer median hospital stay, which was approximately twice as long when compared to non-AKI patients[59]. One study found that mortality is about ten times higher in patients with moderate-to-severe COVID-19 who developed AKI in comparison to those who did not[55]. Another observational study concluded that AKI is almost 2.5 times more frequent in non-survivors than in survivors of critical COVID-19 cases[60]. It is also stated that AKI is an independent risk factor for 30 d mortality among COVID-19 patients[52].
Although remission from proteinuria and hematuria is a common outcome for patients with renal COVID-19 manifestations, less than half of AKI patients recover their kidney function[55]. Mortality rates were as high as 35% of AKI patients and use of RRT increases the lethality of the disease to levels over 60%. Furthermore, approximately one in every three RRT patients that were discharged remained RRT-dependent[46,47].
Patients with COVID-19 may experience diverse electrolyte disturbances with clinical impact. The main disorders are hyponatremia, hypokalemia, and hypocalcemia. The pathophysiological mechanisms are diverse and imply changes in the renin angiotensin aldosterone system as well as immuno-inflammatory phenomena underlying the coronavirus, which are generally associated with kidney and/or gastrointestinal damage[61]. Table 1 gives a general overview of the pathophysiology of the most frequent electrolyte disorders associated with COVID-19.
| Electrolyte disorder | Pathophysiological mechanisms |
| Hyponatremia | Syndrome of inappropriate secretion of antidiuretic hormone |
| Hypokalemia | Excessive aldosterone liberation, volume loss, and use of diuretics and glucocorticoids |
| Hypocalcemia | Vitamin D deficiency and parathyroid imbalance |
Hyponatremia is the most frequent electrolyte disorder in clinical practice, with a prevalence of 20% to 30% in hospitalized patients, and is defined by serum sodium levels below 135 mEq/L[62]. The association between pneumonia and hyponatremia was firstly described in 1962, mainly related to community-acquired pneumonia, which was later reported in other respiratory infections[63]. Thus, with the emergence of the COVID-19 pandemic, preliminary studies indicated that hyponatremia was one of the possible complications caused by the viral disease[64]. In general, COVID-19 patients with hyponatremia have more severe forms of the disease, with higher levels of hospitalization, when compared to normonatremic patients, both in infirmary and ICU beds. Most of these patients also have other markers of severity, such as higher levels of C-reactive protein (CRP), ferritin, and IL-6; consolidation lesions more present on chest CT; and greater need for oxygen support[65].
The pathophysiology of hyponatremia in patients with COVID-19 is considered multifactorial, but the main cause is the syndrome of inappropriate antidiuresis (SIAD). SIAD is characterized by hyponatremia (serum sodium less than 135 mEq/L) and elevated urinary osmolarity (> 100 mOsm/kg) compared to plasma osmolarity (< 280 mOsm/kg) in euvolemic patients that have normal renal, thyroid, hepatic, cardiac, and adrenal functions and are not on use of diuretics[66]. Despite its mechanism not being fully understood, SIAD in patients with COVID-19 is apparently related to elevated levels of IL-6, which induce the non-osmotic release of antidiuretic hormone. In addition, these cytokines can damage lung tissue and alveolar cells, generating hypoxic pulmonary vasoconstriction, which may induce SIAD[67]. Evidence demonstrates a directly proportional relationship between the serum sodium level and the PaO2/FiO2 ratio and an inversely proportional relationship between the serum sodium level and the IL-6 level[68]. Furthermore, some other factors may contribute to the secretion of this hormone, such as patients who experience fluid loss from vomiting and diarrhea (reported symptoms of COVID-19)[69].
Hypokalemia corresponds to the most frequent potassium disorder and is characterized by a serum concentration of potassium below 3.5 mEq/L. The presence of hypokalemia can be variable and data in the literature point to an incidence between 10% and 41% of patients who were hospitalized for COVID-19[70,71]. Furthermore, in another study carried out in Italy, hypokalemia was associated with a longer hospital stay[71].
