Published online Mar 19, 2022. doi: 10.5498/wjp.v12.i3.450
Peer-review started: June 30, 2021
First decision: July 28, 2021
Revised: August 13, 2021
Accepted: February 10, 2022
Article in press: February 10, 2022
Published online: March 19, 2022
Processing time: 260 Days and 19.5 Hours
Neuropsychiatric symptoms (NPS) have been insufficiently examined in persons with aphasia (PWA) because most previous studies exclude participants with language and communication disorders.
To report a two-part study consisting of a literature review and an observational study on NPS in post-stroke aphasia.
Study 1 reviewed articles obtained from PubMed, PsycINFO, Google Scholar and Cochrane databases after cross-referencing key words of post-stroke aphasia to NPS and disorders. Study 2 examined language deficits and activities of daily living in 20 PWA (median age: 58, range: 28-65 years; 13 men) with the Western Aphasia Battery-Revised and the Barthel Index, respectively. Informants of these 20 PWA were proxy-evaluated with the Neuropsychiatric Inventory and domain-specific scales, including the Stroke Aphasia Depression Questionnaire-10 item version and the Starkstein Apathy Scale. In addition, an adapted version of the Hospital Anxiety and Depression Scale was directly administered to the PWA themselves. This observational study is based on the baseline assessment of an intervention clinical trial (EudraCT: 2017-002858-36; ClinicalTrials.gov identifier: NCT04134416).
The literature review revealed a broad spectrum of NPS in PWA, including depression, anxiety, apathy, agitation/aggression, eating and sleep disorders, psychosis, and hypomania/mania. These findings alert to the need for improving assessment and treatment approaches of NPS taking into consideration their frequent occurrence in PWA. Study 2 showed that the 20 participants had mild- to-moderate aphasia severity and were functionally independent. A wide range of comorbid NPS was found in the post-stroke aphasic population (median number of NPS: 5, range: 1-8). The majority of PWA (75%) had depressive symptoms, followed by agitation/aggression (70%), irritability (70%), anxiety (65%) and appetite/eating symptoms (65%). Half of them also presented symptoms of apathy, whereas euphoria and psychotic symptoms were rare (5%). Domain-specific scales revealed that 45% of participants had apathy and 30% were diagnosed with depression and anxiety.
Concurrent NPS are frequent in the chronic period of post-stroke aphasia. Therefore, further research on reliable and valid assessment tools and treatment for this aphasic population is strongly warranted.
Core Tip: The literature on neuropsychiatric disorders in persons with aphasia (PWA) is limited, given that this population is usually excluded from neuropsychiatric evaluations. This article provides a state-of-art analysis on the prevalence, nature, pathophysiology, assessment, and treatment of neuropsychiatric symptoms (NPS) in PWA. We also report findings from a proof-of-concept observational study that included 20 PWA after chronic left hemisphere lesions which identified a spectrum of NPS, primarily depression, irritability, agitation, anxiety, and apathy.
- Citation: Edelkraut L, López-Barroso D, Torres-Prioris MJ, Starkstein SE, Jorge RE, Aloisi J, Berthier ML, Dávila G. Spectrum of neuropsychiatric symptoms in chronic post-stroke aphasia. World J Psychiatry 2022; 12(3): 450-469
- URL: https://www.wjgnet.com/2220-3206/full/v12/i3/450.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i3.450
Aphasia, defined as the partial or complete loss of language caused by brain damage is one of the most frequent and devastating sequelae of stroke[1-3]. In fact, about 21% to 38% of acute stroke individuals have aphasia[1] and 25% to 50% of them still show residual language and communication deficits in the chronic period[4]. Before focusing on neuropsychiatric symptoms (NPS) associated with post-stroke aphasia (PSA), below we present a brief overview on the impact of aphasia in the acute and chronic stroke periods as well as the traditional and emerging evaluation approaches used to classify language deficits and to implements adequate therapies.
PSA may be associated with worse outcomes in acute/subacute (onset to week 12), chronic (week 13-week 52) and very chronic (week 53 onwards) periods than strokes unassociated with aphasia[5,6]. This is the consequence of the increased length of hospital stay and inpatient medical complications, including those caused by the neurological disability itself. The course of aphasia during the sub-acute and chronic stroke periods may also be complicated by reduced functional independence, longer stays in rehabilitation settings, reduced adherence to aphasia therapy, particularly in older people, and poorer quality of life and activities of daily living[1,5,6].
Stroke lesions causing aphasia usually affect the left hemisphere (dominant for language in most right handed individuals) in the distribution of the middle cerebral artery territory with damage to the perisylvian language core and its subcortical structures (basal ganglia, internal capsule and white matter). The resulting syndromes are chiefly characterized by impaired repetition in the context of impaired spontaneous speech, and variable deficits in auditory comprehension and naming. These syndromes are known as the “classical” or “perisylvian” aphasias (Broca’s, Wernicke’s, conduction and global) which roughly account for 80% of all cases and have poorer prognosis than other types of aphasias[7]. The rest of post-stroke aphasic syndromes, representing 20% of all cases, are associated with infarctions in arterial “borderzone” vascular territories (i.e., the junction between anterior and middle cerebral arteries). These aphasias (motor, sensory and mixed transcorticals and anomic) are characterized by preserved repetition and echolalia with variable deficits in other language domains (spontaneous speech, comprehension and naming) and usually have better long-term prognosis than perisylvian aphasias[8]. Whereas infarctions account for around 80% of cases, hemorrhages are less frequent[1]. The clinical profile of acute and chronic PSA is heterogeneous with a variable degree of involvement of phonology, semantics, fluency and connected speech production. Traditional classifications of aphasia dichotomically separate syndromes (e.g., Broca’s, Wernicke’s, transcortical) on the basis of differences in surface language deficits (fluent/nonfluent speech, impaired/preserved comprehension). In spite of this coarse division, the syndrome-based approach (e.g., Broca’s aphasia) is still retained in clinical practice to predict prognosis, manage recovery in acute clinical settings, and inform patients and relatives[1]. However, using this approach the aphasia profile in more than a quarter of stroke patients is unclassifiable and there is no clear-cut correspondence between lesion location and aphasia profile particularly in chronic cases. Even more important is that clinical labels (e.g., Broca’s aphasia) provide little information on the underlying language and cognitive deficits and knowing the status of these deficits is crucial to select adequate model-based therapies. Therefore, since understanding the neural mechanisms underpinning language processing is important for diagnosis and treatment, current accounts use data-driven approaches for aphasia classification and lesion-based predictions of recovery[9,10]. Moreover, it is well known that persons with aphasia (PWA) have an increased incidence of NPS compared to patients with other chronic diseases[2,11], greatly influencing rehabilitation responses, quality of life, and long-term functional outcomes[12-15]. In general, NPS are a frequent and challenging consequence of stroke, derived from the crossroad of lesion-related brain factors and psychological distress related to the event and its functional impact in daily life[16,17]. Several comprehensive reviews dealing with NPS in post-stroke patients have been reported[18-22] and a recent original study evaluating 518 non-aphasic stroke patients found that half of the sample presented at least one NPS based on Neuropsychiatric Inventory (NPI)[23,24]. However, one relevant limitation of the above studies is that they exclude aphasic participants due to the inherent linguistic-assessment difficulties[25-29]. The aims of the present study were thus twofold. In study 1 the objective was to carry out a narrative review on NPS in PSA, covering data of prevalence, risk factors, assessment tools, pathophysiology, and treatment options. Study 2 reports original data from 20 PWA in the chronic phase after suffering a left hemisphere lesion who were evaluated with the NPI and domain-specific psychiatric scales to examine the frequency and severity of NPS.
Search strategy: The authors conducted a literature search on Medline/PubMed, PsycINFO, Google Scholar and Cochrane databases from inception to June 2021. Key search terms for NPS or disorders were cross-referenced to PSA. The following terms were included: “aphasia” or “PSA” or “acquired language impairment” or “acquired language disorder” or “post-stroke linguistic disorder” or “post-stroke linguistic impairment” AND “neuropsychiatric*” or “neuropsychiatry” “psychy*” or “neurobehav*” or “behavio[u]r*” or “emotion*” or “mood” or “affect*” or “depression” or “depressive” or “dysthym*” or “distress“ or “apathy” or “apathetic” or “motivat*” or “drive” or “indifferen*” or “anxiety” or “anxious*” or “stress” or “phobia” or “fear” “catastrophic reaction” or “disinhibit*” or “impulsiv*” or “agitat*” or “aggress*” or “anger” or “irritab*” or “psycho*” or “hallucination” or “delusion” or “delusive” or “prodrom* or “sleep” or “appetite” or “eating” or “elation” or “pathological laugh*” or “euphoria” or “mania” or “bipolar” or “quality of life”. Articles including the terms “stroke” or “post-stroke” or “cerebrovascular” AND “neuropsychy*” or “neurobehav*” or “emotion” or “depression” were also screened for aphasia terms within its full text and considered for inclusion. This search strategy was analogous to other published reviews on post-stroke depression[30,31].
Titles, abstracts, and full texts were reviewed by 2 independent observers (MB and LE) to assess inclusion criteria and read the selected articles for final incorporation. In addition, references of all selected articles were searched for studies that could also meet inclusion criteria. Possible investigator divergences were compared and resolved through discussion. A third observer (GD) was available for an appeal if disagreements existed. Studies were included if: (1) Participants had a clear assessment of aphasia and presented a single or multiple NPS or disorders; (2) NPS or disorders were assessed with validated scales or through clearly defined criteria; (3) participants were adults (i.e., 18 years or older); (4) participants were only affected by cerebrovascular lesions; and (5) articles were written in English.
This narrative review prioritized manuscripts in the following order: (1) Meta-analysis or systematic reviews; (2) randomized clinical trials; (3) cohort studies; and (4) case-reports. When only case-reports were retrieved, articles including neuroimaging measures were prioritized. Studies including participants with pre-stroke neurodegenerative (e.g., primary progressive aphasia, dementia) or premorbid psychiatric disorders that would make differential diagnoses difficult were excluded from the search.
