Published online Oct 19, 2025. doi: 10.5498/wjp.v15.i10.108884
Revised: May 29, 2025
Accepted: July 21, 2025
Published online: October 19, 2025
Processing time: 154 Days and 4.4 Hours
Sleep disorders are highly prevalent among college students, adversely affecting their physical health, psychological well-being, and academic outcomes. While pharmacotherapy remains a common intervention, its potential for dependency and adverse effects underscores the need for safer alternatives. Physical activity, characterized by accessibility and a favorable cost-effectiveness profile, has gained attention as a non-pharmacological intervention. However, the evidence regar
Core Tip: Exercise is crucial for improving sleep because it enhances sleep quality and provides overall benefits for physical and mental health. This review looks at how exercise helps sleep by focusing on three main points: (1) How different types of exercise compare in effectiveness; (2) The science behind how exercise improves sleep; and (3) What current research is missing and what future studies should focus on. We found that aerobic exercise can really help with sleep, and combining different types of exercise works even better than just one type. Future research should try mixing exercise with other methods to find new ways to help college students sleep better. In short, exercise is a great way to improve sleep and overall health for college students.
- Citation: Fei LL, Zhao SX, Chen YF, Hao CF, Xin YJ. Exercise and sleep health in college students: Efficacy, mechanisms, and implications for practice. World J Psychiatry 2025; 15(10): 108884
- URL: https://www.wjgnet.com/2220-3206/full/v15/i10/108884.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i10.108884
Sleep is a vital physiological process that sustains physical health, mental well-being, and optimal daily functioning. Quality sleep offers a range of benefits, including improved cardiovascular health, enhanced cognitive performance and memory consolidation, support for immune resilience, regulation of hormonal balance, and maintenance of reproductive health[1]. According to the American Academy of Sleep Medicine Manual for the Scoring of Sleep and Associated Events, overnight sleep consists of several distinct stages: Wakefulness (stage W), sleep onset (stage N1), light sleep (stage N2), deep sleep (stage N3), and rapid eye movement (REM) sleep. Stages N1, N2, and N3 are jointly known as non-REM (NREM) sleep. Sleep quality, a comprehensive measure used to evaluate the sleep process and its effects, can be assessed using both subjective and objective methods. Subjective assessments typically involve self-reported measures, whereas objective assessments commonly use polysomnography (PSG). The Pittsburgh Sleep Quality Index (PSQI) is a widely used self-assessment tool that evaluates sleep quality across seven dimensions: Subjective sleep quality, sleep onset latency (SOL), sleep duration, sleep efficiency (SE), sleep disturbances, use of sleep medication, and daytime dysfunction. The PSQI has shown strong internal reliability and validity, making it one of the most frequently used subjective measures of sleep quality[2]. For objective measurement, PSG is recognized as the gold standard for diagnosing sleep disorders[3], as it provides detailed data on sleep architecture, including total sleep time (TST), SOL, SE, and wakefulness after sleep onset (WASO).
College students are particularly vulnerable to sleep disorders, which is a concerning trend given their critical stages of physical, cognitive, and emotional development. College students are at a special stage. Academic and social pressures frequently result in nocturnal wakefulness for studying or socializing, leading to chronic sleep deprivation and circadian misalignment[4]. Lifestyle factors like diets high in processed foods, prolonged sedentary behavior, and excessive screen time disrupt hormonal regulation, particularly the secretion of melatonin and cortisol, and exacerbate endocrine dysfunction, further impairing sleep quality[5]. Environmental stressors, including shared dormitory living characterized by noise pollution and limited personal space, create suboptimal sleep conditions[6,7]. Moreover, the psychological demands of identity formation, autonomy development, and self-efficacy challenges increase susceptibility to anxiety and depressive symptoms, fostering emotional dysregulation that directly destabilizes sleep architecture.
Studies have consistently highlighted the alarming prevalence of sleep disorders in this population[8-12]. To illustrate, a three-year cohort study at the University of Washington involving 271 students found that 26.4% reported poor sleep quality[8]. While in the United Kingdom, over one-third of students failed to meet the recommended 7-hour nightly sleep threshold, largely because of academic demands and exam pressures[9]. This pattern extends beyond Western contexts. A meta-analysis of 35 studies across Africa (16275 participants) revealed a staggering 63.31% prevalence of poor sleep quality among university students[10]. In Jordan, a cross-sectional study of 1308 students found that 73% rated their sleep quality as “fairly bad” or “very bad”[11]. A nationwide survey of 2099 students across six Chinese provinces further underscored these challenges, finding that 62.3% reported poor sleep quality, with higher rates among female (65.0%) than among male (58.0%) respondents[12]. These findings collectively highlight a global public health concern among college students, characterized by interrelated sleep problems as inconsistent sleep timing, inadequate sleep duration, and diminished sleep quality. Targeted interventions informed by region-specific risk factors, like academic stress, lifestyle habits, and sociocultural pressures, are essential to safeguard sleep health in this critical demographic.
Sleep quality is influenced by various complex physiological, psychological, behavioral, gender-related, and social factors. Conditions like chronic obstructive pulmonary disease[13] and recurrent upper respiratory infections[14] are linked to poor sleep. Obesity also increases the risk of sleep disorders, especially for obstructive sleep apnea[15]. Regarding psychological factors, symptoms of depression and anxiety are well-established precursors to insomnia, and longitudinal studies have highlighted the enduring effects of early life adversity. To illustrate, retrospective studies have consistently identified adverse childhood experiences as robust predictors of adult sleep disorders, underscoring the lifelong consequences of psychological stressors[16,17]. Behavioral influences include modifiable lifestyle factors that directly disrupt circadian rhythms and sleep architecture. Specifically, sedentary habits, alcohol consumption, nicotine use, and pre-sleep screen exposure[18] are linked to delayed sleep onset, reduced melatonin production, and diminished sleep quality[19]. Nutritional epidemiology adds another layer to this dynamic. Dietary patterns, particularly macronutrient balance and micronutrient intake (e.g., vitamins B6 and D, magnesium), are increasingly recognized for their regulatory effects on sleep[20]. Regarding gender differences, sleep quality remains debated. While some studies reported poorer sleep quality among women than men[21], which has been attributed to hormonal fluctuations or caregiving roles, others found minimal disparities, noting only marginally better SE in men. These inconsistencies likely stem from variations in study design, sociocultural contexts, and sample sizes, emphasizing the need for context-specific analyses. Finally, social determinants can modulate sleep health. High-quality interpersonal relationships buffer against sleep disturbances[22], whereas strained social connections are strongly correlated with sleep disorders[23]. Ultimately, this bidirectional relationship between social support and sleep underscores the value of integrating psychosocial interventions into sleep medicine frameworks.
According to the European Insomnia Guidelines 2023[24], insomnia treatment encompasses both non-pharmacological and pharmacological approaches. Among non-pharmacological interventions, cognitive behavioral therapy for insomnia (CBT-I) remains the first-line treatment for chronic adult insomnia. CBT-I combines behavioral and psychological strategies, for instance, stimulus control and sleep restriction, and has demonstrated benefits for both sleep quality and overall well-being, with higher patient acceptance compared to medications[25]. While CBT-I can be delivered in person or digitally[26], research variability in control conditions and intervention protocols poses challenges for standardizing outcomes[27]. Pharmacological treatments, including benzodiazepines and benzodiazepine receptor agonists (e.g., eszopiclone and zolpidem), are also utilized. However, these medications have limitations. Long-term use may lead to tolerance[24], dependency, and side effects such as dizziness and nausea[28]. Ultimately, treatment outcomes vary widely due to individual differences in physiology, lifestyle, and comorbidities.
Exercise interventions have recently emerged as a widely studied non-pharmacological treatment for sleep disorders, offering advantages such as accessibility, cost-effectiveness, and non-dependency. While physical activity broadly benefits sleep outcomes across various age groups and genders, the type of exercise plays a critical role in determining specific improvements[29]. Aerobic exercises (AE) (e.g., running, cycling) show greater efficacy in improving SE, likely because of their effects on circadian rhythm regulation and metabolic health[30]. In contrast, mind-body exercises (e.g., yoga and Tai Chi) are particularly effective in enhancing subjective sleep quality[20], as measured by tools namely PSQI. Research indicates that optimizing exercise parameters can further refine outcomes[20,29-34]. A regimen of three weekly sessions[31], each lasting 45 to 60 minutes[32], is most effective for improving sleep quality in adults with sleep disorders. Workout intensity plays a crucial role. Moderate-to-high-intensity exercises yield significant benefits[20], although sessions should ideally conclude at least 1.5 hours before bedtime to avoid sleep disruption from elevated core body temperature (CBT) or adrenaline levels[33]. Notably, both acute bouts of exercise (single sessions)[29] and long-term routines enhance sleep[30], with the latter resulting in more pronounced and lasting improvements. Over time, regular physical activity helps normalize sleep duration and architecture[34], addressing common deficits associated with chronic sleep disorders. Therefore, for optimal results, interventions should be tailored to individual needs by carefully balancing exercise type, timing, and intensity to align with specific sleep-related goals.
Despite growing interest in exercise as a therapeutic intervention, no comprehensive synthesis of its impact on sleep has been provided. To address this gap, we conducted a narrative review to evaluate the methodological rigor and scope of evidence supporting the role of exercise in sleep disorder management. Our analysis focused on three pillars: (1) Comparative efficacy of exercise regimens; (2) Biological mechanisms underlying exercise-induced sleep regulation; and (3) Critical limitations of the current research and priorities for future inquiries. Improving sleep through exercise is crucial, as it not only enhances sleep quality but also provides broader physical and mental health benefits. By integrating evidence-based exercise types with individually tailored parameters, as if timing, intensity, and duration, individuals can achieve meaningful enhancements in sleep architecture, daytime functionality, and overall quality of life. Future studies should prioritize the investigation of personalized exercise prescriptions and long-term outcomes to strengthen the scientific foundation and practical effectiveness of exercise-based interventions.