There are many factors that can generate hypokalemia, so a precise mechanism for this complication in patients with COVID-19 has not yet been determined. However, some hypotheses can be taken into consideration, such as: (1) Viral interaction with its input receptor (ACE2), altering the classic renin-angiotensin-aldosterone pathway and stimulating the release of aldosterone, thus increasing potassium secretion in the urine; (2) Volume loss due to gastrointestinal symptoms caused by the viral infection, mainly diarrhea; and (3) Being secondary to the use of medications, such as diuretics and glucocorticoids[9,71].
The clinical manifestations of symptomatic hypokalemia include muscle weakness and fatigue. However, in more severe cases, low levels of potassium can cause cardiac arrhythmias with alterations on the electrocardiogram tracing, and respiratory muscular weakness[72]. Therefore, it is correct to say that hypokalemia can increase respiratory stress and the risk of cardiac injury[61]. Regarding coronaviruses, hypokalemia was reported upon the onset of SARS-CoV-1 infection, back in 2003, and was also described in some preliminary studies during the beginning of the COVID-19 pandemic[71].
Calcium plays an important role in the mechanism of entering a host cell and viral replication, something that was already reported in the pathophysiology of Ebola and SARS-CoV-1 viruses[73,74]. In addition, hypocalcemia represents an independent factor for increased mortality among critically ill patients with long hospital stay[75].
In a study carried out in China, the incidence of hypocalcemia in COVID-19 patients was 62.6%. Other laboratory findings included lymphocytosis and higher levels of CRP, D-dimer, and IL-6 when compared to the normocalcemic group. In addition, in that same study, the hypocalcemia group was more likely to have a poor outcome in comparison to the normocalcemic group (47.8% vs 25%, respectively)[76]. In another study carried out in Italy, the incidence of hypocalcemia in patients with COVID-19 was 78.6%, and this electrolyte imbalance also had a strong association with ICU admissions and death when compared to patients with normal calcium levels[77].
Parathyroid hormone and vitamin D play a key role in calcium metabolism. Patients with chronic hypovitaminosis D and who are affected by COVID-19 are more predisposed to hypocalcemia, as this vitamin alters calcium metabolism by reducing the intestinal absorption of calcium and phosphorus. These patients may have a compensatory tendency to secondary hyperparathyroidism, but this is not always sufficient to prevent hypocalcemia[78].
COVID-19 hypocalcemia has been associated with higher mortality rates when compared to other patients with respiratory conditions that have similar clinical manifestations. Hypocalcemia is also more incident and quantitatively significant in COVID-19 than in other infections. The main factors responsible for hypocalcemia in hospitalized patients include low dietary intake, hypoparathyroidism, hypoproteinemia, vitamin D deficiency, and drug interaction. However, when it comes to COVID-19, vitamin D deficiency and parathyroid imbalance are identified as the main causes of said electrolyte disorder[75]. Parathyroid gland function can be impaired during critical systemic illness and inflammatory response with increased circulating cytokines[78].
Besides the respiratory complications caused by the SARS-CoV-2 virus, infected patients are also subject to manifestations regarding other systems, such as the renal system. AKI is a multifactorial and fairly common complication in moderate-to-severe COVID-19. Patients that develop AKI due to COVID-19 are usually older males with other comorbidities and are usually admitted to ICUs. Clinical mana-gement involves measurement of kidney function, cardiovascular status assessment, volume expansion, and nephrotoxin exposure limitation, as well as standard AKI care measures. AKI also acts as a risk factor for death in SARS-CoV-2 infected patients, specially concerning those on RRT.