Study design and subject selection: The focus of this study was the assessment of the frequency of NPS at baseline of an intervention trial in PWA after stroke (EudraCT:2017-002858-36; ClinicalTrials.gov identifier: NCT04134416). The study included 20 chronic PWA (median age of participants: 58, range: 28-65 years; 13 men) evaluated at the Unit of Cognitive Neurology and Aphasia at the University of Malaga, Spain. A consecutive series of participants meeting the following criteria were included: (1) Age between 18 and 70 years; (2) right handedness (80 points in the Edinburgh handedness inventory)[32]; (3) Spanish as native language; (4) left-hemisphere stroke lesions; and (5) diagnosis of aphasia established by a score in the aphasia quotient (AQ) of the Western Aphasia Battery-Revised (WAB-R) ≤ 93.8 points[33]. Exclusion criteria were: (1) Dysarthria without aphasia; (2) bilateral lesions; (3) increased risk of a new stroke or unstable neurological condition (e.g., transient ischemic attacks); (4) history of pre-stroke dementia and/or psychiatric disorders (schizophrenia, major depression, bipolar disorder, anxiety disorders); (5) alcohol and substance use or abuse; or (6) coexistence of aphasia with post-stroke dementia. Table 1 shows the demographic and clinical characteristics of the group. Participants with aphasia also underwent comprehensive neurological, neuropsychological, and neuroradiological assessments. Participants who were taking psychotropic drugs (antidepressants or tranquillizers) and/or antiepileptics were not excluded, but all prescribed medications were maintained stable during the study. Written informed consent was obtained from all participants and informants after providing detailed descriptions of the study. None of the participants or informants refused to take part in the investigation. The study was performed in accordance with the Declaration of Helsinki and approved by the Ethical Research of Drugs Committee Provincial of Malaga, Spain and the Spanish Drug and Healthcare Products Agency.
| Patient | Sex/handedness | Age (yr) | Education (yr) | Stroke duration (mo) | Barthel index1 | Lesion volume (cm3) | Aphasia type2 | Antidepressants |
| 1 | F/R | 50 | 12 | 80 | 80 | 113.33 | Conduction | Sertraline |
| 2 | M/R | 61 | 14 | 103 | 90 | 163.02 | Broca | Citalopram |
| 3 | M/R | 49 | 17 | 61 | 90 | 210.38 | Broca | No |
| 4 | M/R | 42 | 11 | 45 | 100 | 99.31 | Anomic | Citalopram |
| 5 | M/R | 63 | 8 | 11 | 85 | 23.16 | Conduction | No |
| 6 | F/R | 58 | 12 | 126 | 95 | 188.76 | Anomic | No |
| 7 | M/R | 60 | 12 | 45 | 60 | 44.96 | Anomic | No |
| 8 | M/R | 54 | 14 | 44 | 90 | 66.97 | Anomic | No |
| 9 | M/R | 51 | 13 | 7 | 80 | 225.69 | Anomic | Amitriptyline |
| 10 | M/R | 54 | 10 | 19 | 90 | 282.59 | Wernicke | No |
| 11 | F/R | 58 | 15 | 66 | 95 | 98.84 | Broca | No |
| 12 | F/R | 61 | 12 | 17 | 45 | 4.47 | Anomic | Sertraline |
| 13 | M/R | 32 | 18 | 10 | 100 | 34.01 | Wernicke | Sertraline |
| 14 | M/R | 49 | 8 | 13 | 80 | 17.45 | Anomic | No |
| 15 | F/R | 28 | 8 | 6 | 100 | 51.26 | Anomic | No |
| 16 | F/R | 65 | 17 | 13 | 100 | 26.10 | Wernicke | No |
| 17 | M/R | 64 | 17 | 120 | 100 | 157.58 | Anomic | No |
| 18 | F/R | 65 | 17 | 13 | 75 | 158.25 | Broca | No |
| 19 | M/R | 58 | 8 | 17 | 100 | 69.66 | Wernicke | No |
| 20 | M/R | 63 | 17 | 10 | 100 | 50.43 | Wernicke | Fluoxetine |
| Median | 58 | 12.5 | 18 | 90 | 84.25 |
Functional evaluation: The Barthel Index was employed to measure the degree of assistance required by each person on 10 items of mobility and self-care regarding activities of daily living. A higher score (maximum: 100 points) reflects a better competence to function independently[34].
Language evaluation: The type and severity of aphasia were evaluated with the WAB-R[33]. The profile of aphasia was made according to the taxonomic criteria of the WAB-R and aphasia severity was rated according to the scoring of the AQ of WAB-R. Lower AQ scores indicate more severe aphasia.
Multidomain neuropsychiatric evaluation: Relatives in close contact with participants were interviewed using the NPI[24]. Although this semi-structured interview was originally developed to evaluate the spectrum of NPS in patients with dementia, its use has later been expanded to assess people with stroke and other neurological conditions[23,35-37]. The NPI assesses the frequency and severity of psychological and behavioral symptoms grouped into 12 categories: delusions, hallucinations, agitation/aggression, depression, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behavior, appetite, and sleep/nighttime disturbances. The questions pertain to changes in the patient’s behavior since the onset of the stroke and, if so, whether the altered behavior was present during the last month. For the purpose of this study, the presence of symptoms was determined by the number of patients with scores > 0 in the respective symptom[23]. Frequency scores range from 1 to 4 (where 1= occasionally, less than once per week; 4 = very frequently, once, or more per day or continuously). Severity scores range from 1 to 3 (where 1 = mild, 2 = moderate, 3 = severe). Each domain´s final composite score is the product of the frequency times the severity, with a maximum score of 12 points.
Domain-specific neuropsychiatric evaluation: Since the NPI does not provide specific cut-off scores for neuropsychiatric diagnoses, domain-specific scales that specifically assessed depression, anxiety, and apathy disorders were also used. These scales were selected because all of them have previously been used in patients with stroke[38-40]. After data interpretation, neuropsychiatric diagnoses were blindly assessed by an expert behavioral neurologist who was blinded to the outcome goals of this study.
Hospital Anxiety and Depression Scale: The Hospital anxiety and depression scale (HADS) is a 14-item instrument evaluating both anxiety and depression (seven items for each subscale)[39]. For each statement, the participant chooses one of four responses (e.g., ‘definitely as much’, ‘not quite as much’, ‘only a little’, ‘hardly at all’)[39]. Scores for each subscale range from 0 to 21 points and a cut-off scores of 8 points are used for each scale. In the present study, the HADS was directly administered to the PWA. To overcome comprehension deficits of participants, each question together with the alternative responses were printed in large font letters on individual pages and the items were read aloud by the examiner who then scored a reliable answer. Cronbach's alpha for HADS-Anxiety varies from 0.68-0.93 and for HADS-Depression from 0.67-0.90[41].
Starkstein Apathy Scale: This scale was developed to assess apathy in patients with neurological diseases including stroke[40,42]. Informants of the participants were requested to answer the Starkstein Apathy Scale (SAS)’s 14 items, each of which scores on a 0–3 scale[40]. The cut-off score of the SAS is 14 (maximum score 42) and higher scores indicate more severe apathy. The scale has an excellent Cronbach’s α of 0.939[43].
Stroke Aphasia Depression Questionnaire: The stroke aphasia depression questionnaire (SADQ)-10 was developed to assess depressed mood in patients with aphasia[38]. It contains 10 items answered on a 0-3 scale by the principal informant on behalf of the PWA. The cut-off score of the SADQ-10 is 14 points (maximum score 30)[44]. Participants are classified as having depression when they score ≥ 14 points[38], or classified with subthreshold depression[45] when the SADQ-10 score is ≥ 6[46]. The questionnaire has an excellent internal consistency, with a Cronbach’s alpha of 0.80 and split-half reliability of r = 0.81[44].
We computed descriptive statistics for demographic and clinical data. In addition, non-parametrical two-tailed Spearman’s correlations on NPI scores and domain-specific instruments (AS, HADS and SAQ21) were performed. Statistical analysis regarding the number of NPS based on demographic and clinical variables were obtained with non-parametric independent samples Mann-Whitney U test and Kruskal-Wallis test. All statistical tests were two-tailed, and the significance threshold was set at P < 0.05. Analyses were carried out using SPSS v.21 and JASP (2020) software.
For the purpose of the current study, only T1-weighted magnetic resonance images (MRI) were acquired at the baseline assessment to delineate the lesion of each participant with aphasia. The MRI sequence was acquired on a 3-T MRI scanner (Philips Intera, Amsterdam, The Netherlands), Release 3.2.3.4, with a MASTER gradient system (nominal maximum gradient strength = 30 mT/m, maximum slew rate = 150 mT/m/ms), equipped with a six-channel Philips SENSE head coil. Lesions were manually drawn in native space by DL-B and MJT-P, who were blind to all clinical data outcomes at the moment of the lesion delineation. Lesion maps were drawn by using Mricron software[47] on a slice-by-slice basis. Lesion overlap maps were created as follow: first, individual lesion maps and T1-weighted images were reoriented according to the anterior commisure. To achieve optimal normalization of the lesions and the T1-weighted images, cost function masking was applied during the preprocessing[48]. T1-weighted images after masking out individual lesions were segmented into different tissues and the resulting parameters were used to normalize both the T1-weighted images and the lesion masks to Montreal Neurological Institute space. Normalized lesion masks were smoothed with a 3 mm FWHM kernel and binarised. The overlap of the resulting binarised masks was performed with ImCalc. All these processing steps were performed with SPM12 (Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/software/spm12).
One hundred fourteen articles covering PSA and NPS (disorders) were included in this narrative review. The studied publications focused primarily on assessment/diagnosis of depression and anxiety (n = 34/29.8%) and on intervention approaches (n = 24/20.5%). Few articles covered prevalence data of NPS in PWA (n = 6/5.2%), risk factors (n = 5/4.2%) or pathophysiological mechanisms (n = 7/6.1%). Over half of all articles covered depression (n = 60/52.6%), 21 articles (18.4%) examined anxiety, and 12 articles (10.5%) referred to quality of life in PWA. Systematic reviews and meta-analysis accounted for 16.6% of all included articles (n = 19). Below, we present the synthesis of published data on depression, anxiety, apathy, agitation/aggression, mania, and psychosis.
Depression: The co-occurrence of stroke and aphasia is a stressful life event resulting in mood alterations and depression[11]. In fact, post-stroke depression has been identified as one of the most frequent and long-term sequela of PWA[11,26,49,50]. The disorder is categorized in the diagnostic and statistical manual for mental disorder-fifth edition (DSM-5) as “mood disorders due to another medical condition” such as stroke with depressive features, major depressive-like episode, or mixed-mood features (simultaneous depression and manic features)[30,51]. A diagnosis of major depression includes depressed mood and/or anhedonia/Loss of interest alongside four other symptoms (weight loss/gain, insomnia/hypersomnia, psychomotor agitation/retardation, fatigue, feelings of worthlessness, diminished concentration, and suicidal ideation) lasting for two or more weeks, and having an impact on daily functioning[51].
Depressive symptoms are associated with communication impairments following stroke, but the prevalence of depression in PWA is seldomly reported[26,28,45,52]. However, if any NPS are reported in PWA it is usually depression. Prevalence of this disorder ranges from 52%[53] to 62%[54] of PWA one year post-stroke, and this incidence is higher than in the overall stroke population[53-56]. As for risk factors, the presence of aphasia augments the chance of developing depressive symptoms[21,29,53,54]. Moreover, depressive symptoms may account for a significant variance in functional communication after acquired aphasia[57] and persons with Broca’s aphasia (non-fluent verbal production with mild impairment in comprehension) are almost 9 times more likely to suffer depression compared to Wernicke’s patients (fluent speech with impaired auditory and reading comprehension)[58]. Regarding aphasia evolution, Herrmann et al[59] compared single left-sided acute to chronic PWA and depression and found no significant between-group differences on depression sum-scores, age, sex, or severity of hemiparesis. On the other hand, Kauhanen et al[60] stated that aphasia increases the risk of developing depression in the chronic phase of stroke (> 6 mo of evolution)[60].
