Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.107839
Revised: April 22, 2025
Accepted: June 7, 2025
Published online: December 18, 2025
Processing time: 235 Days and 8.1 Hours
Brugada syndrome (BS) is a rare disorder affecting approximately 5 in every 10000 people. Reports of kidney donation in individuals with BS are exceptionally uncommon.
The decision to permit live kidney donation places significant responsibility on clinicians. Donors must adapt to life with a single kidney. While the risk of deve
Kidney donation in individuals with BS requires careful evaluation to mitigate the risk of fatal arrhythmias during surgical and anesthetic stress. With comprehensive preoperative assessment and vigilant perioperative management, kidney donation can be performed safely.
Core Tip: This case describes a successful kidney donation in a patient with newly diagnosed Brugada syndrome (BS). This case report describes the ethical dilemma and ensuring donor safety in the long run and shares our experience while managing a kidney donor with BS. To our knowledge, this is the first report describing kidney donation in patients with BS.
- Citation: Khalil MAM, Rajput AS, Khalil MSU, Ullah SR, Ghani R, Daiwajna RG, Hong LK, Tan J. Multidisciplinary care of kidney donation in Brugada syndrome: A case report. World J Transplant 2025; 15(4): 107839
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/107839.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.107839
Brugada syndrome (BS) is an autosomal dominant condition caused by mutations in sodium channels, predominantly affecting males more than females[1]. Incomplete penetrance is common, and up to 60% of the cases can be sporadic in nature[2]. Clinically, BS presents with a spectrum ranging from being asymptomatic to causing life-threatening ventri
Interestingly, BS has been described in patients with kidney dysfunction and kidney transplant recipients[4,5]. Various medications, particularly those administered perioperatively, have been implicated in triggering arrhythmias in individuals with BS. Among these, the use of propofol during anesthesia remains controversial. While some reports associate propofol with arrhythmias in BS patients[6], leading the Brugada Drug Advisory Board to recommend its avoidance[7], other studies suggest its safety in controlled doses. For instance, a prospective trial demonstrated that a single dose of propofol could be safely administered[8]. This conflicting evidence highlights the importance of meticulous anesthetic management in patients with BS. To date, there have been no documented cases of kidney donation from a donor with BS. We present a unique case of a kidney donor with newly diagnosed sporadic BS who successfully donated his kidney to his younger brother. This case illustrates the multidisciplinary approach involving teams from nephrology, cardiology, and anesthesiology to ensure a favorable outcome while mitigating the arrhythmia risks associated with BS.
A 43-year-old gentleman wanted to donate his kidney to his younger brother. At the time of evaluation, he had no complaints.
The patient was first evaluated in the donor clinic. There was no complaint on systemic inquiry, and he was totally asymptomatic.
He had undergone laparoscopic cholecystectomy in 2003. There was no history of chest pain, palpitation, shortness of breath, or syncopal episode.
He was an ex-smoker and quit smoking in 1988. There was no history of alcoholism or drug addiction. His father had type 2 diabetes. There was no family history of cardiac problems, arrhythmias, or syncopal episodes.
On examination, his regular heart rate was 80 beats per minute, and his jugular venous pressure was not elevated. His first and second heart sounds were normal, and his chest was clear. His body mass index was 29 kg/m2. Ambulatory blood pressure monitoring screening for hypertension was normal.
