Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.105905
Revised: April 24, 2025
Accepted: September 24, 2025
Published online: December 18, 2025
Processing time: 271 Days and 12.4 Hours
In France, nitazoxanide is available through compassionate use authorization, as there is no summary of product characteristics for this medication. However, it has been marketed in the United States for several years, with evidence sup
We report the case of a 79-year-old immunocompromised patient, a renal transplant recipient undergoing treatment with mycophenolate mofetil and tacr
This case highlights the potential of nitazoxanide to induce dose-dependent toxic agranulocytosis. While this adverse effect does not necessarily contraindicate reintroduction of the drug, it underscores the necessity for close hematological monitoring in such cases.
Core Tip: Nitazoxanide is a thiazolide compound. The Food and Drug Administration has approved it for the treatment of Giardia intestinalis and Cryptosporidium parvum infections in immunocompromised patients aged 12 years or older. In immunocompromised patients, nitazoxanide use for chronic Norovirus diarrhea has been reported and appears promising, including in solid organ transplant recipients. However, the drug is not yet approved for this indication. We herein report on a patient for which nitazoxanide induced a dose-dependent toxic agranulocytosis.
- Citation: Grépilloux D, Guéneau C, Weinhard J, Richaud R, Chevallier E, Jouve T, Dusserre J, Rostaing L. Toxic agranulocytosis following nitazoxanide treatment for norovirus diarrhea in a kidney transplant recipient: A case report and review of literature. World J Transplant 2025; 15(4): 105905
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/105905.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.105905
Norovirus is a positive-sense, single-stranded RNA virus belonging to the Caliciviridae family. Its primary mode of transmission is fecal-oral, occurring either directly or indirectly. Norovirus is the leading cause of acute gastroenteritis across all age groups and the most common etiological agent in outbreaks of foodborne illnesses[1]. In immunocompetent individuals, infection typically resolves spontaneously and rapidly. However, vulnerability due to frailty or immunosuppression predisposes individuals to chronic norovirus infections, characterized by prolonged diarrhea[2]. Solid organ transplant (SOT) recipients are particularly susceptible to such infections.
Management of chronic norovirus infections in SOT patients remains poorly standardized. Treatment strategies are largely empirical and include reducing immunosuppression when feasible[3] or replacing calcineurin inhibitors with everolimus, which possesses antiviral properties[4,5]. The use of intravenous immunoglobulins has also been explored, with some evidence suggesting it may aid in viral clearance[6].
Nitazoxanide is a thiazolide compound. Its chemical structure is 2-acetyloxy-N-(5-nitro-2-thiazolyl) benzamide. The benzamide structure resembles niclosamide, a drug used to treat tapeworm infections, whereas the nitrothiazolyl ring shares homology with the nitroimidazole drugs metronidazole[7]. Nitazoxanide is so rapidly hydrolyzed to its active metabolite tizoxanide that it is not detectable in the blood. Most of tizoxanide (> 99%) is protein-bound and it does not significantly inhibit cytochrome P450[8]. Therefore, no significant interaction is expected when nitazoxanide is administered concurrently with agents that are metabolized or inhibited by cytochrome P450 enzymes, but it could lead to competition with other drugs that are protein-bound. Tizoxanide is then conjugated to tizoxanide glucuronide, that is inactive. One third of tizoxanide and tizoxanide glucuronide are eliminated by the urinary route and the remaining two-thirds by the biliary route[9].
Nitazoxanide displays a broad anti-infectious action, notably by inhibiting the pyruvate-ferredoxin/flavodoxin oxidoreductase-dependent electron transfer reaction, involved in the anaerobic mechanism of many microorganisms[10]. It has first been approved by the United States Food and Drug Administration for the treatment of Giardia intestinalis and Cryptosporidium parvum infections in immunocompromised patients aged 12 years or older, based on clinical trials demonstrating its efficacy in these indications[11-14]. Nitazoxanide's anti-infective activity appears to extend to other parasites[15,16], bacteria[17,18] and viruses, including enteric viruses such as rotavirus[19] or norovirus[20]. The exact mechanisms of its broad antiviral activity have yet to be specified. For instance, it has demonstrated anti-influenza activity by blocking the maturation of viral hemagglutinin at the post-translational level[21]. In the case of hepatitis C virus (HCV), activation of protein kinase R plays a key role in regulating the host's innate anti-HCV response[22]. In hepatitis B virus infection, nitazoxanide has been shown to inhibit the action of the HBx protein, which increases viral transcription and alters homologous recombination leading to tumorigenesis[23]. Finally, nitazoxanide inhibits Viral Protein 7 (VP7) and alters the formation of rotavirus viroplasms, thereby reducing its replication[24].
