Published online Mar 19, 2024. doi: 10.5498/wjp.v14.i3.380
Peer-review started: December 17, 2023
First decision: January 10, 2024
Revised: January 15, 2024
Accepted: February 4, 2024
Article in press: February 4, 2024
Published online: March 19, 2024
Processing time: 92 Days and 22.1 Hours
Grasping the underlying mechanisms of Alzheimer's disease (AD) is still a work in progress, and existing diagnostic techniques encounter various obstacles. Therefore, the discovery of dependable biomarkers is essential for early detection, tracking the disease's advancement, and steering treatment strategies.
To explore the diagnostic potential of serum CXCL12, sCD22, Lp-PLA2, and their ratios in AD, aiming to enhance early detection and inform targeted treatment strategies.
The study was conducted in Dongying people's Hospital from January 2021 to De
Serum CXCL12 levels were higher in the AD group (47.2 ± 8.5 ng/mL) than the control group (32.8 ± 5.7 ng/mL, P < 0.001), while sCD22 levels were lower (14.3 ± 2.1 ng/mL vs 18.9 ± 3.4 ng/mL, P < 0.01). Lp-PLA2 levels were also higher in the AD group (112.5 ± 20.6 ng/mL vs 89.7 ± 15.2 ng/mL, P < 0.05). Significant di
Serum CXCL12 and Lp-PLA2 levels were significantly increased, while sCD22 were significantly decreased, as well as increases in the ratios of CXCL12/sCD22 and Lp-PLA2/sCD22, are closely related to the onset of AD. These biomarkers and their ratios can be used as potential diagnostic indicators for AD, providing an important clinical reference for early intervention and treatment.
Core Tip: This study uncovers the diagnostic potential of serum CXCL12, sCD22, Lp-PLA2 levels, and their ratios in Alzheimer's disease (AD). The research reveals distinct patterns in these biomarkers among AD patients, providing insight into their roles in neuroinflammation and immune regulation. The findings suggest these serum markers, especially when combined as ratios, could enhance AD diagnosis, offering a non-invasive approach to early detection and intervention.
- Citation: Liu ZL, Hua FF, Qu L, Yan N, Zhang HF. Evaluating serum CXCL12, sCD22, Lp-PLA2 levels and ratios as biomarkers for diagnosis of Alzheimer's disease. World J Psychiatry 2024; 14(3): 380-387
- URL: https://www.wjgnet.com/2220-3206/full/v14/i3/380.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i3.380
Alzheimer's disease (AD), recognized as the most prevalent form of dementia, is a neurodegenerative disorder characterized by a gradual and progressive decline in cognitive function[1-3]. With the aging of the global population ages, AD incidence is rising, posing a significant public health challenge[4,5]. Understanding the pathogenesis of AD remains incomplete, and current diagnostic methods face multiple challenges. Consequently, identifying reliable biomarkers is crucial for early diagnosis, monitoring disease progression, and guiding therapeutic interventions. Recent research has increasingly focused on serum biomarkers to enhance AD diagnosis through simple, non-invasive techniques[6]. Bio