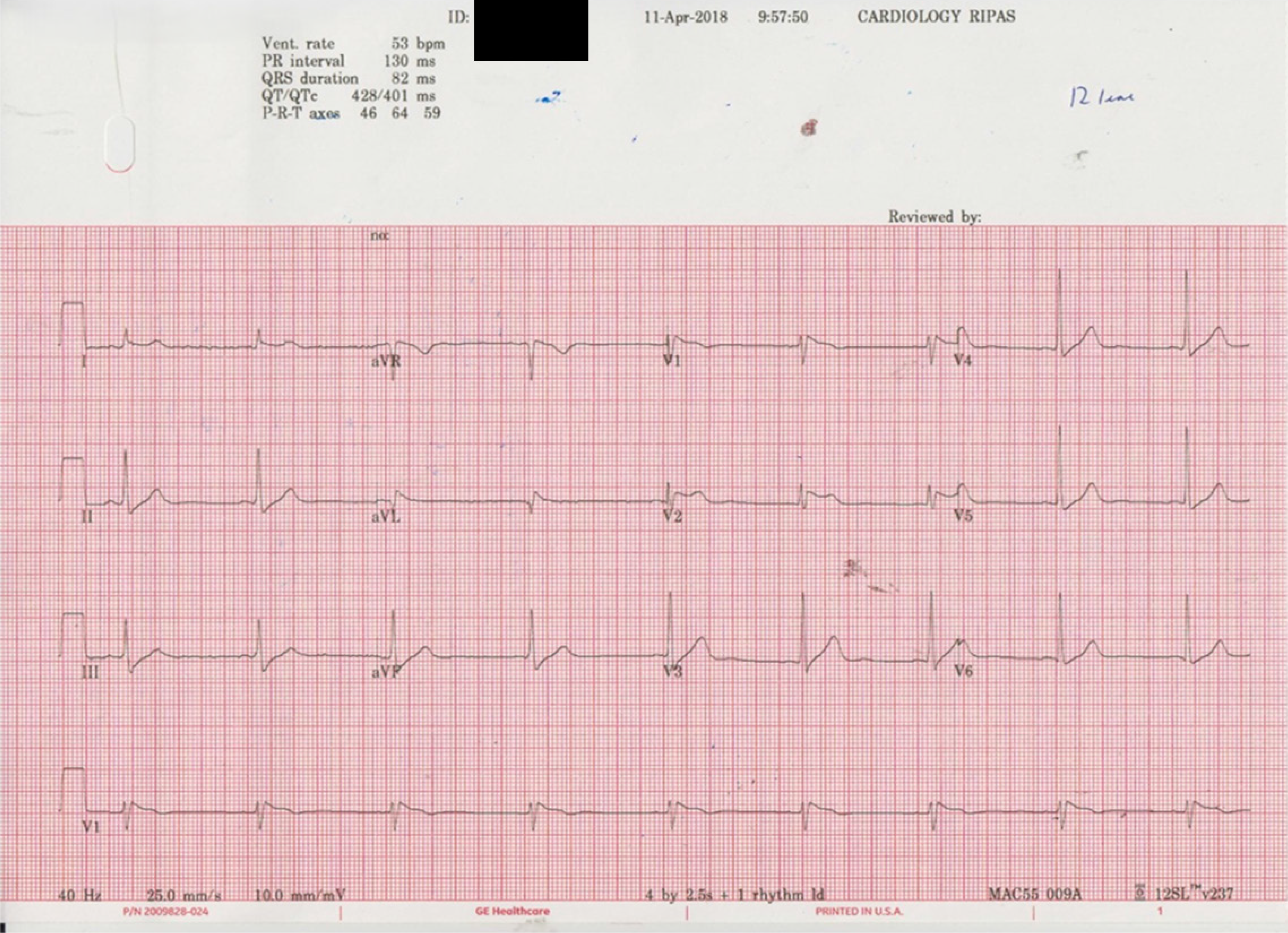
He has mild dyslipidemia, for which lifestyle modification was advised. There was no evidence of proteinuria, and his glomerular filtration rate (GFR) was 125 mL/minute on a 24-hour urine collection. His electrocardiogram showed a Brugada type 1 pattern (Figure 1). A flecainide challenge test with oral 200 mg revealed type 1 BS. There was no evidence of hyperkalemia or hypercalcemia. There was no family history or history of any medication intake. Echocardiography showed no abnormalities, with an ejection fraction of 62%. Holter monitoring was normal. His exercise tolerance test was negative. Electrophysiological testing was done in an angiography suite. A quadripolar catheter was inserted into the right ventricular apex and later moved to the right ventricular outflow tract. Standard 8-beat drive trains were used, and ventricular stimulation was negative up to 3 extras from 2 sites. DNA testing did not reveal mutations in BS-relation genes (SCNA, GPD1 L, CACNA1C, CACNB2, SCN1B, KCNE3, SCN3B, HCN4, KCNJ8, MOG1, KCND3, and KCNE5). After a thorough assessment, the patient was allowed to proceed with the donation.
As part of the cardiac work, a computer tomography angiogram was performed, which revealed normal coronary arteries.
The patient was thoroughly evaluated by a transplant nephrologist, electrophysiologist, anesthetist, and surgical team before being allowed to donate a kidney.
The final diagnosis was sporadic BS type 1 in a healthy kidney donor.
The ethical dilemma in this case was kidney donation in a sporadic BS type in a healthy donor. The transplant team was worried about the potential aggravation of arrhythmias during anesthesia or surgical stress. Secondly, the potential risk of chronic kidney disease (CKD) and electrolyte imbalance was another concern that could aggravate arrhythmias. After a multidisciplinary discussion, the patient was counseled about the pros and cons, and he agreed to donate. Ethical approval was obtained to allow donation. The decision was to take specific precautions during anesthesia and postoperatively observe the patient for 48 hours in the coronary care unit.