Hyponatremia, hypokalemia, and hypocalcemia are the most relevant electrolyte disorders in hospitalized patients with COVID-19. The cause of these laboratory alterations is multifactorial and may be secondary to renal and gastrointestinal lesions caused by inflammatory response, or even by path-ophysiological alterations caused by the entry mechanism of the virus. In patients with COVID-19, electrolyte disorders are associated with worse outcomes, with increased hospitalization length and mortality.
| 1. | Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, Zhang HY, Sun W, Wang Y. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92:577-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 886] [Cited by in RCA: 854] [Article Influence: 142.3] [Reference Citation Analysis (0)] |
| 2. | Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngology. 2020;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1729] [Cited by in RCA: 1755] [Article Influence: 292.5] [Reference Citation Analysis (1)] |
| 3. | Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori-Asenso R. Smell and Taste Dysfunction in Patients With COVID-19: A Systematic Review and Meta-analysis. Mayo Clin Proc. 2020;95:1621-1631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 283] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 4. | Moura-Neto JA, Divino-Filho JC, Ronco C. Nephrology Worldwide: the Vision, the Project, and the Mission. In: Moura-Neto JA, Divino-Filho JC, Ronco C, editors. Nephrology Worldwide. Basel: Springer Nature Switzerland AG, 2021. [DOI] [Full Text] |
| 5. | Nogueira GM, Oliveira MS, Moura AF, Cruz CMS, Moura-Neto JA. COVID-19 in dialysis units: A comprehensive review. World J Virol. 2021;10:264-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (2)] |
| 6. | Zhu J, Ji P, Pang J, Zhong Z, Li H, He C, Zhang J, Zhao C. Clinical characteristics of 3062 COVID-19 patients: A meta-analysis. J Med Virol. 2020;92:1902-1914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 422] [Cited by in RCA: 380] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 7. | Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1-138. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Lippi G, South AM, Henry BM. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann Clin Biochem. 2020;57:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 9. | Lim JH, Jung HY, Choi JY, Park SH, Kim CD, Kim YL, Cho JH. Hypertension and Electrolyte Disorders in Patients with COVID-19. Electrolyte Blood Press. 2020;18:23-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Faour WH, Choaib A, Issa E, Choueiry FE, Shbaklo K, Alhajj M, Sawaya RT, Harhous Z, Alefishat E, Nader M. Mechanisms of COVID-19-induced kidney injury and current pharmacotherapies. Inflamm Res. 2022;71:39-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238-33243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1545] [Cited by in RCA: 1617] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 12. | Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1-E9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2045] [Cited by in RCA: 2209] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 13. | Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure. Circ Res. 2016;118:1313-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 643] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 14. | Pan XW, Xu D, Zhang H, Zhou W, Wang LH, Cui XG. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 2020;46:1114-1116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 426] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 15. | Maruta H, He H. PAK1-blockers: Potential Therapeutics against COVID-19. Med Drug Discov. 2020;6:100039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4113] [Cited by in RCA: 4642] [Article Influence: 201.8] [Reference Citation Analysis (0)] |
| 17. | Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8473] [Cited by in RCA: 7688] [Article Influence: 1281.3] [Reference Citation Analysis (0)] |
| 18. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4193] [Article Influence: 190.6] [Reference Citation Analysis (1)] |