AD is primarily characterized by a progressive decline in memory and cognitive functions, leading to a diminished capacity for daily activities[13]. Pathologically, AD is marked by the deposition of amyloid plaques in neurons and the formation of neurofibrillary tangles, both contributing to altered brain tissue structure and function[14-16]. Currently, AD diagnosis predominantly relies on clinical assessments and neuroimaging, but these methods have limitations, particularly in early detection and disease progression monitoring[1,17,18]. Increasing evidence suggests that AD pathogenesis involves various factors, including neuroinflammation, immune dysregulation, and vascular dysfunction[14]. Conse
CXCL12, also known as SDF-1, produced by bone marrow mesenchymal cells, is a chemokine primarily involved in immune cell migration and tissue repair. Research indicates a significant increase in CXCL12 levels in the cerebrospinal fluid of AD patients, correlating closely with cognitive decline[8,9]. Consequently, understanding how serum CXCL12 levels mirror AD onset and progression is a current research priority. sCD22, a soluble cell adhesion molecule, regulates B cells and is implicated in inflammation in neurological disorders[23]. Notably, sCD22 levels are generally reduced in AD patients[9]. However, further research is required to clarify the specific serum fluctuations of sCD22 and its interactions with other markers such as CXCL12 in AD. Lp-PLA2, an enzyme involved in inflammation and atherosclerosis, is also associated with AD. Higher serum levels of Lp-PLA2 might correlate with the onset of AD, yet the exact mechanisms behind this are not fully understood. Therefore, the diagnostic relevance of serum Lp-PLA2 in AD, as well as its rela
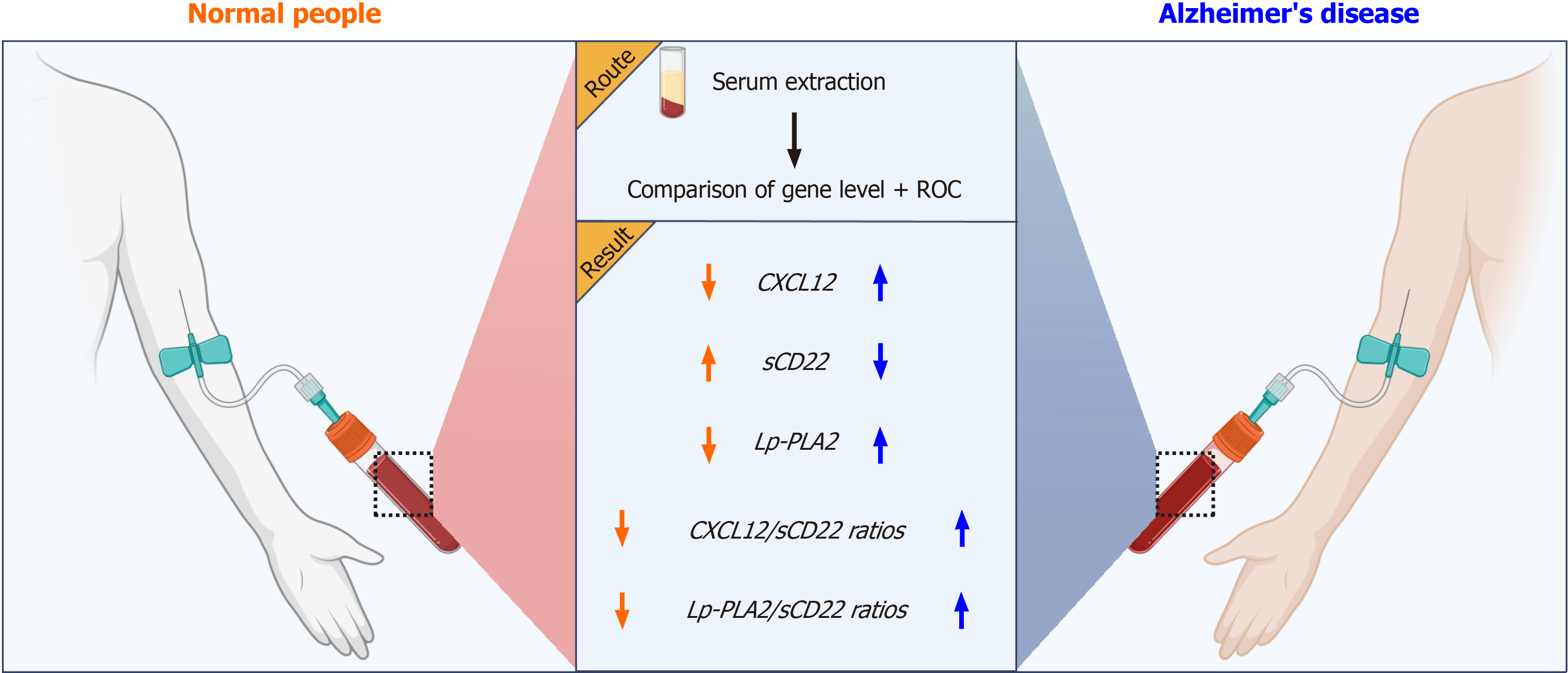
While initial studies have shed light on the roles of CXCL12, sCD22, Lp-PLA2, and other biomarkers in AD, significant uncertainties remain regarding their specific serum levels and interrelationships. Therefore, this study aims to thoroughly investigate the potential diagnostic value of these serum biomarkers and their ratios in AD. Our goal is to establish a more reliable clinical foundation for early diagnosis and treatment of AD (Figure 1).
In our study, we utilized a prospective case-control approach, involving two distinct groups: an AD group and a control group, each consisting of 60 participants. The aim was to measure and contrast the serum markers in both groups to evaluate the diagnostic utility of serum CXCL12, sCD22, and Lp-PLA2 levels, as well as their ratios, in identifying AD.
