Published online Dec 18, 2025. doi: 10.5500/wjt.v15.i4.105649
Revised: May 12, 2025
Accepted: August 20, 2025
Published online: December 18, 2025
Processing time: 291 Days and 11.6 Hours
Cholangiocarcinoma (CCA) is a rare type of cancer which arises from the bile duct epithelium and carries a poor prognosis. One of the main risk factors in the Western world is primary sclerosing cholangitis. Surgical resection has tradi
Core Tip: Liver transplantation (LT) had previously been contraindicated in the treat
- Citation: Affarah L, Kotha S, Berry P. Beyond futility: The history and potential of liver transplantation in cholangiocarcinoma. World J Transplant 2025; 15(4): 105649
- URL: https://www.wjgnet.com/2220-3230/full/v15/i4/105649.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i4.105649
Cholangiocarcinoma (CCA) is a rare type of cancer which arises from the bile duct epithelium. CCA is the second most common primary tumour of the liver following hepatocellular carcinoma (HCC), and has an increasing global incidence and mortality[1]. It is aggressive and carries a poor prognosis with a median survival of 24 months after diagnosis despite treatment[2].
CCA is classified into two subtypes based on anatomical position, extrahepatic and intrahepatic. Extrahepatic CCA makes up 90% of all CCA and can be further divided into distal CCA (dCCA) and hilar CCA (hCCA)[2,3]. dCCA is located between the ampulla and the origin of the cystic duct, whilst hCCA is located above the origin of cystic duct up to the second order biliary ducts[4]. hCCA may also be referred to as perihilar or Klatskin tumours. Intrahepatic CCA (iCCA) is located within the liver parenchyma. Anatomical subtype is important because it dictates management strategies. The main risk factors for CCA include primary sclerosing cholangitis (PSC), cirrhosis, hepatitis B, hepatitis C, choledochal cysts, smoking, diabetes and alcohol[5]. Liver fluke is a common cause of CCA in endemic areas[6].
Surgical resection is the only curative treatment for CCA but is precluded in patients with locally advanced or metastatic disease. Anatomical subtype dictates the approach to surgical resection. dCCA is treated with excision of the extrahepatic bile duct as part of a pancreatoduodenectomy. Surgical resection in iCCA and hCCA is determined by location, radiological extension and whether the remnant liver volume carries a risk of liver failure. Innovative techniques like embolisation of the portal vein and associated liver partition with portal vein ligation for staged hepatectomy have been developed to facilitate removal of larger liver volumes while inducing hypertrophy of the unaffected liver lobe[7]. Advances in surgical techniques in patients with vascular invasion include venous and arterial reconstruction. Although these techniques have higher peri-operative mortality and morbidity, the outcomes are still better than best supportive care[8,9].
Liver transplantation (LT) has evolved as a viable treatment option for patients with CCA with promising outcomes. Despite this, CCA remains a contraindication for transplantation in most countries. This review aims to explore the evo
hCCA is the most common subtype of CCA and is located proximal to the cystic duct origin. It has a complex diagnostic pathway and surgical resection is challenging due to its anatomical location. Incidence varies between 0.3-3.5 per 100000/year in North America and 7.5 per 100000/year in Mediterranean regions[10]. The Bismuth-Corlette classification is used to define the macroscopic appearance to plan surgical approach[11]. One third of hCCA are not resectable at diagnosis and another third are found to have extensive disease at the time of surgical exploration. This led to consideration of LT as a treatment option in this cohort, but due to high recurrence rates and low survival initially, LT was soon contraindicated[12,13].
A study from Kentucky, United States in the late 1990s described an early experience of preoperative chemoradiation followed by resection of extrahepatic CCA. Margin-negative resections were achieved in 100% of patients who received preoperative chemoradiation, compared to 54% in patients who did not[14]. A 1999 study evaluated the results of radiotherapy with various combinations of chemotherapy and surgical resection with selective transplantation in this patient group[15], and concluded that combined modality therapy with complete surgical resection (with or without transplantation) can be curative for this patient group.
Interest in the field grew and other centres started to consider neoadjuvant therapy ahead of LT in this cohort. In 2002, Sudan et al[16] evaluated the effect of neoadjuvant chemoradiation therapy (biliary brachytherapy and intravenous 5-fluorouracil) and orthoptic LT in a selected group of patients with hCCA. Long-term tumour-free survival was achieved in 45% of transplanted patients, providing evidence that hCCA should not be an absolute contraindication for LT.