With regard to norovirus infection, in vitro studies have shown that tizoxanide can stimulate the innate antiviral cellular response, through the production of Interferon-Stimulated Genes, in particular Interferon Regulatory Factor 1 (IRF1). Stimulation of IRF1 was associated with a reduction in replication of Human norovirus replicons, while its kno
The efficacy of nitazoxanide in treating norovirus infections in humans has been evaluated in small randomized, placebo-controlled, double-blind trials involving immunocompetent children and adults, where it significantly reduced symptom duration[26]. In immunocompromised patients, its use for chronic norovirus diarrhea has been reported and appears promising[27,28], including in solid organ transplant recipients[29,30]. However, the drug is not yet approved for this indication.
In France, nitazoxanide is available only through compassionate use authorization, with no summary of product characteristics (SmPC). Consequently, data on the drug’s safety and tolerability remain limited.
We present the case of a 79-year-old immunocompromised kidney transplant patient who developed toxic agranulocytosis following the introduction of nitazoxanide for the treatment of chronic diarrhea caused by norovirus. To our knowledge, this is the first reported case of nitazoxanide-induced toxic agranulocytosis.
The patient complained of chronic diarrhea.
One hundred days after kidney transplantation, the patient consulted a nephrology specialist due to chronic diarrhea complicated by moderate deterioration in graft function.
A 79-year-old male patient presented with a history of end-stage kidney failure secondary to left partial nephrectomy (performed for clear cell renal cell carcinoma) and nephroangiosclerosis. In February 2024, the patient underwent a renal transplant from a brain-dead donor. Induction therapy consisted of Basiliximab (Simulect®), administered at a dose of 20 mg on postoperative days 1 and 4. Maintenance immunosuppressive therapy included Tacrolimus (Prograf®) at a dose of 0.5 mg twice daily (target concentration range: 5–8 µg/L), Mycophenolate mofetil (Cellcept®) at 500 mg twice daily, then decreased to 250 mg twice daily, and Prednisone at 5 mg daily. Serologies for cytomegalovirus (CMV), Epstein-Barr virus (EBV), and toxoplasmosis were positive at the time of transplantation.