In the absence of suitable biological markers, the assessment and diagnosis of depression must rely on the results of clinical evaluations and psychometric testing[61]. To date, only a handful of instruments for the assessment of clinical depression in PWA have been proposed[26,62,63]. Among the most common are the SADQ-21 and its shortened version (SADQ-10)[38], the Aphasic Depression Rating Scale[64], the Signs of Depression scale[65], or the Depression Intensity Scale Circles[66]. Non-verbal measurements, such as the Dynamic Visual Analogue Mood Scale[67] represent an important step forward in assessing mood in people with language impairments[68] while objective acoustic measures related to affective state change in the speech of PWA are also being developed[69]. On the other hand, the ROMA consensus statements highlight the general health questionnaire-12 for the assessment of emotional wellbeing in PWA[70] In addition, in PWA showing mild deficits in auditory comprehension, it is feasible to use well-known testing scales (e.g., Beck Depression Inventory, the Hamilton Depression Rating Scale or the HADS[27,71-73]).
Causal factors of depression after stroke are probably multifactorial. Alterations in monoamine neurotransmitter systems, higher levels of glutamate in the synaptic cleft, hypothalamic–pituitary– adrenal axis abnormalities, anomalous neurotrophic responses, and an excess of proinflammatory cytokines have all been linked to the pathogenesis of depression after stroke[26,31,74]. The idea that the risk of depression after stroke is influenced by lesion location is still controversial[75]. The hypothesis was first proposed over 30 years ago by Robinson’s group reporting that left-hemisphere strokes, especially in frontal region, were associated with depressive disorders[76,77]. Many replication studies have been carried out since then, but results remain inconclusive. Systematic reviews performed by Carson et al[78] and Wei et al[79] found no support for a higher frequency of depression in frontal left-hemisphere stroke lesions. As Wei et al[79] discuss, most patients with severe aphasia are excluded from studies, and the frequency of depression in left-hemisphere patients may be underestimated. However, a multivariate lesion-symptom mapping study found a significant association between the severity of depression scores and lesions affecting the left dorsolateral prefrontal cortex in 39 PWA and chronic stroke[80]. Therefore, a coherent explanatory model, able to integrate the underlying pathophysiological mechanisms of depression in PWA, still remains to be formulated.
Therapeutic interventions to alleviate depression in PWA are still scarce[81]. In fact, less than one percent of PWA receive direct treatment for psychological distress[82]. In a systematic review of rehabilitation interventions for the prevention and treatment of depression in PWA Baker et al[83] highlight that PWA with mild depression may benefit from psychosocial-type treatments, whereas no evidence was found for the treatment of moderate to severe depression. A systematic review by Wray et al[84] for self-management interventions (i.e., decision-making, problem solving, goal setting) could also not clarify whether these approaches were suitable for PWA, especially with moderate or severe aphasia. More recent reports show that the employment of two weeks of Intensive Language-Action Therapy has proven effective in reducing not only language deficits but also low mood in persons with fluent and non-fluent aphasias[71,85]. This is in line with Baker et al[83]’s statement, who suggested that treatment strategies for the improvement of physical, cognitive and communication functions can have a beneficial effect on both rehabilitation and depression outcomes in PWA. A randomized controlled trial for PWA, comparing behavioral therapy and usual care with a usual care control, showed significant improvement of affective symptomatology in the experimental group at three and six months post-intervention[86]. The development of solution-focused psychotherapy approaches, in addition to behavioral activation therapies, specifically tailored for PWA are also under way[82,87,88]. No studies have been published about the pharmacological treatment of depressed PWA. Neuromodulation techniques, such as transcranial direct current stimulation or repetitive transcranial magnetic stimulation have shown promise for the treatment of depression in PWA[89-91].
Anxiety: Adult anxiety comprises a class of conditions that includes generalized anxiety disorder, panic disorder, and phobias[51,92]. In the context of PWA, the DSM-5 classifies these conditions as anxiety disorders due to another medical condition[51] and clinical criteria are disproportionate fear, apprehension of danger, restlessness and day-to-day distress[93-95]. PWA regularly report feeling anxious when employing language to communicate[96]. In some patients, anxiety during language testing can escalate quickly to frustration, transient bursts of tears, eventually leading to requests of interrupting testing (catastrophic reactions)[97-99]. Such reactions are usually associated to non-fluent aphasias due to anterior left-hemisphere or basal ganglia lesions[100,101]. It is noteworthy that anxiety has received comparatively less attention in PWA than depression[94,101]. Impairments in the ability to communicate is one the most significant sources of stress for PWA[102,103]. To date, the prevalence of anxiety among PWA is estimated to be around 44%, in contrast to the 18%-25% of stroke survivors without language disorders[94,104,105]. Schöttke et al[106] however, find a slightly lower prevalence of both anxiety (29%) and depression (38%) in acute PWA and people with post-stroke anomia. As for risk factors, Pompon et al[107] indicates that PWA are at higher risk for experiencing chronic stress, which, in turn, is associated with increases in depression and anxiety.
Cahana-Amitay and colleagues (2011) coined the term “linguistic anxiety”, to describe a person in whom the deliberate, laborious production of language precipitates the apprehension of committing an error, with the anticipation of linguistic failure serving as the trigger[96,108]. Even in mild aphasia, language-based anxiety can interfere with task performance[108]. Indeed, stress reactivity is considerably higher during linguistic in comparison to non-linguistic tasks[55,94,103] and higher anxiety and stress responses are related to non-fluent aphasias[96,102]. PWA also show heightened physiological arousal and anxiety scores in general compared to stroke patients without aphasia[94,109].
Post-stroke anxiety is assessed via questionnaires and/or clinical interviews and PWA are ordinarily excluded from anxiety evaluations[94,104] as scales to assess post-stroke anxiety in aphasia have not yet been developed and validated[104]. Usually, modified versions of the Behavioural Outcomes of Anxiety Scale[110], the HADS[39], the Generalized Anxiety Disorder-7[111], or the Burden of Stroke Scale[112] are employed to rate anxiety in PWA. In addition, the NPI can be proxy-administered[35].
One potential psychological mechanism underlying linguistic anxiety is the overfocus on the language testing (area of worry), coupled with reduced attentional functions. The patient’s fixation on his/her impaired language performance reduces the ability to follow language assignments, which is signaled by heightened physiological stress responses such as heart rate and skin conductance[96,108]. Premorbid personality traits (self-demand attitude, perfectionism) may also favor the emergence of anxiety in PWA[103]. The pathophysiological mechanisms involved in post-stroke anxiety in PWA remain unknown as there are few studies that explore the physiological stress responses in PWA during language examination[96]. An extended cortical and subcortical network was proposed to be involved in the regulation of stress and anxiety responses, including the reticular system of the brainstem, limbic structures (amygdala), and the frontal lobe, activating both the autonomic nervous system and the hypothalamus-pituitary-adrenal axis[96,108]. However, a recent meta-analysis studying post-stroke anxiety and lesion location found no strong associations[104]. Recently, Ryan et al[113] reported a systematic review of non-pharmacological treatment interventions for anxiety in PWA. The authors did find 10 studies (5 randomized controlled studies) and none of them showed significant improvement of anxiety outcomes in PWA[113]. Torres-Prioris et al[103], stressed the usefulness of including adequately trained laypersons/carers in the evaluation and treatment of PWA to overlook the “white coat” effect. Affected individuals usually show reduced anxiety levels towards familiar people in both evaluation and rehabilitation[103]. A beneficial role of the β-blocker agent propranolol in naming has been suggested[114,115]. It is possible that this agent improves anomia by exerting its anxiolytic effects[115,116].
Apathy: Apathy is defined as a multidimensional syndrome of diminished goal-directed behavior, emotion and cognition resulting in a loss of initiative, decreased interaction with their environment, and interest in social life[117-119]. However, the DMS-5 does not categorize apathy as an independent mental illness but as an incipient symptom in other psychiatric and neurocognitive disorders (e.g., energy loss in major depressive disorder)[51]. The prevalence of apathy in PWA is currently unknown as a previous meta-analysis covering post-stroke apathy could not provide any specific data for the aphasic population[120]. However, Kennedy et al[121] evaluated 19 acute PWA with the Apathy Inventory-Clinical Scale[122] and found that 53% of the sample was apathetic[121]. In fact, during the acute post-stroke phase, aphasia correlates with apathy severity and PWA are also less likely to show resolution of such motivation deficits[121]. Apathy is usually proxy-assessed through the SAS[40], the Apathy Evaluation Scale[123], the Apathy Inventory-Clinical Scale[122] or the Dimensional Apathy Scale[124] in addition to the NPI[24]. Actigraphy records from an unaffected arm may serve to measure poststroke apathy in PWA, but should not be used alone[125]. Crucial brain structures for motivated behavior in healthy people include fronto-striatal circuits (including the nucleus accumbens), the dorsal anterior cingulate and the orbitofrontal cortex[126,127]. On the other hand, Starkstein et al[128] recently reviewed the neuroimaging literature and found that lesions of the basal ganglia are the most common correlates of apathy in stroke. However, no studies have specifically evaluated the neuroimaging correlates of apathy in PWA. In addition, there is a lack of high-quality evidence to guide management of post-stroke apathy[117,120,129] and only one case report described the improvement of apathy and behavioral disinhibition with transcranial direct current stimulation combined with speech-language therapy in patient with severe non-fluent aphasia[91]. The recent Canadian Stroke Best Practice Recommendations specifically endorses to offer nonpharmacological interventions, such as exercise and music therapy, to stroke patients with marked apathy (with or without clinical depression), but not special recommendation were given for PWA[130]. Ideally, treatment would begin soon after stroke, as apathy limits the patients’ ability to participate in the intensive rehabilitation programs.
Agitation and aggression: Agitation, inability to control anger and aggression are observed symptoms in PWA[22,36,131]. Anger represents an emotional reaction, whereas aggressiveness is understood as the subsequent behavioral reaction[132]. As everyday functional communication is reduced in PWA, they can become frustrated, less tolerant and irritable, getting easily angry regarding trivial matters[133]. The study of aggression in PWA has traditionally been difficult, and only few articles have been published[132,134-137]. However, it seems that aphasia is associated with higher levels of anger, as well as loneliness and social isolation[131,132]. Angelelli et al[36] observed three times more risk of agitation in PWA and four times more risk of being irritable than those with normal language. Another study evaluating anger in acute stroke patients found that 31% of participants with aphasia (n = 26) were irritable and aggressive[134]. A more recent study, evaluating anger in acute stroke, found that half of PWA (n = 26) and 10 dysarthric participants (n = 44) displayed anger[135]. On studying mild post-stroke aphasia, Choi-Kwon et al[138] found that lesion location was not related to anger. However, participants with moderate to severe aphasia were excluded, thus biasing the results. There are no validated questionnaires for the assessment of anger in PWA. Instruments employed to evaluate anger in non-aphasic population include the state-trait anger expression inventory-2 or the modified Spielberger trait anger scale[139]. In addition, there are no studies targeting the pathophysiology or treatment of agitation and irritability in PWA.