The anesthesia technique was specifically planned. A combination of general anesthesia and intrathecal morphine for intra- and post-operative pain relief was used. He was preloaded with an intravenous compound sodium lactate solution and monitored with an electrocardiogram, non-invasive blood pressure, invasive arterial blood pressure via the radial artery, and pulse oximetry. Spinal anesthesia was performed under a strict aseptic technique using 10 mg of 0.5% heavy bupivacaine with 200 mcg of morphine injected through a 25-G Sprotte spinal needle at the L3-L4 Level. This dosage of bupivacaine and intrathecal morphine is following our departmental guideline for intra- and postoperative analgesia when combined with general anesthesia.
Before induction, adhesive defibrillator pads were applied to the patient. Induction of anesthesia was achieved with fentanyl (150 mcg), ketamine (25 mg), 1.5% sevoflurane, and nitrous oxide, atracurium (40 mg). The trachea was intubated with a size 8 mm cuffed oral endotracheal tube with the aid of a bougie and cricoid pressure. Controlled ventilation was started with a tidal volume of 6-8 mL/kg body weight, and the respiratory rate was titrated to maintain an end-tidal carbon dioxide of 33-38 mmHg. General anesthesia was maintained with sevoflurane and then desflurane, using a carrier gas mixture of oxygen and nitrous oxide (50/50) to keep an inspired oxygen fraction of 0.4-0.5 and a minimal alveolar concentration of 1. Arterial blood gas analysis showed pH 7.374, pressure of CO2 40.6 mmHg, pressure of O2 218 mmHg, HCO3- 23.7 mmol/L, base excess 2, potassium 3.1 mmol/L. One gram of potassium chloride was replaced intravenously.
He underwent a right open donor nephrectomy successfully. Neuromuscular blockade was reversed with neostigmine (2.5 mg) and glycopyrronium (400 mcg). His oxygen saturation, heart rate, mean arterial pressure, and blood pressure remained stable throughout the procedure. He was extubated uneventfully. The patient was monitored in the coronary care unit for 48 hours postoperatively and was then transferred to the general ward.
The patient was discharged on day 6. Post-discharge, the patient was followed up in the transplant and cardiology clinic. Since 2019, he has been regularly monitored in the nephrology clinic with no active issues. During his most recent follow-up in December 2024, his creatinine level was 128 μmol/L, his GFR was 59 mL/minute/1.73 m2, his potassium level was 4.7 mmol/L, his sodium was 129 mmol/L, and the protein-to-creatinine ratio was 13. Table 1 shows the follow-up laboratory workup after donation.
| Date | Creatinine (μmol/L) | eGFR (mL/minute) | Electrolytes (mmol/L) | Protein to creatinine ratio (mg/mmol) | Blood pressure |
| May 18, 2019 | 159.2 | 45 | Potassium 4.3, sodium 138 | 10 | 125/75 |
| September 16, 2019 | 132 | 57 | Potassium 4.8, sodium 138 | 11.2 | 130/70 |
| May 18, 2020 | 135 | 55 | Potassium 4.1, sodium 137 | 11.5 | 120/75 |
| October 4, 2022 | 120 | 64 | Potassium 4.3, sodium 134 | 11 | 115/80 |
| March 31, 2023 | 134 | 56 | Potassium 4.5, sodium 136 | 15 | 125/85 |
| December 25, 2024 | 128 | 59 | Potassium 4.7, sodium 129 | 13 | 120/80 |
Kidney donation in patients with BS is rarely reported. Unlike other surgeries involving various pathological diseases, donor nephrectomy is performed on an otherwise healthy individual. This increases the responsibility of the transplant team to make decisions in the best interest of the kidney donor. Arrhythmias can occur in these patients during exposure to anesthetic agents or postoperative fever. Additionally, the potential future risk of developing CKD further increases the risk of arrhythmia in the future. The Amsterdam forum guidelines, endorsed by the World Health Organization in 2004, published extensive guidelines and emphasized donor safety[9]. Similarly, later on, the Istanbul declaration in 2008 again established guidelines endorsed by the World Health Organization and emphasized donor safety[10].