Participants consisted of AD patients and healthy controls, all recruited from the neurology department of Dongying People's Hospital. The age range participants among 60 to 80 years old. Enrollment in the study required participants to provide written informed consent and agree to the collection and testing of relevant biological samples.
Inclusion Criteria: Diagnosis of AD as per the International Working Group on AD guidelines; Age between 60 and 80 years; Documented disease course and neuroimaging examination results; Willingness to participate and provision of signed informed consent; Absence of other neurological diseases and severe cardiovascular conditions. Exclusion Criteria: Presence of other cognitive impairments, e.g., vascular dementia, Parkinson’s disease; Significant mental illness or severe depression; Severe cardiac, hepatic, or renal dysfunction; Current treatment with anti-inflammatory drugs, immunotherapy, or other medications that could influence study outcomes; Participation in other clinical trials, either currently or previously.
According to the inclusion and exclusion criteria, eligible participants were divided into two groups: the AD group and the control group, with 60 individuals in each. These two groups were matched on basic characteristics such as gender, age, and education level to minimize potential confounding factors.
Participants in the AD group will underwent serum markers collection and testing, which included measuring CXCL12, sCD22, and Lp-PLA2. The control group underwent the same procedure to establish a normal reference range.
The primary observation indicators were the serum levels of CXCL12, sCD22, and Lp-PLA2, as well as their ratios (such as CXCL12/sCD22, Lp-PLA2/sCD22). Collect 3 mL of fasting venous blood from all subjects, place it in EDTA anticoagulant tubes, stand for 30 min, centrifuge at 3000 rpm/min for 10 min, collect the upper serum, freeze it in liquid nitrogen, and wait for further detection. ELISA was used to determine the serum levels of CXCL12 (RHF225CK, Anti
Data analysis was performed using SPSS statistical software. For continuous variables, they are expressed as mean ± SD, and the independent sample t-test is utilized for between-group comparisons. Categorical data are expressed in percen