Hypomania/mania: Elevated mood, hypomania and mania are seldomly reported in PWA, except in aphasic patients with posterior left hemisphere strokes[140]. Mania is defined as an abnormally and persistently raised expansive or irritable mood, thought and speech acceleration, lack of insight, overactivity, and social disinhibition[141]. In the context of PWA, the DSM-5 classifies these conditions as bipolar and related disorders due to another medical condition[51]. In a study conducted by Signers et al[140], one-fifth of participants with chronic fluent aphasia and posterior left hemisphere lesions were elated (a state of extreme happiness or excitement[142]) and unaware of their language impairment[140]. By contrast, elation has not been described among patients with non-fluent aphasia[143], except in a case of mixed transcortical aphasia associated with hypermusia, musicophilia, and compulsive whistling[144]. It seems that mania after left hemisphere damage is rare and according to the sparse published information it is difficult to describe its demographic, clinical and prognostic characteristics[141]. To date, only case reports have been published on mania in PWA[145-147]. These studies suggest that the onset of mania may be delayed up to two years post-stroke[148]. Manic states following stroke are often difficult to treat as brain damage and comorbidities enhance adverse effects and impair efficacy of some antimanic agents[149]. Case reports of post-stroke mania in non-aphasic stroke patients have found lithium, anticonvulsant mood stabilizers (valproate or carbamazepine), atypical antipsychotic drugs (olanzapine, aripiprazole, risperidone), clonazepam and clonidine to be effective[150,151]. However, there are no studies of treatment of hypomania and mania in PWA.
Psychosis: Delusions and hallucinations: Post-stroke psychosis involves the presence of delusions and/or hallucinations[152]. Within the context of PWA, the DSM-5 classifies this conditions as psychotic disorder due to another medical condition[51]. The development of psychosis is considered to be among the most devastating post-stroke syndromes[153]. Delusions in PWA are not rare. Shehata et al[55] evaluated 30 PWA and 31 non-aphasic stroke patients with the Eysenck Personality Questionnaire and found that psychosis was more prominent in PWA. Another study found that 28 PWA out of 61 chronic participants developed delusions, being mostly of persecutory nature[140]. The symptoms were found to be more common with posterior left hemisphere lesions[140], particularly in patients with Wernicke´s aphasia[154], who are more paranoid and aggressive[19,155,156] than patients with anterior lesions who instead may become more frustrated and depressed[133,140]. A detailed language evaluation of Wernicke’s aphasia is desirable because characterization of speech and language deficits can be misinterpreted as psychotic speech disorder[157-159]. Potential explanations for this relationship may include auditory comprehension deficits with misinterpretation of information, in addition to anosognosia for aphasia and psychosis. Up to now, the pathophysiological mechanisms underlying psychosis in PWA are unknown, in part, because these patients are excluded from stroke studies on NPS[25,152]. Treatment approaches for psychosis in PWA are also not currently known. Antipsychotic medication is the main treatment for stroke patients[152] as poststroke and primary psychosis may likely reflect a common mechanism[152,160] but further research is strongly needed for PWA.
Demographic and clinical data: Demographic and clinical data of participants are shown in Table 1. The Barthel Index indicated that most PWA were functionally independent, with a median score of 90 points (range: 45-100). Only one participant (subject 12) with anomic aphasia and a dense right hemiparesis showed high dependency regarding activities of daily living (Barthel Index: 45 points). All participants were in the chronic phase of stroke evolution with a median duration of 18 mo (range: 7-126). Results indicate that 9 patients were diagnosed with anomic aphasia (77.3 ± 6.2 points on the AQ of WAB-R), 5 with Wernicke´s (55.4 ± 15.9 points), 4 with Broca´s (55.7 ± 9.2 points) and 2 with conduction aphasia (64.8 ± 14.9 points). Table 2 displays the number and composite score of NPS in our sample based on the NPI. As can be seen, there was a significant presence of comorbid NPS. In fact, all 20 participants were rated by their informants as exhibiting more than one NPS, except in one participant (subject 15), a female of 28 years of age with mild aphasia, who only showed a high NPI score in changes in appetite/eating behavior. On average, each PWA yielded a median number of 5 NPS (range: 1-8), with a mean composite score of 2 points (range: 1-6), indicating symptoms of mild severity in the chronic phase of stroke evolution.
| NPI symptom | No. of PWA with NPS (max. 20) | Percentage of PWA with NPS (%) | Composite NPI score1 |
| Depression | 15 | 75 | 4 |
| Irritability | 14 | 70 | 2 |
| Agitation | 14 | 70 | 2 |
| Anxiety | 13 | 65 | 2.5 |
| Appetite/eating disorders | 13 | 65 | 1 |
| Apathy | 10 | 50 | 4.5 |
| Disinhibition | 9 | 45 | 1 |
| Sleep/nighttime disturbances | 8 | 40 | 1.5 |
| Euphoria | 1 | 5 | 2 |
| Aberrant motor behavior | 1 | 5 | 2 |
| Hallucinations | 1 | 5 | 1 |
| Delusions | 0 | 0 | 0 |
Based on the result of the NPI, the majority of PWA (75%) had depressive symptoms, followed by agitation and irritability (70%), anxiety and appetite/eating disorders (65%). Half of the sample also showed symptoms of apathy, while sleep disturbances were also relatively frequent (40%). Euphoria and psychotic disorders were rare. The most severe symptoms were apathy, depression, anxiety, agitation, and irritability (see Table 2). Regarding sexes, women had a median number of 6 NPS while men presented 5 NPS. Mann-Whitney U tests showed that there were no statistically significant sex differences concerning the number of NPS (P = 0.841). Antidepressants were taken by 7 patients. Median results showed that participants taking antidepressants were rated with a relatively similar number of NPS (6) compared to the participants without antidepressant intake (5), (P = 0.496). When analyzing the median number of NPS based on aphasia type, participants with Broca´s aphasia presented the highest number of symptoms (6.5) followed by anomic participants (5), conduction aphasia (4.5) and Wernicke´s aphasia (3). However, non-parametric Kruskal-Wallis test showed no statistically significant differences (P = 0.508).
Specific-domain scales revealed that 30% of PWA were above the cut-off score for depression and anxiety (based on the SADQ-10, HADS-anxiety), 40% of patients were diagnosed with subthreshold depression (SADQ-10)[45,85] and 45% of participants had apathy (SAS) (see Table 3). However, percentages of diagnosis of the proxy-administered SADQ-10 stands in contrast to HADS-Depression results. Average scores of these domain-specific scales point into mild disorder severity. There were significant correlations between two domain-specific scales (SADQ-10 and SAS) and the most frequently reported NPI domains (e.g., depression, anxiety, apathy, agitation, and irritability) (see Table 4). No significant correlations were found between both neuropsychiatric scales (NPS and domain-specific scales) and aphasia severity (measured with the AQ of WAB-R), fluency, comprehension, or repetition scores.
| Domain-specific scale (range) | No. of PWA with diagnoses | Percentage of PWA with diagnosis (%) | Median score (range) |
| SADQ-10, depression (0-30) | 6 | 30 | 15 (14-19) |
| SADQ-10, subthreshold depression (0-30) | 9 | 45 | 14 (13-19) |
| SAS, apathy (0-42) | 9 | 45 | 12 (1-35) |
| HADS, anxiety (0-21) | 6 | 30 | 5 (0-12) |
| HADS, depression (0-21) | 3 | 15 | 5.5 (1-14) |
| Domain-specific scale | NPI-subdomains | Spearman correlation (rs) |
| SADQ-10 | NPI-depression | 0.67, P < 0.001a |
| NPI-anxiety | 0.60, P < 0.005a | |
| NPI-apathy | 0.43, P = 0.540 | |
| NPI-agitation | 0.60, P < 0.005a | |
| NPI-irritability | 0.63, P < 0.003a | |
| HADS | NPI-depression | 0.38, P = 0.820 |
| NPI-anxiety | 0.27, P = 0.240 | |
| NPI-apathy | 0.27, P = 0.243 | |
| NPI-agitation | 0.14, P = 0.540 | |
| NPI-irritability | 0.11, P = 0.620 | |
| SAS | NPI-depression | 0.50, P < 0.023b |
| NPI-anxiety | 0.30, P = 0.192 | |
| NPI-apathy | 0.58, P < 0.006a | |
| NPI-agitation | 0.18, P = 0.440 | |
| NPI-irritability | 0.09, P = 0.700 |
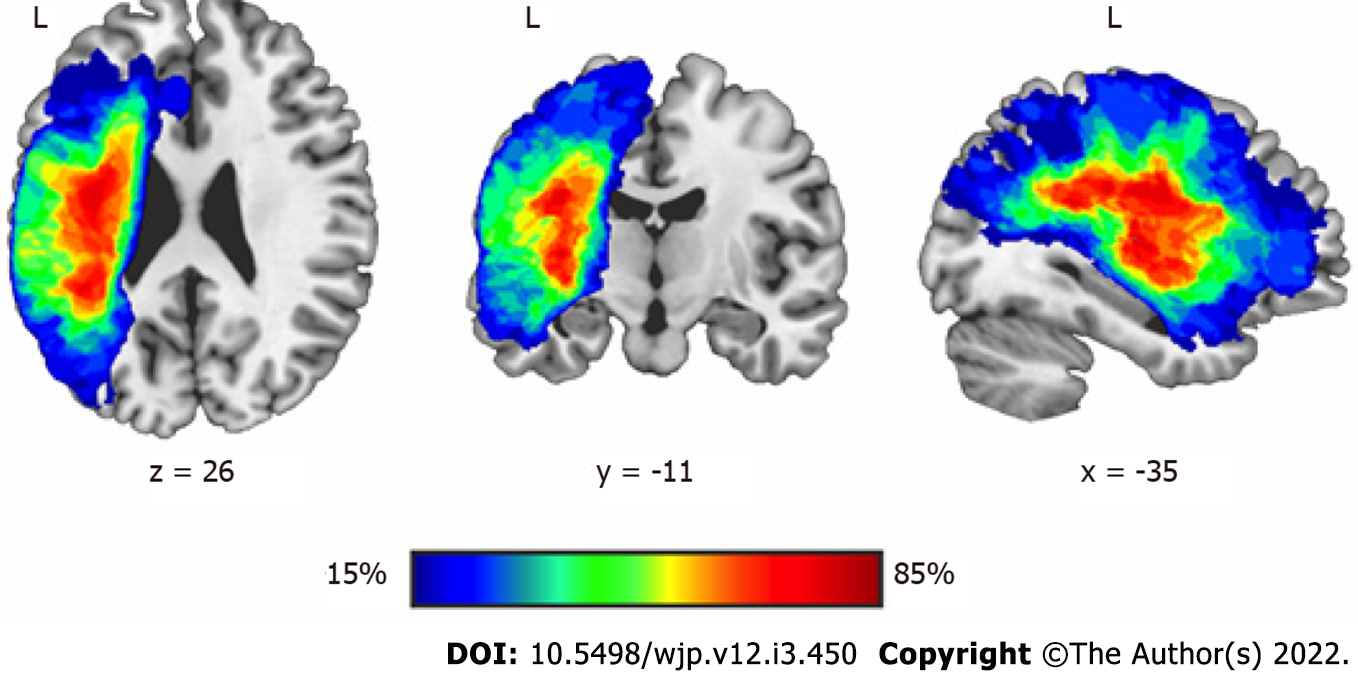
Lesion size and location: The MRIs of participants showed a wide range of lesion volumes (Table 1). Lesion location showed that the maximum areas of overlap comprised regions of the long and the anterior segments of the arcuate fasciculus, the insula and the putamen in the left hemisphere. Involvement of different sectors of the left anterior cingulate gyrus were seen in six participants. The overlay of lesions is shown in Figure 1.