Patients with BS generally have an unremarkable perioperative course. However, conducting a thorough preoperative evaluation is essential to minimize the risk of arrhythmia. Electrophysiology evaluation should be performed to avoid tachyarrhythmias and sudden cardiac death[1]. It is crucial to take a detailed medication history to exclude drugs that can aggravate BS. Preoperative electrolyte panels are recommended to rule out hyperkalemia or hypercalcemia, as well as multi-lead electrocardiogram monitoring, temperature probes, invasive blood pressure monitoring, and a central venous line during surgery, to ensure close monitoring and safety[8]. During anesthesia, the vasogenic effects of medications, electrolyte imbalance, hemodynamic variations, and thermal changes can lead to potential arrhythmias[3]. These patients should be vigilantly monitored during anesthesia for heart rate and rhythm, body temperature, oxygen saturation, and blood pressure. Before induction, it is highly recommended to apply adhesive defibrillator pads[8]. Although there were initial safety concerns about the use of propofol[5,6], recent reports indicate its safe use in patients with BS[8,11]. A randomized controlled trial of 98 patients with established BS received either propofol or etomidate for the induction of anesthesia and did not find any significant difference in electrocardiographic changes in patients with BS[11]. Another prospective interventional study, having a sample size of 36 patients, also established propofol’s safety in BS[12]. There is, however, a need for future larger, prospective, multicenter, randomized control studies to investigate anesthetic agents and safety in patients with BS. In this case, induction of anesthesia was achieved with fentanyl 150, ketamine 25 mg, 1.5% sevoflurane, and nitrous oxide, and atracurium 40 mg. General anesthesia was maintained with sevoflurane and then desflurane, with a carrier gas mixture of oxygen and nitrous oxide (50/50) to keep an inspired oxygen fraction of 0.5 initially, then reduced to 0.4 and a minimal alveolar concentration of 1. The patient did not experience any complications during surgery, in the coronary care unit, or during his ward stay.
After donor nephrectomy, there is typically a 25%-40% reduction in GFR in the early postoperative period. The GFR loss never reaches 50% due to the compensatory hyperfiltration by the remaining kidney[13,14]. Kidney donors are among the healthiest individuals. Post-donation kidney donors are at a higher risk of developing CKD compared to healthy non-kidney donors[15]. Despite a decline in GFR, kidney donors fare better than patients with CKD who have the same GFR. Therefore, some argue that kidney donors should not be considered as CKD[16]. However, further research is needed to establish whether the decline in GFR in kidney donors is, in fact, CKD. Hyperkalemia typically occurs at a GFR of less than 15 mL/minute[17]. Although the future risk of GFR decreasing to this level is rare, it remains a legitimate concern. Therefore, discussing the potential risk of CKD and future arrhythmias is essential. Major guidelines such as the Amsterdam forum guidelines[9] and Kidney Disease: Improving Global Outcomes[18], lacking recommendations regarding rhythm problems, including BS. Consequently, there is a need for research and expert recommendations for patients with rare cardiac rhythm disorders.
There are some limitations in the genetic testing of our case, as it only covered 12 known BS-related genes, thereby posing a risk of missed diagnosis. Since the genetic mechanism of BS is complex, with potential unknown pathogenic genes or rare mutations, there is a possibility that we may have missed a rare mutation. We also acknowledge that the incidence of BS is low, and the successful experience of this case is difficult to verify through repeated studies. It is, therefore, impossible to rule out the possibility of “survivorship bias”.
We recommend the following: (1) Kidney donors with BS should be thoroughly counseled about the risk of arrhythmias during and after surgery; (2) Pre-donation GFR should be adequate to minimize the risk of future renal dysfunction; (3) The future risk of CKD and potential arrhythmias should be explained to the donor; (4) Kidney donors with BS should be evaluated by an electrophysiologist to assess the risk of arrhythmias; (5) A detailed drug history should be taken to avoid medications that are contraindicated in BS; (6) A multidisciplinary meeting including cardiologists, surgeons, nephrologists, and anesthetists should be held to plan for perioperative management; (7) It is essential to screen for electrolyte imbalances, particularly hyperkalemia and hypercalcemia; (8) These patients should be vigilantly monitored during anesthesia for heart rate and rhythm, body temperature, oxygen saturation, and invasive blood pressure; and (9) We recommend keeping the patient in the coronary care unit for 36-48 hours and regularly monitoring his renal function upon discharge.
Kidney donation is safe in kidney donors with asymptomatic BS. A pre-donation electrophysiological evaluation should assess the risk of developing arrhythmias. A multidisciplinary team should manage the patient, including nephrologists, cardiologists, surgeons, and anesthetists. After donation, long-term follow-up with regular monitoring of renal function and electrolytes is crucial.