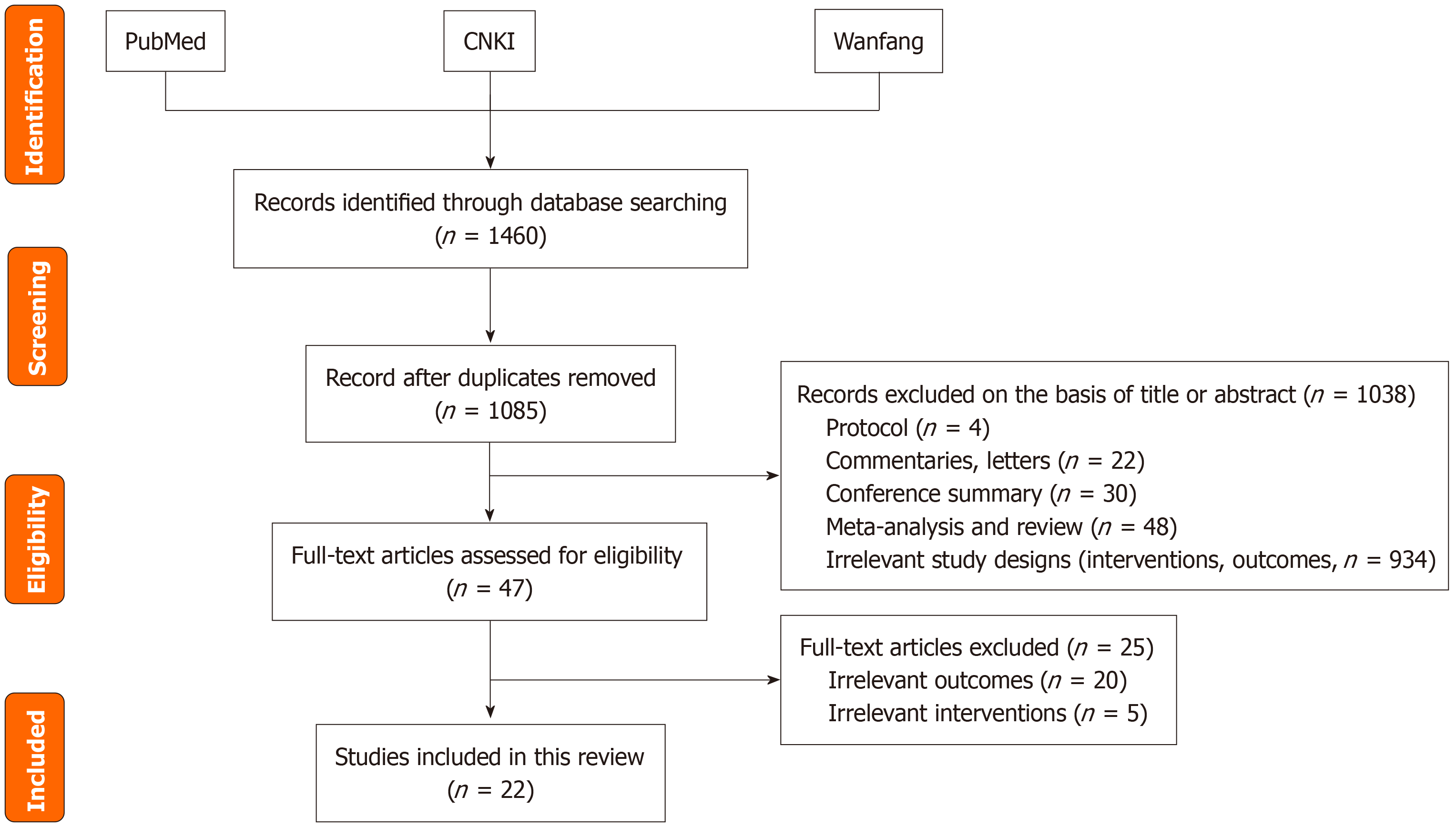
We conducted a narrative review to synthesize evidence on the effect of exercise on sleep quality and its underlying mechanisms. A systematic search was performed using PubMed, CNKI, and the Wanfang Database for studies published between 2015 and February 2025, supplemented by relevant books and manuals. Observational/experimental studies, reviews, and meta-analyses were included, whereas letters, editorials, and case reports were excluded. Keywords included terms related to (either alone or in combination): “Sleep”, “sleep quality”, “sleep disorders”, “insomnia”, “sleep deprivation”, “exercise”, “physical activity”, “sleep-wake cycle”, “sleep structure”, “brain-derived neurotrophic factor”, “interleukin”, “irisin”, “5-hydroxytryptamine (5-HT, serotonin)”, “dopamine”, “norepinephrine”, “peroxisome proliferator-activated receptor-gamma coactivator 1-alpha”, “tumor necrosis factor-alpha”, “core body temperature”, “anxiety”, “depression”, “self-efficacy”, and “college students”. Titles and abstracts were screened for relevance, followed by full-text evaluations. Eligible studies were categorized according to exercise type (e.g., aerobic or resistance), duration, intervention design, and mechanistic pathways. Additional sources were identified from the reference lists of the selected articles. The specific literature screening process is shown in Figure 1.
The following information was extracted by two investigators (Fei LL and Zhao SX) independently using a standardized data collection form: Study research type, sample size, intervention, duration, session duration/frequency, measurement types of exercise, exercise intensity, exercise effects. For study quality, the guideline for STROBE was applied to assess the methodological quality of each study by two investigators (Fei LL and Zhao SX) independently[35]. The STROBE statement is a 22-item checklist developed to improve the reporting of observational studies. This checklist has been used in systematic reviews to evaluate the methodological quality[36,37]. Nine items in methods (items 4-12) were selected, which cover the different aspects of methodology in observational studies. The methodological quality was classified by the number of items that the research met. To be more specific, articles that met 0-3 items, 4-6 items and 7-9 items were regarded as low, moderate and high methodological quality, respectively. Any disagreements were resolved by group discussion with a third investigator (Chen YF).
AE: AE, characterized by rhythmic, low-to-moderate-intensity movements sustained through aerobic metabolism, is a versatile and accessible form of physical activity. A robust body of evidence underscores its efficacy in improving sleep quality across diverse populations, both clinical and non-clinical groups. A 16-week assessor-blinded trial involving 226 patients with advanced lung cancer (mean age = 61.4 ± 8.7 years) compared AE, Tai Chi, and control groups[38]. The AE and Tai Chi groups showed sustained improvements in the PSQI scores at 16 weeks and 1-year follow-up, along with enhanced psychological well-being, physical function, and circadian rhythmicity. A 16-week supervised aerobic program for hypertensive overweight/obese adults (n = 18) demonstrated significant sleep quality improvements via actigraphy (Cole-Kripke algorithm)[39], reinforcing the adaptability of AE to various health contexts. In addition to stand-alone interventions, AE synergizes with pharmacological treatments to amplify therapeutic outcomes. In a study comparing zolpidem monotherapy (n = 45) to zolpidem plus AE (n = 43) for primary insomnia, the combination group exhibited superior outcomes, including reduced SOL, nighttime arousal, and pro-inflammatory markers [tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β and IL-6], as well as increased TST and slow-wave sleep duration[40]. These findings position AE as both a standalone and adjunctive therapy, broadening its clinical utility.
Remarkably, the benefits of AE extend to younger populations facing modern stressors. Growing evidence has highlighted the efficacy of AE in addressing sleep and cognitive challenges among young adults. An 8-week AE intervention involving 41 female college students with sleep disturbances resulted in significantly reduced PSQI scores, as supported by objective sleep-monitoring data[31]. Parallel to this, AE demonstrates acute neurobehavioral benefits. A study of 30 healthy students revealed that a single AE session could alleviate sleep deprivation-induced deficits in cognitive control, such as impaired response inhibition[41]. Improvements in sleep quality also persist long-term. A meta-analysis of 18 AE trials found sustained benefits 3-6 months post-intervention[42], highlighting its durability as a cost-effective, non-pharmacological strategy. Overall, AE has emerged as a dual-action intervention, enhancing chronic sleep quality coupled with acute cognitive recovery, and offering scalable solutions across age groups and health conditions.
Resistance exercise: Resistance exercise (RE), or strength training, involves overcoming external resistance to enhance muscular strength, endurance, and power. Population-based research has underscored its role in improving sleep quality. A German national study involving 23635 participants revealed that regular muscle-strengthening activities can reduce the risk of poor sleep outcomes[43]. This aligns with a systematic review of 13 clinical trials (n = 652), demonstrating that sustained resistance training improves subjective sleep quality, particularly the ability to fall asleep and stay asleep[44]. Extending these findings, longitudinal data from 26205 adolescents revealed that consistent RE (≥ 3 weekly sessions) can increase the likelihood of sufficient sleep duration when compared to inactive peers[45]. Concurrently, this evidence suggests that RE is a scalable, non-pharmacological approach that can optimize sleep health across different age groups.
Controlled trials in targeted populations reinforce these findings. A 3-month cyclic RE program for 43 college students with primary sleep disorders improved sleep latency, TST, SE, and daytime function, alongside mental health metrics[46]. Resistance interval training in 24 sedentary young adults reduced daytime sleepiness without affecting physical activity levels[47], demonstrating RE’s adaptability to modern lifestyles. In addition to chronic adaptations, RE also confers acute sleep benefits. A 3-day crossover trial involving 18 men with delayed sleep-wake phase disorder (DSWPD) compared moderate-intensity RE and AE[48]. RE outperformed AE, accelerated sleep onset, shortened sleep latency, increased TST, reduced nighttime awakenings, and enhanced SE. Mechanistically, RE elevated 6-sulfatoxymelatonin and anti-inflammatory IL-10, while lowering pro-inflammatory IL-6 and biochemical shifts linked to circadian alignment and reduced sleep fragmentation. Although both modalities reduced self-reported anxiety, RE showed more pronounced effects, further highlighting its unique neurophysiological impact.
Mind-body exercise: Mind-body exercise integrates physical activity with mental regulation through slow, controlled movements and focused breathing, emphasizing harmony between the body and mind. Yoga, Tai Chi, and Qigong practices not only improve physical health but also alleviate psychological stress, enhance self-awareness, and foster social interaction. Moreover, they have emerged as effective non-pharmacological interventions for sleep disorders that operate via dual psychological and physiological pathways. A 10-week yoga intervention in young women (18-23 years) reduced total PSQI scores, reflecting improved sleep quality[49]. Chronic insomnia patients also demonstrated objective physiological benefits, including reduced nighttime awakenings, prolonged N3 sleep duration, and enhanced daytime functioning, underscoring yoga’s multifactorial mechanisms[50]. Tai Chi exhibits dose-dependent efficacy. A 24-week trial in older women (60-70 years) showed reduced inflammatory markers (TNF-α, IL-6), increased serotonin, and improved PSQI and depression scores, with longer sessions yielding greater benefits[51]. These anti-inflammatory effects align with findings from a 12-week randomized controlled trial in older adults (mean age 67.3 ± 6.8 years), where Tai Chi outperformed conventional exercise in maintaining SE and reducing sleep latency, with sustained benefits for 24 months[52]. Crucially, a 16-week Tai Chi program in advanced lung cancer patients (n = 226) outperformed AE in sleep quality, circadian rhythm regulation, and survival outcomes[38]. However, its cultural specificity and accessibility may limit its global scalability.
The benefits of mind-body exercises are not limited to older adults or clinical populations. A 12-week Qigong intervention with 37 college students (18-25 years) improved anxiety and sleep quality, with the intervention group (n = 18) practicing for 60 minutes five times per week[53]. This underscores their applicability across age groups, offering scalable strategies for addressing sleep and mental health challenges in young adults. Mind-body practices enhance sleep through interconnected psychological (stress reduction and emotional regulation), physiological (anti-inflammatory effects and circadian alignment), and behavioral (improved sleep architecture) pathways. By bridging ancient traditions with modern evidence, these exercises offer versatile, holistic solutions for improving sleep health.
Combined exercise: Exercise interventions combining multiple modalities consistently outperform single-modality approaches by leveraging synergistic physiological and psychological mechanisms[54]. A trial involving 301 patients with breast cancer undergoing chemotherapy compared three regimens: Standard-dose AE (25-30 minutes), high-dose AE (50-60 minutes), and a combined aerobic-resistance program (50-60 minutes). The combined exercise group demonstrated superior long-term patient-reported outcomes and health-related fitness, highlighting the combined benefits of integrating aerobic and resistance training during and after chemotherapy[55]. A study of college students with sleep dysfunction utilized the PSQI monitoring to evaluate three combined exercise regimens[56] and found distinct mechanistic advantages. AE paired with mind-body practices significantly improved subjective sleep quality by regulating negative emotions, mental stress, and autonomic nervous system balance, while RE combined with mind-body training enhanced objective sleep metrics, likely because of its unique physiological stress-adaptation effects[56]. Crucially, mind-body components were found to universally amplify outcomes across regimens, underscoring their indispensable role in optimizing sleep architecture and reinforcing the value of integrative approaches[56].