Results of study 2 show that our participants presented mild-to-moderate aphasia severity and were functionally independent. We found a spectrum of comorbid NPS in all but one participant with mild anomic aphasia. On average PWA had a median number of 5 NPS (range: 1-8). The most frequent symptoms were depression, irritability, agitation/aggression, and anxiety, followed by appetite/eating disorders, apathy, and sleep disorders, whereas euphoria, delusions/hallucinations were rare. Apathy and depressive symptoms were rated as the most severe by their caregivers, followed by anxiety and agitation. There were no statistically significant differences regarding the number of NPS based on sex, antidepressant intake or aphasia type. When employing domain-specific scales that provide cut-off scores for diagnoses, 30% of participants had mild anxiety and depression, 45% showed subthreshold depression and 45% of participants had mild-to-moderate apathy.
The prevalence of depressive and anxiety symptoms based on the NPI was higher than the frequency of these disorders using the SADQ-10 and HADS. As mentioned, the NPI is an informant-based questionnaire developed to screen for the presence of symptoms, but not to establish diagnoses of mental disorders. Thus, we expected to find a higher number of symptoms with the NPI in contrast to domain-specific scales. Results revealed a higher percentage of depression with the SADQ-10 than with the HADS, therefore showing a low level of congruency between these proxy and self-rated measures. Correlation analyses between NPI subdomains and domain-specific scales showed that the SADQ-10 correlated with a higher number NPI subdomains (depression, anxiety, irritability, and agitation) than the HADS (no associations found). The SAS, on the other hand, showed a significant correlation with NPI subdomains of apathy and depression. In general, it seems that proxy-rated neuropsychiatric instruments (e.g., SADQ-10) are more sensitive to evaluate PWA than directly considering aphasic individuals themselves (e.g., HADS) because of cognitive or communication problems. In support of these findings, outcome differences between proxy-based and directly administered instruments have also been described in other studies regarding PWA[161]. Moreover, family members have generally been found to be reliable informants in areas of emotions, daily activities, well-being, and overall quality of life[162]. In fact, Bourgeois et al[21] advise physicians to give credence to caregivers’ testimonies about the behavior of PWA. Nevertheless, the opinion of informants should not jeopardize the autonomy and self-determination of PWA[163]. In general, more studies regarding the reliability and validity of neuropsychiatric proxy and self-measured instruments in PWA are strongly needed. Lastly, we found a lack of correlation between neuropsychiatric assessment tools (NPI and domain-specific scales) and WAB-R. These results align with findings from another study showing no correlations between WAB-R and depression scores based on the SADQ-21 in PWA and chronic left hemisphere strokes[80].
Structural MRIs in our sample showed a wide range of lesion volumes. There was a predominant involvement of the left perisylvian language core and lesion overlap analysis showed that the region corresponding to the arcuate fasciculus, insula and putamen were affected in 17 participants (85%). The insular cortex together with the striatum and the anterior cingulate gyrus (affected in 6 participants) are intrinsic components of the Salience Network[164]. The Salience Network is composed of two major hubs, anterior insula and dorsal anterior cingulate cortex. It also included three interconnected subcortical hubs: amygdala, ventral striatum, and substantia nigra/ventral tegmental area[165]. This network, among others, contributes to complex brain functions such as communication, social behavior, and self-awareness, by means of integrating of sensory, emotional, and cognitive information[164,165]. Damage to the left Salience Network in our sample may have impaired self-regulation of cognition, behavior, emotion and autonomic arousal favoring the emergence of an array of NPS[164]. Moreover, lesions in the left arcuate fasciculus have been associated with affective symptoms and somatic depressive complaints[166] and preliminary findings show that the lesion load in the left arcuate fasciculus correlates with naming improvement in PWA treated with antidepressants[167]. In any case, the role of the arcuate fasciculus in the NPS of PWA requires further analysis.
Some limitations to the current study should be acknowledged. First, this was a relatively small sample including people with chronic PSA of mild to moderate severity, so that it is not representative of all PWA and stroke. Another limitation is that we only used three domain-specific scales, whereas the NPI assesses twelve NPS. Nevertheless, we have evaluated the three most prevalent neuropsychiatric disorders already found in stroke patients without aphasia. In any case, future studies may include further domain-specific scales targeting other neuropsychiatric disorders. A longitudinal study to evaluate the evolution of NPS from the acute to the chronic phase of stroke survivors is also warranted.
We did find that NPS in PWA are insufficiently investigated. Prevalence of NPS in PWA is unknown, hindering the development of assessment tools and treatment strategies. If reported, most researchers and clinicians tend to focus mostly on diagnosing depression to the extent that there are no reports on symptoms of disinhibition, aberrant motor behavior, appetite-eating disorders, or sleep disturbances, already identified in non-aphasic stroke patients using the NPI. In addition, no pharmacological randomized controlled trials have been published for the reviewed symptoms in PWA. Pharmacotherapy, neuromodulation and behavioral therapies have only been implemented for depression and/or anxiety. Therefore, further research on the prevalence, assessment, pathophysiology, and treatment of NPS in PSA is strongly needed.
The comorbidity of NPS in patients with chronic PSA is very frequent and seems to exceed the prevalence data reported in the non-aphasic stroke population. Therefore, more studies are necessary as NPS are still underdiagnosed in chronic PSA. Our study 1 shows the paucity of reports dealing with NPS diagnosis, assessment, and treatment in PWA. In our study 2, we found high comorbidity of NPS among a small sample of PWA. Findings from study 2 suggest that the NPI may be used as a screening instrument and this assessment can be complemented with domain-specific psychiatric scales. Further aims must attempt to develop structured interviews and guidelines for the diagnosis, treatment, or prevention of comorbid NPS in PWA.
Many important questions regarding the neuropsychiatric spectrum in PWA remain unanswered or unaddressed. What is the frequency of NPS in acute aphasic stroke patients? Which are the best psychometric instruments to evaluate NPS? What is the best combination of self-rated and proxy-based measures depending on the severity of language impairment (production and/or comprehension deficits)? Which are the most important demographic variables that affect the occurrence of NPS in PWA? How do premorbid psychiatric conditions affect the occurrence and clinical phenomenology of NPS and language deficits after stroke? Is there any relationship between anosognosia for aphasia and NPS (hypomania/mania, psychosis)? How do NPS evolve or remit spontaneously? Are psychopharmacological agents including cognitive enhancing drugs useful? What kind of behavioral therapies should be applied for NPS in PWA? Does aphasia therapy positively influence psychiatric outcomes? Does the treatment of one NPS affect the outcome and comorbidity of other symptoms? Should biological treatments be prioritized over behavioral approaches, or should they be combined?
Aphasia due to stroke is associated with worse outcomes than in non-aphasic stroke patients. Worse outcomes in post-stroke aphasia often result from the co-occurrence of neuropsychiatric symptoms (NPS) and disorders.
Persons with aphasia (PWA) are frequently excluded from studies on stroke related NPS because of their language and communication deficits. The exclusion of PWA and stroke hinders obtaining relevant information on prevalence, diagnosis, associated deficits (cognitive impairment, functional disability), assessment, neurobiological mechanisms, and treatment of NPS in this population.
We report a two-part study consisting of a literature review on NPS (study 1) and an observational study on NPS in chronic post-stroke aphasia (study 2).
In study 1, we reviewed the databases after cross-referencing key words of post-stroke aphasia to NPS and disorders. In study 2, we evaluated aphasic deficits, activities of daily living and a spectrum of NPS and disorders using well-validated scales in 20 persons with chronic mild-to-moderate post-stroke aphasia associated with left hemisphere strokes. NPS were evaluated with the 12 symptom domains of the Neuropsychiatric Inventory and with three domain-specific scales for depression, anxiety, and apathy.
The literature review performed in study 1 revealed a spectrum of NPS in PSA including depression, anxiety, apathy, agitation/aggression, psychosis, and hypomania/mania. This broad spectrum of NPS was also found in observational study 2, since all but one PWA has more than one NPS (median number of NPS: 5, range: 1-8).
A spectrum of NPS is highly prevalent in chronic PSA. Therefore, future comprehensive evaluations of NPS using multidomain and domain-specific scales will enable a better characterization of this broad spectrum favoring the design and implementation of adequate therapies.
Since the spectrum of NPS in PWA and stroke is an underexplored research area, there are still many pending issues to be addressed. Essential areas of inquiry include knowing the incidence in acute and chronic stroke periods, risk factors (family and personal history of psychiatric disorders), clinical features, assessment instruments devised to test language and communication impaired patients, impact on quality of life, neurobiological correlates, short- and long-term outcomes, and response to psychological and biological interventions.
