Published online Feb 19, 2025. doi: 10.5498/wjp.v15.i2.99008
Revised: November 27, 2024
Accepted: December 17, 2024
Published online: February 19, 2025
Processing time: 95 Days and 0.9 Hours
Body composition analysis (BCA) is primarily used in the management of conditions such as obesity and endocrine disorders. However, its potential in providing nutritional guidance for patients with Alzheimer’s disease (AD) remains relatively unexplored.
To explore the clinical efficacy of BCA-based dietary nutrition scheme on bone metabolism in AD patients.
This retrospective study included 96 patients with AD complicated by osteo
No significant differences were observed between groups in terms of age, sex, height, BMI, or other baseline data (P > 0.05). In both groups, BMI did not show significant changes after the intervention (P > 0.05), whereas muscle mass and mineral content were significantly increased (P < 0.05). After the intervention, BMI in the PN group did not differ significantly from that of the RD group, but muscle mass and mineral content were significantly higher in the PN group (P < 0.05). After the intervention, a higher proportion of patients in the PN group had a T score > -1 compared to the RD group (P < 0.05). The mini-mental state examination (MMSE) score was similar in both groups before the intervention. However, 12 weeks after the intervention, the MMSE score in the PN group was significantly higher than that in the RD group (P < 0.05). In both groups, the MMSE score significantly increased 12 weeks post-intervention compared to pre-intervention levels (P < 0.05). Before the intervention, the levels of osteocalcin, serum calcium, PINP, β-CTX, and 25-hydroxyvitamin D were not significantly different between the two groups (P > 0.05). After 12 weeks of intervention, the PN group exhibited higher levels of osteocalcin, serum calcium, and 25-hydroxyvitamin D, as well as lower levels of PINP and β-CTX, compared to the RD group (P < 0.05). In both groups, osteocalcin, serum calcium, and 25-hydroxyvitamin D levels were significantly higher, while PINP and β-CTX levels were significantly lower after 12 weeks of intervention compared to baseline (P < 0.05).
The human BCA-based dietary nutrition regimen plays a crucial role in improving BMD and bone metabolism, with effects that surpass those of conventional nutrition strategies. The findings of this study provide strong evidence for the nutritional management of AD patients.
Core Tip: Body composition analysis (BCA) is primarily used in the management of conditions such as obesity and endocrine disorders. However, its potential in providing nutritional guidance for patients with Alzheimer’s disease (AD) remains relatively unexplored. In this study, we found that both routine diet (RD) and personalized nutritional (PN) interventions significantly increased bone mineral density (BMD) and improved bone metabolism indexes in AD patients compared to baseline levels. Furthermore, PN intervention, which is based on human BCA, provided superior bone outcomes compared to RD, as evidenced by higher BMD and better bone metabolic indexes in the PN group. These findings provide strong research evidence supporting the development of targeted nutritional interventions for AD patients.
- Citation: Wang XL, Zhao YR, Yu Y, Mao ZF, Tan SX, Yu SS. Impact of dietary nutrition regimens based on body composition analysis on bone metabolism in Alzheimer’s disease patients. World J Psychiatry 2025; 15(2): 99008
- URL: https://www.wjgnet.com/2220-3206/full/v15/i2/99008.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i2.99008
Alzheimer’s disease (AD), a progressive neurodegenerative disease, is pathologically characterized by excessive accumulation of amyloid plaques, formation of neurofibrillary tangles, and synaptic deficits and loss[1,2]. AD is also a major cause of dementia in the elderly[3]. This disease significantly affects the global elderly population, with estimates suggesting that by 2050, approximately 100 million individuals worldwide will be affected by AD[2]. Apart from affecting the nervous system, AD is also associated with bone metabolism disorders. The pathophysiology between AD and osteoporosis (OP) exhibits several similarities, such as b-amyloid deposition, Wnt/b-cateinin pathway, and estrogen deficiency, all of which are crucial for the onset and progression of both AD and OP[4]. Clinical data indicate that early-stage AD patients have higher serum osteocalcin (OC) levels and lower bone mineral density (BMD) compared to healthy controls, suggesting imbalances in bone metabolism among early-stage AD patients[5]. The crosstalk between AD and bone metabolism impairment may also contribute to an increased fracture risk in AD patients, with evidence suggesting a higher fracture risk in AD patients compared to age-matched controls[6]. This also suggests that AD patients face a higher risk of falls, considerably affecting their daily living and quality of life. Despite the strong connection between AD and bone metabolism disorders, there is still a lack of treatments targeting bone disorders for AD patients. In a clinical evaluation, only 38.5% of AD patients received OP medication[7]. The side effects of antiresorptive, anabolic, and dual-action agents on middle-aged and elderly patients also limit their use in the treatment of AD[6]. Consequently, the search for an effective anti-bone disorder therapy with minimal side effects has become a crucial focus in current research on AD complicated by bone disorders.