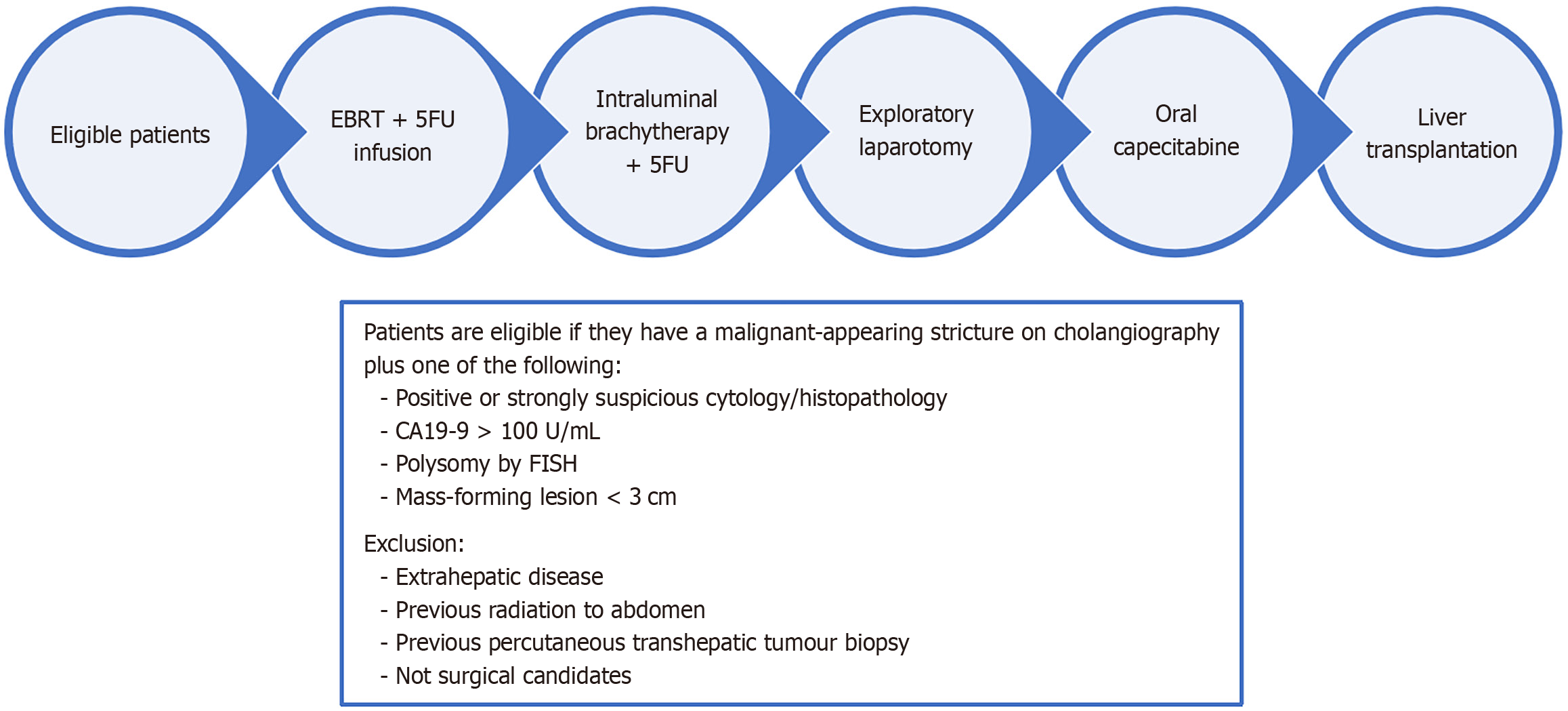
The Mayo Protocol (Figure 1) has proved to be the most successful strategy for the treatment of previously unre
In the Mayo Clinic pilot study, 19 patients were enrolled, 11 proceeded to LT and 8 were followed up for over 12 months at the time of publishing their results[17]. One patient developed disease recurrence but the remainder remained disease-free. They published further results a few years later where 28 patients had neoadjuvant therapy followed by LT, and the survival rate for this group was 82% at 5 years after transplantation[18]. They concluded that neoadjuvant chemoradiotherapy with LT achieved excellent outcomes for patients with locally advanced, regional lymph-node-negative hCCA. The promising outcomes with the Mayo protocol have been attributed to a strict adherence to the selection criteria[19].
A few years later, a multi-center retrospective study by Darwish Murad et al[20], in the United States was performed to assess the overall effectiveness of neoadjuvant therapy followed by LT in patients with hCCA[20]. Their results showed a 5-year disease-free survival rate of 65%. Another multi-center retrospective study, from 2000-2015, compared the outcomes of hCCA patients undergoing resection vs transplantation between[21]. It found that patients with a hCCA meeting criteria for transplantation (< 3 cm, lymph node negative disease) who underwent resection had decreased survival compared to those with unresectable disease who were transplanted; 5-year overall survival rate of 18% vs 64%. Of note, 61% of patients who were transplanted had PSC, compared to 2% of those who had resection[21].
A metanalysis published in 2021 Looked at the survival data following neoadjuvant chemoradiation and LT for patients with unresectable hCCA[22]. The time period covered was 2000-2019, following the publication of the Mayo protocol. The pooled 5-year survival rate following LT for patients with neoadjuvant therapy was 65.1% vs 31.6% in those without. Pooled recurrence of CCA after 3 years was 24.1% in patients with neoadjuvant therapy vs 51.7% in patients without. Patients with PSC appeared to have the most favourable outcomes.
Toronto General hospital recently developed a neoadjuvant regimen modelled on Mayo protocol[23]. The overall survival was 83.3% and 55.5% at 1 and 2 years respectively but 5-year data is not yet available[24]. Currently, CCA is not an accepted indication for LT in the United Kingdom, outside an ongoing pilot programme which is led by the Liver Advisory Group[25].
iCCA is the second most common malignancy of liver and constitutes 10% of CCAs. Most are adenocarcinomas and only a small proportion are resectable at diagnosis[26]. The mean age of diagnosis is over 65 years and it is more common in men[27,28]. Histologically it can be divided into intraductal-infiltrating, mass-forming or periductal[29].