| 19. | Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schröder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB. Multiorgan and Renal Tropism of SARS-CoV-2. N Engl J Med. 2020;383:590-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1254] [Cited by in RCA: 1445] [Article Influence: 240.8] [Reference Citation Analysis (1)] |
| 20. | Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, Zhang C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1054] [Cited by in RCA: 1278] [Article Influence: 213.0] [Reference Citation Analysis (0)] |
| 21. | Diao B, Wang C, Wang R, Feng Z, Zhang J, Yang H, Tan Y, Wang H, Liu L, Liu Y, Wang G, Yuan Z, Hou X, Ren L, Wu Y, Chen Y. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun. 2021;12:2506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 275] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 22. | de Souza SP, Silveira MAD, Souza BSF, Cabral JB, de Melo EBDSG, Nonaka CKV, Coelho FO, da Hora Passos R. Evaluation of urine SARS-COV-2 RT-PCR as a predictor of acute kidney injury and disease severity in patients with critical COVID-19. J Int Med Res. 2021;49:3000605211015555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Wiese OJ, Allwood BW, Zemlin AE. COVID-19 and the renin-angiotensin system (RAS): A spark that sets the forest alight? Med Hypotheses. 2020;144:110231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 805] [Cited by in RCA: 905] [Article Influence: 150.8] [Reference Citation Analysis (0)] |
| 25. | Fraga-Silva RA, Pinheiro SV, Gonçalves AC, Alenina N, Bader M, Santos RA. The antithrombotic effect of angiotensin-(1-7) involves mas-mediated NO release from platelets. Mol Med. 2008;14:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Fraga-Silva RA, Costa-Fraga FP, De Sousa FB, Alenina N, Bader M, Sinisterra RD, Santos RA. An orally active formulation of angiotensin-(1-7) produces an antithrombotic effect. Clinics (Sao Paulo). 2011;66:837-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, Scalia R, Eguchi S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol Rev. 2018;98:1627-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1315] [Cited by in RCA: 1480] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 28. | Long DA, Price KL, Herrera-Acosta J, Johnson RJ. How does angiotensin II cause renal injury? Hypertension. 2004;43:722-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Ahmadian E, Hosseiniyan Khatibi SM, Razi Soofiyani S, Abediazar S, Shoja MM, Ardalan M, Zununi Vahed S. Covid-19 and kidney injury: Pathophysiology and molecular mechanisms. Rev Med Virol. 2021;31:e2176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 223] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 30. | Mohamed MMB, Lukitsch I, Torres-Ortiz AE, Walker JB, Varghese V, Hernandez-Arroyo CF, Alqudsi M, LeDoux JR, Velez JCQ. Acute Kidney Injury Associated with Coronavirus Disease 2019 in Urban New Orleans. Kidney360. 2020;1:614-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 31. | Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, Kochanek M, Böll B, von Bergwelt-Baildon MS. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 727] [Cited by in RCA: 1189] [Article Influence: 148.6] [Reference Citation Analysis (0)] |
| 32. | Chatenoud L, Ferran C, Reuter A, Legendre C, Gevaert Y, Kreis H, Franchimont P, Bach JF. Systemic reaction to the anti-T-cell monoclonal antibody OKT3 in relation to serum levels of tumor necrosis factor and interferon-gamma [corrected]. N Engl J Med. 1989;320:1420-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 197] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30462] [Article Influence: 5077.0] [Reference Citation Analysis (12)] |
| 34. | Law HK, Cheung CY, Ng HY, Sia SF, Chan YO, Luk W, Nicholls JM, Peiris JS, Lau YL. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 2005;106:2366-2374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 374] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 35. | Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10:102-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1000] [Cited by in RCA: 946] [Article Influence: 157.7] [Reference Citation Analysis (0)] |
| 36. | Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 3470] [Article Influence: 578.3] [Reference Citation Analysis (0)] |