We acknowledge the contribution of all team who contributed to the management of this patient.
| 1. | Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, Shimizu W, Schulze-Bahr E, Tan H, Wilde A. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1203] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 2. | Campuzano O, Brugada R, Iglesias A. Genetics of Brugada syndrome. Curr Opin Cardiol. 2010;25:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Li KHC, Lee S, Yin C, Liu T, Ngarmukos T, Conte G, Yan GX, Sy RW, Letsas KP, Tse G. Brugada syndrome: A comprehensive review of pathophysiological mechanisms and risk stratification strategies. Int J Cardiol Heart Vasc. 2020;26:100468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Ortega-Carnicer J, Benezet J, Ruiz-Lorenzo F, Alcázar R. Transient Brugada-type electrocardiographic abnormalities in renal failure reversed by dialysis. Resuscitation. 2002;55:215-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Davis E. Brugada Syndrome Unmasked by Fever but not Flecainide Challenge in a Renal Transplant Patient. Heart Lung Circ. 2017;26:S159. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Robinson JD, Melman Y, Walsh EP. Cardiac conduction disturbances and ventricular tachycardia after prolonged propofol infusion in an infant. Pacing Clin Electrophysiol. 2008;31:1070-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Postema PG, Wolpert C, Amin AS, Probst V, Borggrefe M, Roden DM, Priori SG, Tan HL, Hiraoka M, Brugada J, Wilde AA. Drugs and Brugada syndrome patients: review of the literature, recommendations, and an up-to-date website (www.brugadadrugs.org). Heart Rhythm. 2009;6:1335-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 8. | Sorajja D, Ramakrishna H, Poterack AK, Shen WK, Mookadam F. Brugada syndrome and its relevance in the perioperative period. Ann Card Anaesth. 2015;18:403-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Ethics Committee of the Transplantation Society. The consensus statement of the Amsterdam Forum on the Care of the Live Kidney Donor. Transplantation. 2004;78:491-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | International Summit on Transplant Tourism and Organ Trafficking. The Declaration of Istanbul on Organ Trafficking and Transplant Tourism. Clin J Am Soc Nephrol. 2008;3:1227-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Flamée P, Varnavas V, Dewals W, Carvalho H, Cools W, Bhutia JT, Beckers S, Umbrain V, Verborgh C, Forget P, Chierchia GB, Brugada P, Poelaert J, de Asmundis C. Electrocardiographic Effects of Propofol versus Etomidate in Patients with Brugada Syndrome. Anesthesiology. 2020;132:440-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Ciconte G, Santinelli V, Brugada J, Vicedomini G, Conti M, Monasky MM, Borrelli V, Castracane W, Aloisio T, Giannelli L, Di Dedda U, Pozzi P, Ranucci M, Pappone C. General Anesthesia Attenuates Brugada Syndrome Phenotype Expression: Clinical Implications From a Prospective Clinical Trial. JACC Clin Electrophysiol. 2018;4:518-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Kasiske BL, Anderson-Haag T, Israni AK, Kalil RS, Kimmel PL, Kraus ES, Kumar R, Posselt AA, Pesavento TE, Rabb H, Steffes MW, Snyder JJ, Weir MR. A prospective controlled study of living kidney donors: three-year follow-up. Am J Kidney Dis. 2015;66:114-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 14. | Garg AX, Muirhead N, Knoll G, Yang RC, Prasad GV, Thiessen-Philbrook H, Rosas-Arellano MP, Housawi A, Boudville N; Donor Nephrectomy Outcomes Research (DONOR) Network. Proteinuria and reduced kidney function in living kidney donors: A systematic review, meta-analysis, and meta-regression. Kidney Int. 2006;70:1801-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 284] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 15. | Muzaale AD, Massie AB, Wang MC, Montgomery RA, McBride MA, Wainright JL, Segev DL. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 735] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 16. | Matas AJ, Ibrahim HN. The unjustified classification of kidney donors as patients with CKD: critique and recommendations. Clin J Am Soc Nephrol. 2013;8:1406-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Palmer BF, Clegg DJ. Hyperkalemia across the Continuum of Kidney Function. Clin J Am Soc Nephrol. 2018;13:155-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Lentine KL, Kasiske BL, Levey AS, Adams PL, Alberú J, Bakr MA, Gallon L, Garvey CA, Guleria S, Li PK, Segev DL, Taler SJ, Tanabe K, Wright L, Zeier MG, Cheung M, Garg AX. KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation. 2017;101:S1-S109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/