Liver resection is currently the only accepted curative treatment for iCCA. Liver resection is considered appropriate in iCCA patients if a free-margin resection is possible (R0) and if there is sufficient volume of the remnant liver to avoid post-operative liver failure[30], i.e. early disease. Early diagnosis in this patient group can be tricky, as most patients with iCCA will only develop symptoms such as abdominal pain, weight loss and jaundice with advanced disease; the majority of patients will present with advanced disease which is unresectable[30,31]. Even in patients undergoing a liver resection for iCCA, the median overall survival is about 40 months, with a 5-year overall survival of 25%-40%[30,32-34].
LT had been largely contraindicated in this group of patients due to poor initial results, with earlier studies showing a 2-year survival rate of only 30%[35-37]. The second ever successful transplant by Thomas Strazl in 1963 was for iCCA and the patient died on post operative day 7[38].
However, the paradigm began to change: Patient selection criteria was proving to be essential in improving the survival in early-stage HCC patients undergoing LT[30,39] and promising results were seen in patients with hCCA receiving LT. This, alongside the advancements in neoadjuvant chemo and radiotherapy, reopened the door to considering LT in iCCA.
In 2014, a multicentre retrospective cohort study reviewed cirrhotic patients who had undergone LT and had an iCCA in their liver explant[40]. The study aimed to identify risk factors for recurrence in these patient as well as assess the outcomes of patients with ‘very early’ iCCA, defined as a single tumour ≤ 2 cm. Their results identified the following as risk factors: Larger tumour volume, microscopic vascular invasion and poor degree of differentiation. The ‘very early’ iCCA group showed no tumour recurrence and achieved excellent overall survival rates (73% at 5 years), and the study recommended that validating these findings may alter the current exclusion of these patients from LT.
The same group carried out a larger retrospective multicentre study in 2016, and reviewed all patients with an iCCA in their liver explant who were transplanted for either an HCC or decompensated cirrhosis[41]. The patients were classified as ‘very early’ iCCA, defined as a single tumour ≤ 2 cm and ‘advanced’ iCCA, defined as either a single tumour > 2 cm or multi-focal disease, and their 1, 3 and 5-year recurrence and overall survival rates were compared. The study similarly showed that cirrhotic patients with ‘very early’ iCCA had significantly lower rates of recurrence and higher survival rates, and concluded that they may be considered for LT.
A retrospective study in 2011 reviewed patients transplanted for an advanced iCCA and hCCA to identify risk factors for recurrence and to develop a prognostic scoring system for risk stratification in this group[42]. Risk stratification was divided into low, intermediate and high-risk based on a scoring system. Factors that were found to be predictive for tumour recurrence included multifocal tumour, perineural and lymphovascular invasion, lack of neoadjuvant and adjuvant therapy and a history of PSC. Recurrence-free survival was significantly higher in the low-risk group, and there was a survival benefit in the intermediate group when compared to the high-risk group. The study concluded that there were grounds for expanding LT criteria in selected patients with locally advanced iCCA.
A prospective case-series in a single-center published their data in 2018, specifically looking at the efficacy of LT in patients with locally advanced iCCA who had undergone neoadjuvant therapy[40]. The group used their own protocol for neoadjuvant therapy which consisted of gemcitabine-based chemotherapy as first-line treatment. Patients with radiological response or stability following at least 6 months of neoadjuvant therapy were listed for transplantation (n = 6). Overall survival rates were 100%, 83.3% and 83.3% at 1, 3 and 5 years respectively. The study concluded that selected patients with locally advanced iCCA with stable disease on neoadjuvant therapy may benefit from LT.
Further data was published by the same group in 2022[43] when they had performed LT on 18 patients on neoadjuvant therapy. Overall survival rates were 100%, 71% and 57% at 1, 3 and 5 years respectively. The data supported the group’s hypothesis that stable disease on neoadjuvant therapy was a surrogate measure for long-term survival after liver transplantation.
Another center published their experience with LT for CCA[44] and showed that multimodal neoadjuvant treatment (chemotherapy and local therapy) was associated with improved survival in LT for locally advanced iCCA, regardless of tumour size.
It is imperative to touch upon the various advances in oncological therapy and the potential role they may play in the future of LT in CCA patients. Immunotherapy and immune checkpoint inhibitors (ICI) have revolutionised cancer management and are now being used in advanced CCA, in combination with chemotherapy[45].
The most commonly used agents are in CCA are Pembrolizumab, an anti-programmed death-1 (PD1) antibody and Durvalumab, an anti-programmed death ligand 1 (PD-L1) antibody. Chemotherapy regimens with either Pembrolizumab or Durvalumab have recently been approved by both the United States Food and Drug Administration and the European Medicines Agency as first-line combinations for advanced CCA[45-48]. The role of using targeted therapy to further enhance the effects of ICI is also being explored; for example, anti-vascular endothelial growth factor agents and tyrosine kinase inhibitors and their potential to increase tumour response to PD-L1 inhibitors and anti-PD1 agents[45,49-51]. Next generation sequencing technologies have also enabled the molecular analysis of tumours such as CCA, therefore identifying specific genetic alterations that can be targeted by small molecule inhibitors[45,52].