A trial involving 44 female college students (18-29 years) that tested mindful self-hypnosis (MSH) paired with RE[57] further reinforced this synergy. The participants were randomized into MSH + RE, RE-only, and control groups. Both exercise groups completed a 5-week RE program (3 times per week, 45-60 minutes/session), with the MSH + RE group receiving additional 5-minute MSH sessions pre- and post-workout. The combined MSH + RE intervention not only reduced stress but also outperformed RE-alone in improving sleep quality and overall well-being, likely through synergistic hypothalamic-pituitary-adrenal axis modulation[57]. These studies demonstrate that multimodal interventions, whether they combine exercise types or integrate physical and psychological strategies, capitalize on complementary mechanisms (e.g., autonomic regulation, anti-inflammatory effects, and hypothalamic-pituitary-adrenal axis balance) to achieve superior, holistic sleep improvements. This evidence supports the use of personalized, integrated approaches in sleep medicine.
Exercise intensity, defined as the degree of effort exerted during physical activity and quantified using metrics including heart rate or perceived exertion, is pivotal in shaping sleep outcomes. Low-intensity exercise enhances endurance and basal metabolic rate but offers gradual cardiopulmonary improvements, whereas moderate-intensity exercise balances cardiopulmonary gains with muscular strength and endurance. High-intensity exercise accelerates cardiopulmonary and muscular adaptation but requires baseline fitness to mitigate injury and fatigue risks. Moderate-to-high exercise is most effective for sleep enhancement, likely because it induces physical fatigue and reduces stress. Recent studies have validated intensity-dependent effects across populations. An 8-week trial with 40 non-athlete college freshmen found that both moderate and high intensity exercise improved sleep quality (assessed using the PSQI and activity questionnaires), although high-intensity exercise yielded greater gains[58]. Intriguingly, a crossover study of nine healthy young men revealed that a single vigorous session subjectively disrupted sleep perception but objectively enhanced sleep function, as assessed via PSG[59]. High-intensity exercise boosted delta wave energy during slow-wave sleep, promoting restorative sleep[59]. Although promising, these findings are limited by small, gender-specific samples, warranting broader validation.
Guidelines emphasize dose-intensity balance for optimal health. Physical Activity Guidelines for Chinese (2021) recommend that adults engage in approximately 150-300 minutes of moderate or 75-150 minutes of vigorous weekly AE[60]. Supporting this, a 15-year cohort study of 341248 adults linked 25-65 minutes of moderate exercise daily to reduced mortality risks from poor sleep patterns, whereas inactivity exacerbated these risks[61]. Undoubtedly, the intensity benefits extend to clinical populations. A meta-analysis found that high-intensity exercise can improve sleep architecture in patients with Parkinson’s disease[54], while an 8-week moderate aerobic program enhanced PSQI scores in co
Exercise timing impacts sleep quality, with variations depending on intensity, duration, and individual chronobiology. Acute high-intensity exercise 2-4 hours before bedtime generally does not disrupt sleep in healthy adults, though sessions ending ≤ 1 hour pre-sleep may impair sleep by delaying cardiovascular recovery and reducing parasympathetic activity[63]. A crossover study of 12 healthy men found evening moderate-intensity AE or RE did not alter sleep parameters (e.g., latency, efficiency, REM/NREM stages), as cognitive arousal normalized 90 minutes post-exercise[33]. However, chronotype modulates outcomes: Evening high-intensity exercise reduced SE and increased latency in 42 male adolescent athletes, except for evening-type individuals resilient to such disruptions[64]. Vigorous morning or evening runs (45-60 minutes at 70% maximum aerobic velocity) in 13 recreational runners extended NREM sleep (N2 stage), likely via exercise-induced hormonal shifts aiding recovery, with evening exercise within 2 hours of bedtime showing no subjective/objective sleep impairment[65].
Despite these findings, prolonged high-intensity exercise risks elevating CBT, delaying recovery, and triggering insomnia[66], while even brief evening exercise may disrupt circadian rhythms in susceptible individuals[67]. Critically, methodological limitations (e.g., small samples of healthy young males) and inconsistent protocols complicate generalizability. The interaction between exercise timing and sleep is thus multifactorial, influenced by intensity, recovery intervals, and chronotype. Tailoring exercise schedules to circadian preferences - particularly avoiding evening workouts in morning/intermediate chronotypes - and ensuring adequate pre-sleep recovery windows may optimize sleep and training adaptations.
Frequency and duration of individual exercise sessions: Exercise duration and consistency are critical determinants of sleep improvement, particularly among college students. Most studies recommend regimens of three weekly sessions lasting 40-60 minutes, with evidence from broader populations reinforcing this framework[32,55]. A trial involving 301 patients with breast cancer demonstrated a direct correlation between exercise duration and sleep quality[55]. Sessions ≤ 30 minutes yielded no significant improvements, whereas 50-60 minutes sessions enhanced slow-wave sleep, REM sleep, and TST[55]. Extending the duration further amplified the benefits, suggesting a dose-response relationship. This aligns with a meta-analysis (n = 670) concluding that 45-60 minutes sessions are optimal for improving sleep architecture in adults with sleep disorders[32], underscoring the universal benefits of moderate-to-long durations.
These principles translate effectively to college students. An 8-week intervention combining AE and yoga in 70 female undergraduates (3 times weekly, 40 minutes/session) significantly improved PSQI scores and reduced anxiety and negative emotions compared to non-exercising controls[68]. The detailed characteristics of the included research literature are shown in Table 1. A step aerobics program (3 times weekly, 55 minutes/session) in 41 female students with sleep disorders enhanced both subjective (PSQI) and objective (portable monitor) sleep metrics[31]. Both studies employed multidimensional assessments to evaluate sleep quality, emotional states, and physiological markers and capture holistic improvements. These findings emphasize that consistent moderate-duration exercise (40-60 minutes, 3 times weekly) reliably enhances sleep quality across populations, from clinical cohorts to college students. By adhering to evidence-based duration guidelines, interventions can optimize sleep architecture while addressing comorbid psychological stressors, thereby offering a practical blueprint for campus health initiatives.
| Ref. | Research type | Sample size | Intervention duration | Session duration/frequency | Measurement | Types of exercise | Exercise intensity | Exercise effects | Study quality |
| Liu et al[31], 2023 | RCT | EG: 29; CG: 12 | 8 weeks | 55 minutes/session, 3 times/week | PSQI, portable sleep monitor, Actigraph-GT3X, Inbody BSM330 | AE | Moderate to high | Subjective: Improved PSQI total score and subscales (sleep quality, efficiency, disorders, daytime function). Objective: Reduced sleep-wake ratio, increased deep sleep ratio and SE. Energy metabolism: Increased lean body mass and basal metabolic rate | High |
| Miller et al[33], 2020 | Crossover study | 12 | AE: 1 day; RE: 1 day; control: 1 day | 30 minutes/session, only one session of each exercise | KSS, PSG, ingestible temperature capsules | AE, RE | Moderate | Sleep: Moderate-intensity evening aerobic/resistance exercise ending 90 minutes before bedtime did not impair sleep in healthy young males. Core body temperature: Returned to pre-exercise levels by bedtime, with no differences in sleep metrics compared to control | High |
| Takemura et al[38], 2024 | RCT | AE group: 75; Tai chi group: 76; CG: 75 | 16 weeks | AE: 60 minutes/session, 2 times/month for group exercise and 150 minutes/week for home-based exercise; Tai chi: 60 minutes/session, 2 times/week | PSQI, BFI, HADS, EORTC QLQ-C30, actigraphy, physical function test | AE, mind-body exercise | AE: Moderate; Tai chi: Low | Sleep quality: Both AE and Tai chi showed significant PSQI reduction vs CG at 16 weeks and 1 year; Tai chi had greater improvement than AE. Secondary outcomes: Both interventions improved anxiety, depression, physical function, step count, and diurnal cortisol slope; Tai chi showed greater reduction in fatigue and better balance vs AE/CG | High |
| Martinez Aguirre-Betolaza et al[39], 2020 | RCT | EG: 109; CG: 37 | 16 weeks | 60 minutes/session, 2 times/week | IPAQ, STOP-Bang Questionnaire, Actigraph-GT3X+ | AE | Individually tailored | Both groups improved SE and TST; EG had longer bedtime on weekdays and better weekend SE | High |
| Zhao et al[40], 2022 | RCT | EG: 43; CG: 45 | 8 weeks | 30 minutes/session,7 times/week | PSQI, PSG | AE | Moderate to high | PSG: EG showed greater improvements in TST, SE, NREM III/IV stages, and reductions in SOL, WASO vs control. Inflammatory factors: Post-intervention TNF-α, IL-1β, IL-6 Lower in EG (P < 0.01) | High |
| Liu and Zhang[41], 2022 | Crossover study | 30 | - | 30 minutes, single acute session | PSG | AE | Moderate | Cognitive control ability improved immediately, and at 30 minutes and 1-hour post-exercise; blood 5-HT levels increased (P < 0.01) at all time points; no change in glucose levels was observed | High |
| Xu et al[46], 2022 | RCT | EG: 43; CG: 43 | 12 weeks | 3 sets/session, 3 times/week | PSQI, SCL-90 | RE | Low | PSQI: Both groups improved post-intervention, with EG showing greater reduction. SCL-90: Both groups reduced total scores, with EG showing better improvement | High |
| Kowalsky et al[47], 2023 | Crossover study | 24 | CON: 7 days; REX: 7 days | REX: 8 hours/day, 1 break/hour | KSS, PDFQ, ActivPAL Micro 3 device | RE | Low to moderate | Significant improvement in overall discomfort and sleepiness, not significant improvement in mental fatigue and physical fatigue | High |
| Zhang[49], 2022 | RCT | EG: 24; CG: 24 | 10 weeks | 80 minutes/session, 2 times/week | PSQI, SDS | Mind-body exercise | Low to moderate | Sleep quality: EG’s PSQI score decreased (P < 0.01), with significant improvements in sleep duration, sleep latency, and daytime dysfunction (P < 0.05). Depression: Experimental group SDS score decreased from 60.52 ± 4.08 to 44.63 ± 4.78 (P < 0.01), significant difference vs control group (P < 0.01) | High |