In recent years, the impact of nutrients on bone health has gradually attracted attention. A cross-sectional study that included 1570 elderly women revealed a significant positive correlation between the intake of animal-derived proteins and the BMD of the participants[8]. A balanced diet, such as the mediterranean diet, has also been proven to help maintain human BMD[9]. The nutritional approach is vital for enhancing bone metabolism and sustaining bone homeostasis. Additionally, this dietary model may aid in restoring bone homeostasis in patients. Currently, ongoing research is investigating the influence of dietary patterns on fracture management in AD patients. For instance, a randomized controlled clinical trial is designed to examine the effects of the mediterranean diet on BMD, gait, balance, and fall risk in AD patients through the implementation of a comprehensive physical exercise program[10]. While the potential of nutritional therapy for AD is widely recognized, a consensus on its specific implementation remains elusive. Fortunately, body composition analysis (BCA) provides a solution to this challenge. BCA is a detection method based on bioelectrical impedance analysis, which works by introducing a specific frequency of current into the body to measure its resistance. This enables the indirect assessment of body fat content, fat-free mass content, and the proportions of various body components[11]. BMD has been shown to be positively correlated with fat-free mass and muscle content, and this correlation is more significant in the elderly population[12]. Human BCA indicators can serve as indirect measures of the body’s BMD and nutritional status, offering a theoretical foundation for subsequent nutritional interventions[13]. Therefore, nutritional management based on human BCA is expected to be used for bone metabolism treatment of AD patients.
Currently, human BCA is primarily used in treating and intervening in conditions such as obesity, hypertension, and endocrine disorders; however, there is limited research on its role in providing nutritional guidance for AD patients. To address this gap, we enrolled 96 AD patients and randomly assigned them to two groups: A routine diet (RD) group that received standard dietary guidance, and a personalized nutrition (PN) group that underwent BCA-based individualized dietary interventions. By comparing BMD, OC, 25-hydroxyvitamin D (25-OH-D), procollagen type I N-terminal pro
This retrospective study included 96 AD patients complicated by OP admitted to The Third Hospital of Quzhou from January 2023 to December 2024. The patients were divided into two groups: A PN group and a RD group, with 48 cases in each group.
Inclusion criteria: (1) Age ≥ 60 years; (2) Diagnosis of AD based on the criteria set by the American Academy of Neurology and the National Institute of Neurological and Communicative Disorders and Stroke, and the AD and Related Disorders Association; (3) Diagnosis of senile OP; (4) Mini-mental state examination (MMSE) score ≤ 26; and (5) No significant swallowing dysfunction, with energy needs primarily met through oral intake.
Exclusion criteria: (1) Severe dysfunction of the heart, liver, or kidneys, as indicated by organ functional tests; (2) Severe disturbance of consciousness; (3) Incomplete or missing case data; (4) Poor patient compliance and inability to cooperate with therapeutic interventions; (5) Dysphagia that interfered with oral food intake; and (6) Vegetarianism with special dietary restrictions or food allergies.
All patients underwent collection of clinical history and general data, along with a physical examination and clinical diagnosis performed by qualified physicians. The collected general information included the patient’s gender, age, occupation, personal history, allergy history, medical history, dietary recall, and other pertinent details.
Based on data from previous similar studies, 48 patients were randomly divided into two groups: The RD group and the PN group. The intervention period lasted 12 weeks during which BMD and indicators for bone metabolism and biochemistry were measured before and after intervention. Intragroup comparison, before and after the intervention, as well as inter-group comparison were performed to evaluate BMD and bone metabolism indexes. Patients in the RD group received standard dietary guidance, while those in the PN group were provided with personalized dietary interventions based on human BCA.
RD guidance: Health and nutrition education was provided to patients and their families to help them rationally plan their diet, ensure balanced nutrition, and increase protein and calcium intake. Patients were instructed to prepare their diets according to the following guidelines: (1) Increase consumption of calcium-rich foods such as dairy products, tofu, beans, shrimp, and nuts; (2) Supplement vitamin D appropriately by eating more foods rich in vitamin D, such as cod liver oil, animal liver, lean meat, and egg yolk; (3) Eat more fresh fruits and vegetables, which are rich in vitamins and trace elements that aid calcium absorption, and consume an appropriate amount of protein; (4) Supplement protein primarily with fresh fish, eggs, and bean products; and (5) Quit smoking and drinking, and control caffeine intake.
BCA-based individualized dietary intervention measures: A body composition analyzer (X-SCAN PLUS-II, Jawon, Korea) was used to measure the patient’s height, weight, body mass index (BMI), muscle mass, mineral content, and other parameters before and 12 weeks after the intervention, resulting in a comprehensive analysis report. Using standard dietary guidelines, an individualized dietary plan was created based on the detailed analysis of the patient’s body composition[14].
