Published online Oct 19, 2023. doi: 10.5498/wjp.v13.i10.784
Peer-review started: June 5, 2023
First decision: July 4, 2023
Revised: July 15, 2023
Accepted: August 7, 2023
Article in press: August 7, 2023
Published online: October 19, 2023
Processing time: 128 Days and 17.8 Hours
Depression is a common mental disorder among college students. The main symptoms include being persistent low mood, sad emotional experiences, lack of pleasure, listlessness, and impaired cognitive function accompanied by tendencies of self-harm and suicide.
To clarify the pathways and effects of the behavioral activation system between physical activity and depressive symptoms in college students with depressive symptoms.
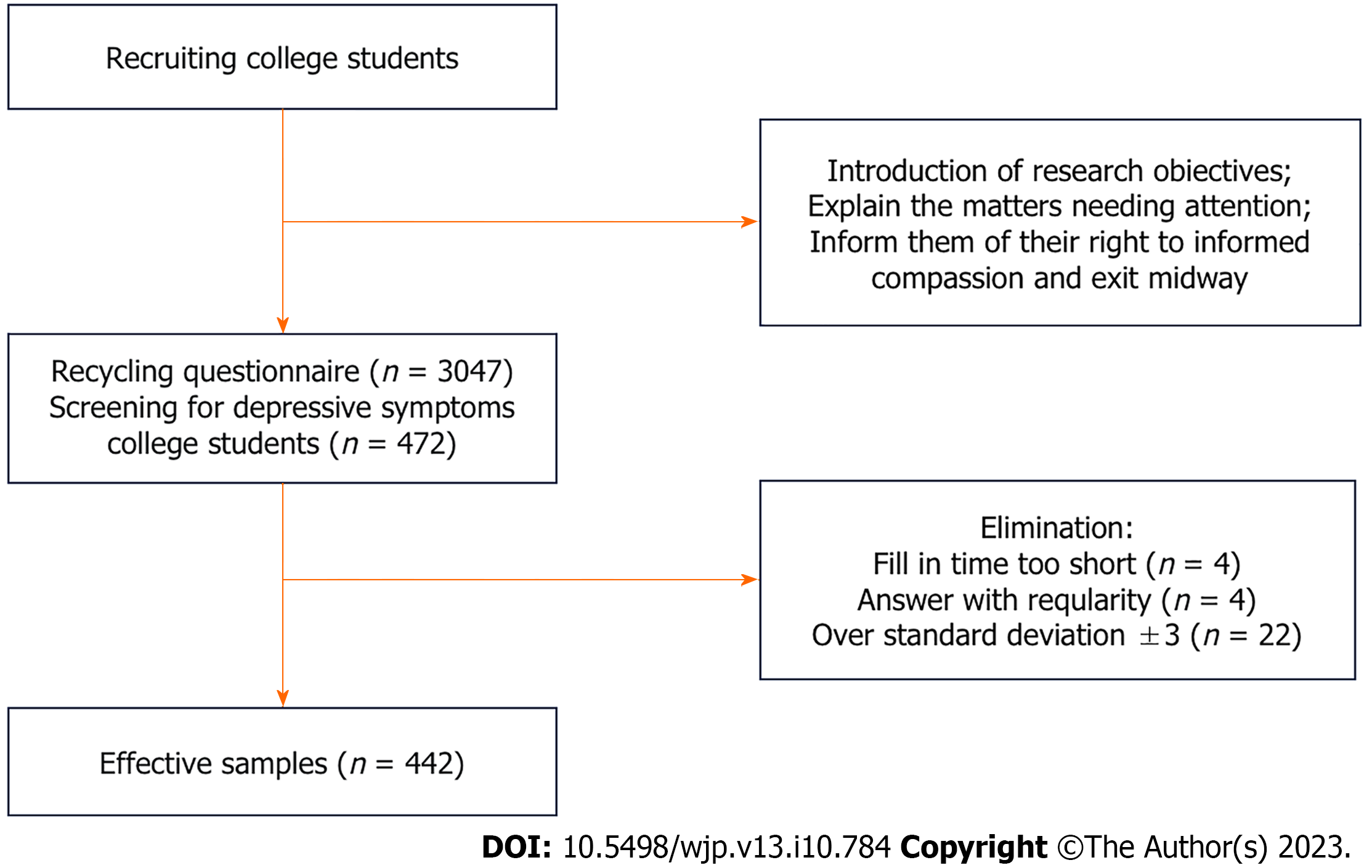
This cross-sectional research screened 3047 college students. Of these, 472 had depressive symptoms, with a depression detection rate of 15.49%. Furthermore, 442 college students with depressive symptoms were analyzed. A one-way analysis of variance and Pearson’s correlation, linear regression, and structural equation modeling analyses were used to explore the correlations and pathways of the interactions between the variables.
