Published online Mar 19, 2024. doi: 10.5498/wjp.v14.i3.370
Peer-review started: October 18, 2023
First decision: December 6, 2023
Revised: December 21, 2023
Accepted: January 15, 2024
Article in press: January 15, 2024
Published online: March 19, 2024
Processing time: 153 Days and 1.1 Hours
Dexmedetomidine and propofol are two sedatives used for long-term sedation. It remains unclear whether dexmedetomidine provides superior cerebral protection for patients undergoing long-term mechanical ventilation.
To compare the neuroprotective effects of dexmedetomidine and propofol for sedation during prolonged mechanical ventilation in patients without brain injury.
Patients who underwent mechanical ventilation for > 72 h were randomly assi
A total of 52 and 63 patients were allocated to the dexmedetomidine group and propofol group, respectively. Baseline data were comparable between groups. No significant differences were identified between groups within the median duration of study drug infusion [52.0 (IQR: 36.0-73.5) h vs 53.0 (IQR: 37.0-72.0) h, P = 0.958], the median dose of remifentanil [4.5 (IQR: 4.0-5.0) μg/kg/h vs 4.6 (IQR: 4.0-5.0) μg/kg/h, P = 0.395], the median percentage of time in the target RASS range without rescue sedation [85.6% (IQR: 65.8%-96.6%) vs 86.7% (IQR: 72.3%-95.3), P = 0.592], and the median frequency within the target RASS range without rescue sedation [72.2% (60.8%-91.7%) vs 73.3% (60.0%-100.0%), P = 0.880]. The proportion of patients in the dexmedetomidine group who required rescue sedation was higher than in the propofol group with statistical significance (69.2% vs 50.8%, P = 0.045). Serum S100-β and NSE levels in the propofol group were higher than in the dexmedetomidine group with statistical significance during the first six and five days of mechanical ventilation, respectively (all P < 0.05).
Dexmedetomidine demonstrated stronger protective effects on the brain compared to propofol for long-term mechanical ventilation in patients without brain injury.
Core Tip: In this study, we designed a single center, prospective, randomized controlled study to compare the brain protective effect of dexmedetomidine vs propofol for sedation during prolonged mechanical ventilation in non-brain injured patients.
- Citation: Yuan HX, Zhang LN, Li G, Qiao L. Brain protective effect of dexmedetomidine vs propofol for sedation during prolonged mechanical ventilation in non-brain injured patients. World J Psychiatry 2024; 14(3): 370-379
- URL: https://www.wjgnet.com/2220-3206/full/v14/i3/370.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i3.370
Patients who require intensive care may experience a strong stress response due to their own serious illness, leading to long-term negative emotions such as anxiety and irritability. In addition, most of these patients also necessitate mech
Dexmedetomidine and propofol are two sedatives used for long-term sedation[4]. Dexmedetomidine, an adrenergic receptor agonist, possesses analgesic, sedative, and inhibitory effects on sympathetic nervous activity[5,6], contributing to enhanced patient safety and comfort during long-term sedation[5,6]. Previous studies have demonstrated that compared to propofol or midazolam, dexmedetomidine can reduce the incidence of coma and delirium, as well as decrease mechanical ventilation time in ICU patients[6,7]. A multicenter randomized controlled trial from Europe revea
In this study, we designed a single-center, prospective, randomized controlled study to compare the brain-protective effects of dexmedetomidine versus propofol for sedation during prolonged mechanical ventilation in non-brain-injured patients.
This single-center, prospective, randomized controlled study was approved by the Ethics Committee of Peking University International Hospital (Approval No. 2021-KY-0037-01). Patients or their legal representatives signed an agreement to voluntarily participate in the present study.
The inclusion criteria of patients included: (1) Age ≥ 18 years and ≤ 75 years; (2) mechanical ventilation time ≥ 72 h and sedation time ≥ 24 h; and (3) patients without brain injuries.
Exclusion criteria: (1) Body mass index (BMI) < 18 kg/m2 or > 30 kg/m2; (2) acute severe neurological disorders; (3) brain injury, including head trauma, cerebral hemorrhage, cerebral infarction, and neurosurgery; and (4) acute hepatitis or serious hepatic dysfunction (Child-Pugh class C); (5) chronic kidney disease with glomerular filtration rate < 60 mL/min/1.73 m2; (6) alcohol consumption or drug addiction; (7) myasthenia gravis, pregnancy or lactation, study drug alle
Eligible patients received sedative drugs by doctors who were blind to the research details. The patients were unaware of the sedative medications administered as well.
All patients received analgesia at a dosage ranging from 4.0 to 9.0 μg/kg/h. Patients in the dexmedetomidine group received dexmedetomidine hydrochloride injection (0.1-1.2 μg/kg/h) (H20183219, Yangzijiang Pharmaceutical Group Co., Ltd, China) for sedation, while patients in the propofol group were given propofol medium long chain fat emulsion injection (0.3-4.0 mg/kg/h) (HJ20150655, Beijing Feisenyuskabi Pharmaceutical Co., Ltd, China) for sedation.
Serum S100-β and neuron-specific enolase (NSE) levels were measured to assess brain function. Briefly, venous blood was collected every 24 h during mechanical ventilation, followed by centrifugation (1000 × g, room temperature, 10 min) to separate the serum. The central laboratory detects serum S100-β and NSE levels using enzyme-linked immunosorbent assay.
The secondary outcomes included the remifentanil dosage, the proportion of patients receiving rescue sedation, and the time and frequency of Richmond Agitation Sedation Scale (RASS) within the target range. Briefly, patients eventually included in the analysis recorded the dose of remifentanil used during the study. If a patient’s RASS score was above the target range (-3 to 0) and required rescue sedation, the patient was recorded as requiring rescue sedation. RASS scores were assessed every 4 h prior to any administration of rescue therapy.
Due to a lack of assumptions, sample size estimation was not conducted in this study. Data were collected using an Excel table and analyzed by SPSS 25.0 (IBM, United States). Continuous data were presented as median and interquartile range (IQR). Differences between groups were compared utilizing Student’s t-test or the Mann-Whitney U test, based on the results of the Kolmogorov-Smirnov test. Count data were expressed as percentages (%), and differences between groups were compared utilizing the chi-square test or Fisher’s exact test. Statistical significance was set at P < 0.05.
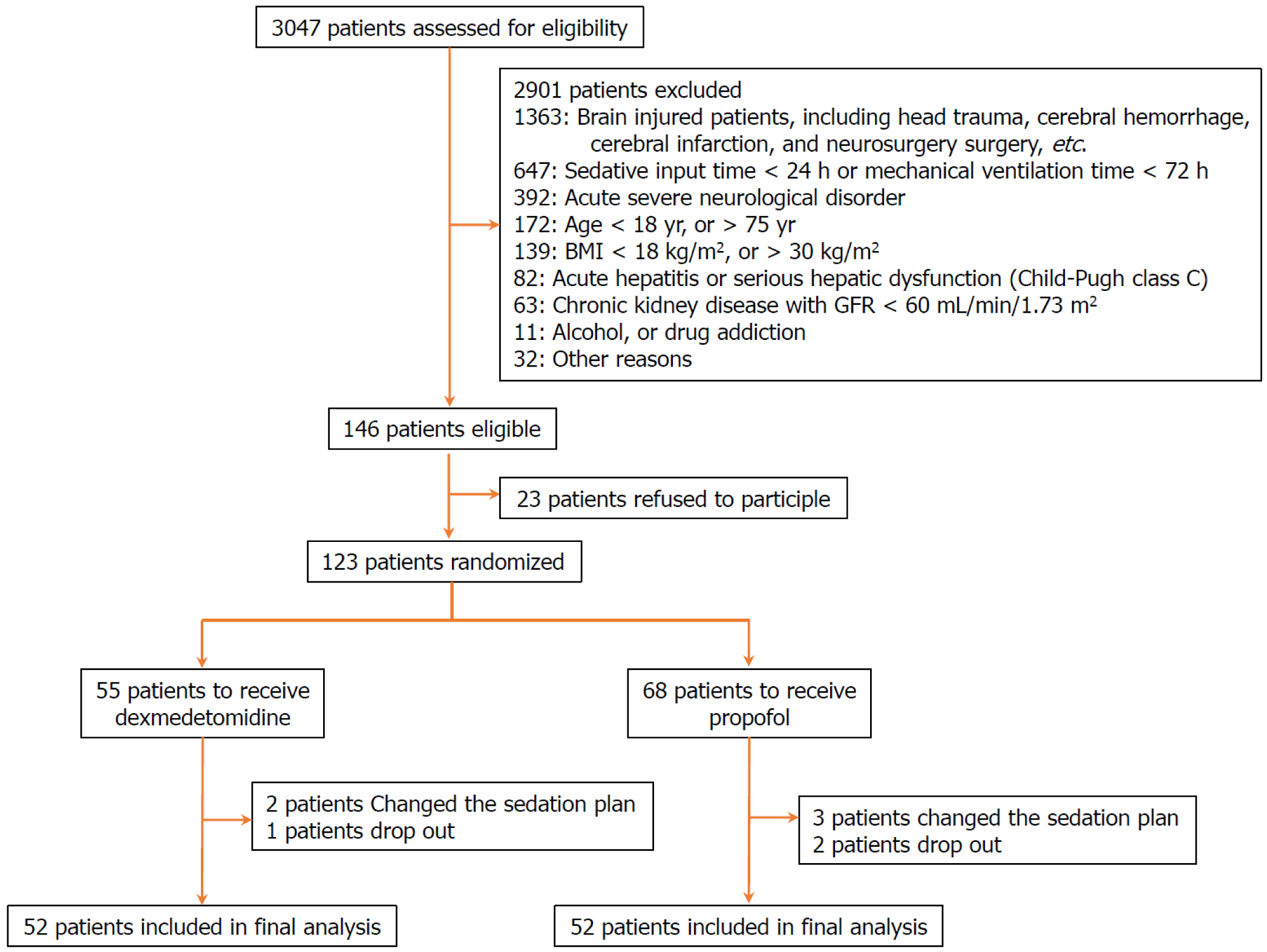
We screened 3047 ICU patients and ultimately included 115 patients in the final analysis: 52 in the dexmedetomidine group and 63 in the propofol group (Figure 1). Their median age was 61.0 years (IQR: 54.00-65.00), with 69 male patients (60.0%) and a median BMI of 21.32 kg/m2 (IQR: 19.35-22.98). No significant differences were observed in the baseline clinical characteristics between groups, such as the SAPS II score, the main reason for ICU admission, infection at ICU admission, SOFA score of organs (including respiratory, cardiovascular, renal, coagulation, and liver), total SOFA score, RASS score at enrollment, and time from ICU admission to drug initiation (Table 1).
| Dexmedetomidine (n = 52) | Propofol (n = 63) | P value | |
| Age (yr), median (IQR) | 61.0 (55.0-64.0) | 61.0 (53.0-66.0) | 0.663 |
| Male | 30 (57.7) | 39 (61.9) | 0.646 |
| BMI (kg/m2), median (IQR) | 21.8 (19.6-24.3) | 21.1 (19.0-22.3) | 0.191 |
| SAPS II, median (IQR) | 46.0 (38.0-54.0) | 46.0 (36.0-53.0) | 0.675 |
| Main reason for ICU | |||
| Medical | 37 (71.2) | 44 (69.9) | 0.983 |
| Surgical | 10 (19.2) | 13 (20.6) | |
| Trauma | 5 (9.6) | 6 (9.5) | |
| Infection at ICU admission | 24 (46.2) | 30 (47.6) | 0.875 |
| SOFA score of organ > 2 | |||
| Respiratory | 30 (57.7) | 35 (55.6) | 0.818 |
| Cardiovascular | 26 (50.0) | 27 (42.9) | 0.444 |
| Renal | 8 (15.4) | 10 (15.9) | 0.943 |
| Coagulation | 4 (7.7) | 6 (9.5) | 0.729 |
| Liver | 1 (1.9) | 1 (1.6) | 0.891 |
| Total SOFA score, median (IQR) | 7.0 (4.0-9.0) | 6.0 (3.0-9.0) | 0.954 |
| RASS score at enrollment, median (IQR) | -2 (-3 to -1) | -3 (-3 to -1) | 0.247 |
| Time from ICU admission to drug initiation (h), median (IQR) | 32.0 (20.0-35.0) | 31.0 (20.0-42.0) | 0.798 |
The median infusion time of dexmedetomidine in the dexmedetomidine group was 52.0 (IQR: 36.0-73.5) hours, and the median infusion time of propofol in the propofol group was 53.0 (IQR: 37.0-72.0) hours, with no significant difference between groups (P = 0.958) (Table 2). Meanwhile, there was also no significant difference in the dose of remifentanil between groups (P = 0.395). However, the proportion of patients undergoing rescue sedation in the dexmedetomidine group was significantly higher in contrast with that in the propofol group (69.2% vs 50.8%, P = 0.045, Table 2).
| Dexmedetomidine (n = 52) | Propofol (n = 63) | P value | |
| Duration of study drug infusion (h), median (IQR) | 52.0 (36.0-73.5) | 53.0 (37.0-72.0) | 0.958 |
| Dose of study drug (μg or mg/kg/h), median (IQR) | 0.58 (0.34-0.79) | 0.82 (0.65-1.32) | - |
| Dose of remifentanil (μg/kg/h), median (IQR) | 4.5 (4.0-5.0) | 4.6 (4.0-5.0) | 0.395 |
| Receiving rescue sedation, n (%) | 36.0 (69.2) | 32.0 (50.8) | 0.045 |
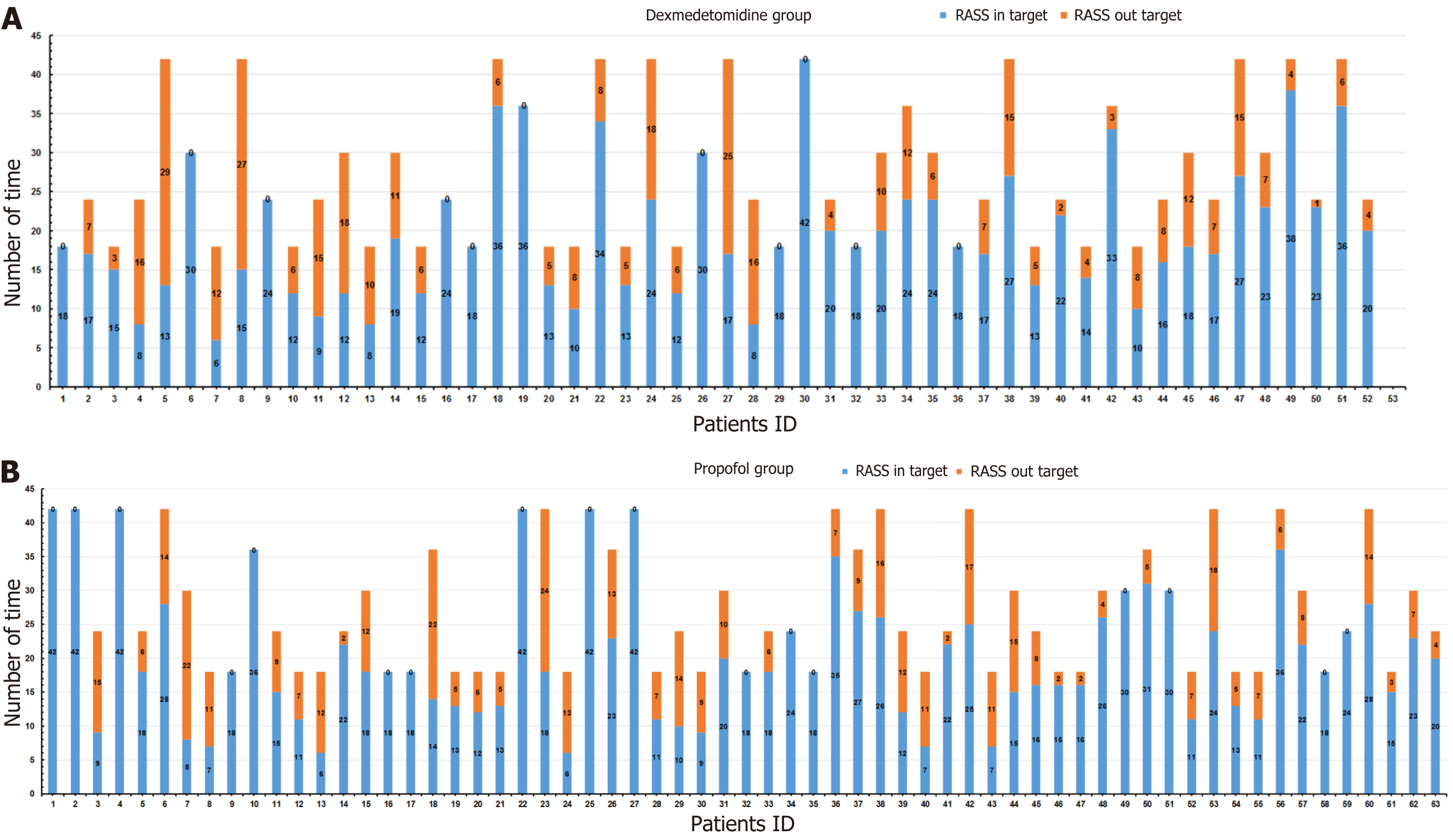
During the absence of rescue sedation, the median percentage of time within the target RASS in the dexmedetomidine group was similar to the propofol group [85.6% (IQR: 65.8%-96.6%) vs 86.7% (IQR: 72.3%-95.3%), P = 0.592] (Table 3). Patients in the dexmedetomidine group underwent 1428 RASS evaluations, with 1031 (72.2%) reaching the target RASS range (-3 to 0) (Figure 2A), and patients in the propofol group underwent a total of 1740 RASS evaluations, with 1297 (74.5%) patients in the target RASS range (Figure 2B). The median percentage of the target RASS score in the dexme
| Dexmedetomidine (n = 52) | Propofol (n = 63) | P value | |
| Percentage of time within the target RASS (%), median (IQR) | 85.6 (65.8-96.6) | 86.7 (72.3-95.3) | 0.592 |
| Percentage of target RASS score (%), median (IQR) | 72.2 (60.8-91.7) | 73.3 (60.0-100.0) | 0.880 |
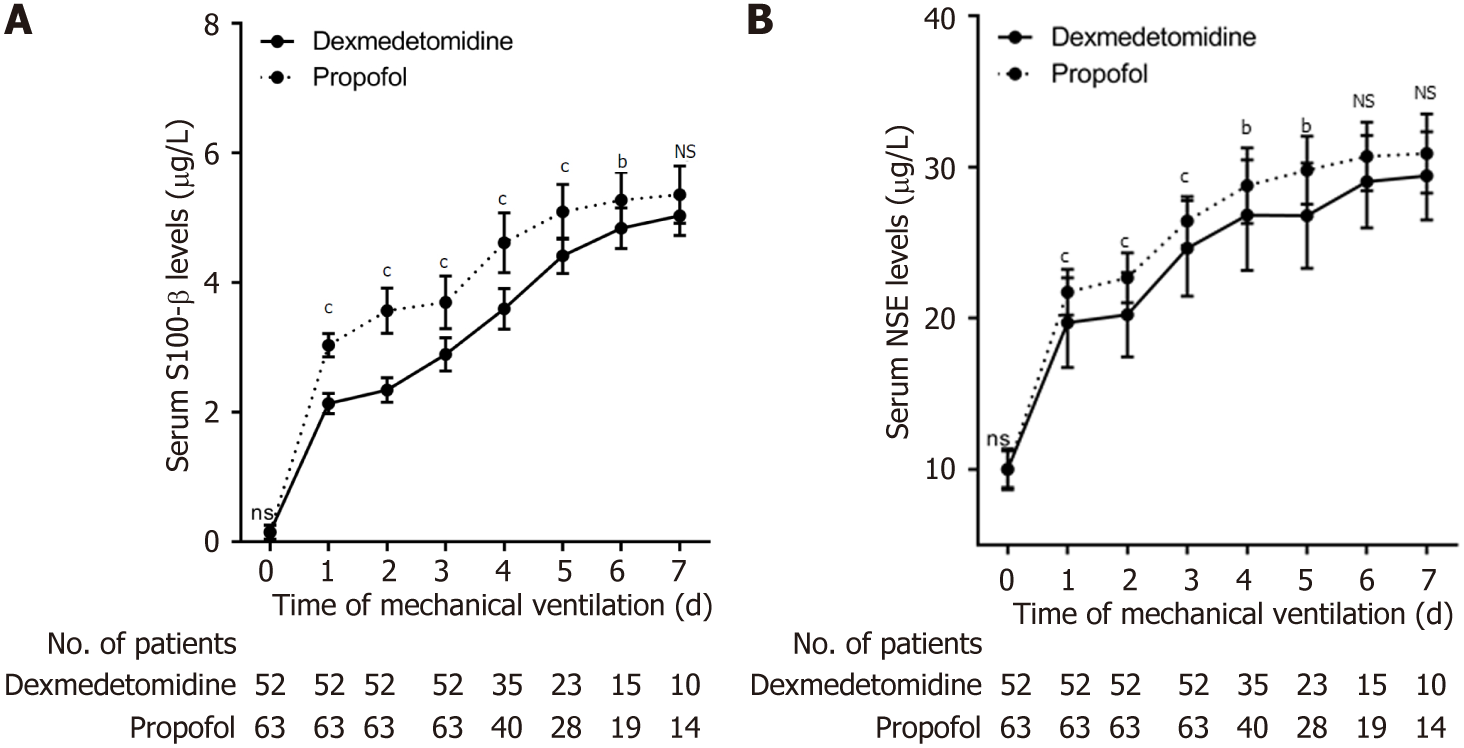
Starting with mechanical ventilation, sedation, and analgesia, we evaluated the brain function of all patients every 24 h by measuring serum S100-β and NSE levels. Serum S100-β levels in patients in the propofol group were higher in contrast with those in the dexmedetomidine group during the first 7 d of mechanical ventilation and were significantly higher from day 1 to day 6, with no significant difference on day 7 (Table 4, Figure 3A). The levels of serum NSE in patients in the propofol group were also higher in contrast with those in the dexmedetomidine group during the first 7 d of mechanical ventilation and were significantly higher from day 1 to day 5, with no significant difference from day 6 to day 7 (Table 5, Figure 3B).
| Time | Dexmedetomidine | Propofol | P value | ||
| n | S100-β | n | S100-β | ||
| Day 0 | 52 | 0.12 (0.06-0.18) | 63 | 0.14 (0.08-0.23) | 0.4080 |
| Day 1 | 52 | 2.12 (2.03-2.22) | 63 | 3.02 (2.92-3.18) | < 0.001 |
| Day 2 | 52 | 2.30 (2.18-2.48) | 63 | 3.53 (3.32-3.85) | < 0.001 |
| Day 3 | 52 | 2.88 (2.67-3.05) | 63 | 3.62 (3.39-4.06) | < 0.001 |
| Day 4 | 35 | 3.58 (3.36-3.85) | 40 | 4.70 (4.35-4.97) | < 0.001 |
| Day 5 | 22 | 4.46 (4.34-4.58) | 28 | 4.98 (4.86-5.44) | < 0.001 |
| Day 6 | 15 | 4.83 (4.68-5.03) | 19 | 5.33 (4.98-5.65) | 0.0026 |
| Day 7 | 10 | 5.06 (4.81-5.32) | 14 | 5.38 (5.19-5.67) | 0.0562 |
| Time | Dexmedetomidine | Propofol | P value | ||
| n | NSE | n | NSE | ||
| Day 0 | 52 | 9.95 (9.08-10.65) | 63 | 9.86 (9.35-10.56) | 0.9570 |
| Day 1 | 52 | 20.09 (17.63-21.43) | 63 | 21.42 (20.71-23.08) | < 0.001 |
| Day 2 | 52 | 20.35 (17.96-21.50) | 63 | 22.35 (21.38-23.92) | < 0.001 |
| Day 3 | 52 | 24.89 (21.87-26.85) | 63 | 26.25 (25.15-27.35) | < 0.001 |
| Day 4 | 35 | 26.62 (23.43-29.35) | 40 | 29.17 (26.61-31.14) | 0.0082 |
| Day 5 | 22 | 26.75 (24.93-29.37) | 28 | 29.66 (27.72-31.14) | 0.0047 |
| Day 6 | 15 | 28.93 (26.35-30.52) | 19 | 30.72 (28.65-31.98) | 0.0774 |
| Day 7 | 10 | 28.34 (26.95-31.23) | 14 | 30.54 (28.90-32.46) | 0.2060 |
In this study, we initially observed that the sedative effects of dexmedetomidine and propofol during prolonged mechanical ventilation in patients without brain injury were similar. There were no significant differences in remifentanil dosage, RASS target range time ratio, and frequency. However, it is important to note that the proportion of patients in the dexmedetomidine group requiring rescue sedation was significantly higher than that in the propofol group. These research results were in accordance with previous studies; for instance, Jakob et al[4] found that the dexmedetomidine/propofol ratio in time at target sedation was 1.00 (95% confidence interval: 0.92-1.08), and the proportion of patients undergoing rescue sedation in the dexmedetomidine group was significantly higher in contrast with that in the propofol group (72.5% vs 64.4%, P = 0.05).
In addition, we found some unreported results: Serum S100-β and NSE levels in the propofol group were higher in contrast with those in the dexmedetomidine group during prolonged mechanical ventilation in patients without brain injury. As a marker of glial cells, S100-β protein is a calcium-binding protein mainly present in mature perivascular astrocytes. It is primarily found in glial cells and Schwann cells, released from the cytoplasm into the cerebrospinal fluid after central nervous system cell injury, and then enters the bloodstream via the damaged blood-brain barrier[12,13]. NSE represents a marker enzyme for neuronal damage and is a key enzyme in the glycolytic pathway. It is specifically localized within neurons and predominantly exists in the cytoplasm of brain nerve cells as well as neuroendocrine cells[14,15]. The content of NSE in body fluids is very low under normal circumstances, but a large amount of NSE quickly leaks out of damaged neurons in the case of nerve cell damage and passes through the blood-brain barrier, entering the cerebrospinal fluid and bloodstream[16,17]. Therefore, serum S100-β and NSE levels can be utilized to evaluate the degree of brain injury, particularly the brain-protective effects of anesthetic drugs in non-cerebral injury[18,19].
We observed that serum levels of S100-β (first 6 d) as well as NSE (first 5 d) in the propofol group were obviously higher in contrast with those in the dexmedetomidine group during the early stage of mechanical ventilation and sedation. However, as the 7-d mechanical ventilation observation period progressed, although these levels remained higher in the propofol group compared to the dexmedetomidine group, the difference was not statistically significant. Therefore, our results indicate that dexmedetomidine has a stronger brain protective effect in the early stages of prolonged mechanical ventilation and sedation compared to propofol in patients. Studies have demonstrated that dexmedetomidine are neuroprotective based on various pathways, including binding to α2-adrenal receptor subtype binding[20], reducing the brain metabolic rate[21,22], curtailing excitatory amino acid release[23], mitigating intracellular calcium overload[24], and regulating apoptotic protein expression to inhibit neuronal apoptosis[25,26]. On one hand, uncontrolled inflammation is the main cause of neuronal apoptosis/necrosis, and dexmedetomidine has been proven to exert anti-inflammatory effects by inhibiting the production of pro-inflammatory factors and microglial M1 phenotype, inhibiting neuroinflammation, and protecting neurons from apoptosis caused by inflammatory factors[27,28]. On the other hand, dexmedetomidine can inhibit oxidative stress and cell apoptosis by regulating the NRF2/ARE pathway and Trx1 dependent Akt pathway. Dexmedetomidine can also eliminate excess oxygen free radicals in the body by reducing the content of malondialdehyde and reactive oxygen species, increasing the activity of superoxide dismutase, and alleviating the damage caused by the chain reaction caused by oxygen free radicals, It has a protective effect on oxidative stress and neuronal apoptosis triggered by ischemia-reperfusion injury[29,30]. Moreover, our results suggested that the brain-protective effect of dexmedetomidine was not markedly superior to that of propofol in the later stages of mechanical ventilation and sedation. However, given that only a small number of patients (10 in the dexmedetomidine group and 14 in the propofol group) completed the full 7-d mechanical ventilation, we believe that the findings regarding the brain protective effect in the later stage of mechanical ventilation and sedation may be biased.
There were several limitations in this study. Firstly, as a single-center randomized controlled study, its generalizability is limited, and the results require further validation with a larger sample size from multiple centers. Secondly, hundreds of nursing staff members randomly participated in the care of all patients, eliminating the impact of nursing practices. Lastly, due to the distinct nature of propofol, patient allocation was not blinded to healthcare professionals.
Overall, dexmedetomidine exhibited stronger protective effects on the brain than propofol for long-term mechanical ventilation in patients without brain injury.
Dexmedetomidine and propofol are two sedatives used for long-term sedation. It remains unclear whether dexme
In this study, we designed a single-center, prospective, randomized controlled study to compare the brain-protective effects of dexmedetomidine versus propofol for sedation during prolonged mechanical ventilation in non-brain-injured patients.
To compare the neuroprotective effects of dexmedetomidine and propofol for sedation during prolonged mechanical ventilation in patients without brain injury.
Patients who underwent mechanical ventilation for > 72 h were randomly assigned to receive sedation with dexme
The sedative effects of dexmedetomidine and propofol during prolonged mechanical ventilation in patients without brain injury were similar. Serum S100-β and NSE levels in the propofol group were higher in contrast with those in the dexmedetomidine group during prolonged mechanical ventilation in patients without brain injury. Serum levels of S100-β (first 6 d) as well as NSE (first 5 d) levels in the propofol group were obviously higher in contrast with those in the dexmedetomidine group during the early stage of mechanical ventilation and sedation.
Dexmedetomidine exhibited stronger protective effects on the brain than propofol for long-term mechanical ventilation in patients without brain injury.
We believe that the findings regarding the brain protective effect in the later stage of mechanical ventilation and sedation may be biased.
| 1. | Jacobs JM, Marcus EL, Stessman J. Prolonged Mechanical Ventilation: Symptomatology, Well-Being, and Attitudes to Life. J Am Med Dir Assoc. 2021;22:1242-1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Tetiker S, Türktan M, Esquinas AM. Predictors of survival after prolonged weaning from mechanical ventilation. J Crit Care. 2021;63:269. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Pearson SD, Patel BK. Evolving targets for sedation during mechanical ventilation. Curr Opin Crit Care. 2020;26:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, Bratty JR, Takala J; Dexmedetomidine for Long-Term Sedation Investigators. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307:1151-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 666] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 5. | Ojha S, Abramson J, Dorling J. Sedation and analgesia from prolonged pain and stress during mechanical ventilation in preterm infants: is dexmedetomidine an alternative to current practice? BMJ Paediatr Open. 2022;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW, Rocha MG; SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1146] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 7. | Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, Stiles RA, Dittus RS, Bernard GR, Ely EW. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644-2653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1014] [Cited by in RCA: 1003] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 8. | Goettel N, Bharadwaj S, Venkatraghavan L, Mehta J, Bernstein M, Manninen PH. Dexmedetomidine vs propofol-remifentanil conscious sedation for awake craniotomy: a prospective randomized controlled trial. Br J Anaesth. 2016;116:811-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Li Y, Wang C, Bi M, Gao J, Zhang X, Tian H. Effect of dexmedetomidine on brain function and hemodynamics in patients undergoing lung cancer resection. Oncol Lett. 2020;20:1077-1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Chen Z, Ding Y, Zeng Y, Zhang XP, Chen JY. Dexmedetomidine reduces propofol-induced hippocampal neuron injury by modulating the miR-377-5p/Arc pathway. BMC Pharmacol Toxicol. 2022;23:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Lv J, Wei Y, Chen Y, Zhang X, Gong Z, Jiang Y, Gong Q, Zhou L, Wang H, Xie Y. Dexmedetomidine attenuates propofol-induce neuroapoptosis partly via the activation of the PI3k/Akt/GSK3β pathway in the hippocampus of neonatal rats. Environ Toxicol Pharmacol. 2017;52:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Kanner AA, Marchi N, Fazio V, Mayberg MR, Koltz MT, Siomin V, Stevens GH, Masaryk T, Aumayr B, Vogelbaum MA, Barnett GH, Janigro D. Serum S100beta: a noninvasive marker of blood-brain barrier function and brain lesions. Cancer. 