The BMD, bone metabolism indexes, and body composition data of the two groups after intervention were statistically analyzed.
BMD determination: The BMD (g/cm2) of different parts of the research participants, including the lumbar vertebrae (L1-L4), femoral neck (neck), trochanter (troch), and Ward’s triangle (Ward’s), was assessed using a PRIMUS dual-energy X-ray absorptiometry device produced by OSTEOSYS, Korea. A phantom test was performed prior to each measurement to ensure accuracy.
Detection of bone metabolism indexes: Serum bone metabolism markers, including OC, 25-OH-D, serum calcium, PINP, and β-CTX, were measured using an automatic enzyme immunoassay analyzer (Thermo, United States).
BCA and detection: The PLUS-II body composition analyzer (Jawon, Korea) was used to measure the patient’s height, weight, BMI, muscle mass, mineral content, and other related data.
MMSE scale: This scale was used to evaluate the cognitive function of patients both before the intervention and 12 weeks after. The scores range from 0 to 30 points. Specifically, a score of 27-30 points indicates normal cognition, 21-26 points reflects mild cognitive impairment, 10-20 points signifies moderate cognitive impairment, and 0-9 points represents severe cognitive impairment.
After reviewing all research data, the information was compiled, and EXCEL tables were created to organize the basic details of the research subjects along with various test results. These data were then imported into statistical product and service solutions 20.0 for statistical analysis and processing. A t-test was performed for the measurement data presented as “mean ± SD”, while the χ² test was used for counting data expressed as “number of cases, (%)”. A significance level of α = 0.05 was established, where P < 0.05 indicated statistical significance.
Analysis using the independent sample t-test and χ2 test revealed no significant differences between the two groups in terms of age (RD vs PN: 69.17 ± 6.77 vs 68.42 ± 6.36, t = 0.559, P = 0.577), sex [RD vs PN (male/female): 27/21 vs 25/23, χ² = 0.168, P = 0.682], height (RD vs PN: 169.28 ± 11.08 vs 170.67 ± 12.42, t = 0.579, P = 0.564), BMI (RD vs PN: 21.98 ± 1.46 vs 22.09 ± 1.35, t = 0.385, P = 0.701), and other general data (P > 0.05). These findings suggest the feasibility of follow-up research. For specific results, see Table 1.
| Characterization | Routine diet group | Personalized nutrition group | χ2/t value | P value |
| Age | 69.17 ± 6.77 | 68.42 ± 6.36 | 0.559 | 0.577 |
| Sex | 0.168 | 0.682 | ||
| Male | 27 (56.25) | 25 (52.08) | ||
| Female | 21 (43.75) | 23 (47.92) | ||