| 37. | Panitchote A, Mehkri O, Hastings A, Hanane T, Demirjian S, Torbic H, Mireles-Cabodevila E, Krishnan S, Duggal A. Factors associated with acute kidney injury in acute respiratory distress syndrome. Ann Intensive Care. 2019;9:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 38. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6231] [Cited by in RCA: 6704] [Article Influence: 1117.3] [Reference Citation Analysis (1)] |
| 39. | Joannidis M, Forni LG, Klein SJ, Honore PM, Kashani K, Ostermann M, Prowle J, Bagshaw SM, Cantaluppi V, Darmon M, Ding X, Fuhrmann V, Hoste E, Husain-Syed F, Lubnow M, Maggiorini M, Meersch M, Murray PT, Ricci Z, Singbartl K, Staudinger T, Welte T, Ronco C, Kellum JA. Lung-kidney interactions in critically ill patients: consensus report of the Acute Disease Quality Initiative (ADQI) 21 Workgroup. Intensive Care Med. 2020;46:654-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 40. | Husain-Syed F, Slutsky AS, Ronco C. Lung-Kidney Cross-Talk in the Critically Ill Patient. Am J Respir Crit Care Med. 2016;194:402-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 187] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 41. | Rangaswami J, Bhalla V, Blair JEA, Chang TI, Costa S, Lentine KL, Lerma EV, Mezue K, Molitch M, Mullens W, Ronco C, Tang WHW, McCullough PA; American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Clinical Cardiology. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e840-e878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 877] [Article Influence: 146.2] [Reference Citation Analysis (0)] |
| 42. | Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal Syndrome [Internet]. J Am Coll Cardiol. 2008;52. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1271] [Cited by in RCA: 1479] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 43. | Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394:1949-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 1307] [Article Influence: 186.7] [Reference Citation Analysis (0)] |
| 44. | Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020;8:738-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 457] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 45. | Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1757] [Cited by in RCA: 1835] [Article Influence: 305.8] [Reference Citation Analysis (0)] |
| 46. | Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 939] [Cited by in RCA: 1037] [Article Influence: 172.8] [Reference Citation Analysis (1)] |
| 47. | Gupta S, Coca SG, Chan L, Melamed ML, Brenner SK, Hayek SS, Sutherland A, Puri S, Srivastava A, Leonberg-Yoo A, Shehata AM, Flythe JE, Rashidi A, Schenck EJ, Goyal N, Hedayati SS, Dy R, Bansal A, Athavale A, Nguyen HB, Vijayan A, Charytan DM, Schulze CE, Joo MJ, Friedman AN, Zhang J, Sosa MA, Judd E, Velez JCQ, Mallappallil M, Redfern RE, Bansal AD, Neyra JA, Liu KD, Renaghan AD, Christov M, Molnar MZ, Sharma S, Kamal O, Boateng JO, Short SAP, Admon AJ, Sise ME, Wang W, Parikh CR, Leaf DE; STOP-COVID Investigators. AKI Treated with Renal Replacement Therapy in Critically Ill Patients with COVID-19. J Am Soc Nephrol. 2021;32:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 48. | Fominskiy EV, Scandroglio AM, Monti G, Calabrò MG, Landoni G, Dell'Acqua A, Beretta L, Moizo E, Ravizza A, Monaco F, Campochiaro C, Pieri M, Azzolini ML, Borghi G, Crivellari M, Conte C, Mattioli C, Silvani P, Mucci M, Turi S, Tentori S, Baiardo Redaelli M, Sartorelli M, Angelillo P, Belletti A, Nardelli P, Nisi FG, Valsecchi G, Barberio C, Ciceri F, Serpa Neto A, Dagna L, Bellomo R, Zangrillo A; COVID-BioB Study Group. Prevalence, Characteristics, Risk Factors, and Outcomes of Invasively Ventilated COVID-19 Patients with Acute Kidney Injury and Renal Replacement Therapy. Blood Purif. 2021;50:102-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 49. | Chen YT, Shao SC, Hsu CK, Wu IW, Hung MJ, Chen YC. Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Crit Care. 2020;24:346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 50. | Fisher M, Neugarten J, Bellin E, Yunes M, Stahl L, Johns TS, Abramowitz MK, Levy R, Kumar N, Mokrzycki MH, Coco M, Dominguez M, Prudhvi K, Golestaneh L. AKI in Hospitalized Patients with and without COVID-19: A Comparison Study. J Am Soc Nephrol. 2020;31:2145-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 51. | Kunutsor SK, Laukkanen JA. Renal complications in COVID-19: a systematic review and meta-analysis. Ann Med. 2020;52:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (1)] |
