Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.107728
Revised: April 14, 2025
Accepted: May 18, 2025
Published online: December 18, 2025
Processing time: 236 Days and 11.2 Hours
Pediatric kidney transplantation is the treatment of choice for children with end-stage renal disease; however, access to transplantation remains limited in low- and middle-income countries. Uzbekistan had no prior institutional experience in performing pediatric living donor kidney transplantation (LDKT).
To report the implementation, surgical protocols, and clinical outcomes of the first pediatric LDKT program in Uzbekistan.
This retrospective single-center study analyzed the first 20 pediatric LDKTs performed between April 2023 and February 2025. All donors were related family members who underwent either open or laparoscopic hand-assisted nephrectomy. Pre-transplant immunologic workup included HLA typing and anti-HLA anti
Donors included 13 women and 7 men (median age: 38 years; range: 31–50). Median operative times were 182.5 minutes for open nephrectomy and 198.5 minutes for laparoscopic nephrectomy. No major intraoperative complications occurred; one donor developed a postoperative wound seroma. All recipients (aged 87–207 months) exhibited immediate graft function, with no delayed graft function observed. Median cold and warm ischemia times were 15 minutes (range: 10–138) and 35 minutes (range: 18–40), respectively. Median serum creatinine decreased from 198 μmol/L on postoperative day 1 to 54 μmol/L by day 7. Three rejection episodes were reported, two of which occurred in sensitized recipients. Two graft losses were attributed to late rejection. One patient died from hemorrhagic stroke six months post-transplant. At 24 months, patient and graft survival rates were 95% and 90%, respectively.
The successful implementation of a pediatric living donor kidney transplantation program in Uzbekistan yielded favorable short- and intermediate-term outcomes, with high graft survival and low complication rates. This experience may provide a practical framework for initiating similar programs in other resource-constrained heal
Core Tip: This is the first report on the establishment of a pediatric kidney transplantation program from living-related donors in Uzbekistan. We present detailed surgical techniques, immunological screening protocols, and early clinical outcomes based on the first 20 transplantations. All recipients demonstrated immediate graft function without delayed graft function. At 24 months, patient survival was 95% and graft survival was 90%. This study demonstrates the feasibility, safety, and effectiveness of introducing pediatric kidney transplantation in a limited-resource setting, and may serve as a practical model for developing similar programs in other low- and middle-income countries.
- Citation: Semash K, Akhmedov A, Dzhanbekov T, Umarov Q, Dustmurodov J. Implementation of a pediatric kidney transplantation program in Uzbekistan: Feasibility and early outcomes. World J Transplant 2025; 15(4): 107728
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/107728.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.107728
End-stage chronic kidney disease (CKD 5) in children presents a significant medical challenge, requiring renal replacement therapy. Despite advancements in peritoneal dialysis and hemodialysis, kidney transplantation remains the gold standard of treatment, significantly improving both life expectancy and quality of life for pediatric patients[1,2]. In recent decades, the implementation of modern immunosuppressive protocols, advancements in surgical techniques, and improved post-transplant management have led to high 1- and 5-year graft survival rates in children[3].
Uzbekistan has already established a successful kidney and liver transplantation program, which has significantly improved access to organ transplantation and clinical outcomes in adult patients[4,5]. However, pediatric kidney transplantation has remained an underdeveloped field. One of the key challenges of pediatric transplantation in low-resource countries includes delayed CKD diagnosis, a shortage of donor organs, and the necessity for long-term post-transplant monitoring[6]. These challenges are also relevant for Uzbekistan, where pediatric kidney transplantation is only beginning to be systematically practiced.
This study represents the first systematic analysis of 20 pediatric kidney transplants performed in Uzbekistan at the National Children’s Medical Center. The primary objective is to evaluate early surgical and functional outcomes, analyze the incidence of postoperative complications, and identify factors influencing graft function. Furthermore, we aim to compare the obtained results with international data and highlight the key challenges faced by pediatric kidney transplantation in the region.
We have carefully started the pediatric living-related donor kidney transplant (LDKT) program in the Republic of Uzbekistan since April 2023. A retrospective review and analysis were performed on prospectively collected data from our database on surgeries performed from July 2023 to February 2025. The median follow-up period was 17.5 months (range 2–24 months). The study was approved by the local ethics committee.
During the study period, we performed 20 LDKT in pediatric patients. There were 12 (60%) male and 8 (40%) female recipients. Median age was 167 (87-207) months old. The causes of kidney failure were congenital anomalies of the kidney and urinary tract in 8 (40%) patients, tubulointerstitial nephropathies in 5 (25%) patients, glomerular diseases in 5 (25%) patients, toxic kidney injury in 1 сase (5%), and renal hypoplasia associated with Russell–Silver syndrome in 1 (5%) patient. The majority of patients received maintenance hemodialysis as the initial modality of renal replacement therapy. Comorbid conditions, such as symptomatic arterial hypertension, chronic diseases, and infectious disorders, were evaluated and managed conservatively before the transplant procedure. Additionally, all recipients were tested for preformed antibodies, crossmatch compatibility, and HLA typing with the potential donor to assess the risk of immunological complications following kidney transplantation. The purpose of this assessment was to identify sensitized patients at increased immunological risk and to guide immunosuppressive management and donor-recipient compatibility. No ABO-incompatible transplantations were performed in this series. Patient baseline demographic and clinical characteristics are summarized in Table 1.