While these novel therapies have found their place in the management of advanced CCA, their role in peri and post-LT patients is yet to be determined[53]. The use of ICIs in patients with HCC is being explored to downstage disease to result in eligibility for a transplant, but also to control disease burden whilst a transplant is awaited[39,54]. There is also the question of ICI use in transplanted patients with tumour recurrence. The concern however is that ICIs are associated with multiple immune-related adverse events, such as colitis, hepatitis/hepatic necrosis and pneumonitis, and in the context of LT, graft-rejection[55,56]. Further data are required to understand the benefits vs risks of these treatments and to fully understand the role they may play in both the pre and post-transplantation period.
PSC is a rare condition caused by biliary inflammation resulting in strictures and ultimately liver fibrosis[57]. PSC is more common in men[58], is strongly associated with inflammatory bowel disease and can be seen in conjunction with autoimmune hepatitis[59]. The risk of CCA in PSC is more than 100 times that of the general population and is highest in the first year following a diagnosis of PSC[60,61].
Diagnosing CCA in PSC remains a challenge. This is often due to overlap of symptoms with benign progression of disease, difficulty in differentiating benign from malignant strictures and poor sensitivity of sampling from strictures[62,63]. Although there is widespread adoption of annual imaging in this cohort, a recent prospective surveillance study found it to be ineffective in early diagnosis of CCA[64].
hCCA in PSC can be treated curatively with a liver resection if liver function can be preserved with good liver volumes after radical resection. This can be challenging in PSC patients who have parenchymal fibrosis resulting in low liver volumes and therefore high mortality and morbidity[65].
With increasingly favourable outcomes, LT is emerging as a potentially curative alternative to resection in hCCA with PSC. A multi-centre study by Ethun et al[21] reported an 18% vs 64% 5-year survival in PSC patients with hCCA undergoing liver resection vs LT, respectively.
The Mayo protocol has also been adopted in these patients with favourable results. In a highly selected group, the Mayo protocol reported a 5-year survival of 82%[18]. Various centres have adopted versions of the Mayo protocol and the success has led to an award of model for end-stage liver disease exception points for this indication[20,22]. In the Mayo protocol group, long-term data showed better long-term survival in perihilar CCA (pCCA) and PSC (74%) compared to de-novo pCCA (58%)[66]. Despite the improving 5-year survival, it needs to be recognised that PSC recurs in 15%-25% of patients in post-transplant period[67], and it is imperative to use strict diagnostic and selection criteria in this cohort.
It is also worth noting that PSC patients are often diagnosed younger, have fewer co-morbidities and are often transplanted earlier in their disease course compared to other liver conditions. This generally means that they have better post-LT outcomes compared with other liver diseases, and therefore dominate LT cohorts. But it is important to acknowledge and consider this selection bias when interpreting data and results[68].
iCCA can potentially be curable with resection if adequate liver volumes can be preserved. LT is still not established for this indication but studies have shown favourable outcomes in iCCA discovered incidentally in explants of PSC patients following transplantation[69]. Small studies have shown improved survival following adjuvant chemotherapy[40] but more trials are needed and are underway.
Novel therapies with check point inhibitors have been used in CCA with PSC with some promising results[48,70].
There are a number of ongoing clinical trials which will shed further light on the role of LT in the treatment of CCA.
The clinical trial NCT02232932 (TRANSPHIL) is a prospective randomized trial in France, which aims to compare 5-year survival and recurrence-free survival at 3 years in patients having liver resection (control group) compared to those receiving neoadjuvant chemo-radiotherapy and then a liver transplant[71]. A prospective study is being undertaken in Toronto: NCT02878473, to evaluate the effectiveness of LT as a treatment for patients with early iCCA and cirrhosis, as measured by 5-year survival and disease recurrence rates[72].
Another single-centre study in Toronto (NCT04195503) is evaluating the effectiveness of LT in patients with locally advanced, unresectable, non-metastatic iCCA in patients who have had 6-months of stable disease on neoadjuvant chemotherapy. Outcomes will again be overall survival and disease recurrence[73].
Lastly, there is a prospective trial being conducted at Oslo University in Norway: The TESLA trial NCT04556214, which looks at LT in locally advanced, unresectable non-metastatic iCCA, treated with neoadjuvant systemic therapy for at least 6 months[74].
CCAs are a challenging group of aggressive tumours which require a multi-disciplinary approach for both diagnosis and treatment. The role of LT in CCA has evolved significantly with numerous centers in North America and Europe adopting this as standard of care. Organ scarcity makes LT in this group controversial, but strict selection criteria could ensure good outcomes without disadvantaging other groups. Currently there are no standardized oncological protocols for this cohort. The future for this would be to develop personalised models for these patients to achieve the best out
In view of the substantial evidence-base that now exists, patients with CCA who cannot access assessment in LT programmes may be unfairly disadvantaged. When carefully selected, outcomes approach those of conventional indications for LT. Additionally, rapid progress in personalised oncological treatment, including immunotherapy and agents that target individual molecular and genetic characteristics, may herald an era of substantially improved post-LT survival. The progress described in this review appears to justify greater access to LT in appropriately managed pro
| 1. | Van Dyke AL, Shiels MS, Jones GS, Pfeiffer RM, Petrick JL, Beebe-Dimmer JL, Koshiol J. Biliary tract cancer incidence and trends in the United States by demographic group, 1999-2013. Cancer. 2019;125:1489-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 2. | Ilyas SI, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215-1229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1070] [Cited by in RCA: 1012] [Article Influence: 77.8] [Reference Citation Analysis (6)] |
| 3. | Sapisochin G, Javle M, Lerut J, Ohtsuka M, Ghobrial M, Hibi T, Kwan NM, Heimbach J. Liver Transplantation for Cholangiocarcinoma and Mixed Hepatocellular Cholangiocarcinoma: Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104:1125-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 4. | Mertens J. Clinical diagnosis and management of perihilar cholangiocarcinoma. Clin Liver Dis (Hoboken). 2014;3:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Petrick JL, Yang B, Altekruse SF, Van Dyke AL, Koshiol J, Graubard BI, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based study in SEER-Medicare. PLoS One. 2017;12:e0186643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 6. | Prueksapanich P, Piyachaturawat P, Aumpansub P, Ridtitid W, Chaiteerakij R, Rerknimitr R. Liver Fluke-Associated Biliary Tract Cancer. Gut Liver. 2018;12:236-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Chan A, Zhang WY, Chok K, Dai J, Ji R, Kwan C, Man N, Poon R, Lo CM. ALPPS Versus Portal Vein Embolization for Hepatitis-related Hepatocellular Carcinoma: A Changing Paradigm in Modulation of Future Liver Remnant Before Major Hepatectomy. Ann Surg. 2021;273:957-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 8. | Alikhanov R, Dudareva A, Trigo MÁ, Serrablo A. Vascular Resection for Intrahepatic Cholangiocarcinoma: Current Considerations. J Clin Med. 2021;10:3829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Sugiura T, Uesaka K, Okamura Y, Ito T, Yamamoto Y, Ashida R, Ohgi K, Otsuka S, Nakagawa M, Aramaki T, Asakura K. Major hepatectomy with combined vascular resection for perihilar cholangiocarcinoma. BJS Open. 2021;5:zrab064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D; ESMO Guidelines Committee. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v28-v37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 502] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 11. | Paul A, Kaiser GM, Molmenti EP, Schroeder T, Vernadakis S, Oezcelik A, Baba HA, Cicinnati VR, Sotiropoulos GC. Klatskin Tumors and the Accuracy of the Bismuth-Corlette Classification. Am Surg. 2011;77:1695-1699. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 353] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Robles R, Figueras J, Turrión VS, Margarit C, Moya A, Varo E, Calleja J, Valdivieso A, Valdecasas JC, López P, Gómez M, de Vicente E, Loinaz C, Santoyo J, Fleitas M, Bernardos A, Lladó L, Ramírez P, Bueno FS, Jaurrieta E, Parrilla P. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 223] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 14. | McMasters KM, Tuttle TM, Leach SD, Rich T, Cleary KR, Evans DB, Curley SA. Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am J Surg. 1997;174:605-8; discussion 608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 147] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Urego M, Flickinger JC, Carr BI. Radiotherapy and multimodality management of cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 1999;44:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Sudan D, DeRoover A, Chinnakotla S, Fox I, Shaw B Jr, McCashland T, Sorrell M, Tempero M, Langnas A. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2:774-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 220] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 17. | De Vreede I, Steers JL, Burch PA, Rosen CB, Gunderson LL, Haddock MG, Burgart L, Gores GJ. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl. 2000;6:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 246] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 18. | Heimbach JK, Gores GJ, Haddock MG, Alberts SR, Nyberg SL, Ishitani MB, Rosen CB. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin Liver Dis. 2004;24:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 199] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Villard C, Jorns C, Bergquist A. Treatment of cholangiocarcinoma in patients with primary sclerosing cholangitis: a comprehensive review. eGastroenterology. 2024;2:e100045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Darwish Murad S, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, Botha JF, Mezrich JD, Chapman WC, Schwartz JJ, Hong JC, Emond JC, Jeon H, Rosen CB, Gores GJ, Heimbach JK. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88-98.e3; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 407] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 21. | Ethun CG, Lopez-Aguiar AG, Anderson DJ, Adams AB, Fields RC, Doyle MB, Chapman WC, Krasnick BA, Weber SM, Mezrich JD, Salem A, Pawlik TM, Poultsides G, Tran TB, Idrees K, Isom CA, Martin RCG, Scoggins CR, Shen P, Mogal HD, Schmidt C, Beal E, Hatzaras I, Shenoy R, Cardona K, Maithel SK. Transplantation Versus Resection for Hilar Cholangiocarcinoma: An Argument for Shifting Treatment Paradigms for Resectable Disease. Ann Surg. 2018;267:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 22. | Cambridge WA, Fairfield C, Powell JJ, Harrison EM, Søreide K, Wigmore SJ, Guest RV. Meta-analysis and Meta-regression of Survival After Liver Transplantation for Unresectable Perihilar Cholangiocarcinoma. Ann Surg. 2021;273:240-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 23. | Loveday BPT, Knox JJ, Dawson LA, Metser U, Brade A, Horgan AM, Gallinger S, Greig PD, Moulton CA. Neoadjuvant hyperfractionated chemoradiation and liver transplantation for unresectable perihilar cholangiocarcinoma in Canada. J Surg Oncol. 2018;117:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, Chahal P, Chang DT, Cloyd J, Covey AM, Glazer ES, Goyal L, Hawkins WG, Iyer R, Jacob R, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Sahai V, Schefter T, Singh G, Stein S, Vauthey JN, Venook AP, Yopp A, McMillian NR, Hochstetler C, Darlow SD. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 651] [Article Influence: 130.2] [Reference Citation Analysis (2)] |
| 25. | Hakeem AR, Isaac J, Thorburn D, Heaton N, Prasad R. The role of liver transplant for intrahepatic cholangiocarcinoma: the UK NHSBT liver advisory group pilot programme. Hepatoma Res. 2023;9:38. [DOI] [Full Text] |
| 26. | Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol. 2010;2:419-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 240] [Article Influence: 15.0] [Reference Citation Analysis (5)] |
| 27. | Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 606] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 28. | Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 722] [Cited by in RCA: 710] [Article Influence: 47.3] [Reference Citation Analysis (1)] |
| 29. | Chung YE, Kim MJ, Park YN, Choi JY, Pyo JY, Kim YC, Cho HJ, Kim KA, Choi SY. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics. 2009;29:683-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 281] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 30. | Mazzaferro V, Gorgen A, Roayaie S, Droz Dit Busset M, Sapisochin G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72:364-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 270] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 31. | Amini N, Ejaz A, Spolverato G, Kim Y, Herman JM, Pawlik TM. Temporal trends in liver-directed therapy of patients with intrahepatic cholangiocarcinoma in the United States: a population-based analysis. J Surg Oncol. 2014;110:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Hyder O, Marques H, Pulitano C, Marsh JW, Alexandrescu S, Bauer TW, Gamblin TC, Sotiropoulos GC, Paul A, Barroso E, Clary BM, Aldrighetti L, Ferrone CR, Zhu AX, Popescu I, Gigot JF, Mentha G, Feng S, Pawlik TM. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience. JAMA Surg. 2014;149:432-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 290] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 33. | Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 804] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 34. | de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM, Aldrighetti L, Ferrone CR, Zhu AX, Bauer TW, Walters DM, Gamblin TC, Nguyen KT, Turley R, Popescu I, Hubert C, Meyer S, Schulick RD, Choti MA, Gigot JF, Mentha G, Pawlik TM. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140-3145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 572] [Article Influence: 38.1] [Reference Citation Analysis (2)] |
| 35. | Goldstein RM, Stone M, Tillery GW, Senzer N, Levy M, Husberg BS, Gonwa T, Klintmalm G. Is liver transplantation indicated for cholangiocarcinoma? Am J Surg. 1993;166:768-71; discussion 771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 122] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Pichlmayr R, Weimann A, Oldhafer KJ, Schlitt HJ, Klempnauer J, Bornscheuer A, Chavan A, Schmoll E, Lang H, Tusch G. Role of liver transplantation in the treatment of unresectable liver cancer. World J Surg. 1995;19:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 108] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Becker NS, Rodriguez JA, Barshes NR, O'Mahony CA, Goss JA, Aloia TA. Outcomes analysis for 280 patients with cholangiocarcinoma treated with liver transplantation over an 18-year period. J Gastrointest Surg. 2008;12:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Cataldo Doria. Contemporary Liver Transplantation. Switzerland: Springer, Cham, 2016. |
| 39. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5395] [Article Influence: 179.8] [Reference Citation Analysis (7)] |