| Hu et al[48], 2025 | Crossover study | 18 | 3 days for each exercise type | 40 minutes/session | PSQI, MEQ, SAS, SDS, PARS-3, Actigraph-GT3X+, sleep watch actigraph, Cosmed K5 Metabolic Gas Analyser | AE, RE | AE: Moderate to high; RE: Moderate | Sleep: RE improved SOT, SOL, TST, WASO and SE; AE improved SOT and SE. RE had better TST and SE than AE. Melatonin: Both increased aMT6s, with RE more effective. Inflammation: Both reduced IL-6 and increased IL-10; RE had greater IL-6 reduction. Mood: Both reduced SAS scores; AE improved SDS scores, while RE showed no significant SDS change | High |
| Turmel et al[50], 2022 | Prospective single-group pre-post study | 21 | 14 weeks | 5-30 minutes/session, 2-3 times/day | PSQI, ESS, HADS, PS, PSG, Actigraphy | Mind-body exercise | Low to moderate | Subjective: Significant improvements in all scales (PSQI: P < 0.01; HADS-A: P < 0.05; HADS-D: P < 0.01; ESS: P < 0.05; PS: P < 0.01). Physiological: Actigraphy showed reduced nighttime arousals (P < 0.01); PSG showed no changes in most parameters, but PSQI improvement correlated with increased N3 sleep stage (P < 0.01) | High |
| Chang et al[51], 2024 | RCT | SG: 31; MG: 30; LG: 32; CG: 31 | 24 weeks | SG: 30 minutes/session; MG: 45 minutes/session; LG: 60 minutes/session; all groups: 5 times/week | PSQI, BDI | Mind-body exercise | Moderate | Within-group: All exercise groups showed reduced TNF-α/IL-6 and increased 5-HT at 12/24 weeks vs baseline; LG had significant BDI/PSQI reductions at 24 weeks. Between-group: At 24 weeks, LG had lower BDI/PSQI and TNF-α than CG/SG/MG; MG had lower TNF-α than SG. Sleep duration improved most in LG | High |
| Siu et al[52], 2021 | RCT | Exercise group: 105; Tai chi group: 105; CG: 110 | 12 weeks | 1 hour/session, 3 times/week | PSQI, ISI, Actigraph-wGT3X-BT | Conventional exercise: Aerobic + resistance training; Tai chi: Mind-body exercise | Moderate | Actigraphy outcomes: SE increased, with effects sustained at the 24-month follow-up; WASO and number of awakenings decreased. Subjective outcomes: PSQI and ISI scores reduced in both intervention groups; insomnia remission rate: 34.4% (Tai chi) vs 19.4% (exercise) post-intervention (P < 0.05) | High |
| Sun et al[53], 2024 | RCT | EG: 18; CG: 19 | 12 weeks | 60 minutes/session, 5 times/week | PSQI, HAM-A, FS-14, SF-36 | Mind-body exercise | Low to moderate | The intervention reduced total and mental anxiety, improved sleep quality, and alleviated bodily pain | High |
| An et al[55], 2020 | RCT | STAN: 96; HIGH: 101; COMB: 104 | 12-18 weeks, median 17 weeks | STAN: 25-30 minutes/session; HIGH: 50-60 minutes/session; COMB: 25-30 minutes/session AE + 25-30 minutes/session RE; all groups: 3 times/week | PSQI, SF-36, FACT-B, FACT-F, FACT-ES, FACT-T, PSS, HM, RSES, CES-D, SSAI | STAN/HIGH: AE; COMB: AE + RE | AE: High; RE: Low to moderate | Subjective: COMB had better sleep quality (vs STAN, P < 0.05) at 6 months; group-by-time interactions for happiness, anxiety, fatigue, stress, sleep. Physiological: Compared to the HIGH group, the COMB group demonstrated superior upper-body muscular endurance at 12 months (P < 0.05) and lower-body strength (P < 0.05) | High |
| Yuan et al[56], 2022 | RCT | AR group: 10; AM group: 8; RM group: 9; CG: 8 | 8 weeks | 60 minutes/session, 3 times/week | PSQI, Actigraph-GT3X+ | AR: AE + RE; AM: AE + mind-body; RM: RE + mind-body | Aerobic: Moderate to high; resistance/mind-body: Low to moderate | Subjective: All exercise groups showed significant reductions in PSQI total score, sleep quality score; AR group reduced sleep latency, AM group reduced daytime dysfunction, RM group reduced sleep duration. Objective: All exercise groups increased TST and SE; AM and RM groups reduced number of awakenings, SOL and WASO. Group differences: AM group had greater subjective improvements; RM group had greater objective improvements | High |
| Lin Latt et al[57], 2024 | RCT | MSH + RE: 11; RE: 8; CG: 11 | 5 weeks | RE: 45-60 minutes/session, MSH + RE: 50-65 minutes/session, both groups: 3 times/week | ISI, PSS, PDP, FFMQ, WHO-5, HES-7, EHS | RE, mind-body exercise | High | Perceived stress: Significant reduction in MSH + RE (P < 0.01) and RE (P < 0.05); MSH + RE > CG (P < 0.05). Mindfulness/well-being/sleep: Significant improvements in MSH + RE only (FFMQ, WHO-5, ISI, P < 0.05). Strength: Significant increases in MSH + RE (P < 0.01) and RE (P < 0.01); MSH + RE > RE (P < 0.01) and CG (P < 0.01) | High |
| Park et al[59], 2021 | Crossover study | 9 | - | 60 minutes/session, single session per trial | PSG, EEG, metabolic chamber, core body temperature sensor | AE | Moderate | Metabolic: Increased energy expenditure during post-exercise sleep (P < 0.05); no significant change in mean core body temperature. Subjective: Worse “refreshness” (P < 0.05) and more “frequent dreaming/nightmares” (P < 0.05) in exercise trial; no other differences. Objective sleep: Shorter REM latency (P < 0.05) and reduced SWS duration (P < 0.01); increased δ power in SWS (P < 0.05) and improved SWS stability (P < 0.05) | High |
| Goldberg et al[65], 2024 | Crossover study | 13 | - | 45-60 minutes/session, single session per condition (morning/evening/rest) | SSI, EEG, Actigraph-wGT3X-BT | AE | Moderate to high | Objective sleep: Both morning and evening exercise increased NREM sleep duration compared to rest, primarily due to extended N2 sleep. No significant effects on SOL, WASO, N1, N3, REM, or TST. Subjective sleep: No differences in SSI scores between conditions | High |
| Barrett et al[62], 2020 | RCT | AE group: 137; MBSR group: 138; CG: 138 | 8 weeks | MBSR: Weekly 2.5-hour classes + 5-hour weekend retreat + daily home practice (20-45 minutes). AE: Weekly supervised sessions (matched to MBSR in contact hours) | PSQI, SF-12, PHQ-9, PSS | AE, mind-body exercise | Moderate | PSQI scores: Both AE and MBSR decreased, with AE showing significant benefit. Perceived sleep quality: Improved in both groups. Daily disturbances: MBSR improved more than AE. SE: No significant improvements in either group. Sustainability: Benefits persisted over 7-month follow-up | High |
| Saidi et al[64], 2023 | Crossover study | M-type: 12; I-type: 14; E-type: 16 | 3 days | 2 hours/session | PSQI, KSS, POMS-A, Hooper questionnaire, PSG, Actigraph-GT3X+ | AEX: AE; EEX: AE | High | Whole sample: EEX showed lower SE, longer SOL, higher N1/N2 stages, lower N3, and more cortical arousals vs AEX. Chronotype differences: M-type and I-type: Lower SE, longer SOL/WASO, more cortical arousals and higher mood disturbances in EEX vs AEX. E-type: No significant sleep or mood changes between AEX and EEX. Next-day wellness: M-type reported higher stress/sleep disturbances after EEX; E-type had lower pre-sleep sleepiness | High |
| Gong et al[68], 2019 | RCT | EG: 34; CG: 36 | 8 weeks | 40 minutes/session, 3 times/week | PSQI, PANAS, SAS | AE, mind-body exercise | Moderate | After intervention, EG’s sleep quality, anxiety, and negative emotion scores were improved. Negative emotions played a partial mediating role in the effect of exercise on sleep quality | High |
Acute exercise intervention vs long-term regular exercise intervention: Exercise intervention duration distinctly influences sleep outcomes. Acute exercise (single sessions) induces immediate benefits, in particular reduced blood glucose, elevated melatonin/cortisol secretion, improved SE, and decreased SOL and WASO[69]. A meta-analysis of 41 acute exercise studies confirmed small-to-medium enhancements in TST, SOL, SE, and slow-wave sleep, albeit with slight REM sleep reduction[70]. These findings position acute exercise as a short-term solution for rapid sleep improvement. In contrast, sustained exercise programs yield enduring, multifaceted benefits. The same meta-analysis[70] revealed that long-term interventions (25 studies) achieved sleep improvements comparable to clinical insomnia treatments, including TST, SE, SOL, and overall quality. A cross-sectional study (n = 843) further demonstrated lifelong impacts: Adults active during adolescence had 46% lower poor sleep risk, rising to 49% for those maintaining activity into adulthood[71], emphasizing early habitual exercise for lifelong sleep health.
Regular exercise is particularly beneficial for college students. A questionnaire-based study found that students who exercised regularly reported better PSQI scores[72]. A meta-analysis of intervention durations reinforced this, indicating that programs lasting ≥ 12 weeks yield optimal sleep improvements[73]. While acute exercise offers quick relief, these results support sustained structured programs, such as 12-week regimens, to address chronic sleep challenges in college students. Exercise duration operates on a spectrum. Acute sessions provide immediate but transient benefits, whereas regular long-term practice fosters lasting sleep improvements. For college students, integrating urgent interventions for acute sleep issues and sustained programs for chronic dysfunction may offer a balanced path to optimal sleep health.
Circadian rhythm regulation: Exercise modulates sleep through circadian regulation, molecular signaling, and sleep architecture remodeling. Animal studies show exercise restores circadian rhythmicity in arrhythmic conditions, surpassing pharmacological interventions in resetting circadian synchronization under darkness[74]. Human trials corroborate these findings: Timed exercise accelerates sleep-wake cycle re-entrainment in sleep-deprived individuals, though melatonin rhythms remain unaffected, suggesting melatonin-independent pathways[75]. In DSWPD patients, moderate-intensity AE or RE advanced sleep onset by approximately 1 hour, alleviating circadian misalignment[48]. RE upregulates skeletal muscle clock genes (Cry1, Per2, Bmal1), indicating peripheral circadian regulation independent of the central suprachiasmatic nucleus[76]. Heavy-load exercise in rats activated the nicotinamide phosphoribosyltransferase/nicotinamide adenine dinucleotide/sirtuin 1 pathway, linking circadian energy metabolism to exercise-induced synchronization of metabolic and circadian rhythms[77]. These findings highlight exercise’s dual role in central and peripheral clock synchronization.
Exercise reshapes sleep architecture by optimizing sleep stages and continuity. A meta-analysis revealed that exercise can reduce SOL and wake after sleep onset, decrease light sleep (N1/N2), and increase slow-wave sleep[32]. AE excels at shortening SOL, whereas mind-body exercise reduces WASO, implying modality-specific mechanisms. Subjectively, exercise improves SE, TST[78], and insomnia severity across modalities, including moderate aerobic training, strength training, and mind-body practices. These structural improvements align with objective metrics, as observed in studies using PSG and actigraphy[78]. Overall, exercise enhances sleep through three synergistic axes: Circadian synchronization, molecular pathway activation, and sleep-stage optimization. By integrating these mechanisms, exercise offers a holistic, non-pharmacological strategy to address sleep disorders.