| 1. | Berthier ML. Poststroke aphasia: epidemiology, pathophysiology and treatment. Drugs Aging. 2005;22:163-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 244] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Hilari K, Needle JJ, Harrison KL. What are the important factors in health-related quality of life for people with aphasia? Arch Phys Med Rehabil. 2012;93:S86-S95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 3. | Code C, Hemsley G, Herrmann M. The emotional impact of aphasia. Semin Speech Lang. 1999;20:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Flowers HL, Skoretz SA, Silver FL, Rochon E, Fang J, Flamand-Roze C, Martino R. Poststroke Aphasia Frequency, Recovery, and Outcomes: A Systematic Review and Meta-Analysis. Arch Phys Med Rehabil. 2016;97:2188-2201.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 287] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 5. | Lazar RM, Boehme AK. Aphasia As a Predictor of Stroke Outcome. Curr Neurol Neurosci Rep. 2017;17:83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Berthier ML, Dávila G, García-Casares N, Moreno-Torres I. Post-stroke Aphasia. In: Schweizer TA, Macdonald RL. The Behavioral Consequences of Stroke. New York: Springer, 2014: 95-118. |
| 7. | Gleichgerrcht E, Kocher M, Nesland T, Rorden C, Fridriksson J, Bonilha L. Preservation of structural brain network hubs is associated with less severe post-stroke aphasia. Restor Neurol Neurosci. 2016;34:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Flamand-Roze C, Cauquil-Michon C, Roze E, Souillard-Scemama R, Maintigneux L, Ducreux D, Adams D, Denier C. Aphasia in border-zone infarcts has a specific initial pattern and good long-term prognosis. Eur J Neurol. 2011;18:1397-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Landrigan JF, Zhang F, Mirman D. A data-driven approach to post-stroke aphasia classification and lesion-based prediction. Brain. 2021;144:1372-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Halai AD, Woollams AM, Lambon Ralph MA. Using principal component analysis to capture individual differences within a unified neuropsychological model of chronic post-stroke aphasia: Revealing the unique neural correlates of speech fluency, phonology and semantics. Cortex. 2017;86:275-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 11. | Baker C, Worrall L, Rose M, Ryan B. ‘It was really dark’: the experiences and preferences of people with aphasia to manage mood changes and depression. Aphasiology. 2020;34:19-46. [DOI] [Full Text] |
| 12. | Worrall LE, Hudson K, Khan A, Ryan B, Simmons-Mackie N. Determinants of living Well With Aphasia in the First Year Poststroke: A Prospective Cohort Study. Arch Phys Med Rehabil. 2017;98:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Code C, Herrmann M. The relevance of emotional and psychosocial factors in aphasia to rehabilitation. Neuropsychol Rehabil. 2003;13:109-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Manning M, MacFarlane A, Hickey A, Franklin S. Perspectives of people with aphasia post-stroke towards personal recovery and living successfully: A systematic review and thematic synthesis. PLoS One. 2019;14:e0214200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Lam JM, Wodchis WP. The relationship of 60 disease diagnoses and 15 conditions to preference-based health-related quality of life in Ontario hospital-based long-term care residents. Med Care. 2010;48:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Nemani K, Gurin L. Neuropsychiatric Complications after Stroke. Semin Neurol. 2021;41:85-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Bullier B, Cassoudesalle H, Villain M, Cogné M, Mollo C, De Gabory I, Dehail P, Joseph PA, Sibon I, Glize B. New factors that affect quality of life in patients with aphasia. Ann Phys Rehabil Med. 2020;63:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Hackett ML, Köhler S, O'Brien JT, Mead GE. Neuropsychiatric outcomes of stroke. Lancet Neurol. 2014;13:525-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 219] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 19. | Ferro JM, Caeiro L, Figueira ML. Neuropsychiatric sequelae of stroke. Nat Rev Neurol. 2016;12:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 20. | Zhang S, Xu M, Liu ZJ, Feng J, Ma Y. Neuropsychiatric issues after stroke: Clinical significance and therapeutic implications. World J Psychiatry. 2020;10:125-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (5)] |
| 21. | Bourgeois JA, Hilty DM, Chang CH, Wineinger MA, Servis ME. Poststroke Neuropsychiatric Illness: An Integrated Approach to Diagnosis and Management. Curr Treat Options Neurol. 2004;6:403-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Ferro JM, Santos AC. Emotions after stroke: A narrative update. Int J Stroke. 2020;15:256-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 23. | Wong A, Lau AY, Yang J, Wang Z, Liu W, Lam BY, Au L, Shi L, Wang D, Chu WC, Xiong YY, Lo ES, Law LS, Leung TW, Lam LC, Chan AY, Soo YO, Leung EY, Wong LK, Mok VC. Neuropsychiatric Symptom Clusters in Stroke and Transient Ischemic Attack by Cognitive Status and Stroke Subtype: Frequency and Relationships with Vascular Lesions, Brain Atrophy and Amyloid. PLoS One. 2016;11:e0162846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5000] [Cited by in RCA: 5532] [Article Influence: 172.9] [Reference Citation Analysis (1)] |
| 25. | Townend E, Brady M, McLaughlan K. Exclusion and inclusion criteria for people with aphasia in studies of depression after stroke: a systematic review and future recommendations. Neuroepidemiology. 2007;29:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Laures-Gore JS, Dotson VM, Belagaje S. Depression in Poststroke Aphasia. Am J Speech Lang Pathol. 2020;29:1798-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Døli H, Helland T, Helland WA. Self-reported symptoms of anxiety and depression in chronic stroke patients with and without aphasia. Aphasiology. 2017;31:1392-1409. [DOI] [Full Text] |
| 28. | Brady MC, Fredrick A, Williams B. People with aphasia: capacity to consent, research participation and intervention inequalities. Int J Stroke. 2013;8:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Perrain R, Mekaoui L, Calvet D, Mas JL, Gorwood P. A meta-analysis of poststroke depression risk factors comparing depressive-related factors versus others. Int Psychogeriatr. 2020;32:1331-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Robinson RG, Jorge RE. Post-Stroke Depression: A Review. Am J Psychiatry. 2016;173:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 730] [Article Influence: 73.0] [Reference Citation Analysis (1)] |
| 31. | Medeiros GC, Roy D, Kontos N, Beach SR. Post-stroke depression: A 2020 updated review. Gen Hosp Psychiatry. 2020;66:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 362] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 32. | Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26222] [Cited by in RCA: 28235] [Article Influence: 513.4] [Reference Citation Analysis (0)] |
| 33. | Kertesz A, Raven JC. Western Aphasia Battery Revised. San Antonio: PsychCorp, 2007. |
| 34. | Mahoney FI, Barthel DW. Functional Evaluation: The Barthel Index. Md State Med J. 1965;14:61-65. [PubMed] |
| 35. | Frey KL, Newman JK, Arciniegas DB, Anderson CA, Ramsberger G. Assessing neuropsychiatric disturbances associated with post-stroke aphasia. J Neuropsychiatry Clin Neurosci. 2011;23:E4-E5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 36. | Angelelli P, Paolucci S, Bivona U, Piccardi L, Ciurli P, Cantagallo A, Antonucci G, Fasotti L, Di Santantonio A, Grasso MG, Pizzamiglio L. Development of neuropsychiatric symptoms in poststroke patients: a cross-sectional study. Acta Psychiatr Scand. 2004;110:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Knutson KM, Dal Monte O, Schintu S, Wassermann EM, Raymont V, Grafman J, Krueger F. Areas of Brain Damage Underlying Increased Reports of Behavioral Disinhibition. J Neuropsychiatry Clin Neurosci. 2015;27:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Sutcliffe LM, Lincoln NB. The assessment of depression in aphasic stroke patients: the development of the Stroke Aphasic Depression Questionnaire. Clin Rehabil. 1998;12:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28548] [Cited by in RCA: 32884] [Article Influence: 764.7] [Reference Citation Analysis (0)] |
| 40. | Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci. 1992;4:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 865] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 41. | Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6329] [Cited by in RCA: 7483] [Article Influence: 311.8] [Reference Citation Analysis (0)] |
| 42. | Starkstein SE, Fedoroff JP, Price TR, Leiguarda R, Robinson RG. Apathy following cerebrovascular lesions. Stroke. 1993;24:1625-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 255] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 43. | Morita H, Kannari K. Reliability and validity assessment of an apathy scale for home-care patients with Parkinson's disease: a structural equation modeling analysis. J Phys Ther Sci. 2016;28:1724-1727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Leeds L, Meara RJ, Hobson JP. The utility of the Stroke Aphasia Depression Questionnaire (SADQ) in a stroke rehabilitation unit. Clin Rehabil. 2004;18:228-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Ashaie SA, Hurwitz R, Cherney LR. Depression and Subthreshold Depression in Stroke-Related Aphasia. Arch Phys Med Rehabil. 2019;100:1294-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Bennett HE, Thomas SA, Austen R, Morris AM, Lincoln NB. Validation of screening measures for assessing mood in stroke patients. Br J Clin Psychol. 2006;45:367-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1813] [Cited by in RCA: 2084] [Article Influence: 83.4] [Reference Citation Analysis (0)] |
| 48. | Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14:486-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 713] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 49. | Wipprecht M, Grötzbach H. Poststroke Depression bei Aphasie: Diagnose und Behandlungsmöglichkeiten. Neuro Geriatrie. 2013;10:149-159. [DOI] [Full Text] |
| 50. | Baker C, Worrall L, Rose M, Ryan B. Experiences of mood changes and depression after post-stroke aphasia. Aphasiology. 2018;32:11-12. [DOI] [Full Text] |
| 51. | American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA, 2013. |
| 52. | Hilari K. The impact of stroke: are people with aphasia different to those without? Disabil Rehabil. 2011;33:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 53. | Mitchell AJ, Sheth B, Gill J, Yadegarfar M, Stubbs B, Meader N. Prevalence and predictors of post-stroke mood disorders: A meta-analysis and meta-regression of depression, anxiety and adjustment disorder. Gen Hosp Psychiatry. 2017;47:48-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 262] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 54. | Kauhanen ML, Korpelainen JT, Hiltunen P, Määttä R, Mononen H, Brusin E, Sotaniemi KA, Myllylä VV. Aphasia, depression, and non-verbal cognitive impairment in ischaemic stroke. Cerebrovasc Dis. 2000;10:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 279] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 55. | Shehata GA, El Mistikawi T, Risha AS, Hassan HS. The effect of aphasia upon personality traits, depression and anxiety among stroke patients. J Affect Disord. 2015;172:312-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 56. | Cruice M, Worrall L, Hickson L. Health-related quality of life in people with aphasia: implications for fluency disorders quality of life research. J Fluency Disord. 2010;35:173-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 57. | Fucetola R, Connor LT, Perry J, Leo P, Tucker F, Corbetta M. Aphasia severity, semantics, and depression predict functional communication in acquired aphasia. Aphasiology. 2006;20:449-461. [DOI] [Full Text] |
| 58. | Robinson RG, Boston JD, Starkstein SE, Price TR. Comparison of mania and depression after brain injury: causal factors. Am J Psychiatry. 1988;145:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 59. | Herrmann M, Bartels C, Wallesch CW. Depression in acute and chronic aphasia: symptoms, pathoanatomical-clinical correlations and functional implications. J Neurol Neurosurg Psychiatry. 1993;56:672-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Kauhanen M, Korpelainen JT, Hiltunen P, Brusin E, Mononen H, Määttä R, Nieminen P, Sotaniemi KA, Myllylä VV. Poststroke depression correlates with cognitive impairment and neurological deficits. Stroke. 1999;30:1875-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 248] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 61. | Carota A, Staub F, Bogousslavsky J. Emotions, behaviours and mood changes in stroke. Curr Opin Neurol. 2002;15:57-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Townend E, Brady M, McLaughlan K. A systematic evaluation of the adaptation of depression diagnostic methods for stroke survivors who have aphasia. Stroke. 2007;38:3076-3083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | van Dijk MJ, de Man-van Ginkel JM, Hafsteinsdóttir TB, Schuurmans MJ. Identifying depression post-stroke in patients with aphasia: a systematic review of the reliability, validity and feasibility of available instruments. Clin Rehabil. 2016;30:795-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 64. | Benaim C, Cailly B, Perennou D, Pelissier J. Validation of the aphasic depression rating scale. Stroke. 2004;35:1692-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Hammond MF, O'Keeffe ST, Barer DH. Development and validation of a brief observer-rated screening scale for depression in elderly medical patients. Age Ageing. 2000;29:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Turner-Stokes L, Kalmus M, Hirani D, Clegg F. The Depression Intensity Scale Circles (DISCs): a first evaluation of a simple assessment tool for depression in the context of brain injury. J Neurol Neurosurg Psychiatry. 2005;76:1273-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Barrows PD, Thomas SA. Assessment of mood in aphasia following stroke: validation of the Dynamic Visual Analogue Mood Scales (D-VAMS). Clin Rehabil. 2018;32:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Barrows PD, Thomas SA, Van Gordon W. Assessing Self-Reported Mood in Aphasia Following Stroke: Challenges, Innovations and Future Directions. J Stroke Cerebrovasc Dis. 2021;30:105425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Gillespie S, Laures-Gore J, Moore E, Farina M, Russell S, Haaland B. Identification of Affective State Change in Adults With Aphasia Using Speech Acoustics. J Speech Lang Hear Res. 2018;61:2906-2916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 70. | Wallace SJ, Worrall L, Rose T, Dorze G, Le Breitenstein C, Hilari K, Babbitt E, Bose A, Brady M, Cherney LR, Copland D, Cruice M, Enderby P, Hersh D, Howe T, Kelly H, Kiran S, Rochon E. A core outcome set for aphasia treatment research : The ROMA consensus statement. Int. J. Stroke 2019 14, 180–185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 71. | Mohr B, Stahl B, Berthier ML, Pulvermüller F. Intensive Communicative Therapy Reduces Symptoms of Depression in Chronic Nonfluent Aphasia. Neurorehabil Neural Repair. 2017;31:1053-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Wallace SJ, Worrall L, Le Dorze G, Brandenburg C, Foulkes J, Rose TA. Many ways of measuring: A scoping review of measurement instruments for use with people with aphasia. Aphasiology. 2020;1-66. [DOI] [Full Text] |
| 73. | Berg A, Lönnqvist J, Palomäki H, Kaste M. Assessment of depression after stroke: a comparison of different screening instruments. Stroke. 2009;40:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 74. | Murakami T, Hama S, Yamashita H, Onoda K, Kobayashi M, Kanazawa J, Yamawaki S, Kurisu K. Neuroanatomic pathways associated with poststroke affective and apathetic depression. Am J Geriatr Psychiatry. 2013;21:840-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 75. | Feng C, Fang M, Liu XY. The neurobiological pathogenesis of poststroke depression. ScientificWorldJournal. 2014;2014:521349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 76. | Robinson RG, Starkstein SE. Current research in affective disorders following stroke. J Neuropsychiatry Clin Neurosci. 1990;2:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 92] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 77. | Robinson RG, Kubos KL, Starr LB, Rao K, Price TR. Mood changes in stroke patients: relationship to lesion location. Compr Psychiatry. 1983;24:555-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 133] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 78. | Carson AJ, MacHale S, Allen K, Lawrie SM, Dennis M, House A, Sharpe M. Depression after stroke and lesion location: a systematic review. Lancet. 2000;356:122-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 374] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 79. | Wei N, Yong W, Li X, Zhou Y, Deng M, Zhu H, Jin H. Post-stroke depression and lesion location: a systematic review. J Neurol. 2015;262:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 80. | Grajny K, Pyata H, Spiegel K, Lacey EH, Xing S, Brophy C, Turkeltaub PE. Depression Symptoms in Chronic Left Hemisphere Stroke Are Related to Dorsolateral Prefrontal Cortex Damage. J Neuropsychiatry Clin Neurosci. 2016;28:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 81. | Allida S, Cox KL, Hsieh CF, Lang H, House A, Hackett ML. Pharmacological, Psychological, and Noninvasive Brain Stimulation Interventions for Treating Depression After Stroke. Stroke. 2020;51:e259-e260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 82. | Santo Pietro MJ, Marks DR, Mullen A. When Words Fail: Providing Effective Psychological Treatment for Depression in Persons with Aphasia. J Clin Psychol Med Settings. 2019;26:483-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 83. | Baker C, Worrall L, Rose M, Hudson K, Ryan B, O'Byrne L. A systematic review of rehabilitation interventions to prevent and treat depression in post-stroke aphasia. Disabil Rehabil. 2018;40:1870-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 84. | Wray F, Clarke D, Forster A. Post-stroke self-management interventions: a systematic review of effectiveness and investigation of the inclusion of stroke survivors with aphasia. Disabil Rehabil. 2018;40:1237-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 85. | Berthier ML, Edelkraut L, Mohr B, Pulvermüller F, Starkstein SE, Green-Heredia C, Dávila G. Intensive aphasia therapy improves low mood in fluent post-stroke aphasia: Evidence from a case-controlled study. Neuropsychol Rehabil. 2022;32:148-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 86. | Thomas SA, Walker MF, Macniven JA, Haworth H, Lincoln NB. Communication and Low Mood (CALM): a randomized controlled trial of behavioural therapy for stroke patients with aphasia. Clin Rehabil. 2013;27:398-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 87. | Northcott S, Thomas S, James K, Simpson A, Hirani S, Barnard R, Hilari K. Solution Focused Brief Therapy in Post-Stroke Aphasia (SOFIA): feasibility and acceptability results of a feasibility randomised wait-list controlled trial. BMJ Open. 2021;11:e050308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 88. | Campanella W, Pedrini R, Vestito L, Marinelli L, Trompetto C, Mori L. Transcranial Direct Current Stimulation in the Treatment of Subacute Post-Stroke Thalamic Aphasia. Eur J Case Rep Intern Med. 2020;7:001794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 89. | Northcott S, Burns K, Simpson A, Hilari K. 'Living with Aphasia the Best Way I Can': A Feasibility Study Exploring Solution-Focused Brief Therapy for People with Aphasia. Folia Phoniatr Logop. 2015;67:156-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 90. | Khedr EM, Abo El-Fetoh N, Ali AM, El-Hammady DH, Khalifa H, Atta H, Karim AA. Dual-hemisphere repetitive transcranial magnetic stimulation for rehabilitation of poststroke aphasia: a randomized, double-blind clinical trial. Neurorehabil Neural Repair. 2014;28:740-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 91. | Valiengo L, Casati R, Bolognini N, Lotufo PA, Benseñor IM, Goulart AC, Brunoni AR. Transcranial direct current stimulation for the treatment of post-stroke depression in aphasic patients: a case series. Neurocase. 2016;22:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 92. | Rafsten L, Danielsson A, Sunnerhagen KS. Anxiety after stroke: A systematic review and meta-analysis. J. Rehabil. Med.. 50:, 769-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 93. | Naghavi FS, Koffman EE, Lin B, Du J. Post-stroke neuronal circuits and mental illnesses. Int J Physiol Pathophysiol Pharmacol. 2019;11:1-11. [PubMed] |
| 94. | Morris R, Eccles A, Ryan B, Kneebone II. Prevalence of anxiety in people with aphasia after stroke. Aphasiology. 2017;31:1410-1415. [DOI] [Full Text] |
| 95. | Kim JS. Post-stroke Mood and Emotional Disturbances: Pharmacological Therapy Based on Mechanisms. J Stroke. 2016;18:244-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 96. | Cahana-Amitay D, Albert ML, Pyun SB, Westwood A, Jenkins T, Wolford S, Finley M. Language as a Stressor in Aphasia. Aphasiology. 2011;25:593-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 97. | Carota A, Rossetti AO, Karapanayiotides T, Bogousslavsky J. Catastrophic reaction in acute stroke: a reflex behavior in aphasic patients. Neurology. 2001;57:1902-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 98. | Berthier ML, Starkstein SE. Catastrophic reaction in crossed aphasia. Aphasiology. 1994;8:89-95. [DOI] [Full Text] |
| 99. | Auerbach S, Karow CM. Neurobehavioral assessment of mood and affect in patients with neurological disorders. Semin Speech Lang. 2003;24:131-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 100. | Starkstein SE, Fedoroff JP, Price TR, Leiguarda R, Robinson RG. Catastrophic reaction after cerebrovascular lesions: frequency, correlates, and validation of a scale. J Neuropsychiatry Clin Neurosci. 1993;5:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 101. | Sagen U, Finset A, Moum T, Mørland T, Vik TG, Nagy T, Dammen T. Early detection of patients at risk for anxiety, depression and apathy after stroke. Gen Hosp Psychiatry. 2010;32:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 102. | Laures-Gore JS, Buchanan TW. Aphasia and the neuropsychobiology of stress. J Clin Exp Neuropsychol. 2015;37:688-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 103. | Torres-Prioris MJ, López-Barroso D, Paredes-Pacheco J, Roé-Vellvé N, Dawid-Milner MS, Berthier ML. Language as a Threat: Multimodal Evaluation and Interventions for Overwhelming Linguistic Anxiety in Severe Aphasia. Front Psychol. 2019;10:678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 104. | Campbell Burton CA, Murray J, Holmes J, Astin F, Greenwood D, Knapp P. Frequency of anxiety after stroke: a systematic review and meta-analysis of observational studies. Int J Stroke. 2013;8:545-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 266] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 105. | Knapp P, Dunn-Roberts A, Sahib N, Cook L, Astin F, Kontou E, Thomas SA. Frequency of anxiety after stroke: An updated systematic review and meta-analysis of observational studies. Int J Stroke. 2020;15:244-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 106. | Schöttke H, Giabbiconi CM. Post-stroke depression and post-stroke anxiety: prevalence and predictors. Int Psychogeriatr. 2015;27:1805-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 107. | Hunting-Pompon R, Smith AN, Baylor C, Kendall D. Exploring associations between a biological marker of chronic stress and reported depression and anxiety in people with aphasia. J Speech Lang Hear Res. 2019;62:4119-4130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 108. | Cahana-Amitay D, Oveis AC, Sayers JT, Pineles SL, Spiro A 3rd, Albert ML. Biomarkers of "Linguistic Anxiety" in aphasia: a proof-of-concept case study. Clin Linguist Phon. 2015;29:401-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 109. | Ayerbe L, Ayis SA, Crichton S, Wolfe CD, Rudd AG. Natural history, predictors and associated outcomes of anxiety up to 10 years after stroke: the South London Stroke Register. Age Ageing. 2014;43:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 110. | Linley-Adams B, Morris R, Kneebone I. The Behavioural Outcomes of Anxiety scale (BOA): a preliminary validation in stroke survivors. Br J Clin Psychol. 2014;53:451-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11947] [Cited by in RCA: 20847] [Article Influence: 1042.4] [Reference Citation Analysis (0)] |
| 112. | Doyle PJ, McNeil MR, Mikolic JM, Prieto L, Hula WD, Lustig AP, Ross K, Wambaugh JL, Gonzalez-Rothi LJ, Elman RJ. The Burden of Stroke Scale (BOSS) provides valid and reliable score estimates of functioning and well-being in stroke survivors with and without communication disorders. J Clin Epidemiol. 2004;57:997-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 113. | Ryan BJ, Clunne SM, Baker CJ, Shiggins C, Rose ML, Kneebone II. A systematic review of non-drug interventions to prevent and treat anxiety in people with aphasia after stroke. Disabil Rehabil. 2021;1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 114. | Tanaka Y, Cahana-Amitay D, Añbert M, Fujita K, Chieko N. Miyazaki, M. Treatment of anxiety in aphasia. Procedia Soc Behav Sci. 2010;6:252-253. [DOI] [Full Text] |
| 115. | Beversdorf DQ, Sharma UK, Phillips NN, Notestine MA, Slivka AP, Friedman NM, Schneider SL, Nagaraja HN, Hillier A. Effect of propranolol on naming in chronic Broca's aphasia with anomia. Neurocase. 2007;13:256-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 116. | Cahana-Amitay D, Albert ML, Oveis A. Psycholinguistics of Aphasia Pharmacotherapy: Asking the Right Questions. Aphasiology. 2014;28:133-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 117. | Tay J, Morris RG, Markus HS. Apathy after stroke: Diagnosis, mechanisms, consequences, and treatment. Int J Stroke. 2021;16:510-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 118. | Jorge RE, Starkstein SE, Robinson RG. Apathy following stroke. Can J Psychiatry. 2010;55:350-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 119. | Starkstein SE, Manes F. Apathy and depression following stroke. CNS Spectr. 2000;5:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 120. | Caeiro L, Ferro JM, Costa J. Apathy secondary to stroke: a systematic review and meta-analysis. Cerebrovasc Dis. 2013;35:23-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 121. | Kennedy JM, Granato DA, Goldfine AM. Natural History of Poststroke Apathy During Acute Rehabilitation. J Neuropsychiatry Clin Neurosci. 2015;27:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 122. | Robert PH, Clairet S, Benoit M, Koutaich J, Bertogliati C, Tible O, Caci H, Borg M, Brocker P, Bedoucha P. The apathy inventory: assessment of apathy and awareness in Alzheimer's disease, Parkinson's disease and mild cognitive impairment. Int J Geriatr Psychiatry. 2002;17:1099-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 272] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 123. | Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38:143-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1277] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 124. | Quang H, Wong S, Husain M, Piguet O, Hodges JR, Irish M, Kumfor F. Beyond language impairment: Profiles of apathy in primary progressive aphasia. Cortex. 2021;139:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 125. | Goldfine AM, Dehbandi B, Kennedy JM, Sabot B, Semper C, Putrino D. Quantifying Poststroke Apathy With Actimeters. J Neuropsychiatry Clin Neurosci. 2016;28:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 126. | Husain M, Roiser JP. Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat Rev Neurosci. 2018;19:470-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 424] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 127. | Le Heron C, Apps MAJ, Husain M. The anatomy of apathy: A neurocognitive framework for amotivated behaviour. Neuropsychologia. 2018;118:54-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 262] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 128. | Starkstein SE, Brockman S. The neuroimaging basis of apathy: Empirical findings and conceptual challenges. Neuropsychologia. 2018;118:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 129. | van Dalen JW, Moll van Charante EP, Nederkoorn PJ, van Gool WA, Richard E. Poststroke apathy. Stroke. 2013;44:851-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 130. | Lanctôt KL, Lindsay MP, Smith EE, Sahlas DJ, Foley N, Gubitz G, Austin M, Ball K, Bhogal S, Blake T, Herrmann N, Hogan D, Khan A, Longman S, King A, Leonard C, Shoniker T, Taylor T, Teed M, de Jong A, Mountain A, Casaubon LK, Dowlatshahi D, Swartz RH; Management of Mood, Cognition and Fatigue Following Stroke Best Practice Writing Group, the Heart & Stroke Canadian Stroke Best Practices and Quality Advisory Committee; in collaboration with the Canadian Stroke Consortium. Canadian Stroke Best Practice Recommendations: Mood, Cognition and Fatigue following Stroke, 6th edition update 2019. Int J Stroke. 2020;15:668-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 131. | Osa García A, Brambati SM, Brisebois A, Désilets-Barnabé M, Houzé B, Bedetti C, Rochon E, Leonard C, Desautels A, Marcotte K. Predicting Early Post-stroke Aphasia Outcome From Initial Aphasia Severity. Front Neurol. 2020;11:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 132. | Ramos-Perdigués S, Mané-Santacana A, Pintor-Pérez L. [Prevalence and associated factors of anger post stroke: a systematic review]. Rev Neurol. 2015;60:481-489. [PubMed] |
| 133. | Benson DF. Psychiatric aspects of aphasia. Br J Psychiatry. 1973;123:555-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 64] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 134. | Santos CO, Caeiro L, Ferro JM, Albuquerque R, Luísa Figueira M. Anger, hostility and aggression in the first days of acute stroke. Eur J Neurol. 2006;13:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 135. | Santos AC, Ferro JM. Profile of Anger in Acute Stroke: A Multifactorial Model of Anger Determinants. J Neuropsychiatry Clin Neurosci. 2019;31:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 136. | Kim JS, Choi S, Kwon SU, Seo YS. Inability to control anger or aggression after stroke. Neurology. 2002;58:1106-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 137. | Chan KL, Campayo A, Moser DJ, Arndt S, Robinson RG. Aggressive behavior in patients with stroke: association with psychopathology and results of antidepressant treatment on aggression. Arch Phys Med Rehabil. 2006;87:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 138. | Choi-Kwon S, Han K, Cho KH, Choi S, Suh M, Nah HW, Kim JS. Factors associated with post-stroke anger proneness in ischaemic stroke patients. Eur J Neurol. 2013;20:1305-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 139. | Spielberger CD, Reheiser EC, Sydeman SJ. Measuring the experience, expression and control of anger. Issues Compr. Pediatr. Nurs.. 18:, 207-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 107] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 140. | Signer S, Cummings JL, Benson DF. Delusions and mood disorders in patients with chronic aphasia. J Neuropsychiatry Clin Neurosci. 1989;1:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 141. | Santos CO, Caeiro L, Ferro JM, Figueira ML. Mania and stroke: a systematic review. Cerebrovasc Dis. 2011;32:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 142. | Cambridge University Press. Elation: a state of extreme happiness or excitement. Cambridge International Dictionary of English. [cited 23 June 2021]. Available from: https://dictionary.cambridge.org/dictionary/english/elation. |
| 143. | Code C. Catastrophic reaction and anosognosia in anterior-posterior and left-right models of the cerebral control of emotion. Psychol Res. 1986;48:53-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 144. | Jacome DE. Aphasia with elation, hypermusia, musicophilia and compulsive whistling. J Neurol Neurosurg Psychiatry. 1984;47:308-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 145. | Liu CY, Wang SJ, Fuh JL, Yang YY, Liu HC. Bipolar disorder following a stroke involving the left hemisphere. Aust N Z J Psychiatry. 1996;30:688-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 146. | Ahmed AI. A manic episode in a 64-year-old man: an adverse effect of varenicline. Gen Hosp Psychiatry. 2011;33:200.e9-200.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 147. | Jampala VC, Abrams R. Mania secondary to left and right hemisphere damage. Am J Psychiatry. 1983;140:1197-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 148. | Ferreira M, Machado C, Machado Á, Santos B. Clinical difficulties in post-stroke mania. Rev Psiquiatr Clin. 2016;43:17. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 149. | Thomas P. Delusion, mania, and personality changes. In: Godefroy O. The Behavioral and Cognitive Neurology of Stroke. Cambridge: Cambridge Univerisy Press, 2013; 351–362. |
| 150. | Huffman J, Stern TA. Acute psychiatric manifestations of stroke: a clinical case conference. Psychosomatics. 2003;44:65-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 151. | Starkstein SE, Fedoroff P, Berthier ML, Robinson RG. Manic-depressive and pure manic states after brain lesions. Biol Psychiatry. 1991;29:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 108] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 152. | Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89:879-885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 153. | Torrisi M, De Luca R, Pollicino P, Leonardi S, Marino S, Maresca G, Maggio MG, Piccolo A, Bramanti P, Calabrò RS. Poststroke delusions: What about the neuroanatomical and neurofunctional basis? Appl Neuropsychol Adult. 2019;26:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 154. | Ross ED. Acute agitation and other behaviors associated with Wernicke aphasia and their possible neurological bases. Cogn Behav Neurol. 1993;6:9-18. |
| 155. | Braun CM, Suffren S. A general neuropsychological model of delusion. Cogn Neuropsychiatry. 2011;16:1-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 156. | Capron DJ. Retentissement psychiatrique de l’AVC. The psychiatric consequences of stroke. Neurol Psychiatr Geriatr. 2015;15:353-358. [DOI] [Full Text] |
| 157. | Sambunaris A, Hyde TM. Stroke-related aphasias mistaken for psychotic speech: two case reports. J Geriatr Psychiatry Neurol. 1994;7:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 158. | Burch EA Jr, Groene BM. Aphasic syndromes and "psychiatric" symptoms: diagnostic dilemmas. South Med J. 1986;79:1234-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 159. | Jilani AQ, Agarwal A, Bharti S, Srivastava S. Psychosis or Wernicke’s aphasia, and response of speech therapy in wernicke’s aphasia: A case report. ERA´s J Med Res. 2019;6:1-3. [DOI] [Full Text] |
| 160. | Joyce EM. Organic psychosis: The pathobiology and treatment of delusions. CNS Neurosci Ther. 2018;24:598-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 161. | Hilari K, Owen S, Farrelly SJ. Proxy and self-report agreement on the Stroke and Aphasia Quality of Life Scale-39. J Neurol Neurosurg Psychiatry. 2007;78:1072-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 162. | Cruice M, Worrall L, Hickson L, Murison R. Measuring quality of life: Comparing family members’ and friends’ ratings with those of their aphasic partners. Aphasiology. 2005;19:111-119. [DOI] [Full Text] |
| 163. | Nicholas M, Jennelle L, Connor LT, Haynes C, Zipse L. Do caregiver proxy reports and congruence of client-proxy activity participation goals relate to quality of life in people with aphasia? Int J Lang Commun Disord. 2020;55:373-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 164. | Peters SK, Dunlop K, Downar J. Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Front Syst Neurosci. 2016;10:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 435] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 165. | Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2116] [Cited by in RCA: 2954] [Article Influence: 196.9] [Reference Citation Analysis (0)] |
| 166. | Pujol J, Bello J, Deus J, Cardoner N, Martí-Vilalta JL, Capdevila A. Beck Depression Inventory factors related to demyelinating lesions of the left arcuate fasciculus region. Psychiatry Res. 2000;99:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 167. | Fridriksson J, den Ouden DB, Hillis AE, Hickok G, Rorden C, Basilakos A, Yourganov G, Bonilha L. Anatomy of aphasia revisited. Brain. 2018;141:848-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 250] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Azocar I, Lin SK S-Editor: Zhang H L-Editor: A P-Editor: Zhang H