| 40. | Lunsford KE, Javle M, Heyne K, Shroff RT, Abdel-Wahab R, Gupta N, Mobley CM, Saharia A, Victor DW, Nguyen DT, Graviss EA, Kaseb AO, McFadden RS, Aloia TA, Conrad C, Li XC, Monsour HP, Gaber AO, Vauthey JN, Ghobrial RM; Methodist–MD Anderson Joint Cholangiocarcinoma Collaborative Committee (MMAJCCC). Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol. 2018;3:337-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 212] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 41. | Sapisochin G, Facciuto M, Rubbia-Brandt L, Marti J, Mehta N, Yao FY, Vibert E, Cherqui D, Grant DR, Hernandez-Alejandro R, Dale CH, Cucchetti A, Pinna A, Hwang S, Lee SG, Agopian VG, Busuttil RW, Rizvi S, Heimbach JK, Montenovo M, Reyes J, Cesaretti M, Soubrane O, Reichman T, Seal J, Kim PT, Klintmalm G, Sposito C, Mazzaferro V, Dutkowski P, Clavien PA, Toso C, Majno P, Kneteman N, Saunders C, Bruix J; iCCA International Consortium. Liver transplantation for "very early" intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology. 2016;64:1178-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 272] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 42. | Hong JC, Petrowsky H, Kaldas FM, Farmer DG, Durazo FA, Finn RS, Saab S, Han SH, Lee P, Markovic D, Lassman C, Hiatt JR, Busuttil RW. Predictive index for tumor recurrence after liver transplantation for locally advanced intrahepatic and hilar cholangiocarcinoma. J Am Coll Surg. 2011;212:514-20; discussion 520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | McMillan RR, Javle M, Kodali S, Saharia A, Mobley C, Heyne K, Hobeika MJ, Lunsford KE, Victor DW 3rd, Shetty A, McFadden RS, Abdelrahim M, Kaseb A, Divatia M, Yu N, Nolte Fong J, Moore LW, Nguyen DT, Graviss EA, Gaber AO, Vauthey JN, Ghobrial RM. Survival following liver transplantation for locally advanced, unresectable intrahepatic cholangiocarcinoma. Am J Transplant. 2022;22:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 44. | Ito T, Butler JR, Noguchi D, Ha M, Aziz A, Agopian VG, DiNorcia J 3rd, Yersiz H, Farmer DG, Busuttil RW, Hong JC, Kaldas FM. A 3-Decade, Single-Center Experience of Liver Transplantation for Cholangiocarcinoma: Impact of Era, Tumor Size, Location, and Neoadjuvant Therapy. Liver Transpl. 2022;28:386-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 45. | Zanuso V, Tesini G, Valenzi E, Rimassa L. New systemic treatment options for advanced cholangiocarcinoma. J Liver Cancer. 2024;24:155-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 46. | Oh DY, Ruth He A, Qin S, Chen LT, Okusaka T, Vogel A, Kim JW, Suksombooncharoen T, Ah Lee M, Kitano M, Burris H, Bouattour M, Tanasanvimon S, McNamara MG, Zaucha R, Avallone A, Tan B, Cundom J, Lee CK, Takahashi H, Ikeda M, Chen JS, Wang J, Makowsky M, Rokutanda N, He P, Kurland JF, Cohen G, Valle JW. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022;1:EVIDoa2200015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 696] [Article Influence: 174.0] [Reference Citation Analysis (1)] |
| 47. | Oh DY, He AR, Bouattour M, Okusaka T, Qin S, Chen LT, Kitano M, Lee CK, Kim JW, Chen MH, Suksombooncharoen T, Ikeda M, Lee MA, Chen JS, Potemski P, Burris HA 3rd, Ostwal V, Tanasanvimon S, Morizane C, Zaucha RE, McNamara MG, Avallone A, Cundom JE, Breder V, Tan B, Shimizu S, Tougeron D, Evesque L, Petrova M, Zhen DB, Gillmore R, Gupta VG, Dayyani F, Park JO, Buchschacher GL Jr, Rey F, Kim H, Wang J, Morgan C, Rokutanda N, Żotkiewicz M, Vogel A, Valle JW. Durvalumab or placebo plus gemcitabine and cisplatin in participants with advanced biliary tract cancer (TOPAZ-1): updated overall survival from a randomised phase 3 study. Lancet Gastroenterol Hepatol. 2024;9:694-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 161] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 48. | Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, Yau T, Klümpen HJ, Chan SL, Ozaka M, Verslype C, Bouattour M, Park JO, Barajas O, Pelzer U, Valle JW, Yu L, Malhotra U, Siegel AB, Edeline J, Vogel A; KEYNOTE-966 Investigators. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401:1853-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 672] [Article Influence: 224.0] [Reference Citation Analysis (0)] |
| 49. | Hack SP, Zhu AX, Wang Y. Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities. Front Immunol. 2020;11:598877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 208] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 50. | Song Y, Fu Y, Xie Q, Zhu B, Wang J, Zhang B. Anti-angiogenic Agents in Combination With Immune Checkpoint Inhibitors: A Promising Strategy for Cancer Treatment. Front Immunol. 2020;11:1956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 196] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 51. | Zhang Q, Liu X, Wei S, Zhang L, Tian Y, Gao Z, Jin M, Yan S. Lenvatinib Plus PD-1 Inhibitors as First-Line Treatment in Patients With Unresectable Biliary Tract Cancer: A Single-Arm, Open-Label, Phase II Study. Front Oncol. 2021;11:751391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 52. | Lamarca A, Barriuso J, McNamara MG, Valle JW. Molecular targeted therapies: Ready for "prime time" in biliary tract cancer. J Hepatol. 2020;73:170-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 281] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 53. | Ortiz V, Loeuillard E. Rethinking Immune Check Point Inhibitors Use in Liver Transplantation: Implications and Resistance. Cell Mol Gastroenterol Hepatol. 2025;19:101407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 54. | Tabrizian P, Florman SS, Schwartz ME. PD-1 inhibitor as bridge therapy to liver transplantation? Am J Transplant. 2021;21:1979-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 55. | Gumusay O, Callan J, Rugo HS. Immunotherapy toxicity: identification and management. Breast Cancer Res Treat. 2022;192:1-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 56. | Kayali S, Pasta A, Plaz Torres MC, Jaffe A, Strazzabosco M, Marenco S, Giannini EG. Immune checkpoint inhibitors in malignancies after liver transplantation: A systematic review and pooled analysis. Liver Int. 2023;43:8-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 57. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on sclerosing cholangitis. J Hepatol. 2022;77:761-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 225] [Article Influence: 56.3] [Reference Citation Analysis (1)] |
| 58. | Rupp C, Rössler A, Zhou T, Rauber C, Friedrich K, Wannhoff A, Weiss KH, Sauer P, Schirmacher P, Süsal C, Stremmel W, Gotthardt DN. Impact of age at diagnosis on disease progression in patients with primary sclerosing cholangitis. United European Gastroenterol J. 2018;6:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 59. | Gregorio GV, Portmann B, Karani J, Harrison P, Donaldson PT, Vergani D, Mieli-Vergani G. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: a 16-year prospective study. Hepatology. 2001;33:544-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 417] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 60. | Lundberg Båve A, Bergquist A, Bottai M, Warnqvist A, von Seth E, Nordenvall C. Increased risk of cancer in patients with primary sclerosing cholangitis. Hepatol Int. 2021;15:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 61. | Boberg KM, Bergquist A, Mitchell S, Pares A, Rosina F, Broomé U, Chapman R, Fausa O, Egeland T, Rocca G, Schrumpf E. Cholangiocarcinoma in primary sclerosing cholangitis: risk factors and clinical presentation. Scand J Gastroenterol. 2002;37:1205-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 214] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 62. | Stiehl A, Rudolph G, Klöters-Plachky P, Sauer P, Walker S. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: outcome after endoscopic treatment. J Hepatol. 2002;36:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 182] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 63. | Kipp BR, Stadheim LM, Halling SA, Pochron NL, Harmsen S, Nagorney DM, Sebo TJ, Therneau TM, Gores GJ, de Groen PC, Baron TH, Levy MJ, Halling KC, Roberts LR. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am J Gastroenterol. 2004;99:1675-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 256] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 64. | Villard C, Friis-Liby I, Rorsman F, Said K, Warnqvist A, Cornillet M, Kechagias S, Nyhlin N, Werner M, Janczewska I, Hagström T, Nilsson E, Bergquist A. Prospective surveillance for cholangiocarcinoma in unselected individuals with primary sclerosing cholangitis. J Hepatol. 2023;78:604-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 65. | Komaya K, Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, Yamaguchi J, Nagino M. Recurrence after curative-intent resection of perihilar cholangiocarcinoma: analysis of a large cohort with a close postoperative follow-up approach. Surgery. 2018;163:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 66. | Azad AI, Rosen CB, Taner T, Heimbach JK, Gores GJ. Selected Patients with Unresectable Perihilar Cholangiocarcinoma (pCCA) Derive Long-Term Benefit from Liver Transplantation. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | Visseren T, Erler NS, Polak WG, Adam R, Karam V, Vondran FWR, Ericzon BG, Thorburn D, IJzermans JNM, Paul A, van der Heide F, Taimr P, Nemec P, Pirenne J, Romagnoli R, Metselaar HJ, Darwish Murad S; European Liver and Intestine Transplantation Association (ELITA). Recurrence of primary sclerosing cholangitis after liver transplantation - analysing the European Liver Transplant Registry and beyond. Transpl Int. 2021;34:1455-1467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 68. | Molinaro A, Engesæter LK, Jorns C, Nordin A, Rasmussen A, Line PD, Pall V, Ericzon BG, Bennet W, Hov JR, Melum E. Outcome of Liver Retransplantation in Patients With Primary Sclerosing Cholangitis. Liver Int. 2025;45:e16214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 69. | 69 De Martin E, Rayar M, Golse N, Dupeux M, Gelli M, Gnemmi V, Allard MA, Cherqui D, Sa Cunha A, Adam R, Coilly A, Antonini TM, Guettier C, Samuel D, Boudjema K, Boleslawski E, Vibert E. Analysis of Liver Resection Versus Liver Transplantation on Outcome of Small Intrahepatic Cholangiocarcinoma and Combined Hepatocellular-Cholangiocarcinoma in the Setting of Cirrhosis. Liver Transpl. 2020;26:785-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 70. | Zhu AX, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DVT, Borad MJ, Bridgewater JA, Harris WP, Murphy AG, Oh DY, Whisenant JR, Lowery MA, Goyal L, Shroff RT, El-Khoueiry AB, Chamberlain CX, Aguado-Fraile E, Choe S, Wu B, Liu H, Gliser C, Pandya SS, Valle JW, Abou-Alfa GK. Final Overall Survival Efficacy Results of Ivosidenib for Patients With Advanced Cholangiocarcinoma With IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol. 2021;7:1669-1677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 358] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 71. | Vibert E. Liver resection versus radio-chemotherapy-transplantation for hilar cholangiocarcinoma (TRANSPHIL). [accessed 2025 Aug 12]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/study/NCT02232932 ClinicalTrials.gov Identifier: NCT02232932. |
| 72. | Sapisochin G, Bruix J. Liver transplantation for early intrahepatic cholangiocarcinoma (LT for iCCA). [accessed 2025 Aug 12]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02878473 ClinicalTrials.gov Identifier: NCT02878473. |
| 73. | Sapisochin G. Liver transplant for stable advanced intrahepatic cholangiocarcinoma. [accessed 2025 Aug 12]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04195503 ClinicalTrials.gov Identifier: NCT04195503. |
| 74. | Smedman M, Yaqub S. Liver transplantation for non-resectable intrahepatic cholangiocarcinoma: a prospective exploratory trial (TESLA Trial). [accessed 2025 Aug 12]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04556214 ClinicalTrials.gov Identifier: NCT04556214. |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/