| 52. | Jewell PD, Bramham K, Galloway J, Post F, Norton S, Teo J, Fisher R, Saha R, Hutchings S, Hopkins P, Smith P, Joslin J, Jayawardene S, Mackie S, Mudhaffer A, Holloway A, Kibble H, Akter M, Zuckerman B, Palmer K, Murphy C, Iatropoulou D, Sharpe CC, Lioudaki E. COVID-19-related acute kidney injury; incidence, risk factors and outcomes in a large UK cohort. BMC Nephrol. 2021;22:359. [PubMed] |
| 53. | Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, Paranjpe I, Somani S, Richter F, Miotto R, Lala A, Kia A, Timsina P, Li L, Freeman R, Chen R, Narula J, Just AC, Horowitz C, Fayad Z, Cordon-Cardo C, Schadt E, Levin MA, Reich DL, Fuster V, Murphy B, He JC, Charney AW, Böttinger EP, Glicksberg BS, Coca SG, Nadkarni GN; Mount Sinai COVID Informatics Center (MSCIC). AKI in Hospitalized Patients with COVID-19. J Am Soc Nephrol. 2021;32:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 498] [Article Influence: 99.6] [Reference Citation Analysis (0)] |
| 54. | Shemies RS, Nagy E, Younis D, Sheashaa H. Renal replacement therapy for critically ill patients with COVID-19-associated acute kidney injury: A review of current knowledge. Ther Apher Dial. 2022;26:15-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, Ma Z, Huang Y, Liu W, Yao Y, Zeng R, Xu G. Renal Involvement and Early Prognosis in Patients with COVID-19 Pneumonia. J Am Soc Nephrol. 2020;31:1157-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 559] [Article Influence: 93.2] [Reference Citation Analysis (0)] |
| 56. | Nadim MK, Forni LG, Mehta RL, Connor MJ Jr, Liu KD, Ostermann M, Rimmelé T, Zarbock A, Bell S, Bihorac A, Cantaluppi V, Hoste E, Husain-Syed F, Germain MJ, Goldstein SL, Gupta S, Joannidis M, Kashani K, Koyner JL, Legrand M, Lumlertgul N, Mohan S, Pannu N, Peng Z, Perez-Fernandez XL, Pickkers P, Prowle J, Reis T, Srisawat N, Tolwani A, Vijayan A, Villa G, Yang L, Ronco C, Kellum JA. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. 2020;16:747-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Costa RLD, Sória TC, Salles EF, Gerecht AV, Corvisier MF, Menezes MAM, Ávila CDS, Silva ECF, Pereira SRN, Simvoulidis LFN. Acute kidney injury in patients with Covid-19 in a Brazilian ICU: incidence, predictors and in-hospital mortality. J Bras Nefrol. 2021;43:349-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 58. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5573] [Article Influence: 928.8] [Reference Citation Analysis (1)] |
| 59. | Piñeiro GJ, Molina-Andújar A, Hermida E, Blasco M, Quintana LF, Rojas GM, Mercadal J, Castro P, Sandoval E, Andrea R, Fernández J, Badia JR, Soriano A, Poch E; Hospital Clínic Critical Care COVID-19 working group (CCCC). Severe acute kidney injury in critically ill COVID-19 patients. J Nephrol. 2021;34:285-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 60. | Ferrando C, Mellado-Artigas R, Gea A, Arruti E, Aldecoa C, Bordell A, Adalia R, Zattera L, Ramasco F, Monedero P, Maseda E, Martínez A, Tamayo G, Mercadal J, Muñoz G, Jacas A, Ángeles G, Castro P, Hernández-Tejero M, Fernandez J, Gómez-Rojo M, Candela Á, Ripollés J, Nieto A, Bassas E, Deiros C, Margarit A, Redondo FJ, Martín A, García N, Casas P, Morcillo C, Hernández-Sanz ML; de la Red de UCI Española para COVID-19. Patient characteristics, clinical course and factors associated to ICU mortality in critically ill patients infected with SARS-CoV-2 in Spain: A prospective, cohort, multicentre study. Rev Esp Anestesiol Reanim (Engl Ed). 2020;67:425-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Pourfridoni M, Abbasnia SM, Shafaei F, Razaviyan J, Heidari-Soureshjani R. Fluid and Electrolyte Disturbances in COVID-19 and Their Complications. Biomed Res Int. 2021;2021:6667047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 62. | Rondon-Berrios H, Agaba EI, Tzamaloukas AH. Hyponatremia: pathophysiology, classification, manifestations and management. Int Urol Nephrol. 2014;46:2153-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 63. | STORMONT JM, WATERHOUSE C. Severe hyponatremia associated with pneumonia. Metabolism. 