The study successfully enrolled 60 patients in each of the two groups: AD group and control group. Fundamental characteristics including age, gender, and education level were similar across both groups. No statistically significant diffe
| Feature | AD group (n = 60) | Control group (n = 60) | P value |
| Age (yr) | 73.5 ± 6.2 | 72.8 ± 5.9 | 0.452 |
| Gender (male/female) | 28/32 | 30/30 | 0.743 |
| Years of education | 11.4 ± 2.3 | 11.8 ± 2.1 | 0.321 |
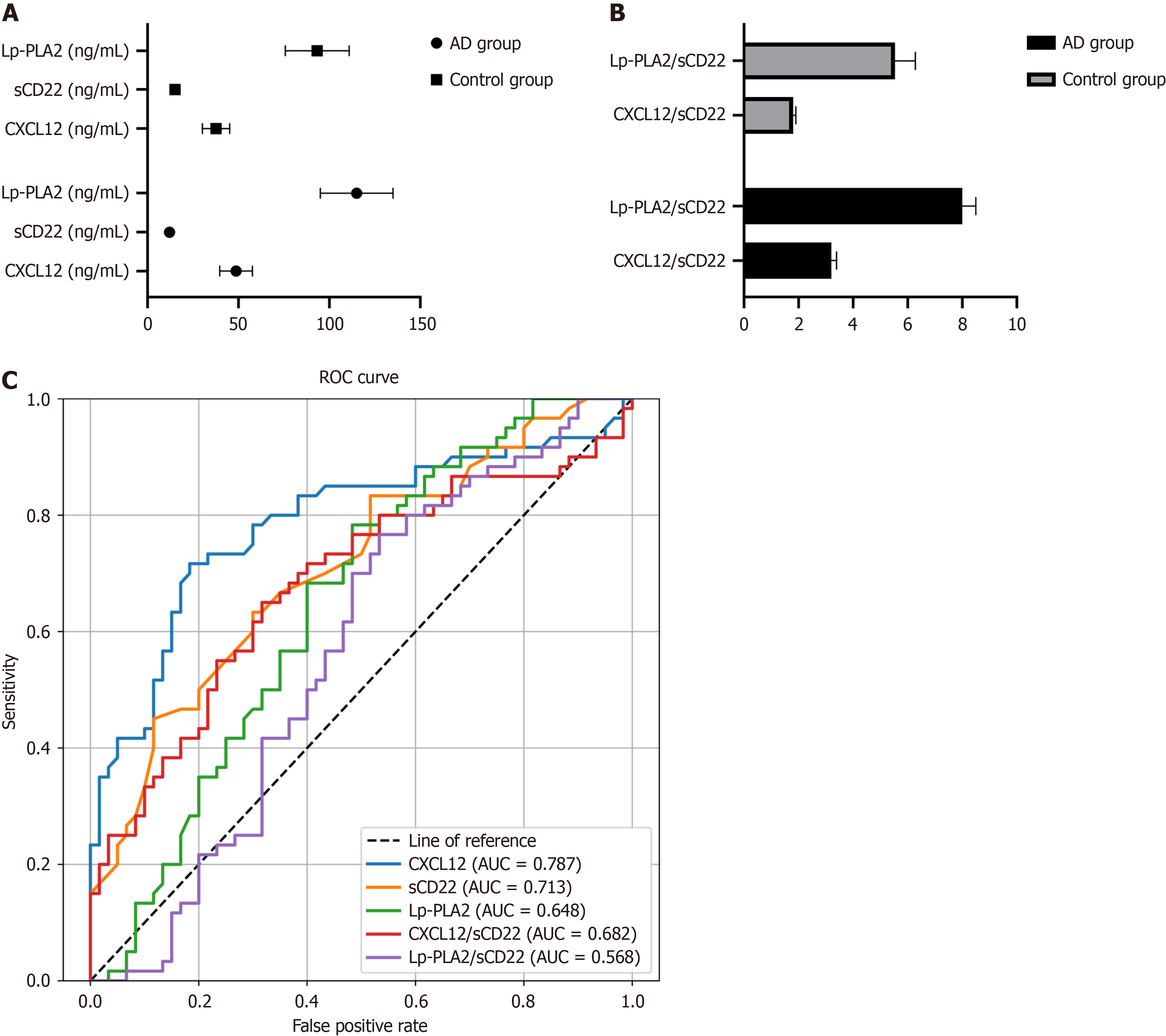
In the AD group, the average serum level of CXCL12 was 47.2 ± 8.5 ng/mL, significantly higher than the 32.8 ± 5.7 ng/mL observed in the control group (P < 0.001). Conversely, the sCD22 level in the AD group averaged 14.3 ± 2.1 ng/mL, significantly lower than 18.9 ± 3.4 ng/mL in the control group (P < 0.01). As for Lp-PLA2, the level in the AD group was 112.5 ± 20.6 ng/mL, which was significantly higher than the 89.7 ± 15.2 ng/mL in the control group (P < 0.05; Table 2, Figure 2A).
| Serum indicators | AD group (n = 60) | Control group (n = 60) | P value |
| CXCL12 (ng/mL) | 47.2 ± 8.5 | 32.8 ± 5.7 | < 0.001 |
| sCD22 (ng/mL) | 14.3 ± 2.1 | 18.9 ± 3.4 | < 0.01 |
| Lp-PLA2 (ng/mL) | 112.5 ± 20.6 | 89.7 ± 15.2 | < 0.05 |
Further analysis of the CXCL12/sCD22 and Lp-PLA2/sCD22 ratios revealed significant differences between the AD and control groups. In the AD group, the CXCL12/sCD22 ratio was 3.3 ± 0.6, notably higher than the control group's 1.7 ± 0.4 (P < 0.001). Similarly, the Lp-PLA2/sCD22 ratio in the AD group was 8.0 ± 1.2, compared to 5.2 ± 0.9 in the control group, demonstrating a statistically significant difference (P < 0.05; Table 3, Figure 2B).
| Ratio indicator | AD group (n = 60) | Control group (n = 60) | P value |
| CXCL12/sCD22 | 3.3 ± 0.6 | 1.7 ± 0.4 | < 0.001 |
| Lp-PLA2/sCD22 | 8.0 ± 1.2 | 5.2 ± 0.9 | < 0.05 |
ROC curve analysis was employed to evaluate the sensitivity and specificity of CXCL12, sCD22, Lp-PLA2 and their ratio in the diagnosis of AD. The analysis revealed that the AUC of CXCL12 was 0.787, that of sCD22 was 0.713, and for Lp-PLA2 was 0.648. The ratios of CXCL12/sCD22 and Lp-PLA2/sCD22 showed AUCs of 0.682 and 0.568, respectively. These findings indicate that these biomarkers and their ratios are highly sensitive and specific in differentiating AD patients from control subjects (Table 4, Figure 2C).
