©The Author(s) 2025.
World J Psychiatry. Dec 19, 2025; 15(12): 108867
Published online Dec 19, 2025. doi: 10.5498/wjp.v15.i12.108867
Published online Dec 19, 2025. doi: 10.5498/wjp.v15.i12.108867
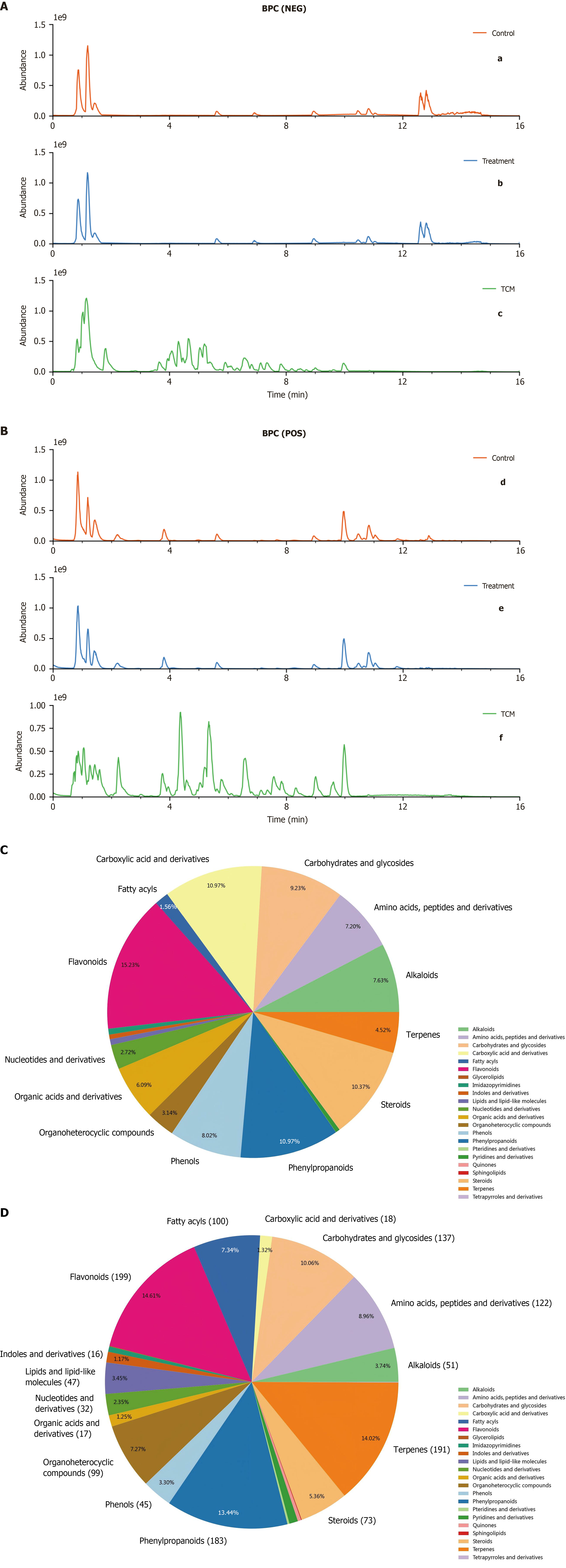
Figure 1 Characterization of chemical constituents in Sini-Suanzaoren decoction and rat serum.
Base peak chromatogram of Sini-Suanzaoren decoction (SNSZRD) obtained by liquid chromatography-mass spectrometry analysis. A: Negative-ion scan (a: Control serum, b: SNSZRD-containing serum, c: SNSZRD sample); B: Positive-ion scan (d: Control serum, e: SNSZRD-containing serum, f: SNSZRD sample); C: Distribution map of SNSZRD component classification content; D: Distribution map of SNSZRD component classification quantity. BPC: Base peak chromatogram; NEG: Negative-ion; POS: Positive-ion; TCM: Traditional Chinese medicine.