Immunomodulation: Exercise improves sleep quality by modulating immune function and reducing systemic inflammation[79]. This relationship is evident in population-level data. A study of 22599 participants from the National Health and Nutrition Examination Survey linked sedentary behavior to heightened sleep disturbances, whereas physical activity counteracted these effects, particularly in individuals with severe inactivity[80]. Mechanistically, exercise reduces pro-inflammatory cytokines critical to sleep regulation. A 4-month trial including 40 patients with primary insomnia found that both AE and AE + RE improved sleep metrics (e.g., TST and NREM) and lowered inflammatory markers (IL-1β, IL-6, and TNF-α)[81]. Prominently, the AE + RE group outperformed the AE group, suggesting the synergistic benefits of multimodal training[81]. Comparative analyses have revealed modality-specific immune and sleep effects. In a study of 18 male college students with DSWPD, RE surpassed AE in advancing sleep onset time, improving SE, and reducing IL-6 Levels[48]. However, both modalities elevated melatonin and anti-inflammatory IL-10 Levels, highlighting the unique advantage of RE in immune modulation[48]. An 8-week trial comparing integrative body-mind-spirit and Qigong interventions (n = 281) was found to reduce IL-6 and IL-1β and improve sleep quality, emphasizing the anti-inflammatory potential of holistic practices[82].
Exercise-induced anti-inflammatory mechanisms have been increasingly elucidated at the molecular level. In male C57BL/6 mice, 8 weeks of treadmill training elevated irisin levels[83], which competitively binds myeloid differentiation factor 2, blocking its interaction with toll-like receptor 4 and suppressing inflammatory signaling[84]. Human studies have corroborated this, with resistance training found to reduce the pro-inflammatory IL-6:IL-10 ratio, thus promoting a robust anti-inflammatory state[85], in a sample of 12 men. Cumulatively, exercise enhances sleep through dual immune pathways: Reducing pro-inflammatory cytokines (e.g., IL-6 and TNF-α) and amplifying anti-inflammatory signals (e.g., IL-10 and irisin). Interventions can optimize immunological and sleep outcomes by integrating aerobic, resistance, and mind-body modalities.
Neuroendocrine regulation: Exercise enhances sleep quality via neuroendocrine mechanisms mediated by brain-derived neurotrophic factor (BDNF), monoamine neurotransmitters, and skeletal muscle factors. As a widely distributed neurotrophic factor, BDNF supports neuronal/glial development, neuroprotection, and synaptic regulation essential for cognition and memory[86]. It demonstrates protective effects on dopaminergic neurons and shows systemic bioenergetic regulatory capabilities[87]. Physical exercise elevates BDNF levels in blood and brain tissue[88], with acute exercise triggering hippocampal BDNF expression through irisin activation[89]. Exercise has been shown to elevate BDNF levels in muscles and plasma[90]. A meta-analysis including 910 participants revealed that BDNF concentration in the blood acutely increases in response to AE but not RE[91]. This increase in BDNF is particularly relevant to sleep quality, as low proportions of stage N3 and REM sleep have been associated with low serum BDNF[92]. Thus, exercise-induced increases in BDNF may improve sleep quality by promoting neuroplasticity and neuroprotection in brain regions involved in sleep regulation.
Monoamine systems mediate the exercise-induced enhancement of various brain functions. Dopamine (DA), norepinephrine, and 5-hydroxytryptamine (5-HT) are three major monoamine neurotransmitters regulated by exercise[93]. An 8-week exercise regimen (5 times/week) reduced depressive behaviors and lowered striatal norepinephrine levels and 5-HT turnover in sleep-deprived mice[94]. In humans, a study involving 121 young adults revealed that acute intense exercise significantly elevated serum 5-HT levels compared to controls[95]. Elevated 5-HT from high-intensity exercise may also promote central fatigue post-endurance activity[93]. Subsequently, in male mice, 30 days of voluntary wheel running enhanced striatal DA release and increased dorsal striatal BDNF expression without altering DA tissue content[96]. Six months of high-intensity exercise increased DA transporter and neuromelanin concentrations in early-stage Parkinson’s patients, underscoring exercise’s neuroregulatory role in the dopaminergic system[97]. Together, these findings indicate that exercise modulates monoamine neurotransmitter levels, potentially improving sleep quality through regulation of brain region activity.
Physical activity prompts skeletal muscle to secrete systemic factors like BDNF, irisin, and peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC-1α), which help regulate sleep via metabolic and circadian mechanisms[98]. PGC-1α, a master regulator of mitochondrial biogenesis, is upregulated by both aerobic and resistance training across tissues (e.g., brain, liver, and muscle)[99]. PGC-1α’s long-term effects sustain mitochondrial health and energy dynamics, indirectly supporting sleep by meeting the brain’s high metabolic demands during restorative sleep stages. This muscle-brain crosstalk underscores the holistic impact of exercise by integrating peripheral adaptations with central sleep regulation. Exercise may enhance sleep through three interconnected axes. BDNF-driven neuroplasticity, monoamine-mediated mood stabilization, and muscle-derived metabolic coordination. These pathways act synergistically to optimize sleep architecture and provide resilience against sleep disorders.
Thermoregulation: CBT follows a circadian rhythm, naturally declining approximately 1 hour before sleep onset, reaching its nadir at the end of sleep, and increasing gradually upon waking[100]. This pre-sleep CBT drop is critical for sleep initiation and maintenance, as elevated CBT correlates with reduced SE and increased nighttime awakenings[101]. A study of ten postmenopausal women found that higher pre-sleep CBT predicted lower SE and prolonged wake after sleep onset, directly implicating thermoregulation in sleep quality[102]. Heat dissipation, particularly via distal skin vasodilation, drives this decrease in CBT. Efficient heat loss through glabrous skin (e.g., hands and feet) is essential for sleep onset[103]; impaired dissipation may underlie difficulties falling asleep[104]. A temperature-controlled sleep system study showed that enhancing distal blood flow and heat loss elevates the distal-proximal skin temperature gradient, lowers CBT, and shortens SOL[105]. These findings suggest that distal vasodilation acts as a gatekeeper for sleep initiation. Exercise amplifies thermoregulatory mechanisms to promote sleep. By raising body temperature, exercise triggers peripheral vasodilation and post-exercise heat dissipation, accelerating the CBT decline critical for sleep[106]. Daytime exercise induces a steeper evening CBT decrease, facilitating deeper sleep stages[101]. Even subtle thermal adjustments are important. A study of 24 healthy volunteers found that skin temperature changes within the thermoneutral zone modulate sleep depth, highlighting the interplay between core and peripheral temperature regulation[104].
Disrupted CBT rhythms are hallmarks of circadian sleep disorders[107]. A cross-sectional study linked delayed CBT minima[108] (about 4 hours later) and a prolonged endogenous circadian period (tau)[109] to DSWPD. Similarly, in 28 children with circadian rhythm sleep-wake disorders, CBT declined during sleep, parallel to symptom improvement, suggesting that CBT trajectories could serve as biomarkers for therapeutic monitoring[110]. This evidence suggested that CBT regulation via heat dissipation, exercise timing, and circadian alignment is a linchpin of sleep health. Optimizing these factors offers a non-pharmacological avenue for addressing sleep disorders rooted in thermal or circadian dysregulation.
Physical exercise enhances sleep quality through interconnected psychological mechanisms, including anxiety reduction, depression alleviation, and bolstered self-efficacy. A study in China identified anxiety and depression as sequential mediators between physical activity and sleep quality[111], a finding reinforced by a study of 673 college students, which found that exercise could reduce anxiety and depression while elevating self-efficacy[112], a key predictor of sleep health. Further surveys of college students have clarified these relationships. AE directly improves sleep quality[113], while exercise also enhances psychological well-being through social self-efficacy, fostering resilience against stressors that disrupt sleep[114]. Thus, exercise cultivates emotional balance and social confidence, creating psychological conditions conducive to restorative sleep.
Systematic reviews have reinforced these psychological benefits across diverse populations. A meta-analysis of 15 prospective cohort studies (n = 191130) revealed a dose-dependent relationship, in which meeting 50% of the recommended physical activity lowered depression risk by 18%, and full adherence reduced it by 25%[115]. Tai Chi has been found to improve anxiety, depression, stress, and sleep quality in college students, demonstrating the unique psychosocial benefits of mind-body practices[116]. A synthesis of 49 studies (about 267000) found a correlation between higher physical activity and reduced depression risk across all ages, strengthening the position of exercise as a universal protective factor[117]. These findings underscore the scalability of exercise as a non-pharmacological intervention for sleep and mental health[118].
Despite growing evidence supporting exercise as a non-pharmacological strategy for enhancing sleep quality in college students, significant challenges persist. Variations across studies in exercise protocols, including type, intensity, duration, frequency, and sample sizes, hinder the generalizability of findings. Although this review synthesized diverse regimens, the mechanisms by which specific exercises influence sleep are not completely understood. Coupled with this, most studies have relied predominantly on self-reported sleep metrics, which are vulnerable to recall bias and personal interpretation. Although objective tools such as actigraphy and PSG yield more precise data, their underutilization in many studies raises concerns about validity. Subsequently, individual factors that may critically shape intervention efficacy, including chronotype, gender, and baseline fitness levels, remain underexplored. For example, chronotype may determine the optimal exercise timing and modality for sleep benefits. Finally, the existing research predominantly focuses on short-term outcomes, underscoring the need for longitudinal studies to evaluate long-term effects.
Future research should address existing limitations by employing large samples in randomized controlled trials that utilize standardized exercise protocols and objective sleep measurements. Specifically, the relationship between physical condition and sleep quality can be systematically explored using digital tools that enable real-time monitoring of physiological indicators such as heart rate, step count, blood pressure, and blood glucose levels. These tools include wearable devices like smartwatches and continuous glucose monitors. Likewise, for the specific population of college students, mobile applications can be employed to send regular reminders encouraging appropriate exercise and reduced screen time to enhance sleep quality. Furthermore, within the context of school-based education, personalized sports training programs can be developed using data from students’ physical fitness tests, physical condition, and personality traits. Studies should also investigate the personalized effects of exercise interventions based on individual characteristics. The development of mobile health applications and wearable devices could facilitate continuous monitoring of sleep and physical activity, providing real-time data and insights into the dynamic relationship between exercise and sleep quality. Finally, exploring the potential synergistic effects of exercise combined with other interventions, like dietary modifications, may offer new avenues for improving sleep health among college students.