The patient’s medical history was notable for atrial fibrillation, severe hypertension, prostatic adenoma, bladder papillary carcinoma treated with intravesical Bacillus Calmette–Guérin therapy, and superficial spreading melanoma excised 6 years ago. The prescription at entry is listed in Table 1.
| Drug | Current dosing | Previous dosing |
| Tacrolimus | 0.5 mg morning and evening | |
| Mycophenolate Mofetil | 250 mg morning and evening | Decreased from 500 mg to 250 mg (D-12 before AD) |
| Cotrimoxazole | 400/80 mg per day (D) | |
| Folic acid | 5 mg twice a day | |
| Apixaban | 2.5 mg morning and evening | |
| Rilmenidine | 10 mg per day | Decreased from 20 mg to 10 mg (D-12 before AD) |
| Nebivolol | 1.25 mg per day | |
| Furosemide | 40 mg per day | |
| Pravastatin | 40 mg per day | |
| Calcium + Cholecalciferol | 500 + 400 UI twice a day | |
| Sodium bicarbonate | 1 gr per day | |
| Darbepoetin alfa | 80 micro gram once a week | |
| Urapidil | 30 mg per day | Stopped on D-12 before AD |
| Prednisone | 5 mg a day | Stopped on D-12 before AD |
| Pantoprazole | 20 mg a day | Stopped on D-12 before AD |
| Nitazoxanide | 500 mg morning and evening | Added on D-18 before AD and stopped on D-4 before AD (total treatment time achieved) |
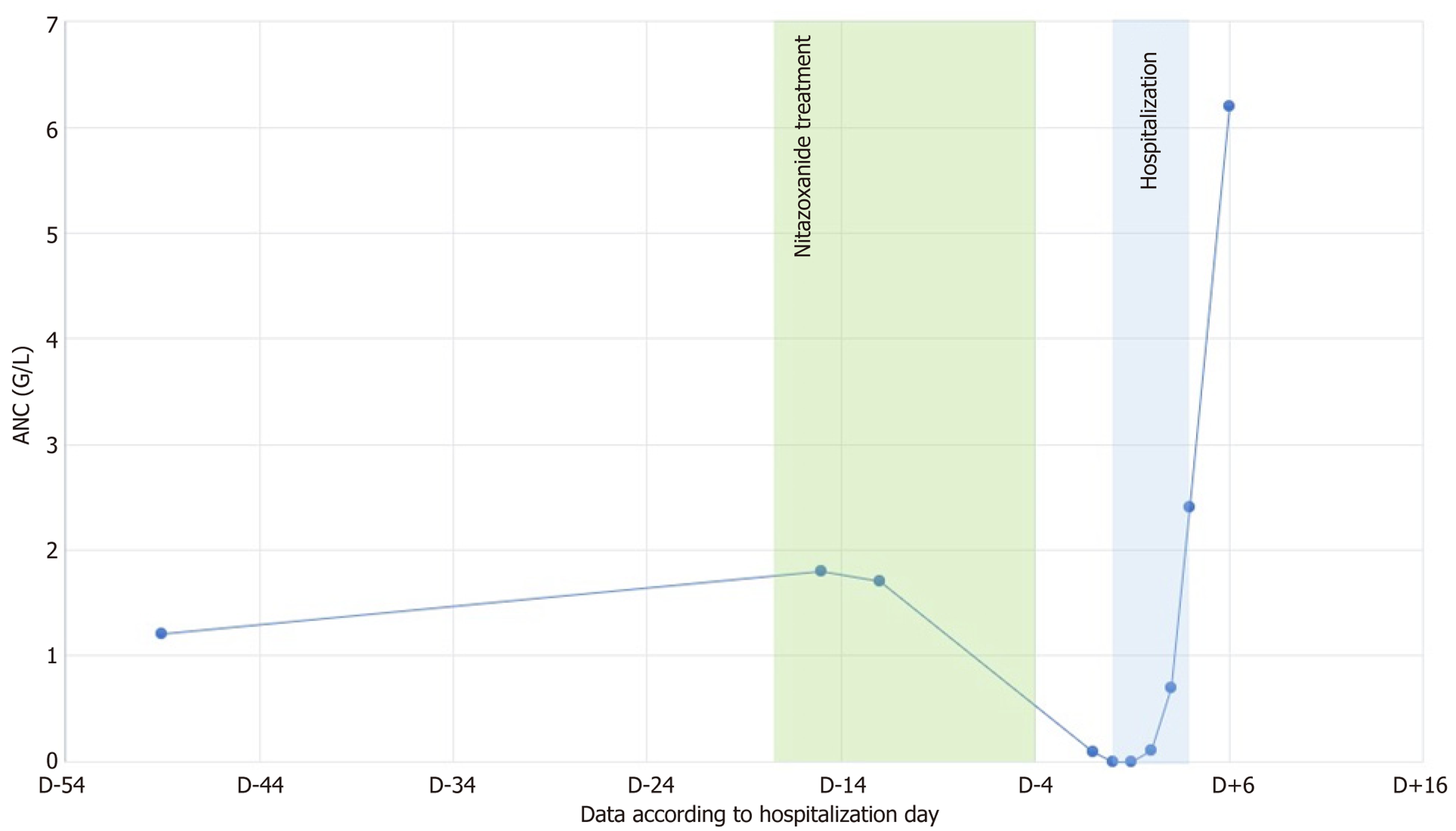
Treatment with nitazoxanide (500 mg twice daily) was initiated for a 14-day course, ending 4 days before admission. At treatment initiation, the absolute neutrophil count (ANC) was 2.93 G/L. Serial monitoring revealed a progressive decline in ANC: 1.8 G/L on day 3 of treatment initiation, and 1.7 G/L on Day 6, with dysgranulopoiesis observed on the blood smear. During a follow-up biology on D+3 following completion of treatment, ANC had decreased to 0.09 G/L, pro
On admission, the patient reported a 6 kg weight loss since the onset of diarrhea and exhibited signs of volume depletion, including orthostatic hypotension. Diarrhea had resolved before admission and did not recur during hospitalization. Clinical examination revealed no signs of infection, symptomatic anemia, or bleeding. Point-of-care ultrasound showed no evidence of urinary obstruction.
Laboratory findings on admission included a leukocyte count of 0.8 G/L, with ANC undetectable. Tacrolimus trough level was slightly above target (9.2 µg/L), while it was on target two weeks before, during nitazoxanide treatment (6.4 µg/L). A myelogram performed on D+7 after nitazoxanide discontinuation revealed blocked granulocyte maturation at the promyelocytic stage, with no involvement of other hematopoietic lineages, consistent with toxic agranulocytosis. Other hematological lineages were present without any observable abnormalities. Bone marrow samples tested negative for EBV, CMV, and human herpesvirus 6 (HHV-6).
With serum creatinine levels increasing to 2.03 mg/dL (180 μmol/L) from a baseline of 1.81 mg/dL (160 μmol/L). Stool analysis revealed a positive norovirus polymerase chain reaction (PCR), with no detection of other viruses, bacteria, or parasites.
It was not necessary.
The regional pharmacovigilance center concluded that nitazoxanide was responsible for the agranulocytosis based on chronology and the absence of other drug introductions.
Management involved hospitalization in a nephrology unit with protective isolation. The patient received two doses of lenograstim (34 MUI). Mycophenolate mofetil and cotrimoxazole were temporarily discontinued, and prednisone was reintroduced to prevent kidney transplant rejection. At discharge, mycophenolate mofetil was resumed at a reduced dose (250 mg twice daily), allowing prednisone discontinuation.
Hematological recovery was rapid: ANC increased to 0.7 G/L on D+7, 2.4 G/L on D+8, and 6.2 G/L on D+10 after nitozoxanide discontinuation. The evolution of ANC over time is shown in Figure 1. The patient remained asymptomatic throughout the episode, with no signs of infection or involvement of other hematological lineages
This report describes a case of agranulocytosis in a kidney transplant recipient following the introduction of nitazoxanide. This adverse effect appears to be exceptional, as evidenced by the limited data available in global pharmacovigilance databases. Only three cases of leukoneutropenia associated with nitazoxanide have been identified in the literature, including resources such as DrugDex, Martindale, the World Health Organization global pharmacovigilance database (VigiLyze), PubMed, and Google Scholar: A French HIV-infected patient treated concurrently with cotrimoxazole for Pneumocystis pneumonia, in which cotrimoxazole was deemed the most likely cause. (VigiLyze – 2008 - Pitié-Salpêtrière University Hospital, Paris, France). A patient in England with acute lymphoblastic leukemia receiving tisagenlecleucel, with Nitazoxanide’s role not excluded (VigiLyze - 2022). A United States case of leukopenia without further details (VigiLyze - 2022). To our knowledge, this is the first documented case of nitazoxanide -induced toxic agranulocytosis in the literature.
The main strength of this observation lies in the straightforward chronology: Nitazoxanide was the only new drug introduced during the weeks preceding the onset of agranulocytosis. However, the patient was concurrently receiving other medications known for their potential bone marrow toxicity, including cotrimoxazole, mycophenolate mofetil, and pantoprazole. These medications had been administered consistently since the transplantation, 4 months before onset of agranulocytosis, with no changes in pantoprazole or cotrimoxazole dosing, and folic acid supplementation was provided alongside cotrimoxazole. The dose of mycophenolate mofetil had recently been reduced, and its reintroduction at discharge did not result in agranulocytosis. Nitazoxanide pharmacokinetics data suggest a low potential for interactions. Hence, no drug interaction was found between nitazoxanide and the other treatments taken by the patient on the same time period. Among them, the following treatments have a high plasma protein binding affinity: Tacrolimus, myco
Other differential diagnoses, such as infectious causes of agranulocytosis were also considered. Viral infections, particularly those caused by EBV, CMV, and HHV-6, are well-known causes of cytopenia. However, PCR testing of blood and bone marrow samples for these viruses yielded negative results.
The mechanism by which nitazoxanide could lead to agranulocytosis is not yet known. This case shows evidence that nitazoxanide-induced agranulocytosis is linked to a direct medullary toxicity, as shown by the specific pattern on the bone marrow findings, rather than by an immune-mediated mechanism. The thiazolide class is chemically close to the nitroimidazole class, whose myelotoxic potential is well known. For example, leukopenia lower than 1.5 G/L is found in 1%-2% of patients treated with metronidazole[31], and cases of agranulocytosis have been described, reporting a direct marrow toxicity of metronidazole[32-34]. Furthermore, the administration of misonidazole, another molecule in the nitroimidazole class, has demonstrated a direct myelotoxic effect by reducing the number of granulocyte progenitor cells in the marrow[35].