| Height (cm) | 169.28 ± 11.08 | 170.67 ± 12.42 | 0.579 | 0.564 |
| Body mass index | 21.98 ± 1.46 | 22.09 ± 1.35 | 0.385 | 0.701 |
| Smoking history | ||||
| With | 6 (12.50) | 8 (16.67) | ||
| Without | 42 (87.50) | 40 (83.33) | ||
| Alcoholism history | 0.103 | 0.749 | ||
| With | 5 (10.42) | 6 (12.50) | ||
| Without | 43 (89.58) | 42 (87.50) | ||
| History of underlying diseases | ||||
| Hypertension | 0.844 | 0.358 | ||
| With | 11 (22.92) | 15 (31.25) | ||
| Without | 37 (77.08) | 33 (68.75) | ||
| Diabetes | 0.182 | 0.670 | ||
| With | 18 (37.50) | 16 (33.33) | ||
| Without | 30 (62.50) | 32 (66.67) | ||
| Mini-mental state examination score | 21.46 ± 3.86 | 21.42 ± 3.74 | 0.054 | 0.957 |
| Serum osteocalcin (ng/L) | 3.45 ± 1.33 | 3.65 ± 1.08 | 0.813 | 0.418 |
| 25-hydroxyvitamin D (ng/mL) | 15.34 ± 4.02 | 15.15 ± 3.87 | 0.236 | 0.814 |
| Serum calcium (mg/dL) | 8.46 ± 2.34 | 8.62 ± 2.12 | 0.352 | 0.726 |
| Procollagen type I N-terminal propeptide (ng/mL) | 73.27 ± 5.28 | 73.18 ± 5.67 | 0.080 | 0.936 |
| Beta C-terminal telopeptide of type I collagen (ng/mL) | 0.76 ± 0.13 | 0.75 ± 0.15 | 0.327 | 0.744 |
| Bone mineral density score | 1.603 | 0.588 | ||
| T score > -1 | 5 (10.42) | 6 (12.50) | ||
| -2.5 ≤ T score ≤ -1 | 29 (60.42) | 24 (50.00) | ||
| T score < -2.5 | 14 (29.16) | 18 (37.50) |
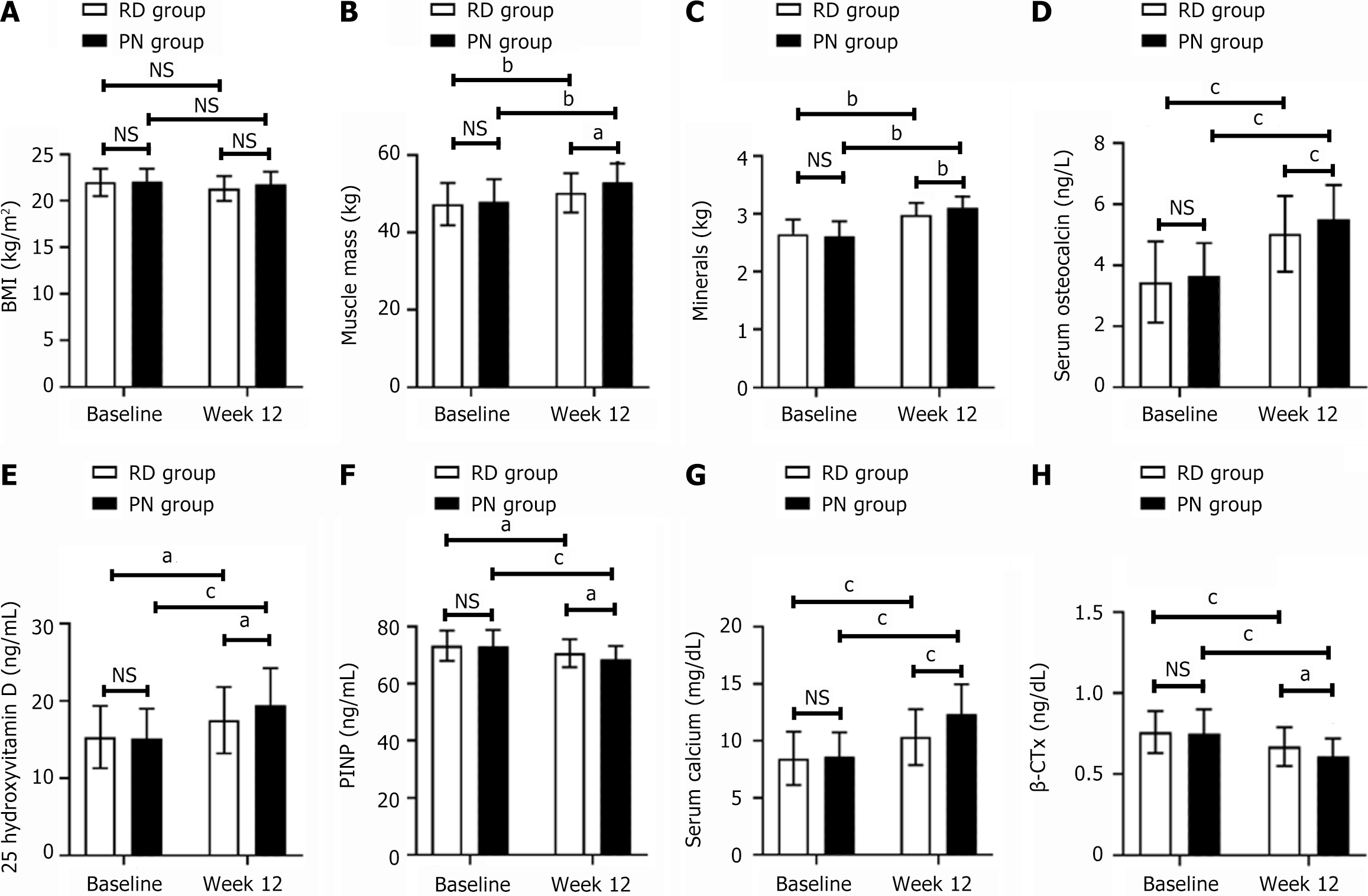
The X-SCAN PLUS-II body composition analyzer, produced by Jawon in Korea, was used to assess body composition in both groups before and 12 weeks following the intervention. As shown in Figure 1A-C, intragroup comparisons showed no significant differences in BMI before and after the intervention (in the RD group, BMI at baseline vs week 12: 21.98 ± 1.46 vs 21.32 ± 1.33, P > 0.05; in the PN group, BMI at baseline vs week 12: 22.09 ± 1.35 vs 21.76 ± 1.37, P > 0.05). However, changes in muscle mass were observed (in the RD group, muscle mass at baseline vs week 12: 47.33 ± 5.43 vs 50.22 ± 5.04, t = 2.703, P = 0.008; in the PN group, muscle mass at baseline vs week 12: 48.01 ± 5.73 vs 52.87 ± 4.90, t = 4.446, P = 0.002), and mineral content (in the RD group, mineral content at baseline vs week 12: 2.65 ± 0.65 vs 2.98 ± 0.21, t = 7.003, P = 0.002; in the PN group, mineral content at baseline vs week 12: 2.61 ± 0.26 vs 3.11 ± 0.19, t = 10.760, P = 0.002) showed significant increases following the intervention. The inter-group comparison revealed a similar BMI but higher muscle mass (RD vs PN at week 12: 50.22 ± 5.04 vs 52.87 ± 4.90, t = 2.612, P = 0.011) and mineral content (RD vs PN at week 12: 2.98 ± 0.21 vs 3.11 ± 0.19, t = 3.180, P = 0.002) in the PN group compared with the RD group after the intervention.
As shown in Table 2, before the intervention, the RD group consisted of 11 cases with a T score < -2.5, 29 cases with -2.5 ≤ T score ≤ -1, and 8 cases with a T score > -1. After 12 weeks of intervention, the RD group had 7 cases with a T score < -2.5, 17 cases with -2.5 ≤ T score ≤ -1, and 24 cases with a T score > -1, showing statistically significant differences in T scores before and after the intervention (P = 0.000). As shown in Table 3, before the intervention, the PN group included 18 patients with a T score < -2.5, 24 patients with -2.5 ≤ T score ≤ -1, and 6 patients with a T score > -1. After 12 weeks of intervention, the group had 4 patients with a T score < -2.5, 14 patients with -2.5 ≤ T score ≤ -1, and 30 patients with a T score > -1. These changes in T scores were statistically significant before and after the intervention in the PN group (P = 0.001). After analysis with the χ2 test, it was determined that the PN group had a higher proportion of patients with a T score > -1 compared to the RD group (RD vs PN: 50.00% vs 62.50%, c² = 6.353, P = 0.042) at week 12 (Table 4).
| After treatment | T score > -1 | -2.5 ≤ T score ≤ -1 | T score < -2.5 | Total | P value |
| Before treatment | 0.000 | ||||
| T score > -1 | 8 (16.67) | 0 (0.00) | 0 (0.00) | 8 (16.67) | |
| -2.5 ≤ T score ≤ -1 | 16 (33.34) | 13 (27.08) | 0 (0.00) | 29 (60.42) | |
| T score < -2.5 | 0 (0.00) | 4 (8.34) | 7 (14.58) | 11 (22.92) | |
| Total | 24 (50.00) | 17 (35.42) | 7 (14.58) | 48 (100) |
| After treatment | T score > -1 | -2.5 ≤ T score ≤ -1 | T score < -2.5 | Total | P value |
| Before treatment | 0.001 | ||||
| T score > -1 | 6 (12.50) | 0 (0.00) | 0 (0.00) | 6 (12.50) | |
| -2.5 ≤ T score ≤ -1 | 13 (27.08) | 11 (22.92) | 0 (0.00) | 24 (50.00) | |
| T score < -2.5 | 11 (22.92) | 3 (6.25) | 4 (8.33) | 18 (37.50) | |
| Total | 30 (62.50) | 14 (29.17) | 4 (8.33) |
| Routine diet group (n = 48) | Personalized nutrition group (n = 48) | χ2 value | P value | |
| 6.353 | 0.042 | |||
| T score > -1 | 24 (50.00) | 30 (62.50) | ||
| -2.5 ≤ T score ≤ -1 | 17 (35.42) | 14 (29.17) | ||
| T score < -2.5 | 7 (14.58) | 4 (8.33) |
Table 5 indicates that the MMSE score did not show a statistically significant difference between the groups before the intervention. However, after 12 weeks, the MMSE score in the PN group was higher than that in the RD group (RD vs PN: 25.39 ± 2.86 vs 26.48 ± 2.27, t = 2.057, P = 0.042). Moreover, both groups experienced a significant increase in MMSE scores after 12 weeks compared to their pre-intervention levels (in the RD group, baseline vs week 12: 21.46 ± 3.86 vs 25.39 ± 2.86, t = 6.266 P = 0.000; in the PN group, baseline vs week 12: 21.42 ± 3.74 vs 26.48 ± 2.27, t = 8.244, P = 0.000).
| Routine diet group (n = 48) | Personalized nutrition group (n = 48) | t value | P value | |
| Before treatment | 21.46 ± 3.86 | 21.42 ± 3.74 | 0.054 | 0.957 |
| After treatment | 25.39 ± 2.86 | 26.48 ± 2.27 | 2.057 | 0.042 |
| t value | 6.266 | 8.244 | ||
| P value | 0.000 | 0.000 |
Figure 1D-H show that the two groups did not exhibit significant differences at pre-intervention in the following parameters: OC (RD vs PN: 3.45 ± 1.33 vs 3.65 ± 1.08, t = 0.809, P = 0.421), serum calcium (RD vs PN: 8.46 ± 2.34 vs 8.62 ± 2.12, t = 0.351, P = 0.726), PINP (RD vs PN: 73.27 ± 5.28 vs 73.18 ± 5.67, t = 0.081, P = 0.936), β-CTX (RD vs PN: 0.76 ± 0.13 vs 0.75 ± 0.15, t = 0.349, P = 0.728), and 25-OH-D (RD vs PN: 15.34 ± 4.02 vs 15.15 ± 3.87, t = 0.236, P = 0.814). However, after 12 weeks of intervention, a significant difference was observed in OC (RD vs PN: 5.03 ± 1.24 vs 5.51 ± 1.12, t = 1.990, P = 0.049). Serum calcium levels were significantly higher in the PN group compared to the RD group (RD vs PN: 10.33 ± 2.45 vs 12.34 ± 2.63, t = 3.874, P = 0.000), as well as 25-OH-D (RD vs PN: 17.51 ± 4.29 vs 19.44 ± 4.82, t = 2.072, P = 0.041). In contrast, both PINP (RD vs PN: 70.67 ± 4.88 vs 68.59 ± 4.59, t = 2.151, P = 0.034) and β-CTX (RD vs PN: 0.67 ± 0.12 vs 0.61 ± 0.11, t = 2.554, P = 0.012) were lower in the PN group (P < 0.05). In both groups, OC (in the RD group, before vs Week 12: 3.45 ± 1.33 vs 5.03 ± 1.24, t = 6.020, P = 0.000; in the PN group, before vs Week 12: 3.65 ± 1.08 vs 5.51 ± 1.12, t = 8.282, P = 0.000), serum calcium (in the RD group, before vs Week 12: 8.46 ± 2.34 vs 10.33 ± 2.45, t = 3.824, P = 0.000; in the PN group, before vs Week 12: 8.62 ± 2.12 vs 12.34 ± 2.63, t = 7.629, P = 0.000), and 25-OH-D (in the RD group, before vs Week 12: 15.34 ± 4.02 vs 17.51 ± 4.29, t = 2.557, P = 0.012; in the PN group, before vs Week 12: 15.15 ± 3.87 vs 19.44 ± 4.82, t = 4.808, P = 0.000) increased significantly while PINP (in the RD group, before vs Week 12: 73.27 ± 5.28 vs 70.67 ± 4.88, t = 2.505, P = 0.014; in the PN group, before vs Week 12: 73.18 ± 5.67 vs 68.59 ± 4.59, t = 4.359, P = 0.000) and β-CTX (in the RD group, before vs Week 12: 0.76 ± 0.13 vs 0.67 ± 0.12, t = 3.524, P = 0.000; in the PN group, before vs Week 12: 0.75 ± 0.15 vs 0.61 ± 0.11, t = 5.214, P = 0.000) decreased after 12 weeks of intervention.
Patients with AD experience disruptions in bone homeostasis[15]. Astrocytes in AD patients are phenotypically abnormal and depleted, which may contribute to calcium loss from the bones, leading to OP[16]. Clinical data indicate a significant positive correlation between AD-related factors, including amyloid precursor protein, and bone regulatory genes such as TRAP and OPG. This suggests that AD and OP may share common molecular pathways in their pathological mechanisms[17]. AD-induced bone loss also increases the risk of fractures and falls in patients[18,19]. Therefore, the remission of AD also helps reduce the incidence of hip fractures and clinical fractures[20]. The bone health challenges faced by AD patients present a significant and pressing burden requiring immediate attention. This clinical controlled trial investigates a potential bone management strategy for AD patients based on nutritional patterns and presents several valuable findings. First, compared to pre-intervention levels, the BMD of patients significantly increased after both conventional and personalized nutritional interventions, along with improvements in bone metabolism markers. Second, compared to conventional nutrition, dietary nutrition based on human BCA yielded better bone outcomes, as evidenced by higher BMD and more favorable bone metabolic markers in the PN group.
Nutritional factors play a crucial role in the reduction of BMD. Calcium ions and vitamin D are vital for maintaining bone metabolic homeostasis, while sufficient calcium intake and increased dietary protein are advantageous in lowering the risk of fractures[21]. An increase in dietary iron intake is significantly associated with a decreased risk of fractures and OP. Additionally, patients with AD often exhibit clinical signs of malnutrition, and diet has been shown to influence the progression of the disease in these individuals[22,23]. Therefore, timely adjustments to the nutritional structure and regimen of AD patients can help mitigate disruptions in their bone homeostasis.
Body composition refers to the relative proportions of fat, muscle, bones, and water in an individual’s body mass. Real-time data on body composition is crucial for personalizing and optimizing body fluid balance, nutritional plans, and medication dosages, as well as aiding in prognostic evaluation[24,25]. Therefore, this study was designed to create a dietary plan focusing on BCA and compare it with a conventional nutrition plan. The aim was to investigate if the former is more effective in managing bone health among AD patients. To assess primary outcomes, this study collected T scores from patients before and 12 weeks after intervention. The number of patients with a T score > -1 was significantly greater after the intervention compared to before in both the RD group and the PN group (RD group: P = 0.000; PN group: P = 0.001). Additionally, the number of patients with a T score < 2.5 was significantly reduced (RD vs PN: P = 0.042), suggesting that adjusting the nutritional structure of AD patients helps in the recovery from OP. Furthermore, after intervention, more patients in the PN group had a T score > -1, and fewer had a T score < 2.5 compared to the RD group.
BCA, based on bioelectrical impedance, is non-invasive, rapid, and cost-effective, making it highly feasible for bedside use. This bioelectrical impedance technique is widely used to evaluate the nutritional status of patients with various diseases[24,26], making it an important reference for developing nutritional programs. In this study, a series of bioelectrical impedance tests were performed before initiating the nutritional intervention in the PN group, allowing for the creation of a personalized dietary nutrition plan for each patient based on their specific body composition (including factors such as body weight, BMI, muscle mass, etc.). These tailored regimens align with the individual characteristics of the patients, resulting in a more pronounced impact and effect, which accounts for the greater bone recovery observed in the PN group compared to the RD group.
In this study, we not only gathered patients’ T scores but also evaluated the changes in bone metabolism before and after the intervention. Both groups demonstrated a significant improvement in bone metabolism indices following the intervention, including OC (RD group: P = 0.000; PN group: P = 0.000), serum calcium (RD group: P = 0.000; PN group: P = 0.000), and 25-OH-D (RD group: P = 0.012; PN group: P = 0.000), PINP (RD group: P = 0.014; PN group: P = 0.000), and β-CTX (RD group: P = 0.000; PN group: P = 0.000). Notably, the PN group exhibited a more pronounced improvement in various bone metabolism indices compared to the RD group after the intervention, including OC (P = 0.049), serum calcium (P = 0.000), 25-OH-D (P = 0.041), PINP (P = 0.034), and β-CTX (P = 0.012), highlighting the critical role of nutritional management in enhancing bone metabolism in AD patients and showcasing the significant benefits of BCA-based dietary nutrition. Furthermore, this study revealed that nutritional intervention also led to an improvement in MMSE scores (RD group: P = 0.000; PN group: P = 0.000), with the PN group achieving higher MMSE scores compared to the RD group (P = 0.042). This indicates that nutritional intervention may also aid in improving the cognitive function of AD patients. The changes observed in MMSE scores further suggest that reducing bone loss or promoting bone metabolic homeostasis is advantageous for AD.
Currently, there are limited studies on BCA-based dietary nutrition interventions for OP in AD patients. This study strongly confirmed the feasibility of developing dietary nutrition based on BCA and its application in bone management for AD patients. However, this controlled interventional trial has some limitations. First, because this study is not a multi-center study, the sample size presented is limited. To overcome this limitation, we will collaborate with other hospitals to validate the findings of this study through multi-center and diverse clinical trials. Second, owing to time and funding constraints, this study could not provide long-term follow-up for patients. Thus, it was not possible to demonstrate the long-term impact of BCA-based dietary nutrition regimens in the results.
In summary, this research assesses the feasibility of using human BCA-based dietary nutrition to alleviate OP in AD patients and confirms that this approach is more effective than conventional nutritional strategies in enhancing BMD and bone metabolism. This provides strong evidence for the nutritional development of AD patients.
| 1. | Griffiths J, Grant SGN. Synapse pathology in Alzheimer's disease. Semin Cell Dev Biol. 2023;139:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 137] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 2. | Monteiro AR, Barbosa DJ, Remião F, Silva R. Alzheimer's disease: Insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs. Biochem Pharmacol. 2023;211:115522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 176] [Reference Citation Analysis (0)] |
| 3. | Zhang C. Etiology of Alzheimer's Disease. Discov Med. 2023;35:757-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 4. | Wang HS, Karnik SJ, Margetts TJ, Plotkin LI, Movila A, Fehrenbacher JC, Kacena MA, Oblak AL. Mind Gaps and Bone Snaps: Exploring the Connection Between Alzheimer's Disease and Osteoporosis. Curr Osteoporos Rep. 2024;22:483-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Pu Z, Tang X, Fei Y, Hou Q, Lin Y, Zha X. Bone metabolic biomarkers and bone mineral density in male patients with early-stage Alzheimer's disease. Eur Geriatr Med. 2020;11:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Zhou BN, Zhang Q, Li M. Alzheimer's disease and its associated risk of bone fractures: a narrative review. Front Endocrinol (Lausanne). 2023;14:1190762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Armstrong P, Kuo YF, Cram P, Westra J, Raji MA. National trends in osteoporosis medication use among Medicare beneficiaries with and without Alzheimer's disease/related dementias. Osteoporos Int. 2023;34:725-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Groenendijk I, Grootswagers P, Santoro A, Franceschi C, Bazzocchi A, Meunier N, Caille A, Malpuech-Brugere C, Bialecka-Debek A, Pietruszka B, Fairweather-Tait S, Jennings A, de Groot LCPGM. Protein intake and bone mineral density: Cross-sectional relationship and longitudinal effects in older adults. J Cachexia Sarcopenia Muscle. 