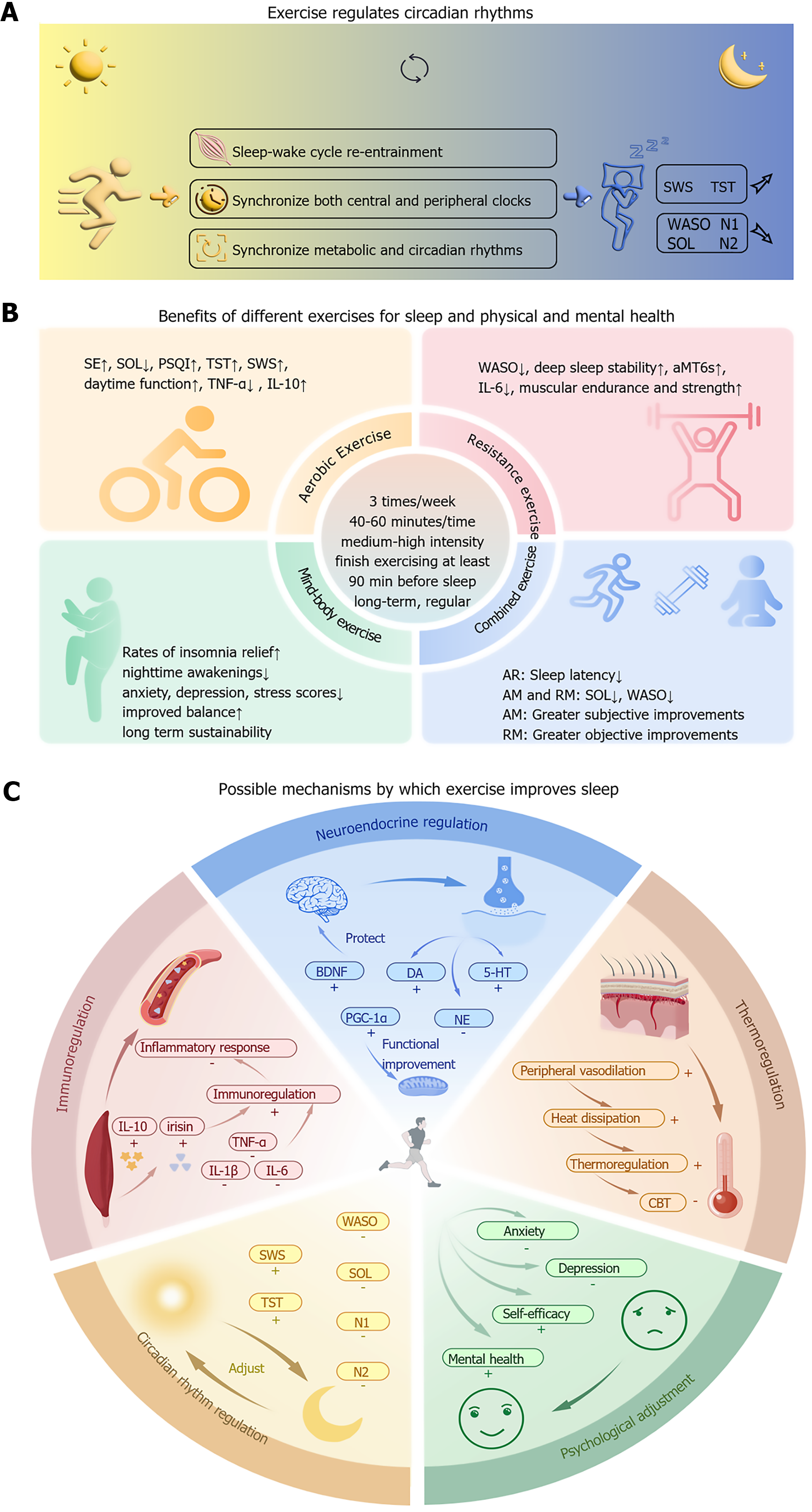
Sleep disorders are highly prevalent among college students, posing significant risks to physical health, academic performance, and psychological well-being. This review synthesizes evidence supporting exercise as a safe, non-pharmacological intervention - to improve sleep quality, emphasizing structured regimens of 3 weekly sessions (40-60 minutes each) while avoiding vigorous activity within 90 minutes of bedtime for optimal outcomes. Exercise enhances sleep through multifaceted physiological mechanisms, including circadian rhythm regulation (aligning sleep-wake cycles), stabilization of sleep architecture (increased slow-wave sleep), immune modulation (reducing pro-inflammatory cytokines), neuroendocrine adjustments (elevating BDNF and melatonin), and thermoregulation (CBT decline), alongside psychological benefits such as alleviating anxiety and depression, bolstering self-efficacy, and enhancing positive emotions. AE, RE, and mind-body exercises (e.g., yoga and Tai Chi) have all demonstrated efficacy, with modality-specific advantages. AE reduces sleep latency, whereas mind-body practices improve subjective sleep quality. Despite robust evidence, gaps remain, necessitating large-scale longitudinal trials to standardize protocols and personalize interventions. Future research should integrate wearable technology for real-time monitoring and explore synergies with complementary therapies (e.g., nutrition and CBT-I) to establish exercise as a cornerstone of holistic sleep health strategies for college students (Figure 2).
| 1. | Baranwal N, Yu PK, Siegel NS. Sleep physiology, pathophysiology, and sleep hygiene. Prog Cardiovasc Dis. 2023;77:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 249] [Reference Citation Analysis (0)] |
| 2. | Fabbri M, Beracci A, Martoni M, Meneo D, Tonetti L, Natale V. Measuring Subjective Sleep Quality: A Review. Int J Environ Res Public Health. 2021;18:1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 347] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 3. | Liu M, Zhu H, Tang J, Chen H, Chen C, Luo J, Chen W. Overview of a Sleep Monitoring Protocol for a Large Natural Population. Phenomics. 2023;3:1-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Beiter R, Nash R, McCrady M, Rhoades D, Linscomb M, Clarahan M, Sammut S. The prevalence and correlates of depression, anxiety, and stress in a sample of college students. J Affect Disord. 2015;173:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 787] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 5. | Lin L, Liang W, Wang R, Rhodes RE, Liu H. Association of 24-hour movement guideline adherence, mental health and quality of life in young adults: the role of e-Health literacy. Front Public Health. 2024;12:1344718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 6. | Wang C, Mattingly S, Payne J, Lizardo O, Hachen DS. The impact of social networks on sleep among a cohort of college students. SSM Popul Health. 2021;16:100937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Li M, Han Q, Pan Z, Wang K, Xie J, Zheng B, Lv J. Effectiveness of Multidomain Dormitory Environment and Roommate Intervention for Improving Sleep Quality of Medical College Students: A Cluster Randomised Controlled Trial in China. Int J Environ Res Public Health. 2022;19:15337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Blouin J, Feek A, Jin Y, Cook J, O'Neal T, Sacheck JM. The Fitness, Rest, and Exercise for Strength and Health (FRESH) Study: A Three-Year Comparison of College Students' Perceived and Measured Health Metrics. Nutrients. 2025;17:217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Norbury R, Evans S. Time to think: Subjective sleep quality, trait anxiety and university start time. Psychiatry Res. 2019;271:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Nakie G, Takelle GM, Rtbey G, Andualem F, Tinsae T, Kassa MA, Tadesse G, Fentahun S, Wassie YA, Segon T, Kibralew G, Melkam M. Sleep quality and associated factors among university students in Africa: a systematic review and meta-analysis study. Front Psychiatry. 2024;15:1370757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 11. | Albqoor MA, Shaheen AM. Sleep quality, sleep latency, and sleep duration: a national comparative study of university students in Jordan. Sleep Breath. 2021;25:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 12. | Chen B, Zhou Y, Huang Z. [Associations of physical activity, sleep quality with anxiety and depressive symptoms among college students]. Zhongguo Xuexiao Weisheng. 2024;45:684-688. [DOI] [Full Text] |
| 13. | Li X, Sun W, Yin M, Dou T, Lv Y, Xu W, Zha Z. [Sleep Quality and Anxiety and Depression in Patients with Chronic Obstructive Pulmonary Disease and Their Influencing Factors: a Multicenter Cross-sectional Study]. Zhongguo Quanke Yixue. 2024;27:2437-2444. [DOI] [Full Text] |
| 14. | Tatar D, Dębski P, Bocian B, Bąkowska M, Będkowska J, Tropiejko M, Główczyński P, Badura-Brzoza K. How do teenagers sleep? Analysis of factors related to sleep disorders in a group of Polish high school students. BMC Pediatr. 2023;23:498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Xanthopoulos MS, Berkowitz RI, Tapia IE. Effects of obesity therapies on sleep disorders. Metabolism. 2018;84:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Yu HJ, Liu X, Yang HG, Chen R, He QQ. The association of adverse childhood experiences and its subtypes with adulthood sleep problems: A systematic review and meta-analysis of cohort studies. Sleep Med. 2022;98:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 17. | Olsen EL, April-Sanders AK, Bird HR, Canino GJ, Duarte CS, Suglia SF. Adverse Childhood Experiences and Sleep Disturbances Among Puerto Rican Young Adults. JAMA Netw Open. 2024;7:e247532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 18. | Schmickler JM, Blaschke S, Robbins R, Mess F. Determinants of Sleep Quality: A Cross-Sectional Study in University Students. Int J Environ Res Public Health. 2023;20:2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 19. | Tang X, Zhang X, Yin Y. [Advances in the correlation between digital eye strain and sleep quality in adolescents]. Zhongguo Xuexiao Weisheng. 2024;45:300-304. [DOI] [Full Text] |
| 20. | Sejbuk M, Mirończuk-Chodakowska I, Witkowska AM. Sleep Quality: A Narrative Review on Nutrition, Stimulants, and Physical Activity as Important Factors. Nutrients. 2022;14:1912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 182] [Article Influence: 45.5] [Reference Citation Analysis (1)] |
| 21. | Zhang Y, Ma C, Cai Y, Sun M, Gu R, Zhang L, Zhou D, Du B, Li H, Chen Y, Liu L, Ping Z. [Dynamic investigation on sleep quality and influencing factors of medical students in a comprehensive university in central China]. Xiandai Yufang Yixue. 2022;49:3776-3781. [DOI] [Full Text] |
| 22. | De Lise F, Bacaro V, Crocetti E. The Social Side of Sleep: A Systematic Review of the Longitudinal Associations between Peer Relationships and Sleep Quality. Int J Environ Res Public Health. 2023;20:2017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Zhang S, Liu X, Chen J, Yang H, Chen J, Li D, Xu H, Wang S, Guo H, Zhang N, Liu Z, Min X, Wu W. Patterns of sleep quality and its influence factors: A latent class model among students of medical university in Hubei Province, China. J Affect Disord. 2024;347:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 24. | Riemann D, Espie CA, Altena E, Arnardottir ES, Baglioni C, Bassetti CLA, Bastien C, Berzina N, Bjorvatn B, Dikeos D, Dolenc Groselj L, Ellis JG, Garcia-Borreguero D, Geoffroy PA, Gjerstad M, Gonçalves M, Hertenstein E, Hoedlmoser K, Hion T, Holzinger B, Janku K, Jansson-Fröjmark M, Järnefelt H, Jernelöv S, Jennum PJ, Khachatryan S, Krone L, Kyle SD, Lancee J, Leger D, Lupusor A, Marques DR, Nissen C, Palagini L, Paunio T, Perogamvros L, Pevernagie D, Schabus M, Shochat T, Szentkiralyi A, Van Someren E, van Straten A, Wichniak A, Verbraecken J, Spiegelhalder K. The European Insomnia Guideline: An update on the diagnosis and treatment of insomnia 2023. J Sleep Res. 2023;32:e14035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 395] [Article Influence: 131.7] [Reference Citation Analysis (0)] |
| 25. | Cheung JMY, Bartlett DJ, Armour CL, Saini B, Laba TL. Patient Preferences for Managing Insomnia: A Discrete Choice Experiment. Patient. 2018;11:503-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Kakazu VA, Assis M, Bacelar A, Bezerra AG, Ciutti GLR, Conway SG, Galduróz JCF, Drager LF, Khoury MP, Leite IPA, Luciano YDM, Poyares D, Tufik S, Pires GN. Insomnia and its treatments—trend analysis and publication profile of randomized clinical trials. npj Biol Timing Sleep. 2024;1:14. [DOI] [Full Text] |
| 27. | Edinger JD, Arnedt JT, Bertisch SM, Carney CE, Harrington JJ, Lichstein KL, Sateia MJ, Troxel WM, Zhou ES, Kazmi U, Heald JL, Martin JL. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2021;17:263-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 28. | De Crescenzo F, D'Alò GL, Ostinelli EG, Ciabattini M, Di Franco V, Watanabe N, Kurtulmus A, Tomlinson A, Mitrova Z, Foti F, Del Giovane C, Quested DJ, Cowen PJ, Barbui C, Amato L, Efthimiou O, Cipriani A. Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: a systematic review and network meta-analysis. Lancet. 2022;400:170-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 246] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 29. | Kline CE, Hillman CH, Bloodgood Sheppard B, Tennant B, Conroy DE, Macko RF, Marquez DX, Petruzzello SJ, Powell KE, Erickson KI. Physical activity and sleep: An updated umbrella review of the 2018 Physical Activity Guidelines Advisory Committee report. Sleep Med Rev. 2021;58:101489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 30. | Zhou X, Kong Y, Yu B, Shi S, He H. Effects of exercise on sleep quality in general population: Meta-analysis and systematic review. Sleep Med. 2025;125:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 31. | Liu M, Wang H, Zhang Y, Tang D. [Effect of aerobic pedal exercise on sleep quality and energy metabolism of female college students with mild sleep disorder]. Zhongguo Xuexiao Weisheng. 2023;44:1692-1696. [DOI] [Full Text] |
| 32. | Gong M, Tan S, Sun Y, Wu Y, Hu X. [Meta-analysis of Exercise Intervention on Sleep Structure in Adults With Sleep Disorders]. Shoudu Tiyuxueyuan Xuebao. 2021;33:276-284. |
| 33. | Miller DJ, Sargent C, Roach GD, Scanlan AT, Vincent GE, Lastella M. Moderate-intensity exercise performed in the evening does not impair sleep in healthy males. Eur J Sport Sci. 2020;20:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Chen JH, Chen JY, Wang YC. The effects of exercise programs on sleep architecture in obstructive sleep apnea: a meta-analysis of randomized controlled trials. J Sci Med Sport. 2024;27:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 35. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5754] [Cited by in RCA: 11717] [Article Influence: 650.9] [Reference Citation Analysis (0)] |