2003;97:2806-2813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 234] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 13. | Kato Y, Yoshida S, Kato T. New insights into the role and origin of pituitary S100β-positive cells. Cell Tissue Res. 2021;386:227-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Hajduková L, Sobek O, Prchalová D, Bílková Z, Koudelková M, Lukášková J, Matuchová I. Biomarkers of Brain Damage: S100B and NSE Concentrations in Cerebrospinal Fluid--A Normative Study. Biomed Res Int. 2015;2015:379071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 15. | Arnason S, Molewijk K, Henningsson AJ, Tjernberg I, Skogman BH. Brain damage markers neuron-specific enolase (NSE) and S100B in serum in children with Lyme neuroborreliosis-detection and evaluation as prognostic biomarkers for clinical outcome. Eur J Clin Microbiol Infect Dis. 2022;41:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Barbu M, Jónsson K, Zetterberg H, Blennow K, Kolsrud O, Ricksten SE, Dellgren G, Björk K, Jeppsson A. Serum biomarkers of brain injury after uncomplicated cardiac surgery: Secondary analysis from a randomized trial. Acta Anaesthesiol Scand. 2022;66:447-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Lindblad C, Nelson DW, Zeiler FA, Ercole A, Ghatan PH, von Horn H, Risling M, Svensson M, Agoston DV, Bellander BM, Thelin EP. Influence of Blood-Brain Barrier Integrity on Brain Protein Biomarker Clearance in Severe Traumatic Brain Injury: A Longitudinal Prospective Study. J Neurotrauma. 2020;37:1381-1391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Andropoulos DB. Effect of Anesthesia on the Developing Brain: Infant and Fetus. Fetal Diagn Ther. 2018;43:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 19. | Gong J, Zhang R, Shen L, Xie Y, Li X. The brain protective effect of dexmedetomidine during surgery for paediatric patients with congenital heart disease. J Int Med Res. 2019;47:1677-1684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Ma D, Hossain M, Rajakumaraswamy N, Arshad M, Sanders RD, Franks NP, Maze M. Dexmedetomidine produces its neuroprotective effect via the alpha 2A-adrenoceptor subtype. Eur J Pharmacol. 2004;502:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 213] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Tang Y, Liu J, Huang X, Ding H, Tan S, Zhu Y. Effect of Dexmedetomidine-Assisted Intravenous Inhalation Combined Anesthesia on Cerebral Oxygen Metabolism and Serum Th1/Th2 Level in Elderly Colorectal Cancer Patients. Front Surg. 2021;8:832646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 22. | Laaksonen L, Kallioinen M, Långsjö J, Laitio T, Scheinin A, Scheinin J, Kaisti K, Maksimow A, Kallionpää RE, Rajala V, Johansson J, Kantonen O, Nyman M, Sirén S, Valli K, Revonsuo A, Solin O, Vahlberg T, Alkire M, Scheinin H. Comparative effects of dexmedetomidine, propofol, sevoflurane, and S-ketamine on regional cerebral glucose metabolism in humans: a positron emission tomography study. Br J Anaesth. 2018;121:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Lin S, Zhou G, Shao W, Fu Z. Impact of dexmedetomidine on amino acid contents and the cerebral ultrastructure of rats with cerebral ischemia-reperfusion injury. Acta Cir Bras. 2017;32:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Ok SH, Bae SI, Shim HS, Sohn JT. Dexmedetomidine-induced contraction of isolated rat aorta is dependent on extracellular calcium concentration. Korean J Anesthesiol. 2012;63:253-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Unchiti K, Leurcharusmee P, Samerchua A, Pipanmekaporn T, Chattipakorn N, Chattipakorn SC. The potential role of dexmedetomidine on neuroprotection and its possible mechanisms: Evidence from in vitro and in vivo studies. Eur J Neurosci. 2021;54:7006-7047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 26. | Liaquat Z, Xu X, Zilundu PLM, Fu R, Zhou L. The Current Role of Dexmedetomidine as Neuroprotective Agent: An Updated Review. Brain Sci. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (1)] |
| 27. | Qiu Z, Lu P, Wang K, Zhao X, Li Q, Wen J, Zhang H, Li R, Wei H, Lv Y, Zhang S, Zhang P. Dexmedetomidine Inhibits Neuroinflammation by Altering Microglial M1/M2 Polarization Through MAPK/ERK Pathway. Neurochem Res. 2020;45:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 28. | Sun Z, Lin Y, Li Y, Ren T, Du G, Wang J, Jin X, Yang LC. The effect of dexmedetomidine on inflammatory inhibition and microglial polarization in BV-2 cells. Neurol Res. 2018;40:838-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Xu D, Zhou C, Lin J, Cai W, Lin W. Dexmedetomidine provides protection to neurons against OGD/R-induced oxidative stress and neuronal apoptosis. Toxicol Mech Methods. 2021;31:374-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Wu ZL, Davis JRJ, Zhu Y. Dexmedetomidine Protects against Myocardial Ischemia/Reperfusion Injury by Ameliorating Oxidative Stress and Cell Apoptosis through the Trx1-Dependent Akt Pathway. Biomed Res Int. 2020;2020:8979270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Amornyotin S, Thailand S-Editor: Chen YL L-Editor: A P-Editor: Zhang YL