It is also important to emphasize that the exact pharmacokinetics of nitazoxanide in cases of impaired renal clearance are not known. Given that one third of tizoxanide’s elimination is via urinary route, it is possible that accumulation of nitazoxanide’s metabolites could increase its toxicity, notably on the bone marrow. Dosage adjustment could therefore be beneficial in these patients but needs to be studied.
Nitazoxanide appears capable of inducing agranulocytosis, highlighting the importance of closely monitoring the absolute neutrophil count following its introduction. This agranulocytosis is likely of toxic origin rather than immuno-allergic, suggesting that reintroduction of nitazoxanide may be feasible with careful monitoring of neutrophil levels.
| 1. | de Graaf M, van Beek J, Koopmans MP. Human norovirus transmission and evolution in a changing world. Nat Rev Microbiol. 2016;14:421-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 349] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 2. | Fernández JM, Gómez JB. [Norovirus infections]. Enferm Infecc Microbiol Clin. 2010;28 Suppl 1:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Westhoff TH, Vergoulidou M, Loddenkemper C, Schwartz S, Hofmann J, Schneider T, Zidek W, van der Giet M. Chronic norovirus infection in renal transplant recipients. Nephrol Dial Transplant. 2009;24:1051-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Boillat Blanco N, Kuonen R, Bellini C, Manuel O, Estrade C, Mazza-Stalder J, Aubert JD, Sahli R, Meylan P. Chronic norovirus gastroenteritis in a double hematopoietic stem cell and lung transplant recipient. Transpl Infect Dis. 2011;13:213-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Bowman LJ, Brueckner AJ, Doligalski CT. The Role of mTOR Inhibitors in the Management of Viral Infections: A Review of Current Literature. Transplantation. 2018;102:S50-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Gäckler A, Struve C, Mülling N, Eisenberger U, Korth J, Babel N, Kribben A, Fiedler M, Witzke O, Rohn H. Norovirus Infections in Kidney Transplant Recipients. Transplantation. 2021;105:2655-2660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Wright JM, Dunn LA, Kazimierczuk Z, Burgess AG, Krauer KG, Upcroft P, Upcroft JA. Susceptibility in vitro of clinically metronidazole-resistant Trichomonas vaginalis to nitazoxanide, toyocamycin, and 2-fluoro-2'-deoxyadenosine. Parasitol Res. 2010;107:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Anderson VR, Curran MP. Nitazoxanide: A Review of its Use in the Treatment of Gastrointestinal Infections. Drugs. 2007;67:1947-1967. [RCA] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Broekhuysen J, Stockis A, Lins RL, De Graeve J, Rossignol JF. Nitazoxanide: pharmacokinetics and metabolism in man. Int J Clin Pharmacol Ther. 2000;38:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Hoffman PS, Sisson G, Croxen MA, Welch K, Harman WD, Cremades N, Morash MG. Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrob Agents Chemother. 2007;51:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 193] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Rossignol JF, Ayoub A, Ayers MS. Treatment of diarrhea caused by Giardia intestinalis and Entamoeba histolytica or E. dispar: a randomized, double-blind, placebo-controlled study of nitazoxanide. J Infect Dis. 2001;184:381-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Amadi B, Mwiya M, Musuku J, Watuka A, Sianongo S, Ayoub A, Kelly P. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet. 2002;360:1375-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 276] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Rossignol JF, Kabil SM, el-Gohary Y, Younis AM. Effect of nitazoxanide in diarrhea and enteritis caused by Cryptosporidium species. Clin Gastroenterol Hepatol. 2006;4:320-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Ortiz JJ, Ayoub A, Gargala G, Chegne NL, Favennec L. Randomized clinical study of nitazoxanide compared to metronidazole in the treatment of symptomatic giardiasis in children from Northern Peru. Aliment Pharmacol Ther. 2001;15:1409-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Favennec L, Jave Ortiz J, Gargala G, Lopez Chegne N, Ayoub A, Rossignol JF. Double-blind, randomized, placebo-controlled study of nitazoxanide in the treatment of fascioliasis in adults and children from northern Peru. Aliment Pharmacol Ther. 2003;17:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Rossignol JF, Kabil SM, Said M, Samir H, Younis AM. Effect of nitazoxanide in persistent diarrhea and enteritis associated with Blastocystis hominis. Clin Gastroenterol Hepatol. 