| 36. | Mikó A, Pótó L, Mátrai P, Hegyi P, Füredi N, Garami A, Illés A, Solymár M, Vincze Á, Balaskó M, Pár G, Sarlós P, Bajor J, Tenk J, Rostás I, Pétervári E. Gender difference in the effects of interleukin-6 on grip strength - a systematic review and meta-analysis. BMC Geriatr. 2018;18:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Liu W, Xin Y, Li Q, Shang Y, Ping Z, Min J, Cahill CM, Rogers JT, Wang F. Biomarkers of environmental manganese exposure and associations with childhood neurodevelopment: a systematic review and meta-analysis. Environ Health. 2020;19:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 38. | Takemura N, Cheung DST, Fong DYT, Lee AWM, Lam TC, Ho JC, Kam TY, Chik JYK, Lin CC. Effectiveness of Aerobic Exercise and Tai Chi Interventions on Sleep Quality in Patients With Advanced Lung Cancer: A Randomized Clinical Trial. JAMA Oncol. 2024;10:176-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 53] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 39. | Martinez Aguirre-Betolaza A, Mujika I, Loprinzi P, Corres P, Gorostegi-Anduaga I, Maldonado-Martín S. Physical Activity, Sedentary Behavior, and Sleep Quality in Adults with Primary Hypertension and Obesity before and after an Aerobic Exercise Program: EXERDIET-HTA Study. Life (Basel). 2020;10:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Zhao C, Ouyang S, Chen L, Su J, Li A. [Effect of aerobic exercise on sleep quality, sleep structure and inflammatory factors in patients with primary insomnia]. Huli Yanjiu. 2022;36:154-157. [DOI] [Full Text] |
| 41. | Liu S, Zhang R. Aerobic Exercise Alleviates the Impairment of Cognitive Control Ability Induced by Sleep Deprivation in College Students: Research Based on Go/NoGo Task. Front Psychol. 2022;13:914568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Takemura N, Cheung DST, Smith R, Deng W, Ho KY, Lin J, Kwok JYY, Lam TC, Lin CC. Effectiveness of aerobic exercise and mind-body exercise in cancer patients with poor sleep quality: A systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2020;53:101334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 43. | Bennie JA, Tittlbach S. Muscle-strengthening exercise and sleep quality among a nationally representative sample of 23,635 German adults. Prev Med Rep. 2020;20:101250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Kovacevic A, Mavros Y, Heisz JJ, Fiatarone Singh MA. The effect of resistance exercise on sleep: A systematic review of randomized controlled trials. Sleep Med Rev. 2018;39:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 45. | Patte KA, Qian W, Leatherdale ST. Modifiable predictors of insufficient sleep durations: A longitudinal analysis of youth in the COMPASS study. Prev Med. 2018;106:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Xu M, Zheng C, Chen Y. [Effect of circulation resistance training on the sleep quality and psychological status of college students with primary sleep disorder]. Shiyong Yufang Yixue. 2022;29:226-229. |
| 47. | Kowalsky RJ, Farney TM, Hearon CM. Resistance Exercise Breaks Improve Ratings of Discomfort and Sleepiness in College Students. Res Q Exerc Sport. 2023;94:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 48. | Hu YX, Liu XM, Zhang NX, Ma ZY, Zhu Z, Cao ZB. The effects of resistance are superior to aerobic exercise in improving delayed sleep-wake phase disorder in male college students. Sleep Med. 2025;128:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 49. | Zhang J. [Effect of yoga exercise intervention on depression and sleep quality of female college students]. Shanghai University of Sport, 2022. |
| 50. | Turmel D, Carlier S, Bruyneel AV, Bruyneel M. Tailored individual Yoga practice improves sleep quality, fatigue, anxiety, and depression in chronic insomnia disorder. BMC Psychiatry. 2022;22:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 51. | Chang S, Cheng L, Liu H. Effects of three-duration Tai-Chi exercises on depression and sleep quality in older women. Eur Geriatr Med. 2024;15:1141-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 52. | Siu PM, Yu AP, Tam BT, Chin EC, Yu DS, Chung KF, Hui SS, Woo J, Fong DY, Lee PH, Wei GX, Irwin MR. Effects of Tai Chi or Exercise on Sleep in Older Adults With Insomnia: A Randomized Clinical Trial. JAMA Netw Open. 2021;4:e2037199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 53. | Sun J, Zhuo J, Chu H, Wang J, Chen T, Li B, Lu T, Zheng H, Xu Y, Dong J, Cicchella A. Effects of 3-month Qigong exercise on heart rate variability and respiration in anxious college students. Scand J Med Sci Sports. 2024;34:e14521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 54. | Cristini J, Weiss M, De Las Heras B, Medina-Rincón A, Dagher A, Postuma RB, Huber R, Doyon J, Rosa-Neto P, Carrier J, Amara AW, Roig M. The effects of exercise on sleep quality in persons with Parkinson's disease: A systematic review with meta-analysis. Sleep Med Rev. 2021;55:101384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 55. | An KY, Morielli AR, Kang DW, Friedenreich CM, McKenzie DC, Gelmon K, Mackey JR, Reid RD, Courneya KS. Effects of exercise dose and type during breast cancer chemotherapy on longer-term patient-reported outcomes and health-related fitness: A randomized controlled trial. Int J Cancer. 2020;146:150-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 56. | Yuan S, Gong M, Ha J, Lu C, Guo Z, Ji Y, Meng Y, Zou G. [Influence of three combined exercise programs on improving sleep quality among college students with sleep disorders]. Zhongguo Xuexiao Weisheng. 2022;43:215-220. [DOI] [Full Text] |
| 57. | Lin Latt CM, Alldredge CT, Williams S, Vinson M, Seiba Moris J, Elkins GR. Mindful Self-Hypnosis Combined with Resistance Training to Reduce Perceived Stress and Improve Other Psychological Factors in Female College Students. Int J Clin Exp Hypn. 2024;72:254-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 58. | Wang B. [A study of the effects of physical activity intensity difference intervention on sleep quality and physical activity of college students of different genders]. Tianjin Normal University, 2023. |
| 59. | Park I, Díaz J, Matsumoto S, Iwayama K, Nabekura Y, Ogata H, Kayaba M, Aoyagi A, Yajima K, Satoh M, Tokuyama K, Vogt KE. Exercise improves the quality of slow-wave sleep by increasing slow-wave stability. Sci Rep. 2021;11:4410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 60. | Zhao W, Li K, Wang Y, Wang J, Liu A, Chen X, Xu J, Yang P, Ding C, Wang M, Wang L, Wang H, Wang Z, Liu D, Liu K, Li L, Qiu J, Zhang T, Zhang P, Yang X, Yang X, Yang Z, Jiang Y, Liang Q, Zhai Y, Chang C, Yang P, Hou W. [Physical Activity Guidelines for Chinese(2021)]. Zhongguo Gonggong Weisheng. 2022;38:129-130. [DOI] [Full Text] |
| 61. | Chen LJ, Hamer M, Lai YJ, Huang BH, Ku PW, Stamatakis E. Can physical activity eliminate the mortality risk associated with poor sleep? A 15-year follow-up of 341,248 MJ Cohort participants. J Sport Health Sci. 2022;11:596-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 62. | Barrett B, Harden CM, Brown RL, Coe CL, Irwin MR. Mindfulness meditation and exercise both improve sleep quality: Secondary analysis of a randomized controlled trial of community dwelling adults. Sleep Health. 2020;6:804-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Frimpong E, Mograss M, Zvionow T, Dang-Vu TT. The effects of evening high-intensity exercise on sleep in healthy adults: A systematic review and meta-analysis. Sleep Med Rev. 2021;60:101535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 64. | Saidi O, Peyrel P, Del Sordo G, Gabriel B, Maso F, Doré É, Duché P. Is it wiser to train in the afternoon or the early evening to sleep better? The role of chronotype in young adolescent athletes. Sleep. 2023;46:zsad099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 65. | Goldberg M, Pairot de Fontenay B, Blache Y, Debarnot U. Effects of morning and evening physical exercise on subjective and objective sleep quality: an ecological study. J Sleep Res. 2024;33:e13996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 66. | Driver HS, Taylor SR. Exercise and sleep. Sleep Med Rev. 2000;4:387-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 491] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 67. | Kim N, Ka S, Park J. Effects of exercise timing and intensity on physiological circadian rhythm and sleep quality: a systematic review. Phys Act Nutr. 2023;27:52-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 68. | Gong Y, Zhang Y, Yang X, Guan Y, Tang D. [Effectiveness of exercise intervention on sleep quality and negative emotion among female college students with anxiety]. Zhongguo Xuexiao Weisheng. 2019;40:542-545. [DOI] [Full Text] |
| 69. | He J, Sun Y, Qiu L. [Effects of different modes of disposable exercise on sleep quality of college students and their physiological mechanisms]. Proceedings of the 12th National Congress of Sport Science; 2022 Mar 25; Rizhao, China. China: Chinese Society of Sport Science, 2022: 150-152. |
| 70. | Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW. The effects of physical activity on sleep: a meta-analytic review. J Behav Med. 2015;38:427-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 829] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 71. | Canhin DDS, Tebar WR, Scarabottolo CC, Silva GCR, Pinto RZ, Gobbo LA, Oliveira CBS, Christofaro DGD. Physical activity across life stages and sleep quality in adulthood - an epidemiological study. Sleep Med. 2021;83:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Liu X, Lang L, Wang R, Chen W, Ren X, Lin Y, Chen G, Pan C, Zhao W, Li T, Han C, He L, Gu Y. Poor sleep quality and its related risk factors among university students. Ann Palliat Med. 2021;10:4479-4485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 73. | Amiri S, Hasani J, Satkin M. Effect of exercise training on improving sleep disturbances: a systematic review and meta-analysis of randomized control trials. Sleep Med. 2021;84:205-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 74. | Lax P, Zamora S, Madrid JA. Coupling effect of locomotor activity on the rat's circadian system. Am J Physiol. 1998;275:R580-R587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 75. | Yamanaka Y, Hashimoto S, Masubuchi S, Natsubori A, Nishide SY, Honma S, Honma K. Differential regulation of circadian melatonin rhythm and sleep-wake cycle by bright lights and nonphotic time cues in humans. Am J Physiol Regul Integr Comp Physiol. 2014;307:R546-R557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 76. | Zambon AC, McDearmon EL, Salomonis N, Vranizan KM, Johansen KL, Adey D, Takahashi JS, Schambelan M, Conklin BR. Time- and exercise-dependent gene regulation in human skeletal muscle. Genome Biol. 2003;4:R61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 195] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 77. | Xia Y, Yao B, Fu Z, Li L, Jin S, Qu B, Huang Y, Ding H. Clock genes regulate skeletal muscle energy metabolism through NAMPT/NAD(+)/SIRT1 following heavy-load exercise. Am J Physiol Regul Integr Comp Physiol. 2023;325:R490-R503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 78. | Tian C, Wei Y, Xu M, Liu J, Tong B, Ning J, Wang Y, Wang Y, Estill J, Ge L. The effects of exercise on insomnia disorders: An umbrella review and network meta-analysis. Sleep Med. 2024;115:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 79. | Qu L, Zhang H, Dong L and Chao X. [Progress of research on aerobic exercise to improve sleep disorders in college students]. Proceedings of the 13th National Congress of Sport Science; 2023 Nov 3-5; Tianjin, China. China: Chinese Society of Sport Science, 2023: 142-143. [DOI] [Full Text] |
| 80. | You Y, Chen Y, Fang W, Li X, Wang R, Liu J, Ma X. The association between sedentary behavior, exercise, and sleep disturbance: A mediation analysis of inflammatory biomarkers. Front Immunol. 2022;13:1080782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 138] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 81. | Yang D. [Intervention Effect of Two Different Exercise Modes on Sleep Quality of Patients with Primary Insomnia]. Shanghai Tiyuxueyuan Xuebao. 2020;44:38-43. [DOI] [Full Text] |
| 82. | Ng SM, Yin MXC, Chan JSM, Chan CHY, Fong TCT, Li A, So KF, Yuen LP, Chen JP, Chung KF, Chan CLW. Impact of mind-body intervention on proinflammatory cytokines interleukin 6 and 1β: A three-arm randomized controlled trial for persons with sleep disturbance and depression. Brain Behav Immun. 2022;99:166-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 83. | Zhu W, Sahar NE, Javaid HMA, Pak ES, Liang G, Wang Y, Ha H, Huh JY. Exercise-Induced Irisin Decreases Inflammation and Improves NAFLD by Competitive Binding with MD2. Cells. 2021;10:3306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 84. | Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777-1782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1571] [Cited by in RCA: 1588] [Article Influence: 58.8] [Reference Citation Analysis (1)] |
| 85. | Lira FS, Conrado de Freitas M, Gerosa-Neto J, Cholewa JM, Rossi FE. Comparison Between Full-Body vs. Split-Body Resistance Exercise on the Brain-Derived Neurotrophic Factor and Immunometabolic Response. J Strength Cond Res. 2020;34:3094-3102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 86. | Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell Mol Neurobiol. 2018;38:579-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 703] [Cited by in RCA: 981] [Article Influence: 122.6] [Reference Citation Analysis (0)] |
| 87. | Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 430] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 88. | Palasz E, Wysocka A, Gasiorowska A, Chalimoniuk M, Niewiadomski W, Niewiadomska G. BDNF as a Promising Therapeutic Agent in Parkinson's Disease. Int J Mol Sci. 2020;21:1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 351] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 89. | Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 1058] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 90. | Edman S, Horwath O, Van der Stede T, Blackwood SJ, Moberg I, Strömlind H, Nordström F, Ekblom M, Katz A, Apró W, Moberg M. Pro-Brain-Derived Neurotrophic Factor (BDNF), but Not Mature BDNF, Is Expressed in Human Skeletal Muscle: Implications for Exercise-Induced Neuroplasticity. Function (Oxf). 2024;5:zqae005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 91. | Dinoff A, Herrmann N, Swardfager W, Liu CS, Sherman C, Chan S, Lanctôt KL. The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS One. 2016;11:e0163037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 92. | Deuschle M, Schredl M, Wisch C, Schilling C, Gilles M, Geisel O, Hellweg R. Serum brain-derived neurotrophic factor (BDNF) in sleep-disordered patients: relation to sleep stage N3 and rapid eye movement (REM) sleep across diagnostic entities. J Sleep Res. 2018;27:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 93. | Lin TW, Kuo YM. Exercise benefits brain function: the monoamine connection. Brain Sci. 2013;3:39-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 240] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 94. | Daniele TMDC, de Bruin PFC, Rios ERV, de Bruin VMS. Effects of exercise on depressive behavior and striatal levels of norepinephrine, serotonin and their metabolites in sleep-deprived mice. Behav Brain Res. 2017;332:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 95. | Zimmer P, Stritt C, Bloch W, Schmidt FP, Hübner ST, Binnebößel S, Schenk A, Oberste M. The effects of different aerobic exercise intensities on serum serotonin concentrations and their association with Stroop task performance: a randomized controlled trial. Eur J Appl Physiol. 2016;116:2025-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 96. | Bastioli G, Arnold JC, Mancini M, Mar AC, Gamallo-Lana B, Saadipour K, Chao MV, Rice ME. Voluntary Exercise Boosts Striatal Dopamine Release: Evidence for the Necessary and Sufficient Role of BDNF. J Neurosci. 2022;42:4725-4736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 97. | de Laat B, Hoye J, Stanley G, Hespeler M, Ligi J, Mohan V, Wooten DW, Zhang X, Nguyen TD, Key J, Colonna G, Huang Y, Nabulsi N, Patel A, Matuskey D, Morris ED, Tinaz S. Intense exercise increases dopamine transporter and neuromelanin concentrations in the substantia nigra in Parkinson's disease. NPJ Parkinsons Dis. 2024;10:34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 98. | Tan X, van Egmond LT, Cedernaes J, Benedict C. The role of exercise-induced peripheral factors in sleep regulation. Mol Metab. 2020;42:101096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 99. | Neto IVS, Pinto AP, Muñoz VR, de Cássia Marqueti R, Pauli JR, Ropelle ER, Silva ASRD. Pleiotropic and multi-systemic actions of physical exercise on PGC-1α signaling during the aging process. Ageing Res Rev. 2023;87:101935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 100. | Haghayegh S, Smolensky MH, Khoshnevis S, Hermida RC, Castriotta RJ, Diller KR. The Circadian Rhythm of Thermoregulation Modulates both the Sleep/Wake Cycle and 24 h Pattern of Arterial Blood Pressure. Compr Physiol. 2021;11:2645-2658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 101. | Atkinson G, Davenne D. Relationships between sleep, physical activity and human health. Physiol Behav. 2007;90:229-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 102. | Murphy PJ, Campbell SS. Sex hormones, sleep, and core body temperature in older postmenopausal women. Sleep. 2007;30:1788-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 103. | Kräuchi K. The thermophysiological cascade leading to sleep initiation in relation to phase of entrainment. Sleep Med Rev. 2007;11:439-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 104. | Van Someren EJ. More than a marker: interaction between the circadian regulation of temperature and sleep, age-related changes, and treatment possibilities. Chronobiol Int. 2000;17:313-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 202] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 105. | Haghayegh S, Khoshnevis S, Smolensky MH, Hermida RC, Castriotta RJ, Schernhammer E, Diller KR. Novel temperature-controlled sleep system to improve sleep: a proof-of-concept study. J Sleep Res. 2022;31:e13662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 106. | Horne JA, Moore VJ. Sleep EEG effects of exercise with and without additional body cooling. Electroencephalogr Clin Neurophysiol. 1985;60:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 107. | Kräuchi K. The human sleep-wake cycle reconsidered from a thermoregulatory point of view. Physiol Behav. 2007;90:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 108. | Tai Y, Obayashi K, Yamagami Y, Saeki K. Association between circadian skin temperature rhythms and actigraphic sleep measures in real-life settings. J Clin Sleep Med. 2023;19:1281-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 109. | Micic G, de Bruyn A, Lovato N, Wright H, Gradisar M, Ferguson S, Burgess HJ, Lack L. The endogenous circadian temperature period length (tau) in delayed sleep phase disorder compared to good sleepers. J Sleep Res. 2013;22:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 110. | Kimura S, Takaoka Y, Toyoura M, Kohira S, Ohta M. Core body temperature changes in school-age children with circadian rhythm sleep-wake disorder. Sleep Med. 2021;87:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 111. | Su Y, Wang SB, Zheng H, Tan WY, Li X, Huang ZH, Hou CL, Jia FJ. The role of anxiety and depression in the relationship between physical activity and sleep quality: A serial multiple mediation model. J Affect Disord. 2021;290:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 112. | Wu J, Zhao X, Zhao W, Chi X, Ji J, Hu J. [Effect of physical exercise on negative emotions of college students: The mediating role of self-efficacy]. Zhongguo Jiankang Xinlixue Zazhi. 2022;30:930-934. |
| 113. | Ezati M, Keshavarz M, Barandouzi ZA, Montazeri A. The effect of regular aerobic exercise on sleep quality and fatigue among female student dormitory residents. BMC Sports Sci Med Rehabil. 2020;12:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 114. | Yang J, Xiang C. [Research on the Path of Improving College Students' Psychological Happiness through Physical Exercise: the Mediating Effect of Social Self-Efficacy]. Chengdu Tiyuxueyuan Xuebao. 2021;47:132-136. [DOI] [Full Text] |
| 115. | Pearce M, Garcia L, Abbas A, Strain T, Schuch FB, Golubic R, Kelly P, Khan S, Utukuri M, Laird Y, Mok A, Smith A, Tainio M, Brage S, Woodcock J. Association Between Physical Activity and Risk of Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2022;79:550-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 746] [Article Influence: 186.5] [Reference Citation Analysis (0)] |
| 116. | Cui J, Lin J, Liu Y, Wang P, Zeng H. [Mental health benefits of Tai Chi for college students: a systematic review using ICF]. Zhongguo Kangfu Lilun Yu Shijian. 2023;29:48-54. [DOI] [Full Text] |
| 117. | Schuch FB, Vancampfort D, Firth J, Rosenbaum S, Ward PB, Silva ES, Hallgren M, Ponce De Leon A, Dunn AL, Deslandes AC, Fleck MP, Carvalho AF, Stubbs B. Physical Activity and Incident Depression: A Meta-Analysis of Prospective Cohort Studies. Am J Psychiatry. 2018;175:631-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 1030] [Article Influence: 128.8] [Reference Citation Analysis (0)] |
| 118. | Wang P, Wang J, Zhao J, Wang X, Xin X, Qiu S, Zang Y, Wang X. [Relationship Between Physical Activity Level and Depressive Symptoms in College Students: A Pathway Analysis Based on EEG]. Shanghai Tiyuxueyuan Xuebao. 2023;47:51-60. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/