Depressive symptoms were significantly negatively correlated with physical activity (r = -0.175, P < 0.001), the behavioral activation system (r = -0.197, P < 0.001), and drive (r = -0.113, P = 0.017). Furthermore, it was negatively correlated with fun-seeking (FS) (r = -0.055, P = 0.251); however, it was not significant. Physical activity was significantly positively correlated with reward responsiveness (RR) (r = 0.141, P = 0.003) and drive (r = 0.124, P = 0.009) and not significantly positively correlated with FS (r = 0.090, P = 0.058). The mediating effect of RR between physical activity and depressive symptoms was significant [B = -0.025, 95% confidence interval (95%CI): -0.051 to -0.008, P = 0.001]. The direct and total effects of physical activity on depressive symptoms and were significant (B = -0.150, 95%CI: -0.233 to -0.073, P < 0.001; B =
As physical activity levels increased, depression scores among college students decreased. The mediating effect of RR between physical activity and depressive symptoms was significant. Therefore, colleges and universities should encourage college students with depression to increase their physical activity and improve their behavioral activation system. Particular attention should be paid to RR, which may reduce the prevalence of depressive symptoms.
Core Tip: This study explored the specificity of the behavioral activation system for physical activity and reward motivation in college students with different depressive symptom scores. Furthermore, the inter-relationships among the three variables were examined via a cross-sectional research design. Pathways of the behavioral activation system that mediated the effect of physical activity level on depressive symptoms in college students with depressive symptoms were clarified.
- Citation: Zhu JH, Li SF, Wang P, Xin X, Zhao Q, Chen SC, Wang X. Correlation and pathways of behavioral activation systems mediating physical activity level and depressive symptoms among college students. World J Psychiatry 2023; 13(10): 784-792
- URL: https://www.wjgnet.com/2220-3206/full/v13/i10/784.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i10.784
Depression, a disabling mental disorder, seriously endangers the lives and health of people and ranks the 13th highest in the number of disability-adjusted life-years among all illnesses and injuries worldwide[1]. It is a common mental disorder among college students, with a detection rate of over 30%[2,3]. The main symptoms include persistent low mood, sad emotional experiences, lack of pleasure, listlessness, and impaired cognitive function, with tendencies of self-harm and suicide. The World Health Organization predicted that depression would rank first in the disease burden worldwide by 2030[4].
The behavioral activation system, also known as reward motivation, is located in the midbrain dopamine loop and refers to the convergent motivation for reward, promoting goal-directed behavior to obtain the reward, and producing positive emotions or hedonic pleasure experiences[5]. It is divided into three factors: Reward responsiveness (RR), drive, and fun-seeking (FS)[6]. Impaired reward function, or anhedonia, is a core symptom of depression, and deficits in the behavioral activation system can serve as functional deficits in depressive symptoms[7,8].
An inter-relationship between physical activity, depressive symptoms, and behavioral activation system exists. Appropriate physical activity significantly alleviates clinical symptoms in people with depressive symptoms[9-11] and reduces anxiety and depression levels in college students[12]. In addition, exercise is also strongly associated with behavioral activation system, such as enhancing the midbrain-striatal dopamine (DA) system and improving the brain reward function in adolescents[13]. Physical activity also positively affects the behavioral activation system, and thereby alleviates depressive symptoms.
Previous studies[7-11,13] examined the two-sided relationship between physical activity, behavioral activation system, and depressive symptoms. Physical activity enhanced the behavioral activation system and reduced depressive symptoms. Furthermore, the behavioral activation system acted as an influencing factor for depressive symptoms. However, whether the behavioral activation system intervened in the relationship between physical activity and depressive symptoms remains unclear. Furthermore, its pathways of actions, how it intervened through the three subdimensions of the behavioral activation system, and whether the effects were consistent also remain unclear. Therefore, this study conducted a cross-sectional research that aimed to provide a theoretical basis for a deeper understanding of the relationship between human behavior, emotion, and the nervous system. Furthermore, we aimed to provide a reference for researchers and college administrators. This study proposed the following research hypotheses: (1) Physical activity and the behavioral activation system would have specificity among college students with different depressive symptom scores; (2) Physical activity, the behavioral activation system, and depressive symptoms would be closely related; and (3) Behavioral activation system would mediate the relationship between physical activity and depressive symptoms with different effects of the sub-dimensions.
This study used a cross-sectional research design. In total, 3047 college students were recruited online based on voluntary participation to complete a questionnaire. College students with depressive symptoms were screened via the Beck Depression Inventory (BDI)-II, and 472 students had depressive symptoms, with a depression detection rate of 15.49% (Figure 1). Inclusion criteria were participants who were college students, aged 18-26 years, had a score of ≥ 14 on the BDI-II, with a scores of 14-19, 20-28, and 29-63 indicating mild, moderate, and severe depression, respectively, and had no other psychiatric disorders and no brain injury. The exclusion criteria were participants who took drugs, such as barbiturates, benzodiazepines, and chloral hydrate, majored in sports, and had contraindications for exercise. After 30 invalid questionnaires with regular responses, a short response time (< 3 min), or outliers that exceeded the standard deviation ± 3 were excluded, 442 valid questionnaires (93.64%) were obtained. This study was approved by the Ethics Committee of the Shanghai University of Sport (102772021RT007).
General information questionnaire: Participants’ basic information, such as age, sex, height, weight, and family status were obtained.
International physical activity questionnaire short form: This 7-item questionnaire has been widely used to measure physical activity among Chinese university students. Of these, six questions enquired regarding individuals’ physical activity, which included high-intensity and moderate-intensity physical activity, and walking, and the frequency of different intensity activities for one week and the cumulative time per day. The weekly physical activity levels were calculated and divided into high, medium, and low groups according to the relevant criteria. The higher the group level, the greater the intensity of daily physical activity. This scale’s retest reliability coefficient was 0.718[14].
Behavioral inhibition/activation system scale: A revised Chinese version by Li et al[15] was adopted with 18 items, which included two dimensions: Behavioral inhibition and activation. The behavioral activation dimension, also known as the behavioral activation system, was selected and contained three subfactors: RR, drive, and FS. Each item was scored on a scale from 1 (fully agree) to 4 (fully disagree). Cronbach's alpha was 0.759[15].
BDI-II: This widely used 21-item self-assessment scale assessed depressive symptoms. Responses were rated on a 4-point Likert scale that ranged from 0 (no symptoms) to 3 (severe symptoms). Total scores of 0-13, 14-19, 20-28, and 29-63 indicated no, mild, moderate, and severe depression, respectively. The internal consistency coefficient was 0.948[16].
Measures were expressed as mean ± SD, and the results were retained to three decimal places. For questionnaire data that were not missing at random, interpolation of the means of the same category was performed to avoid biased estimated coefficients via the simple deletion method. One-way analysis of variance and least significant difference post-hoc multiple tests were applied to compare the specificity of physical activity and behavioral activation system among college students with different depressive symptom scores. Pearson's correlation and linear regression analyses were performed to explore the relationships among physical activity, depressive symptoms, and behavioral activation system. Two-tailed tests were adopted for statistical inference of all parameters, and the test level α was set at 0.05. P < 0.05, P < 0.01, and P < 0.001 all indicated statistical significance.
Harman’s single factor test was used to examine the effects of common method bias. Structural equation modeling was conducted to examine the role of behavioral activation system in mediating the relationship between physical activity and depressive symptoms. The non-parametric percentage bootstrap method was adopted for parameter estimation in the path analysis. Number of samples was set at 5000, with a bias-corrected 95% confidence interval (95%CI) for the product of the mediated paths, without 0 defining the mediating effect as statistically significant. Data calculations were performed using SPSS Statistics version 23.0, and Amos version 23.0.
There were no significant differences in FS behavior, physical activity, RR, and drive (P < 0.05) among college students with different depressive symptom scores. Post-hoc multiple comparisons indicated significant differences in physical activity and RR (P < 0.05) between college students with severe depressive symptoms and those with moderate depressive symptoms. In addition, there were also differences in physical activity, RR, and drive (P < 0.01) between those with severe depressive symptoms and those with mild depressive symptoms. Other indicators had no statistically significant differences. See Table 1 for further details.
| Variables | Whole (442) | Levels of depressive symptoms | F-value | P value | Post hoc multiple comparisons | ||||
| Severe (n = 58) | Moderate (n = 190) | Mild (n = 194) | Severe vs Moderate | Severe vs Mild | Moderate vs Mild | ||||
| Physical activity (MET-min/week) | 1308 ± 954 | 944 ± 617 | 1333 ± 1003 | 1392 ± 967 | 5.149 | 0.006 | 0.006 | 0.002 | 0.535 |
| Reward responsiveness | 11.88 ± 2.033 | 11.03 ± 2.232 | 11.81 ± 2.043 | 12.21 ± 1.885 | 7.865 | < 0.001 | < 0.001 | 0.010 | 0.053 |
| Drive | 11.22 ± 2.064 | 10.71 ± 2.656 | 11.15 ± 2.008 | 11.45 ± 1.888 | 3.129 | 0.045 | 0.154 | 0.016 | 0.152 |
| Fun-seeking | 14.17 ± 2.270 | 13.78 ± 2.968 | 14.10 ± 2.217 | 14.36 ± 2.067 | 1.654 | 0.192 | 0.341 | 0.085 | 0.260 |
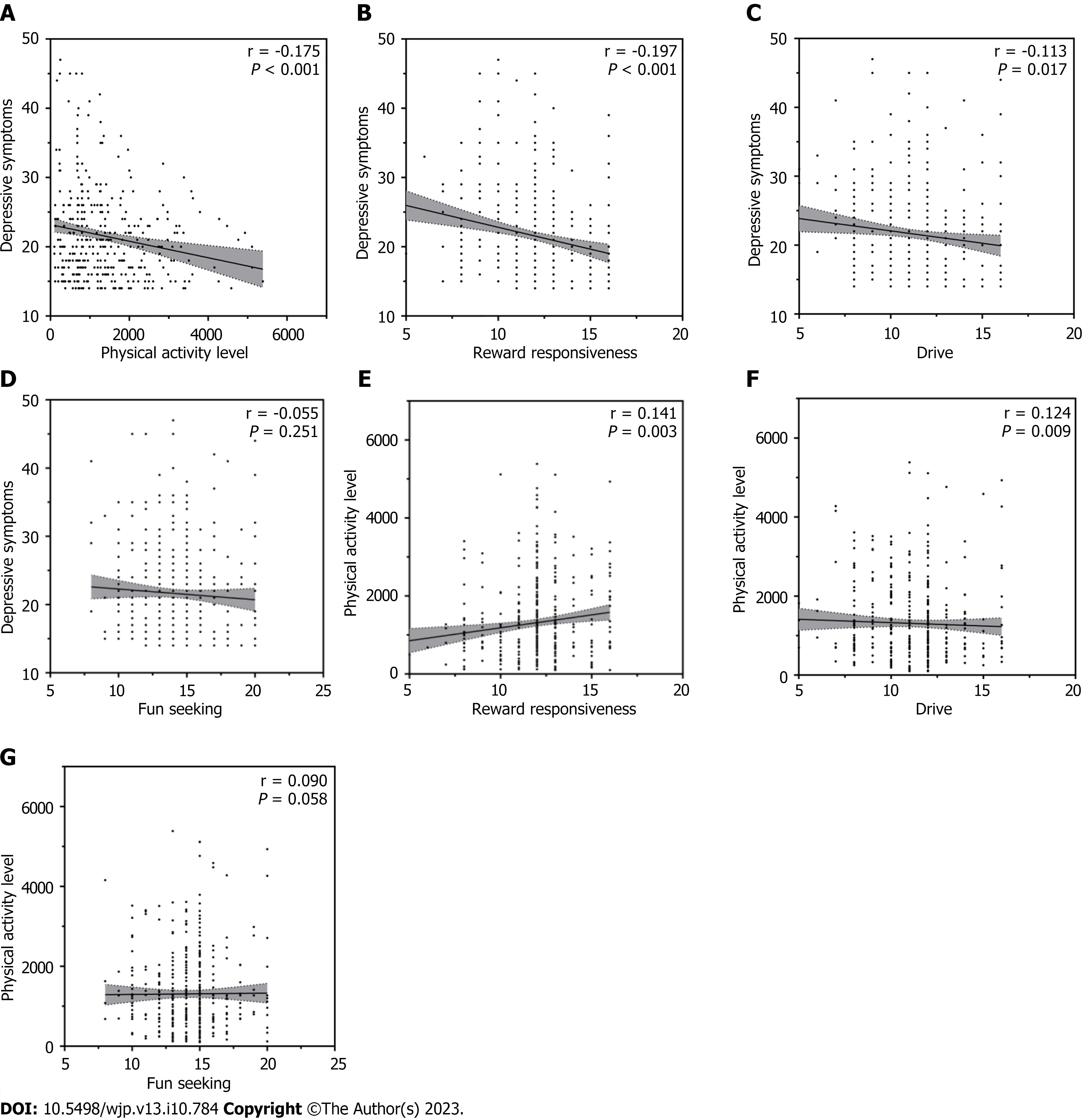
Depressive symptoms were significantly negatively correlated with physical activity (r = -0.175, P < 0.001), RR (r = -0.197, P < 0.001), and drive (r = -0.113, P = 0.017). Furthermore, it was also negatively correlated with FS (r = -0.055, P = 0.251); however, it was not significant. Physical activity was significantly positively correlated with RR (r = 0.141, P = 0.003) and drive (r = 0.124, P = 0.009), and not significantly positively correlated with FS (r = 0.090, P = 0.058). Further details are shown in Figure 2.
To examine the extent of which physical activity and behavioral activation system explained depressive symptoms and explore the feasibility of the structural relationship model, depressive symptoms were considered as dependent variables, and physical activity, RR, drive, and FS as independent variables. Furthermore, a linear regression analysis was performed via stepwise regression. The goodness-of-fit of the prediction model was demonstrated (R = 0.247, R2 = 0.061, adjusted R2 = 0.057, and changed variable F = 10.349), which excluded drive and FS. As shown in Table 2, RR and physical activity were negative influencing factors, with 6.1% explanatory power for depressive symptom scores. The tolerance of each independent variable was > 0.1, and the variance inflation factor was < 5; thus, the effect of multicollinearity was excluded.
| Independent variables | B | 95%CI | Beta | Coefficient significance test | SE | Collinearity diagnostics | |||
| Lower limit | Upper limit | t-value | P value | Tolerance | VIF | ||||
| Reward responsiveness | -0.176 | -0.268 | -0.084 | -0.176 | -3.766 | < 0.001a | 0.047 | 0.980 | 1.020 |
| Physical activity | -0.116 | -0.242 | -0.058 | -0.116 | -3.217 | 0.001 | 0.047 | 0.980 | 1.020 |
To assess for common method bias, a validation factor analysis was conducted on the International Physical Activity Questionnaire, the Behavioral Activation System Scale, and the BDI via Harman’s single factor test, nine factors with characteristic roots greater than 1 were obtained. The amount of variance explained by the first factor was 17.980%, which was less than the critical value of 40%. Therefore, the effect of common method bias was excluded. Based on the inter-relationships among physical activity, behavioral activation system, and depressive symptoms in college students, a model was established with physical activity, depressive symptoms, and behavioral activation system as the independent, dependent, and mediating variables, respectively. Mediated paths with insignificant coefficients were individually removed and recalculated until all mediated path coefficients passed the bootstrap significance test. Discrepancies divided by degrees of freedom (CMIN/df) = 0.286, root mean square residual (RMR) = 0.006, root mean square error of approximation (RMSEA) < 0.001, goodness-of-fit index (GFI) = 1.000, normed fit index (NFI) = 0.999, and comparative fit index (CFI) = 1.000 reached the reference standards of CMIN/df < 3, RMR < 0.05, RMSEA < 0.08, GFI, NFI, and CFI > 0.9[17], which indicated that the structural equation model fit well and was reasonable and reliable. The path analysis is shown in Figure 3, and the results of the mediating effect test are presented in Table 3. The path coefficients of physical activity on FS were not significant. Furthermore, those of FS and driving on depressive symptom scores were not significant, and were excluded. There were significant path coefficient for physical activity on drive (B = 0.124, 95%CI: 0.034 to 0.211, P = 0.007), mediating effect mediated by RR (B = -0.025, 95%CI: -0.051 to -0.008, P = 0.001), direct effect of physical activity on depressive symptoms (B = -0.150, 95%CI: -0.233 to -0.073, P < 0.001), and total effect of physical activity on depressive symptoms (B = -0.175. 95%CI: -0.260 to -0.099, P < 0.001).
| Types of effects | B | SE | Bias-corrected 95%CI | ||
| Lower limit | Upper limit | P value | |||
| Mediating effect of reward responsiveness | -0.025 | 0.011 | -0.051 | -0.008 | 0.001 |
| Path coefficient of physical activity on drive | 0.124 | 0.045 | 0.034 | 0.211 | 0.007 |
| Direct effect | -0.150 | 0.041 | -0.233 | -0.073 | < 0.001 |
| Total effect | -0.175 | 0.040 | -0.260 | -0.099 | < 0.001 |
These results showed that the higher the level of physical activity among college students with depressive symptoms, the higher the behavioral activation system and lower their depressive symptom scores. Furthermore, the direct effect of physical activity on depressive symptoms was significant. The results supported those of previous studies on the relationship between behavioral activation system and depressive symptoms. Furthermore, our findings were generally consistent with previous results. Takagaki et al[18] found a negative association between the behavioral activation system and depressive symptoms among 18-19 years old college students with depressive symptoms in a Japanese University. Absence of pleasure was a core symptom of depression[19] which was related to the dysfunction of the brain's DA reward system[20]. Deficits in the reward system served as a susceptibility factor and predictor of depression, with state independence and heritability[21,22]. A cross-sectional study found that college students with higher levels of physical activity had lower detection rates of depressive symptoms and insufficient physical activity was a risk factor for depressive symptoms among college students. Experimental studies confirmed that increased physical activity was effective in improving depressive symptoms and stimulated the secretion of neurotransmitters, which increased the behavioral activation system and also alleviated depressive symptoms.
This study showed that only RR mediated the relationship between physical activity and depressive symptoms. Physical activity promoted the secretion of neurotransmitters, such as DA in the brain, enhanced the neuroplasticity of the DA system, and improved the reward function. This enhanced the RR and contributed to the maintenance and regulation of good emotions in individuals, promoted the generation of positive emotions, and suppressed negative emotions[23]. High reward responses acted as a protective factor against depression[24]. Furthermore RR purely reflected extroversion and convergent motivation[25], which maintained and regulated individual behavior. Hence, impulsive behaviors that met short-term interests were subordinated to needs more closely related to the individual's long-term interests[26]. High RR prompted a positive response to rewards. It enhanced an individual's ability to obtain pleasurable experiences to avoid the exacerbation of their depressive symptoms[27,28]. Only the RR pathway mediated the relationship between physical activity and depressive symptoms. A possible reason was that RR, as the initial evaluation of reward, measured early "reward interest,” “goal-drive”, and “persistence.” Furthermore, its effect on depressive symptoms may precede the other two dimensions. Reasons why drive and FS did not mediate the relationship between physical activity and depressive symptoms were drive referred to the degree of willingness to exert effort to obtain a reward and measured late "reward responsiveness" and "impulsivity;" FS was a continuous evaluation of the reward. Hence, drive and FS were more significant for major depression, and better predictors of treatment effect[29,30]. Our participants had different conditions, and relatively few individuals reported severe depression symptoms.
This study has some limitations. First, the data were from subjective reports, which may have some bias. Furthermore objective indicators are recommended for future measurements. This study was conducted as a cross-sectional study. Hence, longitudinal studies are required to further confirm the pathways of action.
The higher the level of physical activity, the higher the behavioral activation system and lower the depressive symptom score in college students with depression. Furthermore, there was only one pathway of action in the behavioral activation system, RR, which had a significant mediating effect between physical activity and depressive symptoms. Therefore, colleges and universities should encourage college students with depression to increase their physical activity and improve their behavioral activation system. Particular attention should be paid to RR, which may reduce the prevalence of depressive symptoms.
Depression is a common mental disorder among college students. Key symptoms include persistent depressed mood, sad emotional experiences, lack of pleasure, listlessness, and impaired cognitive function, accompanied by self-harm and suicidal tendencies.
Reduce the prevalence of depressive symptoms in college students.
Elucidating pathways and effects of behavioral activation systems between physical activity and depressive symptoms in college students with depressive symptoms.
One-way analysis of variance and Pearson correlation, linear regression, and structural equation modeling were used to explore the correlation and pathway of interactions between variables.
The mediating effect of reward responses between physical activity and depressive symptoms was significant [B = -0.025, 95% confidence interval (95%CI): -0.051 to -0.008, P = 0.001]. The direct and total effects of physical activity on depressive symptoms were significant ((B = -0.150, 95%CI: -0.233 to -0.073, P < 0.001; B = -0.175, 95%CI: -0.260 to -0.099, P < 0.001, respectively).
Colleges and universities should encourage college students with depression to increase physical activity and improve behavioral activation systems. Particular attention should be paid to the ability to reward responses, which may reduce the prevalence of depressive symptoms.
It is recommended to use objective measurement tools in future measurements; longitudinal studies are needed to further define the course of action.
| 1. | GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9:137-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 436] [Cited by in RCA: 3557] [Article Influence: 889.3] [Reference Citation Analysis (0)] |
| 2. | Zhai WH, Zhang Qi, Yan J. [Meta-analysis of the detection rate of depressive symptoms and related factors among Chinese university students before and after the new crown pneumonia epidemic]. Zhongguo Xuexiao Weisheng. 2022;43:1055-1060. [DOI] [Full Text] |
| 3. | Wang MY, Han FF, Liu J, Huang KL, Peng HY, Huang MT, Zhao ZH. [A meta-analysis of the detection rate of depressive symptoms and related factors among college students]. Zhongguo Xinliweisheng Zazhi. 2020;34:1041-1047. [DOI] [Full Text] |
| 4. | Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7951] [Cited by in RCA: 8177] [Article Influence: 681.4] [Reference Citation Analysis (0)] |
| 5. | Pickering A, Gray JA. Dopamine, appetitive reinforcement, and the neuropsychology of human learning: An individual differences approach. PABST Science Publishers Lengerich, Germany. 2001;113-149. |
| 6. | Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J Pers Soc Psychol. 1994;67:319-333. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4098] [Cited by in RCA: 4167] [Article Influence: 130.2] [Reference Citation Analysis (0)] |
| 7. | Wieman ST, Arditte Hall KA, MacDonald HZ, Gallagher MW, Suvak MK, Rando AA, Liverant GI. Relationships Among Sleep Disturbance, Reward System Functioning, Anhedonia, and Depressive Symptoms. Behav Ther. 2022;53:105-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | McCarron RM, Shapiro B, Rawles J, Luo J. Depression. Ann Intern Med. 2021;174:ITC65-ITC80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 346] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 9. | Cahuas A, He Z, Zhang Z, Chen W. Relationship of physical activity and sleep with depression in college students. J Am Coll Health. 2020;68:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Kandola A, Lewis G, Osborn DPJ, Stubbs B, Hayes JF. Depressive symptoms and objectively measured physical activity and sedentary behaviour throughout adolescence: a prospective cohort study. Lancet Psychiatry. 2020;7:262-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 11. | da Costa BGG, Chaput JP, Lopes MVV, Malheiros LEA, Silva KS. Movement behaviors and their association with depressive symptoms in Brazilian adolescents: A cross-sectional study. J Sport Health Sci. 2022;11:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Gu HB, Wang XY, Ban MJ. [Association between physical activity and trajectories of change in anxiety and depression scores among college students]. Zhongguo Xuexiao Weisheng. 2020;41:1678-1681,1687. [DOI] [Full Text] |
| 13. | Li J, Wang HJ, Chen W. [Advances in research on exercise to improve brain reward function to prevent obesity in children and adolescents]. Zhongguo Ertong Baojian Zazhi. 2021;29:165-168. [DOI] [Full Text] |
| 14. | Qu NN, Li KJ. [Reliability and validity study of the Chinese version of the International Physical Activity Questionnaire]. Zhonghua Liuxingbingxue Zazhi. 2004;(03):87-90. [DOI] [Full Text] |
| 15. | Li YZ, Zhang Y, Jiang Y, Li H, Mi S, Yi GJ, Gu HY. [Reliability analysis of the Chinese version of the Behavioral Inhibition/Activation System Scale]. Zhongguo Xinliweisheng Zazhi. 2008;(08):613-616. [DOI] [Full Text] |
| 16. | Wang Z, Yuan CM, Huang J, Li ZX, Chen J, Zhang HY, Fang YR, Xiao ZP. [Reliability and validity of the Chinese version of the Beck Depression Inventory, Version 2, in patients with depression]. Zhongguo Xinliweisheng Zazhi. 2011;25:476-480. [DOI] [Full Text] |
| 17. | Wen ZL, Hou JT, Herbert M. Structural equation modeling tests:fit indices and chi-square criterion. Acta Psychologica Sinica. 2004;36 :186-194. |
| 18. | Takagaki K, Okamoto Y, Jinnin R, Mori A, Nishiyama Y, Yamamura T, Yokoyama S, Shiota S, Miyake Y, Ogata A, Shimoda H, Kawakami N, Furukawa TA, Yamawaki S. Mechanisms of behavioral activation for late adolescents: Positive reinforcement mediate the relationship between activation and depressive symptoms from pre-treatment to post-treatment. J Affect Disord. 2016;204:70-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Kibitov AO, Mazo GE. [Anhedonia in depression: neurobiological and genetic aspects]. Zh Nevrol Psikhiatr Im S S Korsakova. 2021;121:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Rappaport BI, Kandala S, Luby JL, Barch DM. Brain Reward System Dysfunction in Adolescence: Current, Cumulative, and Developmental Periods of Depression. Am J Psychiatry. 2020;177:754-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Alloy LB, Olino T, Freed RD, Nusslock R. Role of Reward Sensitivity and Processing in Major Depressive and Bipolar Spectrum Disorders. Behav Ther. 2016;47:600-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 22. | Webb CA, Dillon DG, Pechtel P, Goer FK, Murray L, Huys QJ, Fava M, McGrath PJ, Weissman M, Parsey R, Kurian BT, Adams P, Weyandt S, Trombello JM, Grannemann B, Cooper CM, Deldin P, Tenke C, Trivedi M, Bruder G, Pizzagalli DA. Neural Correlates of Three Promising Endophenotypes of Depression: Evidence from the EMBARC Study. Neuropsychopharmacology. 2016;41:454-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Ma K, Liu JM, Fu CY, Zhang H, Jia SH. [Research progress on the intervention effect and mechanism of exercise on depression]. Zhongguo Tiyu Keji. 2020;56:13-24. [DOI] [Full Text] |
| 24. | Markarian SA, Pickett SM, Deveson DF, Kanona BB. A model of BIS/BAS sensitivity, emotion regulation difficulties, and depression, anxiety, and stress symptoms in relation to sleep quality. Psychiatry Res. 2013;210:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Smillie LD, Jackson CJ, Dalgleish LI. Conceptual distinctions among Carver and White’s (1994) BAS scales: A reward-reactivity versus trait impulsivity perspective. Pers Individ Dif. 2006;40:1039-1050. [RCA] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Hamza CA, Willoughby T, Heffer T. Impulsivity and nonsuicidal self-injury: A review and meta-analysis. Clin Psychol Rev. 2015;38:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 184] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 27. | Pinto-Meza A, Caseras X, Soler J, Puigdemont D, Pérez V, Torrubia R. Behavioural Inhibition and Behavioural Activation Systems in current and recovered major depression participants. Pers Individ Dif. 2006;40:215-226. [RCA] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Smillie LD, Pickering AD, Jackson CJ. The new reinforcement sensitivity theory: implications for personality measurement. Pers Soc Psychol Rev. 2006;10:320-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 241] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 29. | Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. J Abnorm Psychol. 2002;111:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Wang XX, Jiang CG, Zhang L, Feng ZZ. [Abnormalities in resting-state brain function in depression and their relationship to behavioral inhibition/activation]. Disan Junyidaxue Xuebao. 2014;36:1600-1603. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Alkhatib AJ, Jordan; Girardi P, Italy; Shalaby MN, Egypt; Stogov MV, Russia S-Editor: Fan JR L-Editor: A P-Editor: Chen YX