2023;14:116-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Rizzoli R, Chevalley T. Bone health: biology and nutrition. Curr Opin Clin Nutr Metab Care. 2024;27:24-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Puente-González AS, Sánchez-González F, Hernández-Xumet JE, Sánchez-Sánchez MC, Barbero-Iglesias FJ, Méndez-Sánchez R. Short and medium-term effects of a multicomponent physical exercise program with a Mediterranean diet on bone mineral density, gait, balance, and fall risk for patients with Alzheimer disease: Randomized controlled clinical trial study protocol. Medicine (Baltimore). 2020;99:e22385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Holmes CJ, Racette SB. The Utility of Body Composition Assessment in Nutrition and Clinical Practice: An Overview of Current Methodology. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 12. | Lins Vieira NF, da Silva Nascimento J, do Nascimento CQ, Barros Neto JA, Oliveira Dos Santo ACS. Association between Bone Mineral Density and Nutritional Status, Body Composition and Bone Metabolism in Older Adults. J Nutr Health Aging. 2021;25:71-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Cornacchia S, La Tegola L, Maldera A, Pierpaoli E, Tupputi U, Ricatti G, Eusebi L, Salerno S, Guglielmi G. Radiation protection in non-ionizing and ionizing body composition assessment procedures. Quant Imaging Med Surg. 2020;10:1723-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Hung KY, Chen TH, Lee YF, Fang WF. Using Body Composition Analysis for Improved Nutritional Intervention in Septic Patients: A Prospective Interventional Study. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 15. | Liu X, Chen C, Jiang Y, Wan M, Jiao B, Liao X, Rao S, Hong C, Yang Q, Zhu Y, Liu Q, Luo Z, Duan R, Wang Y, Tan Y, Cao J, Liu Z, Wang Z, Xie H, Shen L. Brain-derived extracellular vesicles promote bone-fat imbalance in Alzheimer's disease. Int J Biol Sci. 2023;19:2409-2427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 16. | Tsai YL, Yen CT, Wang YF. Astrocyte Dysregulation and Calcium Ion Imbalance May Link the Development of Osteoporosis and Alzheimer's Disease. J Alzheimers Dis. 2022;88:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 17. | Stapledon CJM, Stamenkov R, Cappai R, Clark JM, Bourke A, Bogdan Solomon L, Atkins GJ. Relationships between the Bone Expression of Alzheimer's Disease-Related Genes, Bone Remodelling Genes and Cortical Bone Structure in Neck of Femur Fracture. Calcif Tissue Int. 2021;108:610-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Frame G, Bretland KA, Dengler-Crish CM. Mechanistic complexities of bone loss in Alzheimer's disease: a review. Connect Tissue Res. 2020;61:4-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Dev K, Javed A, Bai P, Murlidhar, Memon S, Alam O, Batool Z. Prevalence of Falls and Fractures in Alzheimer's Patients Compared to General Population. Cureus. 2021;13:e12923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Hosoi T, Yakabe M, Matsumoto S, Fujimori K, Tamaki J, Nakatoh S, Ishii S, Okimoto N, Kamiya K, Akishita M, Iki M, Ogawa S. Relationship between antidementia medication and fracture prevention in patients with Alzheimer's dementia using a nationwide health insurance claims database. Sci Rep. 2023;13:6893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 21. | Rizzoli R, Biver E, Brennan-Speranza TC. Nutritional intake and bone health. Lancet Diabetes Endocrinol. 2021;9:606-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 184] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 22. | Arora S, Santiago JA, Bernstein M, Potashkin JA. Diet and lifestyle impact the development and progression of Alzheimer's dementia. Front Nutr. 2023;10:1213223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 23. | Xu Lou I, Ali K, Chen Q. Effect of nutrition in Alzheimer's disease: A systematic review. Front Neurosci. 2023;17:1147177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 24. | Moonen HPFX, Van Zanten ARH. Bioelectric impedance analysis for body composition measurement and other potential clinical applications in critical illness. Curr Opin Crit Care. 2021;27:344-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 25. | Ceniccola GD, Castro MG, Piovacari SMF, Horie LM, Corrêa FG, Barrere APN, Toledo DO. Current technologies in body composition assessment: advantages and disadvantages. Nutrition. 2019;62:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 171] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 26. | Scislo L, Walewska E, Bodys-Cupak I, Skorus-Zadecka U, Richter P, Szczepanik AM. Selected Body Composition Parameters Analysis Based on Bioelectrical Impedance in Patients Operated for Gastrointestinal Cancer. In Vivo. 2022;36:2936-2944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/