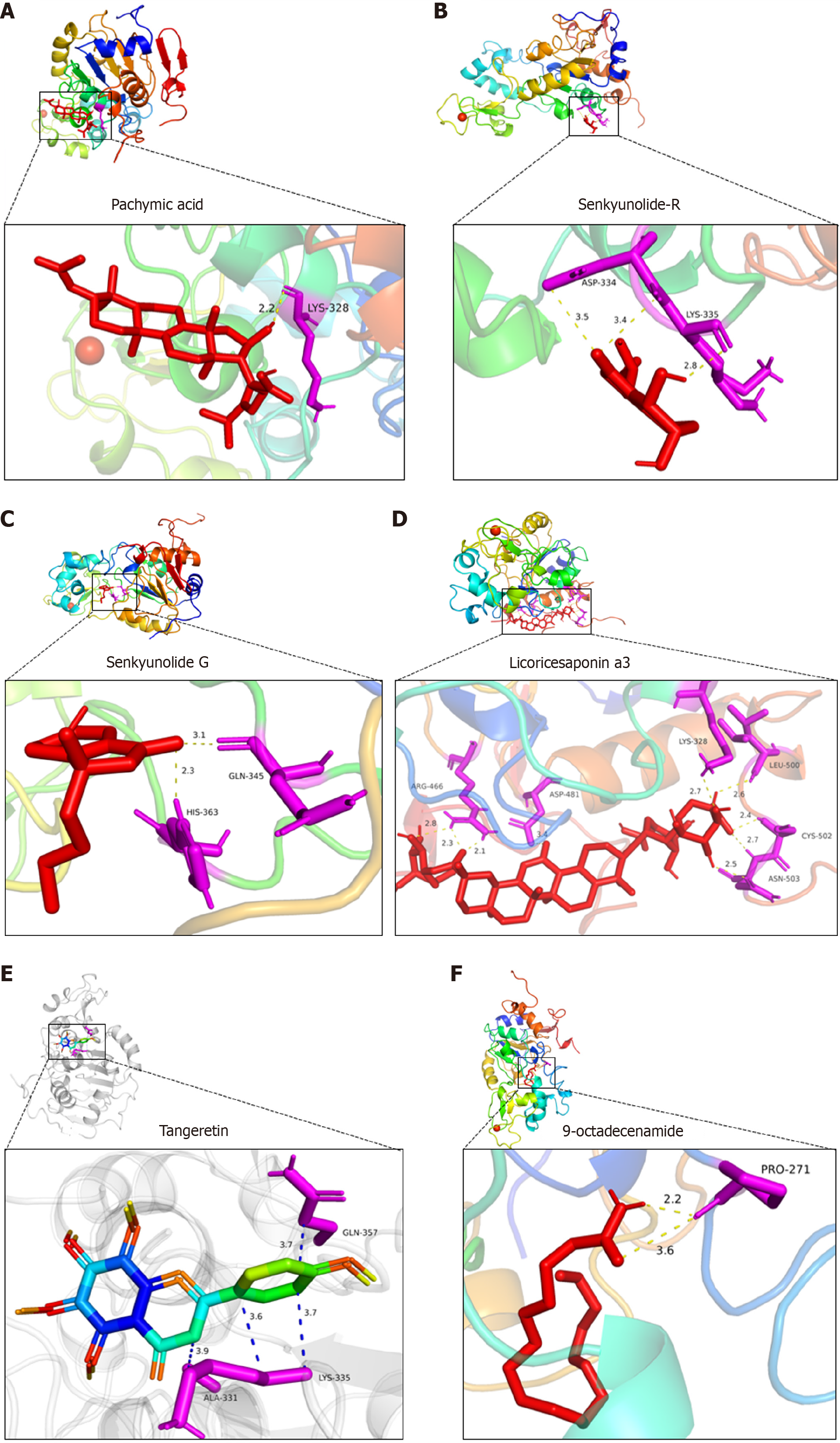
Figure 2 Visualization of molecular docking between the brain-entering components of Sini-Suanzaoren decoction and SIRT1.
A: Pachymic acid; B: Senkyunolide-R; C: Senkyunolide G; D: Licoricesaponin a3; E: Tangeretin; F: 9-octadecenamide.
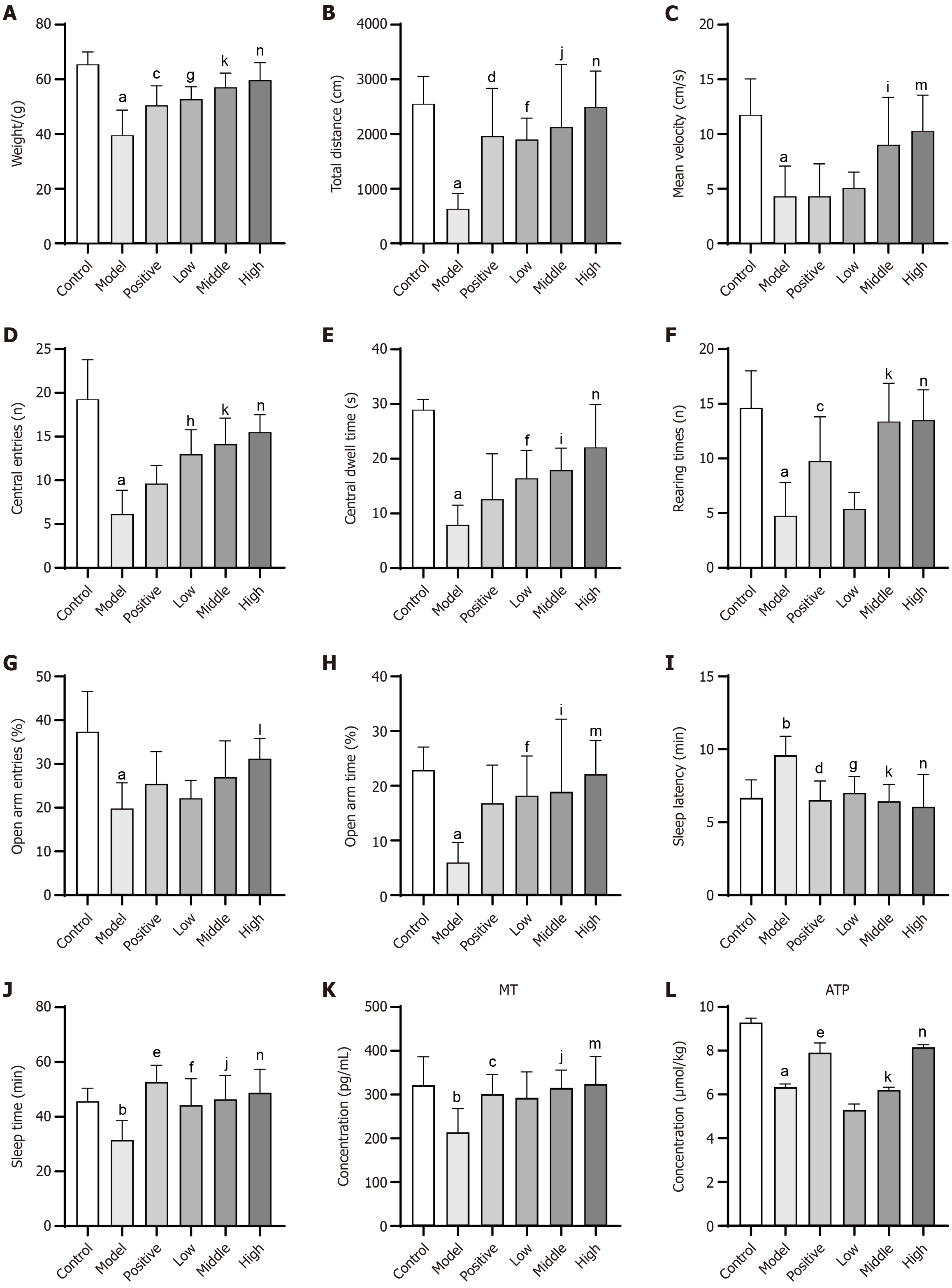
Figure 3 Effect of Sini-Suanzaoren decoction on body weight, behavioral assessments, serum melatonin levels, hypothalamic ATP content in rats.
A: Body weight changes across all groups; B-F: Open field test results; G and H: Elevated plus maze test results; I and J: Pentobarbital-induced sleep test results; K: Serum melatonin content in each group; L: Hypothalamic ATP content in each group. aP < 0.001, model group vs control group; bP < 0.01, model group vs control group; cP < 0.05, positive group vs model group; dP < 0.01, positive group vs model group; eP < 0.001, positive group vs model group; fP < 0.05, Sini-Suanzaoren decoction (SNSZRD) low dose group vs model group; gP < 0.01, SNSZRD low dose group vs model group; hP < 0.001, SNSZRD low dose group vs model group; iP < 0.05, SNSZRD middle dose group vs model group; jP < 0.01, SNSZRD middle dose group vs model group; kP < 0.001, SNSZRD middle dose group vs model group; lP < 0.05, SNSZRD high dose group vs model group; mP < 0.01, SNSZRD high dose group vs model group; nP < 0.001, SNSZRD high dose group vs model group.
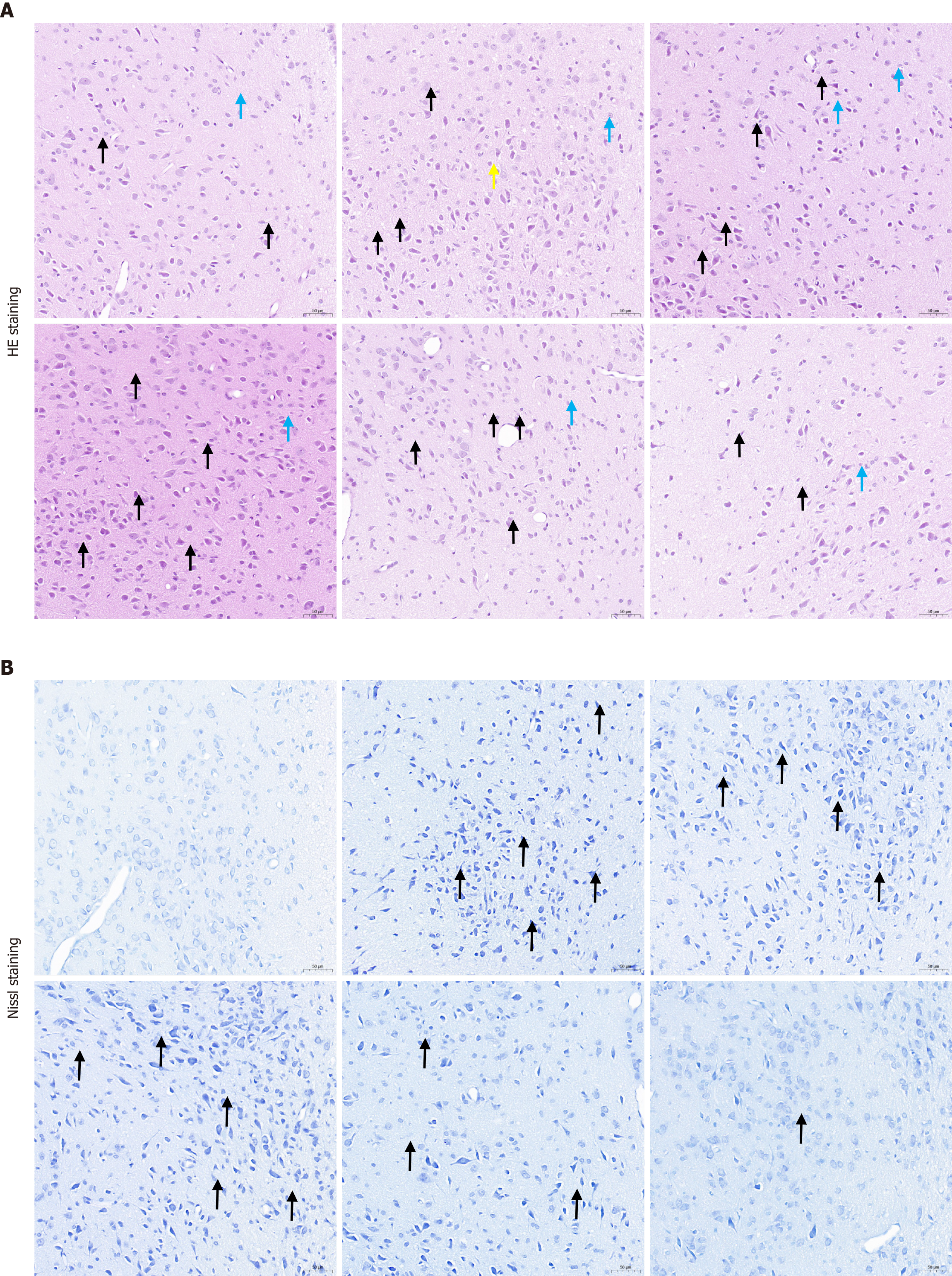
Figure 4 Histological evaluation of the hypothalamus using haematoxylin and eosin staining and Nissl staining.
A: Haematoxylin and eosin staining: Hypothalamic neurons exhibit shrunken and deeply stained cell bodies (indicated by black arrows), increased glial cell density (blue arrows), and occasional neurophagocytosis (yellow arrows); B: Nissl staining: Neuronal cell bodies are shrunken and deeply stained (black arrows), with loss of Nissl bodies in the cytoplasm. HE: Haematoxylin and eosin.
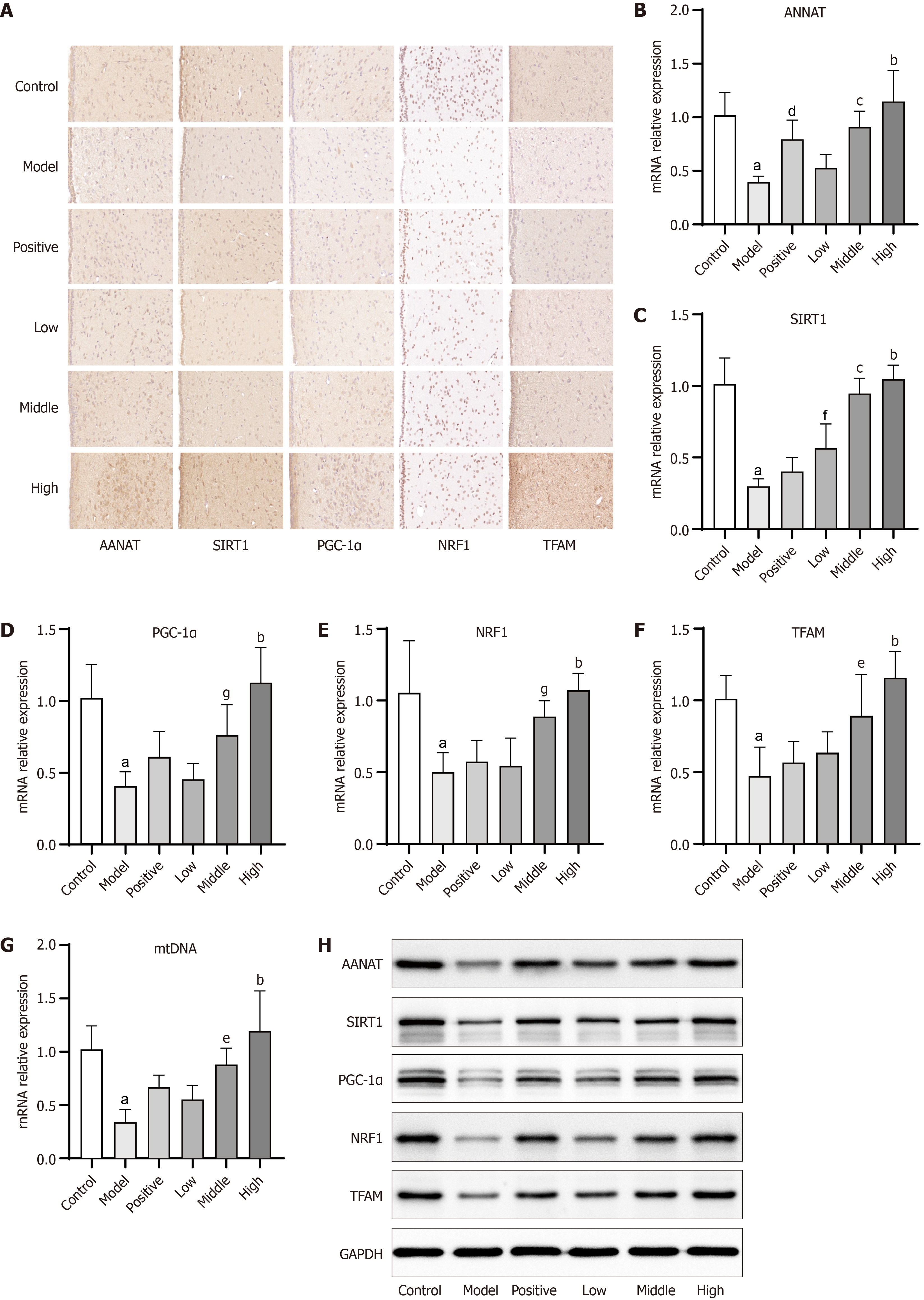
Figure 5 Immunohistochemical, PCR, and Western blot results of rats in each group.
A: Immunohistochemical results; B-G: PCR detection of the mRNA expression of AANAT, SIRT1, PGC-1α, NRF1, TFAM and mtDNA in the hypothalamus; H: Western blot detection of the protein expression of AANAT, SIRT1, PGC-1α, NRF1, TFAM in the hypothalamus. aP < 0.001, model group vs control group; bP < 0.001, Sini-Suanzaoren decoction (SNSZRD) high dose group vs model group; cP < 0.001, SNSZRD middle dose group vs model group; dP < 0.01, positive group vs model group; eP < 0.01, SNSZRD middle dose group vs model group; fP < 0.05, SNSZRD low dose group vs model group; gP < 0.05, SNSZRD middle dose group vs model group.
- Citation: Li RT, Lan BJ, Xiao ZY, Shi QH, Chen XY, Li F. Sini-Suanzaoren decoction regulates mitochondrial biogenesis mediated by MT-SIRT1 in the treatment of insomnia rats. World J Psychiatry 2025; 15(12): 108867
- URL: https://www.wjgnet.com/2220-3206/full/v15/i12/108867.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i12.108867