1962;11:1181-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 64. | Aggarwal S, Garcia-Telles N, Aggarwal G, Lavie C, Lippi G, Henry BM. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): Early report from the United States. Diagnosis (Berl). 2020;7:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 263] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 65. | Atila C, Sailer CO, Bassetti S, Tschudin-Sutter S, Bingisser R, Siegemund M, Osswald S, Rentsch K, Rueegg M, Schaerli S, Kuster GM, Twerenbold R, Christ-Crain M. Prevalence and outcome of dysnatremia in patients with COVID-19 compared to controls. Eur J Endocrinol. 2021;184:409-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 66. | Fajri M, Essafti M, Aloua R, Mouaffak Y. Severe case of COVID -19 pneumonia complicated by SIADH. Ann Med Surg (Lond). 2022;73:103153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 2003;14:523-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 562] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 68. | Gheorghe G, Ilie M, Bungau S, Stoian AMP, Bacalbasa N, Diaconu CC. Is There a Relationship between COVID-19 and Hyponatremia? Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 69. | Khan AA, Ata F, Munir W, Yousaf Z. Fluid Replacement Versus Fluid Restriction in COVID-19 Associated Hyponatremia. Cureus. 2020;12:e9059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Mallow PJ, Belk KW, Topmiller M, Hooker EA. Outcomes of Hospitalized COVID-19 Patients by Risk Factors: Results from a United States Hospital Claims Database. J Health Econ Outcomes Res. 2020;7:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 71. | Alfano G, Ferrari A, Fontana F, Perrone R, Mori G, Ascione E, Magistroni R, Venturi G, Pederzoli S, Margiotta G, Romeo M, Piccinini F, Franceschi G, Volpi S, Faltoni M, Ciusa G, Bacca E, Tutone M, Raimondi A, Menozzi M, Franceschini E, Cuomo G, Orlando G, Santoro A, Di Gaetano M, Puzzolante C, Carli F, Bedini A, Milic J, Meschiari M, Mussini C, Cappelli G, Guaraldi G; Modena Covid-19 Working Group (MoCo19). Hypokalemia in Patients with COVID-19. Clin Exp Nephrol. 2021;25:401-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 72. | Unwin RJ, Luft FC, Shirley DG. Pathophysiology and management of hypokalemia: a clinical perspective. Nat Rev Nephrol. 2011;7:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 73. | Nathan L, Lai AL, Millet JK, Straus MR, Freed JH, Whittaker GR, Daniel S. Calcium Ions Directly Interact with the Ebola Virus Fusion Peptide To Promote Structure-Function Changes That Enhance Infection. ACS Infect Dis. 2020;6:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 74. | Millet JK, Whittaker GR. Physiological and molecular triggers for SARS-CoV membrane fusion and entry into host cells. Virology. 2018;517:3-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 203] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 75. | Martha JW, Wibowo A, Pranata R. Hypocalcemia is associated with severe COVID-19: A systematic review and meta-analysis. Diabetes Metab Syndr. 2021;15:337-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 76. | Liu J, Han P, Wu J, Gong J, Tian D. Prevalence and predictive value of hypocalcemia in severe COVID-19 patients. J Infect Public Health. 2020;13:1224-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 77. | Di Filippo L, Formenti AM, Rovere-Querini P, Carlucci M, Conte C, Ciceri F, Zangrillo A, Giustina A. Hypocalcemia is highly prevalent and predicts hospitalization in patients with COVID-19. Endocrine. 2020;68:475-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 78. | di Filippo L, Allora A, Locatelli M, Rovere Querini P, Frara S, Banfi G, Giustina A. Hypocalcemia in COVID-19 is associated with low vitamin D levels and impaired compensatory PTH response. Endocrine. 2021;74:219-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Brazilian Society of Nephrology; International Society for Hemodialysis; International Society of Nephrology; National Academy of Medicine (Brazil); American Society of Nephrology.
Specialty type: Virology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Naswhan AJ, Qatar; Singh N, United States S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL