Published online Nov 19, 2025. doi: 10.5498/wjp.v15.i11.108288
Revised: July 4, 2025
Accepted: August 26, 2025
Published online: November 19, 2025
Processing time: 151 Days and 19 Hours
Cervical spondylosis (CS) frequently co-occurs with generalized anxiety disorder (GAD), presenting a complex clinical challenge. Managing CS-related pain in pa
To evaluate the efficacy of electroacupuncture (EA) in treating CS-related pain and anxiety in patients with GAD.
This retrospective cohort study analyzed data from 83 patients with CS-related pain and GAD who received EA treatment over 2-year period. Pain intensity was assessed using the visual analog scale, and anxiety symptoms were measured using the Hamilton Anxiety Rating Scale. Additionally, neuroinflammatory markers, including interleukin-6, tumor necrosis factor-alpha, and high-sensitivity C-reactive protein, were examined. Outcomes were evaluated at baseline, after 4 weeks, and after 8 weeks of treatment.
EA treatment significantly reduced CS-related pain (mean visual analog scale re
EA demonstrates significant efficacy in reducing CS-related pain in patients with comorbid GAD, along with concurrent improvements in anxiety symptoms and neuroinflammatory profiles. These findings suggest that EA may offer a valuable integrative approach for managing this complex clinical presentation, potentially addressing both pain and anxiety through the modulation of neuroinflammatory pathways.
Core Tip: This study highlights the effectiveness of electroacupuncture (EA) in reducing cervical spondylosis-related pain and alleviating anxiety symptoms in patients with comorbid generalized anxiety disorders. Over an 8-week treatment period, EA significantly reduced pain, anxiety, and neuroinflammatory markers, such as interleukin-6 and tumor necrosis factor-alpha. These findings suggest that EA may be a promising integrative approach for managing both pain and anxiety by modulating neuroinflammatory pathways, offering valuable clinical benefits for patients with this complex condition.
- Citation: Li Z, Gao Y, Yan D, Feng YH. Efficacy of electroacupuncture for cervical spondylosis-related pain in patients with generalized anxiety disorder. World J Psychiatry 2025; 15(11): 108288
- URL: https://www.wjgnet.com/2220-3206/full/v15/i11/108288.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i11.108288
Cervical spondylosis (CS) is a prevalent degenerative condition of the cervical spine, characterized by the progressive deterioration of the intervertebral discs, facet joints, and surrounding ligamentous structures[1]. With global prevalence rates ranging from 30% to 50% among middle-aged and older populations, CS constitutes a significant healthcare burden[2]. Its clinical manifestations primarily include neck pain, stiffness, radicular symptoms, and, in severe cases, mye
Managing CS-related pain is particularly challenging in the presence of comorbid psychological conditions, among which generalized anxiety disorder (GAD) is among the most frequently encountered psychiatric comorbidities. The prevalence of GAD among patients with chronic cervical pain ranges from 15% to 28%, which is substantially higher than that in the general population[5]. This comorbidity creates a complex bidirectional relationship in which pain exacerbates anxiety symptoms, whereas anxiety amplifies pain perception through central sensitization and altered pain processing pathways[6]. The neurobiological interface between chronic pain and anxiety involves shared neurotransmitter systems, particularly serotonergic and noradrenergic pathways, as well as overlapping neural circuits in the prefrontal cortex, am
Conventional management of CS-related pain typically involves a combination of pharmacological interventions (e.g., non-steroidal anti-inflammatory drugs, muscle relaxants, and antidepressants), physical therapy, and, in refractory cases, surgical procedures[8]. However, these treatment modalities often yield suboptimal outcomes in patients with comorbid anxiety disorders. Pharmacological interventions for pain may interact adversely with anxiolytic medications, and anxiety can diminish patient engagement with rehabilitation programs[9]. Moreover, growing evidence points to neu
Electroacupuncture (EA), an enhanced form of traditional acupuncture that involves the application of small electrical currents between pairs of acupuncture needles, has emerged as a promising intervention for various pain conditions. The neurobiological mechanisms of EA encompass multiple pathways, including the release of endogenous opioids, modulation of autonomic nervous system function, and anti-inflammatory effects[11]. Recent experimental and clinical studies have demonstrated the capacity of EA to suppress pro-inflammatory cytokines, including interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), while upregulating anti-inflammatory mediators through the vagal and sympathetic pathways[12].
The potential of EA to alleviate both pain and anxiety symptoms makes it particularly relevant in the context of CS with comorbid GAD. Preliminary research suggests that EA reduces anxiety symptoms by modulating the hypothalamic-pituitary-adrenal axis function and regulating neurotransmitter levels, thereby complementing its analgesic effects[13]. However, comprehensive studies examining the dual efficacy of EA for CS-related pain in the specific context of comorbid GAD remain limited, particularly regarding its impact on neuroinflammatory markers that may constitute a mechanistic link between the two conditions.
The rationale for investigating EA in this clinical context arises from several considerations. First, integrated treatment approaches that simultaneously address both pain and anxiety symptoms are crucial for optimal management of these comorbid conditions. Second, the growing recognition of neuroinflammation as a shared pathophysiological substrate for both chronic pain and anxiety disorders highlights the potential mechanistic advantages of anti-inflammatory inter
Despite these theoretical advantages, clinical evidence regarding the efficacy of EA in CS with comorbid GAD remains insufficient. Previous studies have primarily focused on either pain reduction or anxiolytic effects in isolation, without adequately considering the interrelationship between these symptoms or underlying inflammatory mechanisms. Furthermore, methodological limitations, including small sample sizes, heterogeneous outcome measures, and a lack of long-term follow-up, have constrained the generalizability of the existing findings[15]. While other integrative app
This study employed a retrospective cohort design to analyze data from patients who received EA treatment for CS-related pain with comorbid GAD between January 2023 and December 2024 at Zhengzhou Orthopedic Hospital, China. The study protocol was approved by the institutional ethics committee, and the requirement for informed consent was waived owing to the retrospective nature of the study. All procedures were conducted in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments.
Inclusion criteria: Patients were included in the analysis if they met the following criteria: (1) Age between 18 and 65 years; (2) Diagnosed with CS based on the clinical practice guidelines of the North American Spine Society, confirmed by radiological evidence (X-ray, computed tomography, or magnetic resonance imaging); (3) Experienced neck pain with or without radicular symptoms for at least 3 months before treatment initiation; (4) Had a concurrent diagnosis of GAD according to Diagnostic and Statistical Manual of Mental Disorders criteria, established by a psychiatrist; (5) Received EA treatment for at least 8 consecutive weeks; and (6) Had complete medical records, including pain assessments, anxiety evaluations, and laboratory examinations at baseline, 4 weeks, and 8 weeks after treatment initiation.
Exclusion criteria: Patients were excluded from the analysis if they had: (1) Cervical myelopathy requiring surgical intervention; (2) A history of cervical spine surgery; (3) Cervical pain attributable to other pathologies (e.g., infection, malignancy, inflammatory arthritis); (4) Severe psychiatric comorbidities beyond GAD (e.g., major depressive disorder, bipolar disorder, schizophrenia); (5) Received acupuncture or EA treatment within 6 months prior to the index treatment; (6) Undergone significant changes in anxiolytic medication regimen during the treatment period; (7) Concurrent use of systemic corticosteroids or immunosuppressants; or (8) Incomplete follow-up data.
Sample size: Based on the eligibility criteria, 96 patients were initially identified from their medical records. After applying the exclusion criteria, 83 patients with complete data were included in the final analysis. This sample size provided 85% power to detect a moderate effect size (Cohen’s d = 0.5) at a significance level of 0.05, based on previous studies on EA in chronic pain conditions.
Acupuncture protocol: The EA protocol was standardized across all patients and administered by licensed acupuncturists with a minimum of 5 years of clinical experience. Treatment sessions were conducted three times a week for 8 consecutive weeks, with each session lasting approximately 30 minutes. Disposable, sterilized stainless steel needles (0.25 mm × 40 mm, Huatuo brand, Suzhou Medical Appliance Factory, China) were used for all the treatments.
Acupuncture points were selected based on traditional Chinese medicine principles for treating cervical pain and anxiety, and included both local and distal points. The standard point prescription included: Fengchi (GB20), Jianjing (GB21), Tianzhu (BL10), Dazhui (GV14), Jianzhongshu (SI15), Hegu (LI4), Lieque (LU7), Shenmen (HT7), and Taichong (LR3). Additional points were permitted at the discretion of the treating acupuncturist, based on individual symptom presentation, and were documented in the medical records.
The needles were inserted to a depth of 10-25 mm, depending on the location of the acupuncture point and the patient’s body habitus. After insertion, the needles were manually manipulated to elicit the sensation of “de qi” (a composite of sensations including soreness, numbness, distention, and heaviness) before electrical stimulation was applied.
Electrical stimulation parameters: Electrical stimulation was delivered using a G6805-2A EA device (Shanghai Medical Instruments High-Tech Co., Ltd., China). Paired electrodes were attached to needles at Fengchi (GB20) and Jianjing (GB21) bilaterally, as well as at Dazhui (GV14) and Jianzhongshu (SI15) bilaterally. The stimulation parameters were as follows: A continuous wave at 2 Hz for the cervical points and a disperse-dense wave with alternating frequencies of 2 Hz and 15 Hz for the distal points. The intensity was individually adjusted for each patient to produce visible muscle contractions without causing discomfort, typically ranging from 0.5 to 3 mA.
Concurrent treatments: Throughout the treatment period, the patients continued their prescribed anxiolytic medications at stable doses. Standard physical therapy for CS, including gentle stretching exercises and postural training, was also permitted. The use of analgesics was documented, and the patients were advised to maintain a consistent dosage when possible and to record any changes in medication use. Non-steroidal anti-inflammatory drugs were permitted as rescue medications, and the frequency of use was recorded.
Pain assessment: The primary outcome measure was the change in pain intensity, assessed using the visual analog scale (VAS). The VAS consists of a 10 cm horizontal line, with endpoints labeled “no pain” (0) and “worst possible pain” (10). Patients were instructed to mark the point on the line that represented their average pain intensity over the preceding week. A change of 2 points or 30% from baseline was considered clinically meaningful, based on established minimal clinically important difference criteria. VAS scores were measured at baseline, after 4 weeks of treatment, and after 8 weeks of treatment.
Additionally, the neck disability index (NDI) was used to evaluate the functional impairment associated with cervical pain. The NDI is a 10-item questionnaire that assesses various aspects of daily activities affected by neck pain, with each item scored from 0 (no disability) to 5 (complete disability). The total score ranges from 0 to 50, with higher scores indicating a greater degree of disability. Scores were interpreted as follows: 0-4 = no disability, 5-14 = mild disability, 15-24 = moderate disability, 25-34 = severe disability, and > 34 = complete disability. A change of 5 points was considered the minimal clinically important difference for the NDI. NDI scores were obtained at the same time points as the VAS assessments.
Anxiety assessment: Anxiety symptoms were evaluated using the Hamilton Anxiety Rating Scale (HAM-A), a clinician-administered tool consisting of 14 items that assess both psychic and somatic anxiety symptoms. Each item is rated on a scale from 0 (not present) to 4 (severe), with a total score range of 0-56. Higher scores indicate more severe anxiety. The HAM-A was administered by trained psychiatrists at baseline, 4 weeks, and 8 weeks after treatment ini
The GAD 7-item scale (GAD-7), a self-reported questionnaire, was also used as a supplementary measure of anxiety severity. The GAD-7 comprises seven items, each rated from 0 (not at all) to 3 (nearly every day), with a total score range of 0-21. Scores of 5, 10, and 15 indicate mild, moderate, and severe anxiety, respectively. Patients completed the GAD-7 at the same time points as HAM-A.
Neuroinflammatory markers: Blood samples were collected from patients after an overnight fast at baseline and 4 and 8 weeks after treatment initiation. Serum IL-6, TNF-α, and high-sensitivity C-reactive protein (hs-CRP) levels were measured as indicators of systemic inflammation.
Serum IL-6 and TNF-α concentrations were determined using enzyme-linked immunosorbent assay kits (RayBiotech Life, Peachtree Corners, GA, United States) according to the manufacturer’s instructions. The detection limits were 0.3 pg/mL for IL-6 and 1.0 pg/mL for TNF-α. Serum hs-CRP was measured using an immunoturbidimetric assay on an automated biochemical analyzer (Beckman Coulter AU5800, Brea, CA, United States), with a detection limit of 0.1 mg/L. All samples were processed within 2 hours of collection, and the analyses were performed in duplicate. The laboratory personnel were blinded to the clinical status of the patients.
Safety assessment: Adverse events potentially related to EA treatment were documented throughout the study period. These included bruising, persistent pain at the needle sites, dizziness, fatigue, and exacerbation of anxiety symptoms. The severity of adverse events was graded as mild (no interference with daily activities), moderate (some interference with daily activities), or severe (complete interference with the daily activities).
Demographic and clinical data, including age, sex, body mass index, CS duration, GAD duration, radiological findings, medications, and comorbidities, were extracted from electronic medical records. Treatment adherence was calculated as the percentage of scheduled EA sessions attended by the patients. Pain, anxiety, and neuroinflammatory marker assessments were conducted at three time points: Baseline (before initiating EA), 4 weeks after treatment, and 8 weeks after treatment. All assessments were performed by trained healthcare professionals, following standardized protocols.
Data processing: Data were anonymized prior to analysis and checked for completeness and accuracy. Continuous variables were tested for normality using the Shapiro-Wilk test. Descriptive statistics were reported as mean ± SD for normally distributed variables, and as median (interquartile range) for non-normally distributed variables. Categorical variables were presented as frequencies and percentages.
Primary analysis: Changes in pain intensity (VAS scores), anxiety symptoms (HAM-A and GAD-7 scores), and neuroinflammatory markers (IL-6, TNF-α, and hs-CRP) from baseline to 4 and 8 weeks after treatment initiation were analyzed using repeated-measures analysis of variance with the Bonferroni correction for multiple comparisons. Effect sizes were calculated using Cohen’s d, with values of 0.2, 0.5, and 0.8 representing small, medium, and large effects, respectively. Additionally, the proportion of patients achieving clinically significant improvements was calculated. Clinically significant improvement was defined as a reduction of ≥ 30% from baseline for VAS scores, a reduction of ≥ 50% from baseline for HAM-A scores, and normalization of neuroinflammatory marker levels.
Secondary analysis: Pearson’s correlation coefficient was used to analyze the relationships between changes in pain intensity, anxiety symptoms, and neuroinflammatory markers. Multiple linear regression analysis was performed to identify predictors of treatment response, with the change in VAS score as the dependent variable and baseline characteristics, anxiety scores, and inflammatory markers as independent variables.
Subgroup analyses were conducted to examine potential differences in treatment effects based on sex, symptom duration, severity of radiological findings, and baseline anxiety levels. Sensitivity analysis was performed by excluding patients with compliance rates below 80%. All statistical analyses were performed using SPSS version 26.0 (IBM Corp., Armonk, NY, United States). A two-sided P value < 0.05 was considered statistically significant. Graphs were created using GraphPad Prism (version 9.0; GraphPad Software, San Diego, CA, United States).
A total of 83 patients with CS-related pain and comorbid GAD were included in the final analysis. The mean age of the cohort was 48.6 ± 9.7 years, with a female predominance (61.4%). The mean duration of CS symptoms was 4.3 ± 2.8 years, and the mean duration of GAD was 3.1 ± 2.2 years. The detailed demographic and clinical characteristics of the study population are presented in Table 1.
| Characteristic | Value |
| Age (years) | 48.6 ± 9.7 |
| Female sex | 51 (61.4) |
| BMI (kg/m²) | 24.3 ± 3.5 |
| Duration of CS symptoms (years) | 4.3 ± 2.8 |
| Duration of GAD diagnosis (years) | 3.1 ± 2.2 |
| Radiological findings | |
| Disc degeneration | 83 (100) |
| Foraminal stenosis | 62 (74.7) |
| Facet joint arthropathy | 48 (57.8) |
| Uncovertebral joint arthropathy | 37 (44.6) |
| Baseline VAS score | 6.8 ± 1.4 |
| Baseline NDI score | 28.3 ± 6.2 |
| Baseline HAM-A score | 21.6 ± 5.3 |
| Baseline GAD-7 score | 14.2 ± 3.8 |
| Neuroinflammatory markers | |
| IL-6 (pg/mL) | 3.42 ± 1.18 |
| TNF-α (pg/mL) | 8.76 ± 2.53 |
| hs-CRP (mg/L) | 2.83 ± 1.17 |
| Concurrent medications | |
| SSRIs/SNRIs | 58 (69.9) |
| Benzodiazepines | 45 (54.2) |
| NSAIDs | 61 (73.5) |
| Muscle relaxants | 42 (50.6) |
| Treatment adherence (%), median (IQR) | 91.7 (83.3-95.8) |
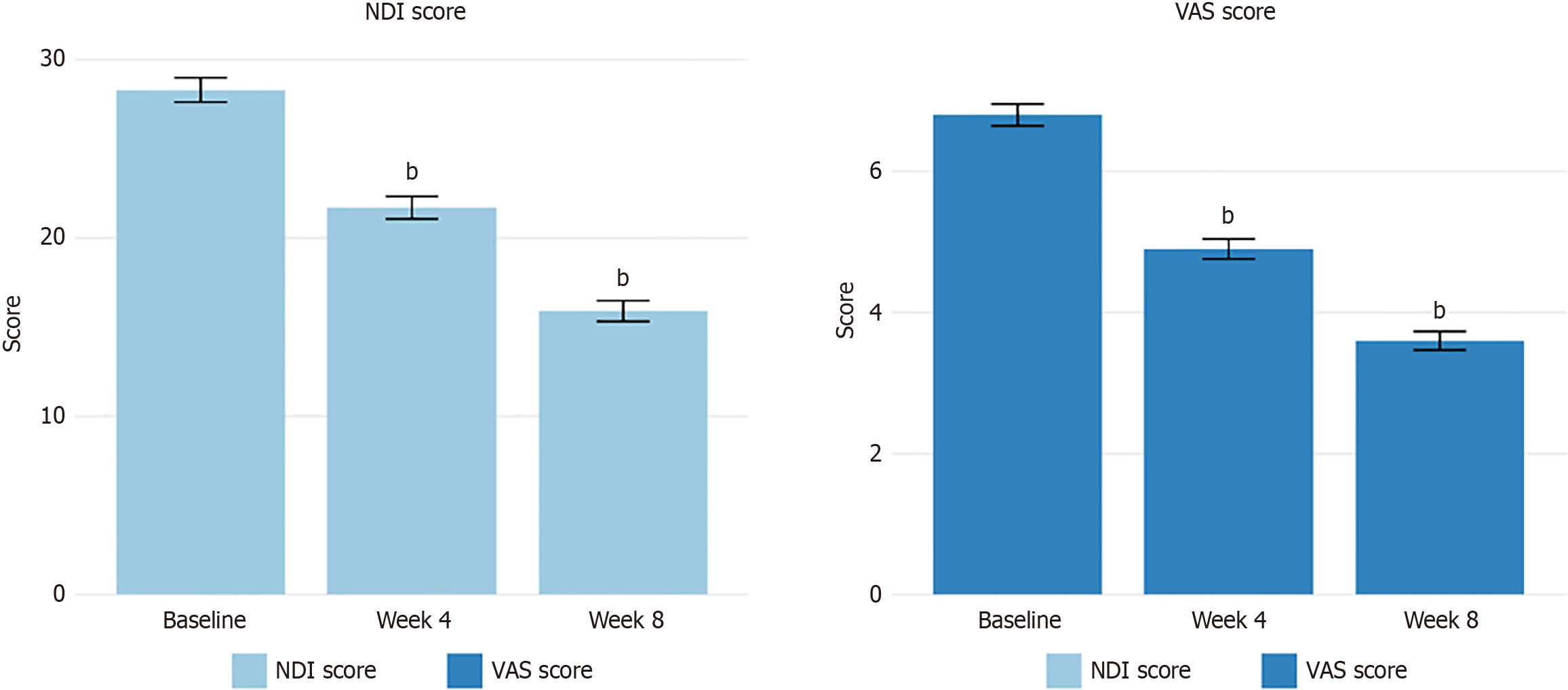
EA treatment resulted in significant reductions in pain intensity, as measured by the VAS scores. The mean VAS score decreased from 6.8 ± 1.4 at baseline to 4.9 ± 1.3 at week 4 (P < 0.001) and further to 3.6 ± 1.2 at week 8 (P < 0.001). The mean reduction in the VAS score from baseline to week 8 was 3.24 ± 1.18, representing a 47.6% decrease in the VAS score. A clinically significant reduction in pain (≥ 30% from baseline) was achieved by 52.4% of patients at week 4 and 78.3% at week 8. Similarly, functional improvement was observed, as indicated by the decrease in NDI scores. The mean NDI score decreased from 28.3 ± 6.2 at baseline to 21.7 ± 5.8 at week 4 (P < 0.001) and to 15.9 ± 5.3 at week 8 (P < 0.001), representing a 43.8% reduction in functional disability by the end of the treatment period. Changes in pain and functional outcomes over the treatment period are summarized in Table 2 and illustrated in Figure 1.
| Outcome measure | Baseline | Week 4 | Week 8 | Change from baseline to week 8 | P value1 |
| Pain and function | |||||
| VAS score | 6.8 ± 1.4 | 4.9 ± 1.3 | 3.6 ± 1.2 | -3.24 ± 1.18 (-47.6%) | < 0.001 |
| NDI score | 28.3 ± 6.2 | 21.7 ± 5.8 | 15.9 ± 5.3 | -12.4 ± 4.7 (-43.8%) | < 0.001 |
| Patients achieving ≥ 30% VAS reduction | 43 (52.4) | 65 (78.3) | |||
| Anxiety symptoms | |||||
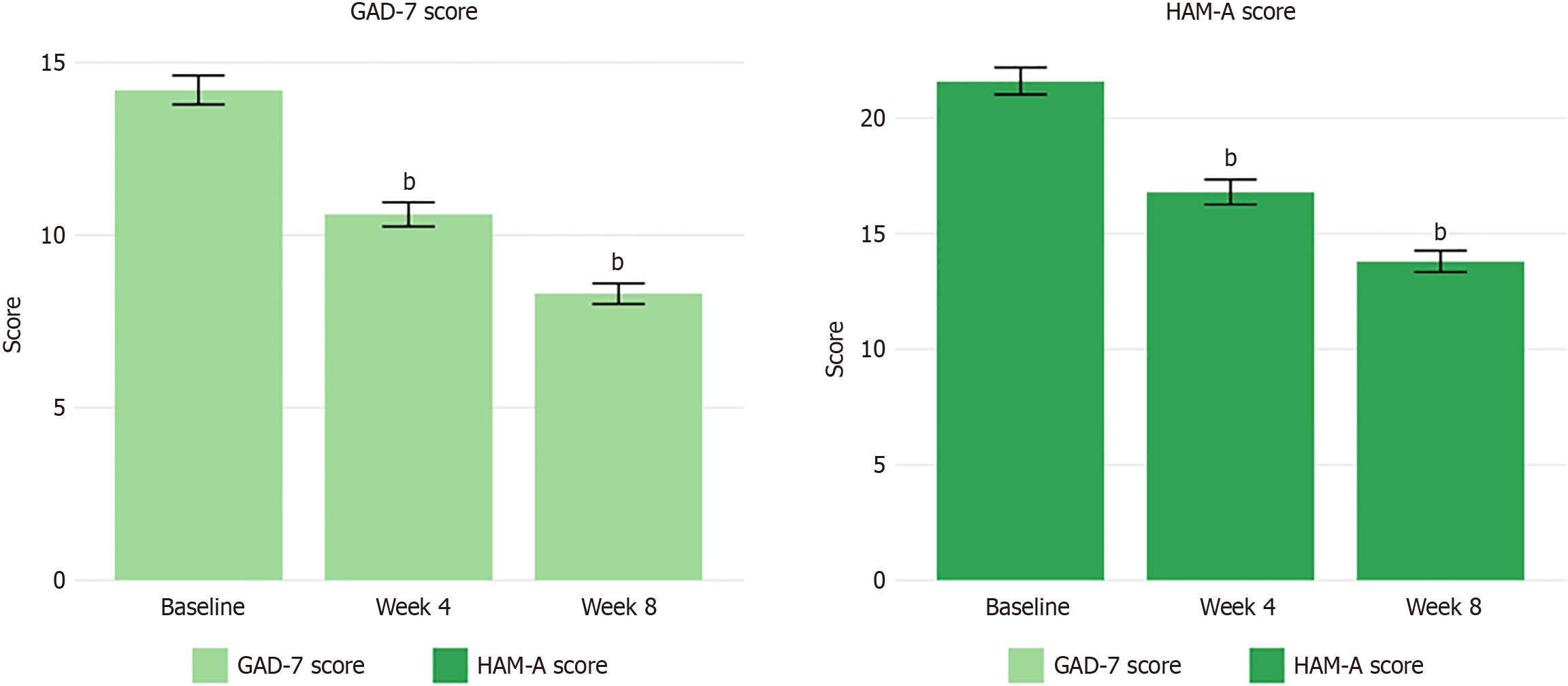
| HAM-A score | 21.6 ± 5.3 | 16.8 ± 4.9 | 13.8 ± 4.2 | -7.83 ± 2.65 (-36.3%) | < 0.001 |
| GAD-7 score | 14.2 ± 3.8 | 10.6 ± 3.2 | 8.3 ± 2.7 | -5.9 ± 2.1 (-41.8%) | < 0.001 |
| Patients achieving ≥ 50% HAM-A reduction | 19 (22.9) | 34 (41.0) | |||
| Neuroinflammatory markers | |||||
| IL-6 (pg/mL) | 3.42 ± 1.18 | 2.83 ± 0.96 | 2.30 ± 0.79 | -1.12 ± 0.51 (-32.7%) | < 0.001 |
| TNF-α (pg/mL) | 8.76 ± 2.53 | 7.25 ± 2.17 | 6.27 ± 1.85 | -2.49 ± 1.02 (-28.5%) | < 0.001 |
| hs-CRP (mg/L) | 2.83 ± 1.17 | 2.36 ± 0.94 | 1.95 ± 0.83 | -0.88 ± 0.42 (-31.1%) | < 0.001 |
| Patients with normal IL-62 | 27 (32.5) | 44 (53.0) | 56 (67.5) | ||
| Patients with normal TNF-α2 | 24 (28.9) | 41 (49.4) | 53 (63.9) | ||
| Patients with normal hs-CRP2 | 30 (36.1) | 48 (57.8) | 60 (72.3) |
Concurrent with improvements in pain, significant reductions in anxiety symptoms were observed following EA treatment. The mean HAM-A score decreased from 21.6 ± 5.3 at baseline to 16.8 ± 4.9 at week 4 (P < 0.001) and further to 13.8 ± 4.2 at week 8 (P < 0.001), representing a 36.3% reduction from the baseline. The proportion of patients achieving a clinically significant improvement in anxiety symptoms (≥ 50% reduction in HAM-A score) was 22.9% and 41.0% at weeks 4 and 8, respectively.
Similar trends were observed in the self-reported GAD-7 scores, which decreased from 14.2 ± 3.8 at baseline to 10.6 ± 3.2 at week 4 (P < 0.001) and to 8.3 ± 2.7 at week 8 (P < 0.001), indicating an overall shift from moderate to mild anxiety levels. This represented a 41.8% reduction in self-reported anxiety symptoms by the end of the treatment period. Changes in anxiety outcomes are presented in Table 2 and illustrated in Figure 2. The temporal pattern of improvement revealed progressive benefits across all domains, with the most substantial changes occurring between weeks 4 and 8. The effect sizes for the primary outcomes were large (Cohen’s d = 1.92 for VAS, d = 1.56 for HAM-A), indicating robust clinical effects. Notably, 67% of patients who achieved clinically significant pain reduction also demonstrated meaningful improvement in anxiety symptoms, supporting the interconnection of these outcomes.
Analysis of neuroinflammatory markers revealed significant reductions in all measured parameters following EA treatment. Serum IL-6 Levels decreased from 3.42 ± 1.18 pg/mL at baseline to 2.83 ± 0.96 pg/mL at week 4 (P < 0.001) and further to 2.30 ± 0.79 pg/mL at week 8 (P < 0.001), representing a 32.7% decrease. Similarly, TNF-α levels decreased from 8.76 ± 2.53 pg/mL at baseline to 7.25 ± 2.17 pg/mL at week 4 (P < 0.001) and to 6.27 ± 1.85 pg/mL at week 8 (P < 0.001), reflecting a 28.5% reduction. Hs-CRP levels also showed significant decreases, from 2.83 ± 1.17 mg/L at baseline to 2.36 ± 0.94 mg/L at week 4 (P < 0.001) and to 1.95 ± 0.83 mg/L at week 8 (P < 0.001), corresponding to a 31.1% reduction. The percentage of patients with neuroinflammatory markers within the normal reference ranges increased from 32.5% at baseline to 67.5% at week 8 for IL-6, from 28.9% to 63.9% for TNF-α, and from 36.1% to 72.3% for hs-CRP. These changes in neuroinflammatory markers are summarized in Table 2.
Correlation analyses revealed significant associations between changes in pain intensity, anxiety symptoms, and neuroinflammatory markers. The change in VAS score from baseline to week 8 showed a strong positive correlation with changes in the HAM-A score (r = 0.68, P < 0.001) and GAD-7 score (r = 0.63, P < 0.001), indicating that reductions in pain were closely associated with improvements in anxiety symptoms. Furthermore, changes in pain intensity were correlated significantly with changes in neuroinflammatory markers, with correlation coefficients of r = 0.54 (P < 0.001) for IL-6, r = 0.49 (P < 0.001) for TNF-α, and r = 0.47 (P < 0.001) for hs-CRP. Similarly, changes in anxiety symptoms (HAM-A score) were significantly correlated with changes in inflammatory markers: r = 0.51 (P < 0.001) for IL-6, r = 0.46 (P < 0.001) for TNF-α, and r = 0.42 (P < 0.001) for hs-CRP.
Multiple linear regression analyses identified baseline anxiety severity (β = 0.36, P = 0.002), baseline IL-6 Level (β = 0.28, P = 0.013), and symptom duration (β = -0.24, P = 0.021) as significant predictors of improvement in pain intensity (change in VAS score). The complete regression model explained 43.7% of the variance in pain improvement (adjusted R² = 0.437, P < 0.001).
Subgroup analyses revealed that patients with moderate-to-severe baseline anxiety (HAM-A score ≥ 18) experienced significantly greater reductions in pain intensity than those with mild anxiety (mean VAS reduction: 3.68 ± 1.25 vs 2.75 ± 1.02, P = 0.012). No significant differences in treatment efficacy were observed based on sex or severity of radiological findings. Patients with a symptom duration of less than 5 years showed greater improvements in both pain intensity (VAS reduction: 3.52 ± 1.21 vs 2.86 ± 1.10, P = 0.024) and anxiety symptoms (HAM-A reduction: 8.43 ± 2.78 vs 6.94 ± 2.36, P = 0.018) than those with longer symptom durations. Sensitivity analysis, excluding patients with treatment adherence < 80% (n = 7), yielded results consistent with the primary analysis, supporting the robustness of the findings.
EA treatment was generally well-tolerated, with minor adverse events reported in 18 patients (21.7%). These included mild bruising at the needle sites (n = 10, 12.0%), temporary exacerbation of pain (n = 5, 6.0%), dizziness during treatment (n = 4, 4.8%), and fatigue following treatment (n = 3, 3.6%). All adverse events were mild and transient, resolving spontaneously without the need for intervention. No serious adverse events were reported during the study period.
This study investigated the efficacy of EA for CS-related pain in patients with comorbid GAD, focusing on changes in anxiety symptoms and neuroinflammatory markers. Our findings demonstrate that an 8-week course of EA treatment was associated with significant reductions in pain intensity, functional disability, anxiety symptoms, and neuroinflammatory markers, with evidence of correlations among these outcomes.
The observed reduction in pain intensity following EA aligns with previous research on acupuncture for cervical pain, which has consistently demonstrated its beneficial effects[16-19]. However, the magnitude of pain reduction in our study (47.6%) exceeded that reported in some earlier investigations, which typically ranged from 30% to 40%[20]. This enhanced effect may be attributable to several factors. First, the application of electrical stimulation may amplify the analgesic effects of traditional acupuncture by more robustly activating endogenous opioid systems and anti-inflammatory pathways[21-23]. Second, the treatment duration and frequency in our protocol (three sessions per week for 8 weeks) were relatively intensive compared to some previous studies, potentially allowing for cumulative therapeutic effects. Third, the concurrent improvement in anxiety symptoms may have contributed to enhanced pain reduction through the modulation of shared neurobiological mechanisms.
The significant improvement in anxiety symptoms observed in our cohort highlights the critical aspect of clinical management in patients with comorbid pain and anxiety. The 36.3% reduction in HAM-A scores and 41.8% reduction in GAD-7 scores indicate meaningful clinical benefits, particularly given that these improvements occurred without adjustments to anxiolytic medications. Previous research has reported variable efficacy of acupuncture for anxiety di
The significant reductions in neuroinflammatory markers (IL-6, TNF-α, and hs-CRP) observed in our study provide valuable insights into the potential mechanisms underlying the therapeutic effects of EA. These findings are consistent with experimental research demonstrating that EA suppresses pro-inflammatory cytokine production through various mechanisms, including activation of the cholinergic anti-inflammatory pathway via vagal stimulation and modulation of sympathetic outflow[28-32]. The observed correlations between changes in inflammatory markers and improvements in both pain and anxiety symptoms support the hypothesis that the modulation of neuroinflammation may represent a shared mechanism by which EA simultaneously addresses these comorbid conditions.
The bidirectional relationship between pain and anxiety was evident in our correlation analyses, which revealed strong associations between changes in pain intensity and anxiety symptoms. This finding supports the concept of a “pain-anxiety-inflammation” cycle, wherein pain exacerbates anxiety, anxiety amplifies pain perception, and both conditions are perpetuated by inflammatory processes[33]. By targeting neuroinflammation, EA may interrupt this cycle, leading to improvements across multiple symptom domains. This perspective aligns with emerging evidence highlighting the role of neuroinflammation in both chronic pain and anxiety disorders, suggesting that anti-inflammatory interventions may offer specific benefits for patients with these comorbidities[34].
The identification of baseline anxiety severity, IL-6 Levels, and symptom duration as predictors of treatment response provides clinically relevant insights for patient selection and for treatment planning. Patients with moderate-to-severe anxiety appeared to derive greater benefit from EA, potentially because of higher baseline inflammation and, conse
From a clinical implementation perspective, EA can be effectively integrated into a stepped-care model for managing CS with comorbid GAD. Within this framework, EA could serve as a second-line intervention for patients who exhibit an inadequate response to first-line treatments (such as education, exercise, and pharmacotherapy) or as a complementary therapy alongside conventional care. EA’s favorable safety profile and dual benefits for pain and anxiety make it a valuable option prior to considering more invasive interventions. Healthcare systems should consider developing integrated pathways in which patients with comorbid presentations are systematically screened and offered EA as part of multimodal treatment plans.
However, this study had some limitations that must be acknowledged. The retrospective design introduces potential selection bias and lacks the methodological rigor of prospective randomized controlled trials. The absence of a control group limits the ability to draw definitive conclusions regarding the specificity of EA effects vs non-specific factors, such as patient expectations, therapeutic attention, and natural symptom progression. Although efforts were made to maintain stable medication regimens throughout the treatment period, unrecorded changes in medication use or dosages may have influenced the outcomes. Additionally, the follow-up period was limited to the 8-week treatment duration, precluding the evaluation of long-term effects. Finally, although systemic inflammatory markers were measured, they may not fully reflect the neuroinflammatory processes occurring within the central nervous system.
This retrospective cohort study demonstrates that EA treatment is associated with significant reductions in pain intensity, functional disability, anxiety symptoms, and neuroinflammatory markers in patients with CS-related pain and comorbid GAD. The observed correlations between improvements in pain, anxiety, and inflammatory parameters suggest that the modulation of neuroinflammation may represent a shared mechanism underlying these therapeutic effects. Although this study had some limitations, these findings support the potential value of EA as an integrative approach for this complex clinical presentation and underscore the importance of addressing inflammatory processes in the management of comorbid pain and anxiety.
| 1. | Luyao H, Xiaoxiao Y, Tianxiao F, Yuandong L, Ping Wang. Management of Cervical Spondylotic Radiculopathy: A Systematic review. Global Spine J. 2022;12:1912-1924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 2. | Yin M, Xu C, Ma J, Ye J, Mo W. A Bibliometric Analysis and Visualization of Current Research Trends in the Treatment of Cervical Spondylotic Myelopathy. Global Spine J. 2021;11:988-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Prablek M, Gadot R, Xu DS, Ropper AE. Neck Pain: Differential Diagnosis and Management. Neurol Clin. 2023;41:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 4. | Kim MW, Kang CN, Choi SH. Update of the Natural History, Pathophysiology, and Treatment Strategies of Degenerative Cervical Myelopathy: A Narrative Review. Asian Spine J. 2023;17:213-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Cezar-Vaz MR, Xavier DM, Bonow CA, Vaz JC, Cardoso LS, Sant'Anna CF, da Costa VZ, Nery CHC, Alves AS, Vettorello JS, de Souza JL, Loureiro HMAM. Musculoskeletal Pain in the Neck and Lower Back Regions among PHC Workers: Association between Workload, Mental Disorders, and Strategies to Manage Pain. Healthcare (Basel). 2023;11:365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | De La Rosa JS, Brady BR, Ibrahim MM, Herder KE, Wallace JS, Padilla AR, Vanderah TW. Co-occurrence of chronic pain and anxiety/depression symptoms in U.S. adults: prevalence, functional impacts, and opportunities. Pain. 2024;165:666-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 102] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 7. | Bailes AH, Navlani R, Koscumb S, Malecky A, Marroquin OC, Wasan AD, Gutstein HB, Delitto A, Zigler C, Vo N, Sowa GA. Use of healthcare resources in patients with low back pain and comorbid depression or anxiety. Spine J. 2021;21:1440-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Borrella-Andrés S, Marqués-García I, Lucha-López MO, Fanlo-Mazas P, Hernández-Secorún M, Pérez-Bellmunt A, Tricás-Moreno JM, Hidalgo-García C. Manual Therapy as a Management of Cervical Radiculopathy: A Systematic Review. Biomed Res Int. 2021;2021:9936981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Asmundson GJ, Coons MJ, Taylor S, Katz J. PTSD and the experience of pain: research and clinical implications of shared vulnerability and mutual maintenance models. Can J Psychiatry. 2002;47:930-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 448] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 10. | Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1671] [Cited by in RCA: 2718] [Article Influence: 271.8] [Reference Citation Analysis (0)] |
| 11. | Lin JG, Kotha P, Chen YH. Understandings of acupuncture application and mechanisms. Am J Transl Res. 2022;14:1469-1481. [PubMed] |
| 12. | Torres-Rosas R, Yehia G, Peña G, Mishra P, del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, Arriaga-Pizano LA, Isibasi A, Ulloa L. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med. 2014;20:291-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 487] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 13. | Zhang M, Shi L, Deng S, Sang B, Chen J, Zhuo B, Qin C, Lyu Y, Liu C, Zhang J, Meng Z. Effective Oriental Magic for Analgesia: Acupuncture. Evid Based Complement Alternat Med. 2022;2022:1451342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Bäumler P, Zhang W, Stübinger T, Irnich D. Acupuncture-related adverse events: systematic review and meta-analyses of prospective clinical studies. BMJ Open. 2021;11:e045961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 15. | Foster NE, Vertosick EA, Lewith G, Linde K, MacPherson H, Sherman KJ, Witt CM, Vickers AJ; Acupuncture Trialists Collaboration. Identifying patients with chronic pain who respond to acupuncture: results from an individual patient data meta-analysis. Acupunct Med. 2021;39:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Berger AA, Liu Y, Mosel L, Champagne KA, Ruoff MT, Cornett EM, Kaye AD, Imani F, Shakeri A, Varrassi G, Viswanath O, Urits I. Efficacy of Dry Needling and Acupuncture in the Treatment of Neck Pain. Anesth Pain Med. 2021;11:e113627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Valera-Calero JA, Fernández-de-Las-Peñas C, Navarro-Santana MJ, Plaza-Manzano G. Efficacy of Dry Needling and Acupuncture in Patients with Fibromyalgia: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2022;19:9904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Vázquez-Justes D, Yarzábal-Rodríguez R, Doménech-García V, Herrero P, Bellosta-López P. Effectiveness of dry needling for headache: A systematic review. Neurologia (Engl Ed). 2020;S0213-4853(19)30144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Hernández-Secorún M, Abenia-Benedí H, Borrella-Andrés S, Marqués-García I, Lucha-López MO, Herrero P, Iguacel I, Tricás-Moreno JM, Hidalgo-García C. Effectiveness of Dry Needling in Improving Pain and Function in Comparison with Other Techniques in Patients with Chronic Neck Pain: A Systematic Review and Meta-Analysis. Pain Res Manag. 2023;2023:1523834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 20. | Fang J, Shi H, Wang W, Chen H, Yang M, Gao S, Yao H, Zhu L, Yan Y, Liu Z. Durable Effect of Acupuncture for Chronic Neck Pain: A Systematic Review and Meta-Analysis. Curr Pain Headache Rep. 2024;28:957-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Araya-Quintanilla F, Cuyúl-Vásquez I, Gutiérrez-Espinoza H. Does acupuncture provide pain relief in patients with osteoarthritis knee? An overview of systematic reviews. J Bodyw Mov Ther. 2022;29:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Atalay SG, Durmus A, Gezginaslan Ö. The Effect of Acupuncture and Physiotherapy on Patients with Knee Osteoarthritis: A Randomized Controlled Study. Pain Physician. 2021;24:E269-E278. [PubMed] |
| 23. | Luo X, Liu J, Li Q, Zhao J, Hao Q, Zhao L, Chen Y, Yin P, Li L, Liang F, Sun X. Acupuncture for treatment of knee osteoarthritis: A clinical practice guideline. J Evid Based Med. 2023;16:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Li M, Liu X, Ye X, Zhuang L. Efficacy of acupuncture for generalized anxiety disorder: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2022;101:e30076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 25. | Ji H, Zhang K, Lu Y, Kong X, Ma X. The efficacy of acupuncture with Lingguibafa acupoint selection in the treatment of insomnia: A PRISMA-compliant meta-analysis. Medicine (Baltimore). 2022;101:e31515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Lee B, Kwon CY, Park MY. Acupuncture for the Treatment of Chronic Rhinosinusitis: A PRISMA-Compliant Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2022;2022:6429836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Kwon CY, Lee B, Ha DJ. Effectiveness and safety of acupuncture in treating sleep disturbance in dementia patients: A PRISMA-compliant systematic review and limitations of current evidence. Medicine (Baltimore). 2021;100:e26871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Li Y, Xu G, Hu S, Wu H, Dai Y, Zhang W, Tang F, Luo H, Shi X. Electroacupuncture alleviates intestinal inflammation and barrier dysfunction by activating dopamine in a rat model of intestinal ischaemia. Acupunct Med. 2021;39:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Guo L, Hu H, Jiang N, Yang H, Sun X, Xia H, Ma J, Liu H. Electroacupuncture blocked motor dysfunction and gut barrier damage by modulating intestinal NLRP3 inflammasome in MPTP-induced Parkinson's disease mice. Heliyon. 2024;10:e30819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 30. | Ma X, Wang Q, Yuan W, Wang Y, Zhou F, Kang K, Tong X, Liu Z. Electroacupuncture Alleviates Neuroinflammation and Motor Dysfunction by Regulating Intestinal Barrier Function in a Mouse Model of Parkinson Disease. J Neuropathol Exp Neurol. 2021;80:844-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 31. | Lei W, Zhao C, Sun J, Jin Y, Duan Z. Electroacupuncture Ameliorates Intestinal Barrier Destruction in Mice With Bile Duct Ligation-Induced Liver Injury by Activating the Cholinergic Anti-Inflammatory Pathway. Neuromodulation. 2022;25:1122-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Felger JC. Imaging the Role of Inflammation in Mood and Anxiety-related Disorders. Curr Neuropharmacol. 2018;16:533-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 319] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 33. | Walker AK, Kavelaars A, Heijnen CJ, Dantzer R. Neuroinflammation and comorbidity of pain and depression. Pharmacol Rev. 2014;66:80-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 363] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 34. | Treede RD, Hoheisel U, Wang D, Magerl W. Central sensitization: clinical utility of a physiological concept for the International Statistical Classification of Diseases and Related Health Problems and for nociplastic pain. Pain. 2022;163:S99-S107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 35. | Napadow V, Makris N, Liu J, Kettner NW, Kwong KK, Hui KK. Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum Brain Mapp. 2005;24:193-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 279] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/