| Variables | Value (n = 20) |
| Age, month, median (range) | 167 (87-207) |
| Sex | |
| Male | 12 (60) |
| Female | 8 (40) |
| Indications for transplant | |
| CAKUT | 8 (40) |
| Tubulointerstitial nephritis | 5 (25) |
| Glomerulonephritis | 5 (25) |
| Toxic kidney injury | 1 (5) |
| Russell-Silver syndrome nephropathy | 1 (5) |
| AH before transplantation | 12 (60) |
| Dialysis before transplantation | 17 (85) |
| Time on dialysis before transplantation, month, median (range) | 5 (2-66) |
| Urea before transplantation, mmol/L, median (range) | 21 (10.9-35) |
| Creatinine before transplantation, µmol/L, median (range) | 503.5 (211-1230) |
| Pre-existing antibodies | 6 (30) |
| Operation time, minutes, median (range) | 237.5 (195-380) |
| Side of graft placement | |
| Right | 18 (90) |
| Left | 2 (10) |
| Native kidneys nephrectomy | 2 (10) |
| Cold ischemia time, minutes, median (range) | 15 (10-138) |
| Warm ischemia time, minutes, median (range) | 35 (18-40) |
| Kidney graft immediate function after reperfusion | 20 (100) |
| Creatinine 1 p/o day, µmol/L, median (range) | 198 (24-695) |
| Creatinine 3 p/o day, µmol/L, median (range) | 57(19-479) |
| Creatinine 5 p/o day, µmol/L, median (range) | 74.5 (21-166) |
| Creatinine 7 p/o day, µmol/L, median (range) | 54 (22-188) |
| Urea 1 p/o day, mmol/L, median (range) | 13.5 (2.9-23.3) |
| Urea 3 p/o day, mmol/L, median (range) | 6.3 (1.6-13.4) |
| Urea 5 p/o day, mmol/L, median (range) | 5.7 (1.9-17.2) |
| Urea 7 p/o day, mmol/L, median (range) | 5.6 (2-10.2) |
| Diuresis 1 p/o day, ml, median (range) | 5080 (3500-13000) |
| Diuresis 3 p/o day, ml, median (range) | 5665 (3240-9770) |
| Diuresis 5 p/o day, ml, median (range) | 3855 (2400-6550) |
| Diuresis 7 p/o day, ml, median (range) | 3000 (2400-7800) |
| Early complications, Clavien–Dindo | |
| Grade 2 (Rejection) | 1 (5) |
| Grade 4a (Hypotension and respiratory insufficiency) | 1 (5) |
| CCI for early complications, median | 0 (0-42.4) |
| Late complications, Clavien–Dindo | |
| Grade 2 (Rejection) | 1 (5) |
| Grade 3b (Rejection, graft nephrectomy) | 1 (5) |
| Grade 5 (Hemorrhagic stroke) | 1 (5) |
| CCI for early complications, median | 0 (0-100) |
| LOS, days, median (range) | 11 (7-15) |
| In-hospital survival | 20 (100) |
| Overall survival | 19 (95) |
| Cause of death, n | |
| Hemorrhagic stroke | 1 |
| Graft survival | 18 (90) |
| Cause of graft loss | |
| Rejection | 2 |
| Follow-up period, month, median (range) | 17 (2-24) |
All the recipients received kidney grafts from their living donors. According to the laws of the Republic of Uzbekistan, only biological relatives of the patient and spouses are eligible to serve as organ donors[6]. All donations were from biological relatives. The degree of relationship between the donors and the recipients was distributed as follows: 12 mothers, 6 fathers, 1 uncle, and 1 aunt.
All living-related kidney donors for pediatric recipients were selected based on rigorous, standardized criteria, optimized for our center[7]. Donor eligibility included first- or second-degree biological relationship, age between 18 and 50 years, voluntary informed consent without coercion or financial incentives, and comprehensive ABO blood group and immunological matching with a mandatory negative cross-match. Medical exclusion criteria encompassed significant chronic conditions, such as uncontrolled hypertension, diabetes mellitus, malignancies, autoimmune diseases, metabolic disorders, and active infections (hepatitis B virus, hepatitis C virus, human immunodeficiency virus, syphilis, tuber
All donors except one were evaluated for left-sided nephrectomy, as the left kidney was the preferred graft due to its longer renal vein, which facilitates vascular anastomosis during transplantation. To assess the vascular anatomy of the left kidney, all donors underwent preoperative CT angiography with intravenous contrast. This imaging modality provided detailed visualization of the renal vasculature, allowing for the identification of potential anatomical variations, such as multiple renal arteries or early arterial branching, which could impact surgical planning.
Potential donors were excluded from kidney donation if they presented with any of the following contraindications. Uncontrolled hypertension, particularly if resistant to treatment, was considered a significant risk factor. ABO incompatibility between the donor and recipient, which could not be resolved through desensitization protocols, was another reason for exclusion. Chronic infectious diseases that posed risks for both the donor and recipient, as well as active or past oncological diseases, were considered contraindications. Significant metabolic disorders, such as diabetes mellitus or severe glucose intolerance, also led to exclusion from donation. Other comorbidities that could impact donor safety or long-term renal function post-nephrectomy were carefully assessed. In addition to medical factors, non-medical concerns, including personal apprehensions, psychological barriers, and familial pressures, were taken into account, as some donors voluntarily opted out before surgery.
For perioperative management of donors, we are using Enhanced Recovery After Surgery Society Recommendations (Guidelines for Perioperative Care for Surgery: Enhanced Recovery After Surgery)[8].
Donor nephrectomy: The graft type in our series was the left kidney in all cases except one. In most cases, donor nephrectomy was performed using the traditional open surgical approach via a lumbotomy incision. The patient was positioned on the right side with a bolster placed under the left flank to optimize access to the kidney. A 12–15 cm incision was made under the 11th–12th rib. The retroperitoneal fat was dissected and mobilized, and the renal fascia was incised. The left kidney was carefully dissected and mobilized, with maximal exposure of the renal vessels and ureter. The renal artery, vein, and ureter were then ligated and clipped, following which the kidney was retrieved and transferred to the preservation table for further processing.
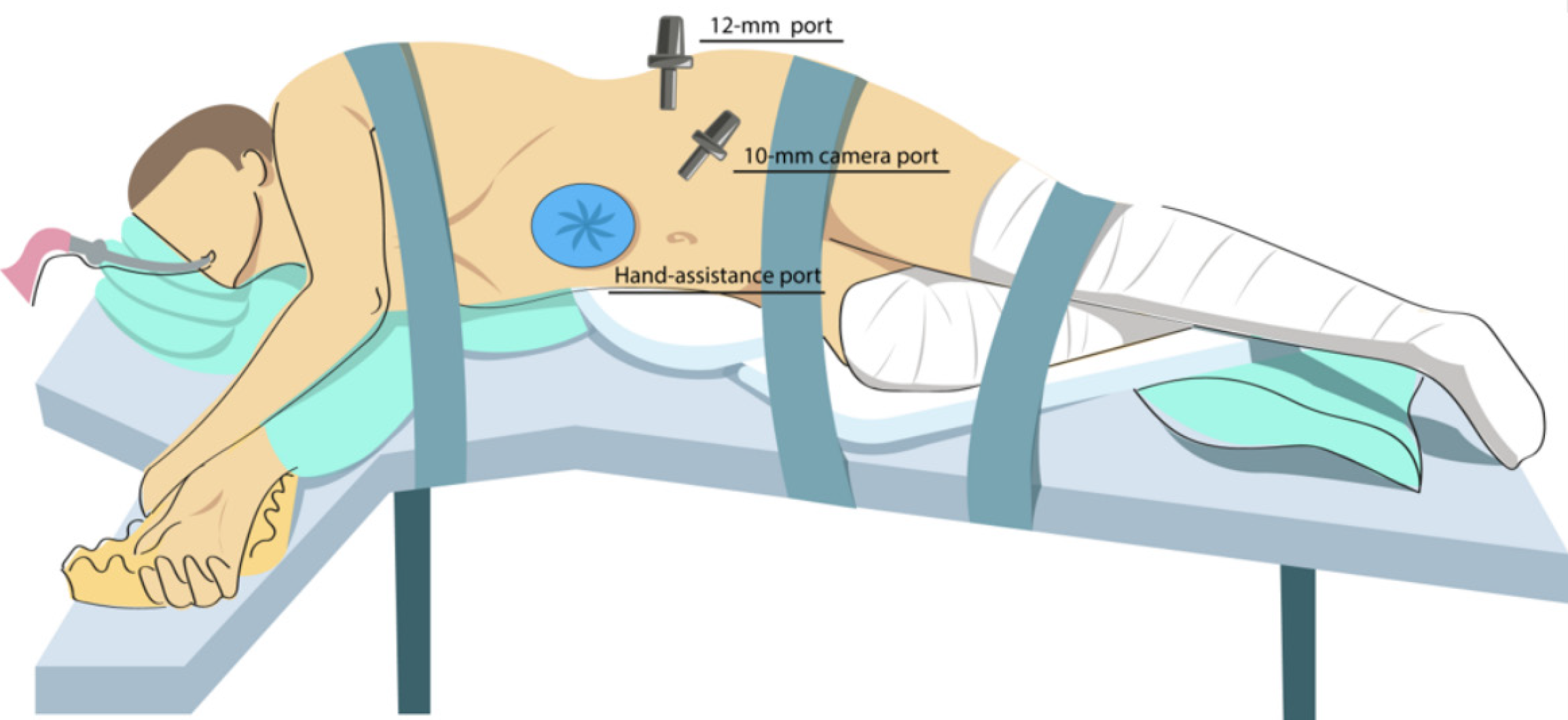
For laparoscopic nephrectomy with hand-assisted technique, the patient was positioned in a right lateral decubitus position with bolsters placed under the lumbar region to enhance exposure. A 7–10 cm paraumbilical incision was made for the insertion of a hand-access port. Subsequently, trocars were placed, as illustrated in Figure 1. Carboxyperitoneum was established, maintaining an intra-abdominal pressure of 12 mmHg. Mobilization of the left kidney was then performed, involving dissection and coagulation of the renal fascia. The ureter and the gonadal vein were dissected along their entire course. The renal artery and vein were also completely mobilized. The ureter was ligated as distally as possible, while the renal artery and vein were clipped at their most distal points using two Hem-o-lok clips. The kidney was then extracted through the hand-assist port and transferred for cold preservation. Hemostasis was ensured, and a drain was placed in the renal bed when indicated.
Histidine-tryptophan-ketoglutarate preservation solution was used in all cases. After flushing with a preservation solution, the renal artery and vein were carefully cleaned of perinephric fat and inspected for any traumatic damage or intimal tears. Any excess perinephric fat was then removed to optimize vascular exposure. The kidney graft was subsequently placed in a sterile container filled with preservation solution at 4°C and transferred for implantation into the recipient.
Surgery in recipients: In kidney transplant recipients, native nephrectomy was not routinely performed, except in cases of chronic kidney infection or severe nephrolithiasis. In most cases, the transplant was implanted in the right iliac fossa. Surgical access to the iliac vessels was achieved through a retroperitoneal pararectal approach, allowing for adequate exposure of the iliac arteries and veins. Once the iliac vessels were dissected and prepared, the kidney graft was placed into the surgical field and surrounded by ice to maintain hypothermia. Arterial anastomosis was performed in an end-to-side configuration using a continuous suture with Prolene 7/0, while venous anastomosis was created in an end-to-side manner using a continuous suture with Prolene 6/0. In cases where the renal allograft had two arteries, two separate arterial anastomoses were performed with the recipient's iliac artery. During reperfusion, efforts were made to increase systolic blood pressure to 130–140 mmHg to optimize graft perfusion. Following reperfusion, the primary function of the graft was assessed. Ureteral reconstruction was performed using the Lich-Gregoir technique, with routine ureteral stenting to ensure adequate drainage and prevent ureteral complications. Finally, a drain was placed in the transplant site to monitor for postoperative fluid collection.
Antithymocyte globulin (ATG) was administered to induce immunosuppression. Thereafter, methylprednisolone (10 mg/kg) was administered after reperfusion. The basic immunosuppressive regimen included tacrolimus and low-dose methylprednisolone. Mycophenolate mofetil was prescribed also. The target serum tacrolimus concentration was 6-9 ng/mL. Side effect profiles of the immunosuppressants were considered for drug discontinuation or conversion.
In the early postoperative period, patients were monitored in the intensive care unit (ICU). Extubation was performed within a few hours after surgery or, in some cases, directly on the operating table. During this phase, continuous cardiac monitoring was conducted, along with daily assessments of body temperature and blood tests to evaluate graft function. Electrolyte levels were monitored every six hours, and hourly urine output was recorded. In cases of polyuria, additional fluid resuscitation was administered to maintain hemodynamic stability. Doppler ultrasound of the kidney graft was performed daily to assess vascular flow and graft perfusion. All patients received thromboprophylaxis with low-molecular-weight heparin starting from the first postoperative day. Additionally, antibiotic prophylaxis was provided using Sulperazone to prevent infections. Patients remained in the ICU until the resolution of the polyuric phase, after which they were transferred to a regular ward for continued recovery. If no complications occurred, they were discharged under ambulatory follow-up. The ureteral stent was removed 21 days postoperatively, provided there were no complications.
Baseline variables, such as age (years), sex, and operation date, were analyzed for the patients. Postoperative complications were assessed using the Clavien–Dindo classification[8], and the comprehensive complication index (CCI) was calculated for these cases[9]. We considered the outcome during hospitalization as short-term results. Long-term outcomes cover over a 2-year follow-up.
Continuous variables are presented as medians and ranges. Categorical variables are expressed as numbers and percentages. Categorical variables were compared using Fisher’s exact test due to the small sample size and low event rate. Patient survival rates were generated using the Kaplan-Meier method. The Mann–Whitney U test was used to compare operative times between groups, as data were not normally distributed and the laparoscopic group size was limited. P values < 0.05 were considered statistically significant. Statistical processing was carried out using IBM SPSS 26 (United States) software.
The median operative time in recipients was 237.5 (195-380) minutes. The median cold ischemia time was 15 minutes (range: 10–138 minutes), and the median warm ischemia time was 35 minutes (range: 18–40 minutes). In 2 cases (10%), the renal graft was implanted on the left side. In one case, the right renal fossa was infected following a nephrectomy due to chronic pyelonephritis associated with vesicoureteral reflux. In the second case, the donor’s left kidney had a short renal artery, and the right kidney was procured; for anatomical congruence, the graft was implanted on the left side. Bilateral native nephrectomy was performed in two patients due to chronic urinary tract infections. In two cases, the graft had two renal arteries, and two separate arterial anastomoses were constructed with the recipient’s iliac artery. Immediate graft function was observed in 100% of cases after reperfusion, as evidenced by prompt urine output.
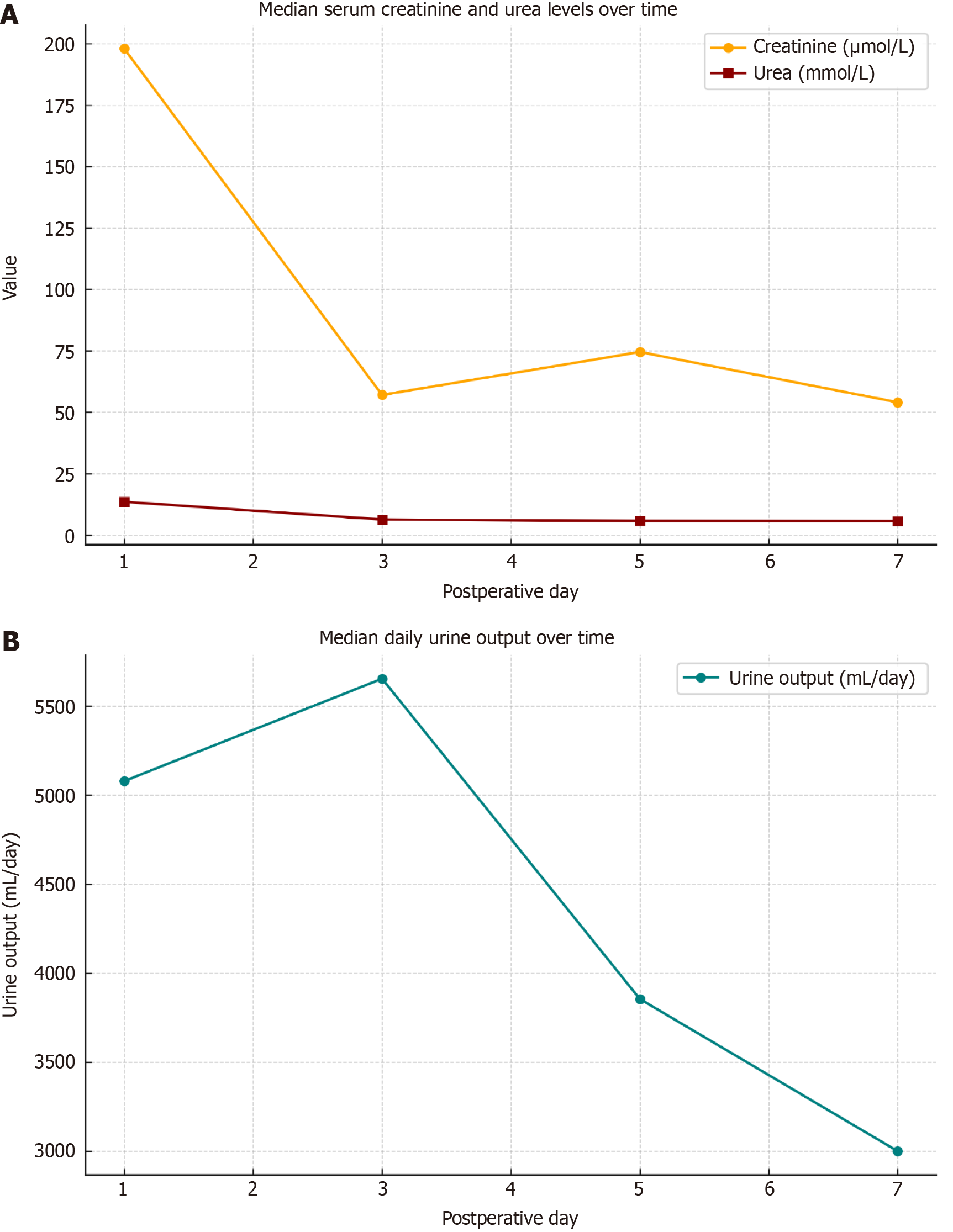
Figure 2 illustrates the dynamics of kidney function recovery during the first postoperative week. The upper panel shows a marked decrease in median serum creatinine from 198 μmol/L on day 1 to 57 μmol/L on day 3, with subsequent stabilization by day 7 (54 μmol/L). Serum urea also demonstrated a consistent downward trend from 13.5 mmol/L on day 1 to 5.6 mmol/L on day 7, indicating early metabolic improvement and graft function.
The lower panel presents the median daily urine output, which remained within the polyuric range throughout the observation period. A peak was observed on postoperative day 3 (5650 mL/day), followed by a gradual decline to 3000 mL/day by day 7. This trend is consistent with the typical polyuric phase of immediate graft function.
Complications: All complications were divided into early and late and are summarized in Table 2. Early postoperative complications occurred in two recipients, with CCI values of 42.4 and 20.9, respectively. Late complications were observed in three recipients, with CCI scores of 33.7, 20.9, and 100. The median CCI for both early and late complications was 0, reflecting the low overall complication rate in the cohort. The mean CCI was 3.2 for early complications and 7.7 for late complications.
| Variables | Value (n = 20) |
| Age, years, median (range) | 38 (31-50) |
| Sex | |
| Male | 7 (35) |
| Female | 13 (65) |
| BMI, kg/m2, median (range) | 27.4 |
| Operation time, minutes, median (range) | |
| Open | 182.5 (110-260) |
| Laparoscopic with manual assistance | 198.5 (190-230) |
| Complications, Clavien–Dindo | |
| Grade 1 | |
| Wound seroma | 1 (5) |
| Median CCI | 0 (0-8.7) |
| LOS, days | 7 (4-12) |
Among the analyzed observations, an acute rejection crisis occurred in one patient on the 5th day after surgery. Rejection was manifested by an increase in serum creatinine and urea levels, accompanied by a decrease in hourly urine output. A positive result of pulse therapy with methylprednisolone was achieved. Chronic rejection occurred in two patients at 4.5- and 6.5-month post-transplantation, respectively. Both patients received treatment with methylprednisolone and ATG, but the therapy was ineffective. Subsequently, they developed anuria, and graft function was lost. At present, both patients are alive, followed up in the outpatient setting, and maintained on chronic hemodialysis.
Pre-existing anti-HLA antibodies were identified in 6 patients (30%). Rejection occurred in 3 patients overall, of whom 2 had pre-existing antibodies. Although a higher proportion of rejection was observed in sensitized recipients (33% vs 7%), this difference did not reach statistical significance (P = 0.202).
No occlusive vascular complications were observed in our series. On postoperative day 4, one patient developed a reduction in graft arterial blood flow as detected by Doppler ultrasound, accompanied by a mild increase in serum creatinine. This was associated with hypotension and respiratory insufficiency, requiring transfer to the ICU. The patient received cardiotonic support and supplemental oxygen. In addition, intravenous heparin boluses were administered on top of standard anticoagulation therapy, resulting in restoration of adequate arterial perfusion and normalization of laboratory parameters. The clinical condition stabilized within 24 hours, and the patient was transferred back to the general ward. This complication was classified as Grade IVa according to the Clavien–Dindo classification.
At 6 months post-transplantation, one patient developed seizures requiring admission to the intensive care unit. Initially, the seizures were attributed to tacrolimus neurotoxicity; however, further evaluation revealed a massive hemorrhagic stroke. Despite intensive treatment, the patient died one month later. Subsequent investigation revealed that the child had a pre-existing diagnosis of epilepsy, which the parents had intentionally withheld prior to transplantation due to fear of being denied the procedure. The patient had not been receiving antiepileptic therapy before or after the transplant, reflecting significant non-compliance.
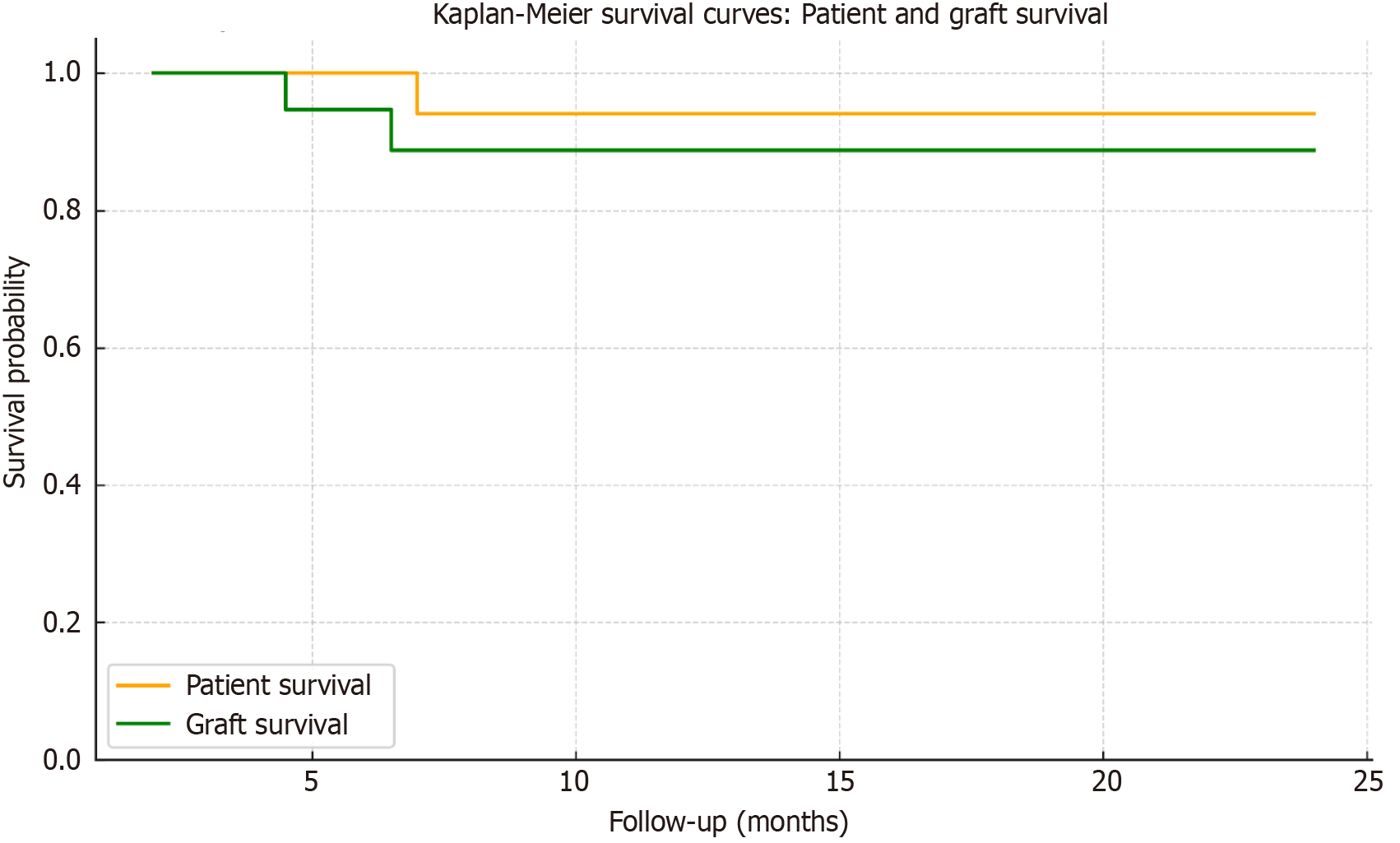
Mortality and graft survival: There was no in-hospital mortality; all patients were discharged. The median length of hospital stay was 11 days (range: 7–15 days). During the follow-up period, one patient died. As described above, the cause of death was a massive hemorrhagic stroke. Two patients experienced graft loss due to chronic rejection. Patient survival and graft survival rates are presented in Figure 3.
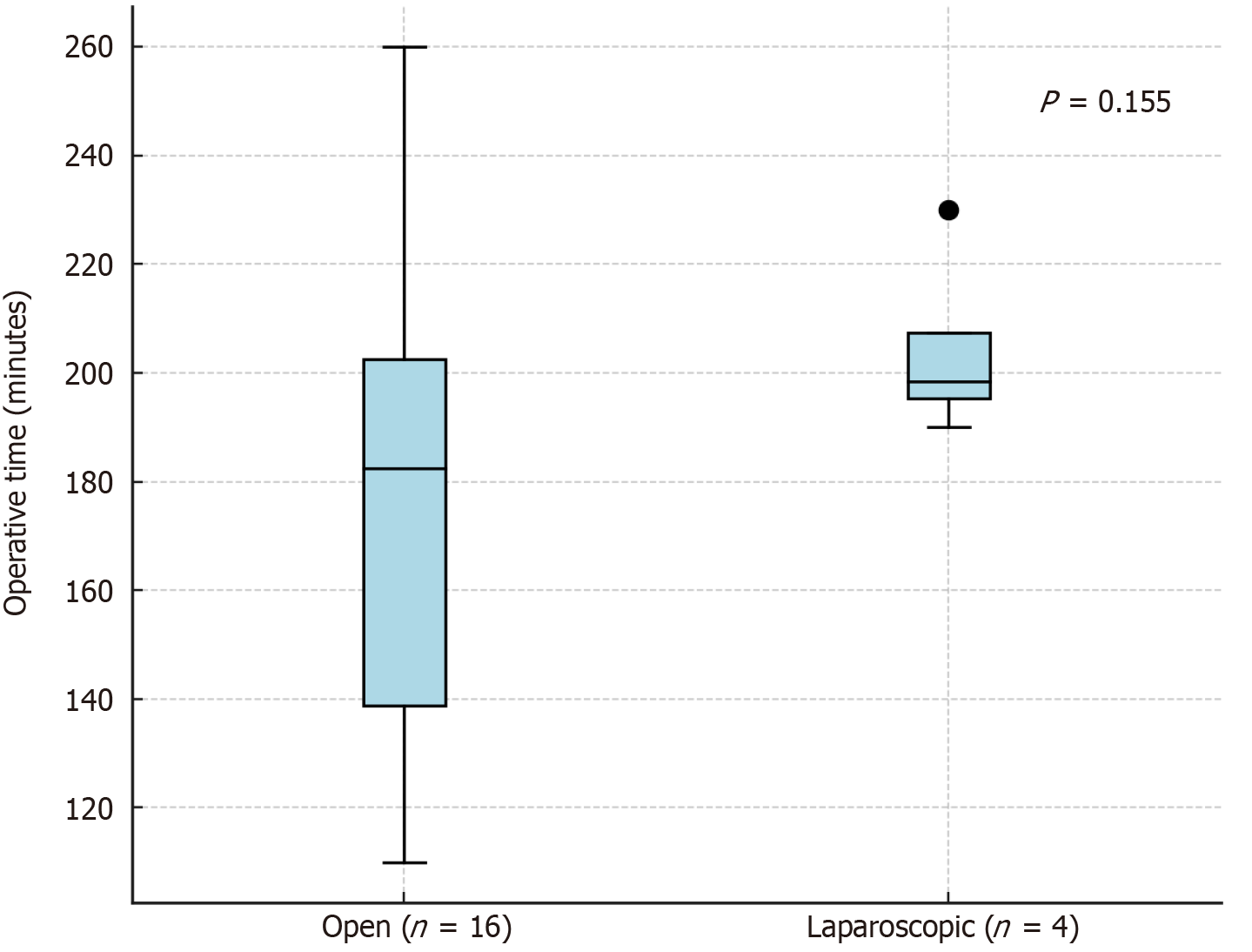
Among the donors, 13 (65%) were women and 7 (35%) were men. The median age of donors was 38 years (range: 31–50 years). The median BMI was 27.4 kg/m2. Regarding perioperative data, the median operative time for open donor nephrectomy was 182.5 minutes (range: 110–260), while for laparoscopic donor nephrectomy with hand assistance, it was 198.5 minutes (range: 190–230) (Figure 4).
No significant complications were observed among the donors. Only one donor experienced a minor postoperative complication—wound seroma at the site of the hand-assist port (Clavien–Dindo Grade I; CCI 8.7), resulting in a median donor CCI of 0 and a mean of 0.4. Median length of stay after donor nephrectomy was 7 (4-12) days. All donors were followed by a nephrologist at our center for three months after discharge, and no late complications were reported during the follow-up period. All perioperative data related to the donors are summarized in Table 2.
To the best of our knowledge, this is the first published report on the implementation of a pediatric living donor kidney transplantation program in Uzbekistan. The presented results demonstrate that establishing such a service is feasible and safe even in a resource-limited setting, with favorable short-term outcomes in terms of both graft function and patient survival.
Our findings confirm the safety and effectiveness of pediatric kidney transplantation from living donors.
Outcomes among pediatric recipients in our cohort were favorable and comparable to those reported by established international centers. All patients demonstrated immediate graft function, with no cases of delayed graft function, which is typically reported in 5%–10% of pediatric recipients depending on donor type and ischemia time[9]. Median serum creatinine decreased from 503.5 μmol/L before transplantation to 54 μmol/L by postoperative day 7, accompanied by a marked decline in serum urea levels and sustained high-volume diuresis, consistent with early graft adaptation and function.
The rate of rejection in our cohort was 15% (n = 3), including two late rejections that ultimately led to graft loss in both cases. These values are within the range reported by large registries such as NAPRTCS, where acute rejection rates vary between 10% and 25% in the first post-transplant year, with long-term graft loss often linked to non-adherence, late immunological events, and limited access to specialized care[1].
Pre-existing donor-specific antibodies are widely recognized in the literature as a significant risk factor for acute and chronic rejection in kidney transplantation[10]. In our cohort, six recipients (30%) had detectable pre-transplant antibodies. Rejection episodes occurred in three patients, two of whom had pre-existing antibodies. However, no statistically significant correlation between preformed antibodies and rejection was observed, likely due to the limited sample size. Nonetheless, these findings emphasize the importance of systematic immunological assessment—including antibody screening and HLA typing—even in newly established pediatric transplant programs, as it allows for better risk stratification and individualized immunosuppression planning.
Graft survival at 24 months was 90%, and patient survival was 95%. These outcomes are comparable to 2-year graft survival rates of 85%–90% and patient survival rates exceeding 95% reported in the NAPRTCS and ESPN/ERA-EDTA registries for pediatric recipients of living donor kidneys[1,9]. Despite being implemented in a resource-limited setting, our program achieved a 2-year graft survival rate of 90%, which is comparable to outcomes reported in high-income countries. In contrast, data from a recent European registry study showed significantly lower access to transplantation and graft survival in low- and middle-income countries, underscoring the importance of structured implementation even in constrained environments[9].
The overall complication rate in our cohort was low, and the median CCI for both early and late complications was 0. Early complications occurred in two patients (10%), including one case of acute rejection (Clavien–Dindo Grade II) and one case of transient hypotension with respiratory insufficiency (Grade IVa). Late complications included one case of fatal hemorrhagic stroke (Grade V) and two rejection-related events. These figures are in line with published pediatric series, which report complication rates ranging from 10% to 30%, depending on definitions used and duration of follow-up[11].
Donor safety was a key focus of the program. All donors underwent thorough preoperative evaluation, with no intraoperative complications observed. Only one minor postoperative complication—a wound seroma classified as Clavien–Dindo Grade I—was recorded. These findings confirm the safety of donor nephrectomy, even during the initial stages of program development.
Laparoscopic donor nephrectomy was gradually introduced as part of the program development. Although laparoscopic procedures demonstrated a tendency toward longer and more uniform operative durations compared to open nephrectomies, the difference did not reach statistical significance (P = 0.155). Given the limited number of cases in the laparoscopic group (n = 4), these findings should be interpreted with caution and considered descriptive in nature.
Our operative times are comparable to those reported in the literature. Open donor nephrectomy is typically associated with operative times ranging from 120 to 180 minutes[12], while purely laparoscopic procedures often range from 180 to 240 minutes, depending on surgical experience[13]. Hand-assisted laparoscopic nephrectomy has been reported as a reasonable compromise, with median durations between 150 and 210 minutes[12,13].
In terms of safety, no intraoperative complications occurred in our donor cohort. Only one donor experienced a minor postoperative event—a wound seroma at the site of the hand-assist port (Clavien–Dindo Grade I). This corresponds to a complication rate of 5%, which is in line with previously published data showing complication rates of 1%–10%, depending on technique and reporting criteria. Large systematic reviews and single-center experiences consistently report low rates of serious morbidity in donor surgery, especially when performed in high-volume centers with experienced teams[14]. These findings further support the safety and reproducibility of both open and laparoscopic approaches to donor nephrectomy, even in the early phases of a program. As surgical experience increases, further transition toward minimally invasive techniques is anticipated.
Despite the positive outcomes and high procedural safety, launching the pediatric kidney transplantation program in Uzbekistan presented several objective challenges. At the time of its initiation, there was no prior clinical or institutional experience with pediatric kidney transplantation in the country. Nonetheless, the program became possible thanks to our team's prior success in developing a National liver transplantation program, which included the creation of clinical protocols, multidisciplinary collaboration, and staff training[5].
Although kidney transplantation is technically less complex than liver transplantation, its implementation still requires substantial effort. One of the advantages that supported program initiation was the fact that our hospital is a tertiary care center with existing pediatric services, including nephrology, urology, anesthesiology, and surgery. However, additional training was necessary to address the specific demands of kidney transplantation, including immunological risk asse
A dedicated focus was placed on establishing HLA typing and immunological monitoring capabilities, as well as training laboratory personnel to ensure high-quality diagnostics. Since the hospital had not previously provided care for adult patients, existing diagnostic and laboratory infrastructure had to be adapted to allow for comprehensive donor screening, HLA typing, and antibody testing. In parallel, institutional regulatory documents were revised and updated to align with the new transplant protocols.
Team training and clinical workflow optimization occurred concurrently with the first transplants, necessitating intensive coordination and continuous involvement of all specialists from preoperative assessment to long-term outpatient follow-up. Implementation of the program also required the creation of a specialized logistics system, including structured referral pathways for pediatric patients with end-stage renal disease, access to dialysis as a pre
The main limitations of this study include the small cohort size and relatively short follow-up duration, which may limit the generalizability of long-term outcomes. Nonetheless, the results provide valuable insight into the feasibility of pediatric transplantation in emerging programs.
As the program evolves, future efforts will focus on expanding access to living and deceased donor transplantation, improving data integration via national registry systems, and strengthening training pathways for transplant professionals.
This study demonstrates that the implementation of a pediatric living donor kidney transplantation program is feasible, safe, and effective even in a resource-limited setting. Despite the absence of prior national experience, our team achieved outcomes comparable with those reported by established international centers, with high patient and graft survival, low complication rates, and universal immediate graft function. The success of this initiative underscores the critical importance of structured multidisciplinary planning, targeted training, and the gradual development of supporting infrastructure, including immunological diagnostics and long-term follow-up systems. Our experience may serve as a reference model for other low- and middle-income countries seeking to establish sustainable pediatric transplantation programs.
The authors would like to thank the administration of the National Children’s Medical Center for their continuous support and institutional commitment to the development of the pediatric kidney transplantation program in Uzbekistan.
We are also grateful to the clinical and laboratory staff involved in the preoperative evaluation, immunological testing, and postoperative care of transplant recipients and donors. Their professionalism and dedication were essential to the successful implementation of this program.
Special thanks are extended to Ivanova Elena for her invaluable contributions to this publication, particularly for creating the illustrations. Her expertise and attention to detail have greatly enhanced the visual quality and clarity of the manuscript.
| 1. | Chua A, Cramer C, Moudgil A, Martz K, Smith J, Blydt-Hansen T, Neu A, Dharnidharka VR; NAPRTCS investigators. Kidney transplant practice patterns and outcome benchmarks over 30 years: The 2018 report of the NAPRTCS. Pediatr Transplant. 2019;23:e13597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 2. | Verghese PS. Pediatric kidney transplantation: a historical review. Pediatr Res. 2017;81:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Lemoine CP, Pozo ME, Superina RA. Overview of pediatric kidney transplantation. Semin Pediatr Surg. 2022;31:151194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 4. | Khadjibaev A, Khadjibaev F, Rakhimova R, Sharipova V, Sultanov P, Ergashev D, Anvarov K, Ruzibakieva M. Three-Year Experience of Kidney Transplantation at a Single Center in Uzbekistan. Exp Clin Transplant. 2022;20:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Semash K, Dzhanbekov T, Akbarov M, Mirolimov M, Usmonov A, Razzokov N, Primov Z, Gaybullaev T, Yigitaliev S. Implementation of a living donor liver transplantation program in the Republic of Uzbekistan: a report of the first 40 cases. Clin Transplant Res. 2024;38:116-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (2)] |
| 6. | Semash K. Evaluation and Management of Living Donors in the Setting of Living Donor Liver Transplant Program in the Republic of Uzbekistan. Exp Clin Transplant. 2024;22:664-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Khadjibaev A, Khadjibaev F, Anvarov K, Sultanov P. Organ Donation in Uzbekistan: Achievements and Prospects for Further Development. Exp Clin Transplant. 2020;18:54-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Byrne MHV, Mehmood A, Summers DM, Hosgood SA, Nicholson ML. A systematic review of living kidney donor enhanced recovery after surgery. Clin Transplant. 2021;35:e14384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Bonthuis M, Cuperus L, Chesnaye NC, Akman S, Melgar AA, Baiko S, Bouts AH, Boyer O, Dimitrova K, Carmo CD, Grenda R, Heaf J, Jahnukainen T, Jankauskiene A, Kaltenegger L, Kostic M, Marks SD, Mitsioni A, Novljan G, Palsson R, Parvex P, Podracka L, Bjerre A, Seeman T, Slavicek J, Szabo T, Tönshoff B, Torres DD, Van Hoeck KJ, Ladfors SW, Harambat J, Groothoff JW, Jager KJ. Results in the ESPN/ERA-EDTA Registry suggest disparities in access to kidney transplantation but little variation in graft survival of children across Europe. Kidney Int. 2020;98:464-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Abbas K, Mubarak M. Expanding role of antibodies in kidney transplantation. World J Transplant. 2025;15:99220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Ashoor IF, Engen RM, Puliyanda D, Hayde N, Peterson CG, Zahr RS, Solomon S, Kallash M, Garro R, Jain A, Harshman LA, McEwen ST, Mansuri A, Gregoski MJ, Twombley KE. Antibody-mediated rejection in pediatric kidney transplant recipients: A report from the Pediatric Nephrology Research Consortium. Pediatr Transplant. 2024;28:e14734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Ruiz-Deya G, Cheng S, Palmer E, Thomas R, Slakey D. Open donor, laparoscopic donor and hand assisted laparoscopic donor nephrectomy: a comparison of outcomes. J Urol. 2001;166:1270-3; discussion 1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Broe MP, Galvin R, Keenan LG, Power RE. Laparoscopic and hand-assisted laparoscopic donor nephrectomy: A systematic review and meta-analysis. Arab J Urol. 2018;16:322-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Lentine KL, Lam NN, Axelrod D, Schnitzler MA, Garg AX, Xiao H, Dzebisashvili N, Schold JD, Brennan DC, Randall H, King EA, Segev DL. Perioperative Complications After Living Kidney Donation: A National Study. Am J Transplant. 2016;16:1848-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/