| Test result variable | AUC | Standard error | 95%CI |
| CXCL12 | 0.787 | 0.043 | 0.702-0.871 |
| sCD22 | 0.713 | 0.047 | 0.621-0.804 |
| LpPLA2 | 0.648 | 0.051 | 0.548-0.747 |
| CXCL12sCD22 | 0.682 | 0.049 | 0.586-0.779 |
| LpPLA2sCD22 | 0.568 | 0.054 | 0.463-0.672 |
Our research was conducted to investigate the diagnostic utility of serum CXCL12, sCD22, and Lp-PLA2 levels, as well as their ratios, in identifying AD. Through a thorough comparison of these serum markers between the AD group and a control group, it was observed that the levels of CXCL12, sCD22, Lp-PLA2, and their respective ratios hold considerable clinical relevance in diagnosing AD[24-28].
Here, we discovered notable variations in the levels of CXCL12, sCD22, and Lp-PLA2 in AD patients. Elevated CXCL12 levels in the AD group align with previous findings, indicating its significant role in AD's pathogenesis. CXCL12, a chemokine critical for immune cell migration and tissue repair[29], showed increased levels in AD patients, potentially linked to heightened neuroinflammation and immune responses. These findings support previous research that links increased levels of CXCL12 in the cerebrospinal fluid with cognitive decline in AD patients. On the other hand, sCD22, a soluble cell adhesion molecule, showed a notable decrease in the AD group. This trend could be due to heightened inflammation and a disturbance in immune regulation. Although the precise role of sCD22 in neurological disorders warrants further exploration, its varying levels in such diseases merit attention. Lp-PLA2, a phospholipase implicated in inflammation and atherosclerosis formation, was also found to be increased in AD patients, reflecting the inflammatory state and abnormal vascular functioning. However, the exact mechanisms behind these changes are still unclear, necessi
Furthermore, we extended our analysis to the ratios of CXCL12/sCD22 and Lp-PLA2/sCD22, discovering a signi
Our findings of this study indicate that serum CXCL12, sCD22, Lp-PLA2 and their ratios have potential clinical signi
Understanding Alzheimer's disease (AD) remains a challenge, and current diagnostic methods face many hurdles, ma
Our research is motivated by the urgent need to improve AD diagnosis through non-invasive methods. Given the in
To investigate the diagnostic potential of serum biomarkers CXCL12, sCD22, Lp-PLA2, and their ratios in AD. We aim to assess their effectiveness in enhancing early detection and informing targeted treatment strategies, thereby contributing to more precise and efficient management of AD.
Our study employed a prospective case-control design. It involved 60 AD patients and 60 healthy individuals (control group). The levels of serum biomarkers CXCL12, sCD22, and Lp-PLA2, along with their ratios, were measured using enzyme-linked immunosorbent assay kits. Statistical methods were applied to analyze the differences between the two groups. Additionally, we constructed specific biomarker ratios to enhance the specificity and sensitivity of AD diagnosis.
Serum CXCL12 and Lp-PLA2 levels were significantly higher in the AD group compared to the control group, while sCD22 levels were lower. Notable differences in the ratios of CXCL12/sCD22 and Lp-PLA2/sCD22, along with high sensitivity and specificity confirmed by ROC analysis, highlight their potential in distinguishing AD.
These biomarkers and their ratios serve as potential diagnostic indicators for AD, offering critical in
This research paves the way for advanced AD diagnosis through serum biomarkers, highlighting the potential for early detection and intervention. It underscores the importance of further exploring AD's pathophysiology for innovative treatment approaches.