2005;3:987-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Pankuch GA, Appelbaum PC. Activities of tizoxanide and nitazoxanide compared to those of five other thiazolides and three other agents against anaerobic species. Antimicrob Agents Chemother. 2006;50:1112-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Musher DM, Logan N, Hamill RJ, Dupont HL, Lentnek A, Gupta A, Rossignol JF. Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis. 2006;43:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Rossignol JF, Abu-Zekry M, Hussein A, Santoro MG. Effect of nitazoxanide for treatment of severe rotavirus diarrhoea: randomised double-blind placebo-controlled trial. Lancet. 2006;368:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Netzler NE, Enosi Tuipulotu D, White PA. Norovirus antivirals: Where are we now? Med Res Rev. 2019;39:860-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 21. | Rossignol JF, La Frazia S, Chiappa L, Ciucci A, Santoro MG. Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level. J Biol Chem. 2009;284:29798-29808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 22. | Elazar M, Liu M, McKenna SA, Liu P, Gehrig EA, Puglisi JD, Rossignol JF, Glenn JS. The anti-hepatitis C agent nitazoxanide induces phosphorylation of eukaryotic initiation factor 2alpha via protein kinase activated by double-stranded RNA activation. Gastroenterology. 2009;137:1827-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Sekiba K, Otsuka M, Funato K, Miyakawa Y, Tanaka E, Seimiya T, Yamagami M, Tsutsumi T, Okushin K, Miyakawa K, Ryo A, Koike K. HBx-induced degradation of Smc5/6 complex impairs homologous recombination-mediated repair of damaged DNA. J Hepatol. 2022;76:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 24. | La Frazia S, Ciucci A, Arnoldi F, Coira M, Gianferretti P, Angelini M, Belardo G, Burrone OR, Rossignol JF, Santoro MG. Thiazolides, a new class of antiviral agents effective against rotavirus infection, target viral morphogenesis, inhibiting viroplasm formation. J Virol. 2013;87:11096-11106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Dang W, Xu L, Ma B, Chen S, Yin Y, Chang KO, Peppelenbosch MP, Pan Q. Nitazoxanide Inhibits Human Norovirus Replication and Synergizes with Ribavirin by Activation of Cellular Antiviral Response. Antimicrob Agents Chemother. 2018;62:e00707-e00718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Rossignol JF, El-Gohary YM. Nitazoxanide in the treatment of viral gastroenteritis: a randomized double-blind placebo-controlled clinical trial. Aliment Pharmacol Ther. 2006;24:1423-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Siddiq DM, Koo HL, Adachi JA, Viola GM. Norovirus gastroenteritis successfully treated with nitazoxanide. J Infect. 2011;63:394-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Morris J, Brown W, Morris CL. Nitazoxanide Is Effective Therapy For Norovirus Gastroenteritis After Chemotherapy and Hematopoietic Stem Cell Transplantation (HSCT). Blood. 2013;122:4581. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Haubrich K, Gantt S, Blydt-Hansen T. Successful treatment of chronic norovirus gastroenteritis with nitazoxanide in a pediatric kidney transplant recipient. Pediatr Transplant. 2018;22:e13186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Hedvat J, Salerno DM, Kovac D, Scheffert JL, Corbo H, Chen JK, Choe JY, Jennings DL, Anamisis A, Liu EC, Lee JH, Shertel T, Lange NW. Nitazoxanide treatment for norovirus infection in solid organ transplant recipients. Clin Transplant. 2022;36:e14594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Lefebvre Y, Hesseltine HC. The peripheral white blood cells and metronidazole. JAMA. 1965;194:15-18. [PubMed] [DOI] [Full Text] |
| 32. | Hosack T, Mandal AK, Griffiths C, Missouris CG. Idiosyncratic metronidazole-induced neutropaenia in an older adult. Br J Hosp Med (Lond). 2023;84:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | McKendrick MW, Geddes AM. Neutropenia associated with metronidazole. Br Med J. 1979;2:795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Smith JA. Neutropenia associated with metronidazole therapy. Can Med Assoc J. 1980;123:202. [PubMed] |
| 35. | Allalunis MJ, Turner AR, Partington JP, Urtasun RC. Effect of misonidazole therapy on human granulopoietic stem cells. Cancer Treat Rep. 1980;64:1097-1102. [PubMed] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/