| 1. | Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM. Alzheimer's disease. Lancet. 2021;397:1577-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1527] [Cited by in RCA: 3364] [Article Influence: 672.8] [Reference Citation Analysis (0)] |
| 2. | Tatulian SA. Challenges and hopes for Alzheimer's disease. Drug Discov Today. 2022;27:1027-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 187] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 3. | Walsh S, Merrick R, Milne R, Brayne C. Aducanumab for Alzheimer's disease? BMJ. 2021;374:n1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 4. | Jucker M, Walker LC. Alzheimer's disease: From immunotherapy to immunoprevention. Cell. 2023;186:4260-4270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 243] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 5. | Calderaro A, Patanè GT, Tellone E, Barreca D, Ficarra S, Misiti F, Laganà G. The Neuroprotective Potentiality of Flavonoids on Alzheimer's Disease. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 97] [Reference Citation Analysis (0)] |
| 6. | Whitwell JL. Alzheimer's disease neuroimaging. Curr Opin Neurol. 2018;31:396-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Kulczyńska-Przybik A, Dulewicz M, Doroszkiewicz J, Borawska R, Słowik A, Zetterberg H, Hanrieder J, Blennow K, Mroczko B. The Relationships between Cerebrospinal Fluid Glial (CXCL12, CX3CL, YKL-40) and Synaptic Biomarkers (Ng, NPTXR) in Early Alzheimer's Disease. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 8. | Gavriel Y, Rabinovich-Nikitin I, Solomon B. Inhibition of CXCR4/CXCL12 signaling: a translational perspective for Alzheimer's disease treatment. Neural Regen Res. 2022;17:108-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Laske C, Stellos K, Eschweiler GW, Leyhe T, Gawaz M. Decreased CXCL12 (SDF-1) plasma levels in early Alzheimer's disease: a contribution to a deficient hematopoietic brain support? J Alzheimers Dis. 2008;15:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Tulbă D, Cozma L, Popescu BO, Davidescu EI. Dysautonomia in Alzheimer's Disease. Medicina (Kaunas). 2020;56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Kosyreva AM, Sentyabreva AV, Tsvetkov IS, Makarova OV. Alzheimer's Disease and Inflammaging. Brain Sci. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 12. | Canevelli M, Vanacore N, Blasimme A, Bruno G, Cesari M. Overtreating Alzheimer's Disease. J Prev Alzheimers Dis. 2021;8:234-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Cervellati C, Zuliani G. Frontier on Alzheimer's Disease. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 14. | Qu L, Liu F, Fang Y, Wang L, Chen H, Yang Q, Dong H, Jin L, Wu W, Sun D. Improvement in Zebrafish with Diabetes and Alzheimer's Disease Treated with Pasteurized Akkermansia muciniphila. Microbiol Spectr. 2023;11:e0084923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Wang L, Liu F, Fang Y, Ma J, Wang J, Qu L, Yang Q, Wu W, Jin L, Sun D. Advances in Zebrafish as a Comprehensive Model of Mental Disorders. Depression Anxiety. 2023;2023:e6663141. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Nicholls RE, Sontag JM, Zhang H, Staniszewski A, Yan S, Kim CY, Yim M, Woodruff CM, Arning E, Wasek B, Yin D, Bottiglieri T, Sontag E, Kandel ER, Arancio O. PP2A methylation controls sensitivity and resistance to β-amyloid-induced cognitive and electrophysiological impairments. Proc Natl Acad Sci U S A. 2016;113:3347-3352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Wang JZ. Alzheimer's disease: from molecule to clinic. Neurosci Bull. 2014;30:169-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Verkhratsky A, Parpura V, Rodriguez-Arellano JJ, Zorec R. Astroglia in Alzheimer's Disease. Adv Exp Med Biol. 2019;1175:273-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Nguyen KV. Special Issue: Alzheimer's disease. AIMS Neurosci. 2018;5:74-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Ono K. Alzheimer's disease as oligomeropathy. Neurochem Int. 2018;119:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | Ferreira D, Wahlund LO, Westman E. The heterogeneity within Alzheimer's disease. Aging (Albany NY). 2018;10:3058-3060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Button EB, Robert J, Caffrey TM, Fan J, Zhao W, Wellington CL. HDL from an Alzheimer's disease perspective. Curr Opin Lipidol. 2019;30:224-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 23. | Clark EA, Giltiay NV. CD22: A Regulator of Innate and Adaptive B Cell Responses and Autoimmunity. Front Immunol. 2018;9:2235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 24. | Maoz R, Garfinkel BP, Soreq H. Alzheimer's Disease and ncRNAs. Adv Exp Med Biol. 2017;978:337-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Zhao S, Zhang L, Yang C, Li Z, Rong S. Procyanidins and Alzheimer's Disease. Mol Neurobiol. 2019;56:5556-5567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Falsetti L. Molecular Research on Alzheimer's Disease. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Goldberg RJ. Alzheimer's disease. Compr Ther. 2007;33:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Loprinzi PD, Frith E, Ponce P. Memorcise and Alzheimer's disease. Phys Sportsmed. 2018;46:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Hwang S, Zimmerman NP, Agle KA, Turner JR, Kumar SN, Dwinell MB. E-cadherin is critical for collective sheet migration and is regulated by the chemokine CXCL12 protein during restitution. J Biol Chem. 2012;287:22227-22240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Soreq L, United Kingdom S-Editor: Lin C L-Editor: A P-Editor: Xu ZH