Published online Dec 9, 2025. doi: 10.5492/wjccm.v14.i4.111054
Revised: July 8, 2025
Accepted: October 10, 2025
Published online: December 9, 2025
Processing time: 160 Days and 3 Hours
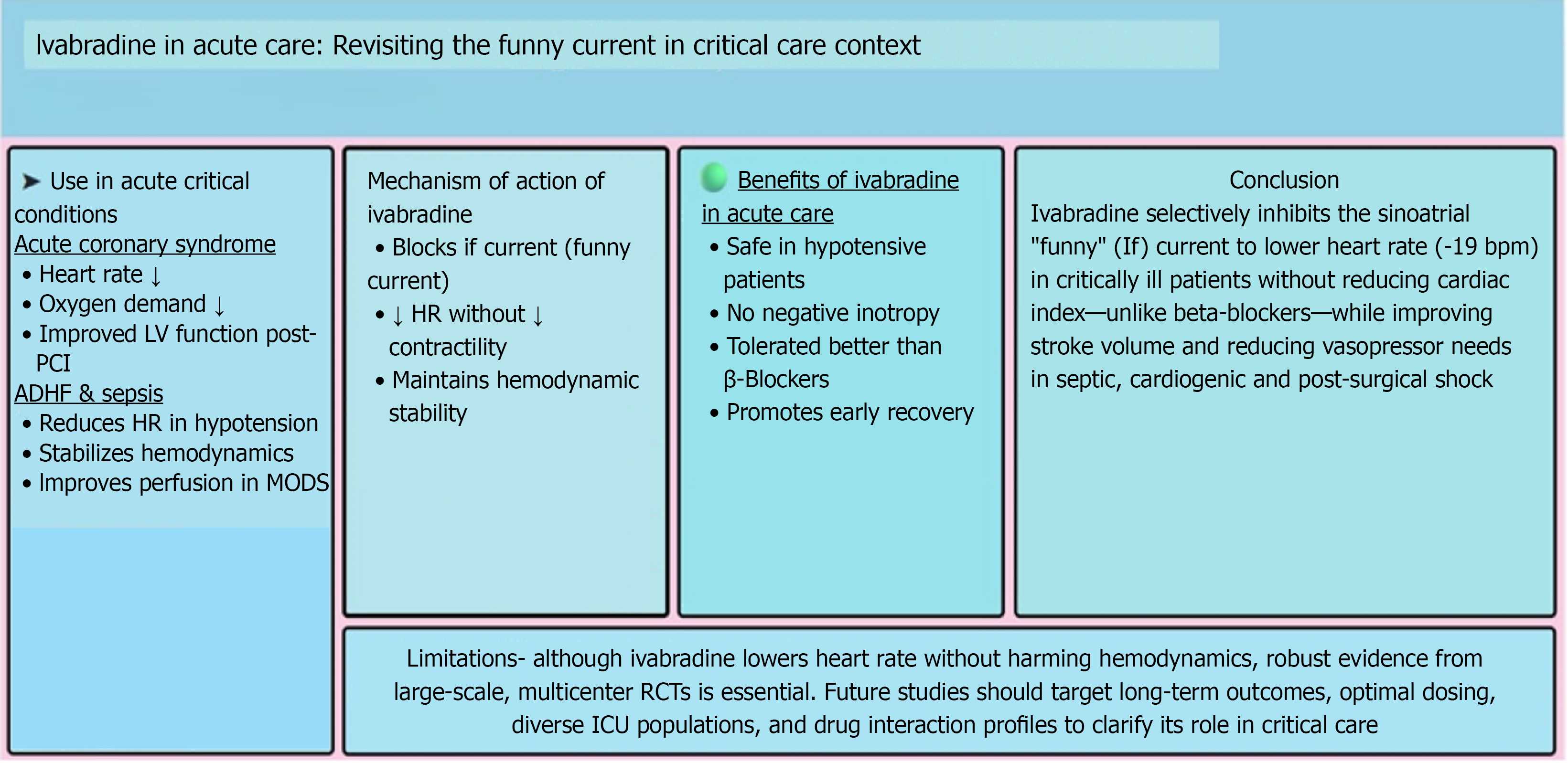
Ivabradine, a selective inhibitor of the funny current in the sinoatrial node, has emerged as a promising agent for heart rate modulation in acute and critical care settings. Unlike beta-blockers, ivabradine reduces heart rate without affecting myocardial contractility, making it a valuable option for patients contraindicated for traditional therapies. This review examines its mechanism of action, clinical applications, comparative efficacy, and safety profile. It incorporates recent lite
Core Tip: Ivabradine reduces heart rate by specifically inhibiting the funny current in the sinoatrial node, without impacting myocardial contractility or blood pressure. This distinctive characteristic renders it an appealing choice for critically sick patients for whom beta-blockers are contraindicated or poorly tolerated. Recent studies corroborate its significance in acute scenarios, including acute decompensated heart failure, acute coronary syndrome, and sepsis-induced tachycardia, where a higher heart rate exacer
- Citation: Mukesh A, Sharma A, Kothari N. Ivabradine in acute care: Revisiting the funny current in critical care context. World J Crit Care Med 2025; 14(4): 111054
- URL: https://www.wjgnet.com/2220-3141/full/v14/i4/111054.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v14.i4.111054
Heart rate control is a critical determinant in managing acute cardiovascular conditions. Traditional agents like beta blockers achieve this but may cause hypotension and bradycardia, limiting their use in acute care, especially in patients with decompensated heart failure[1,2].
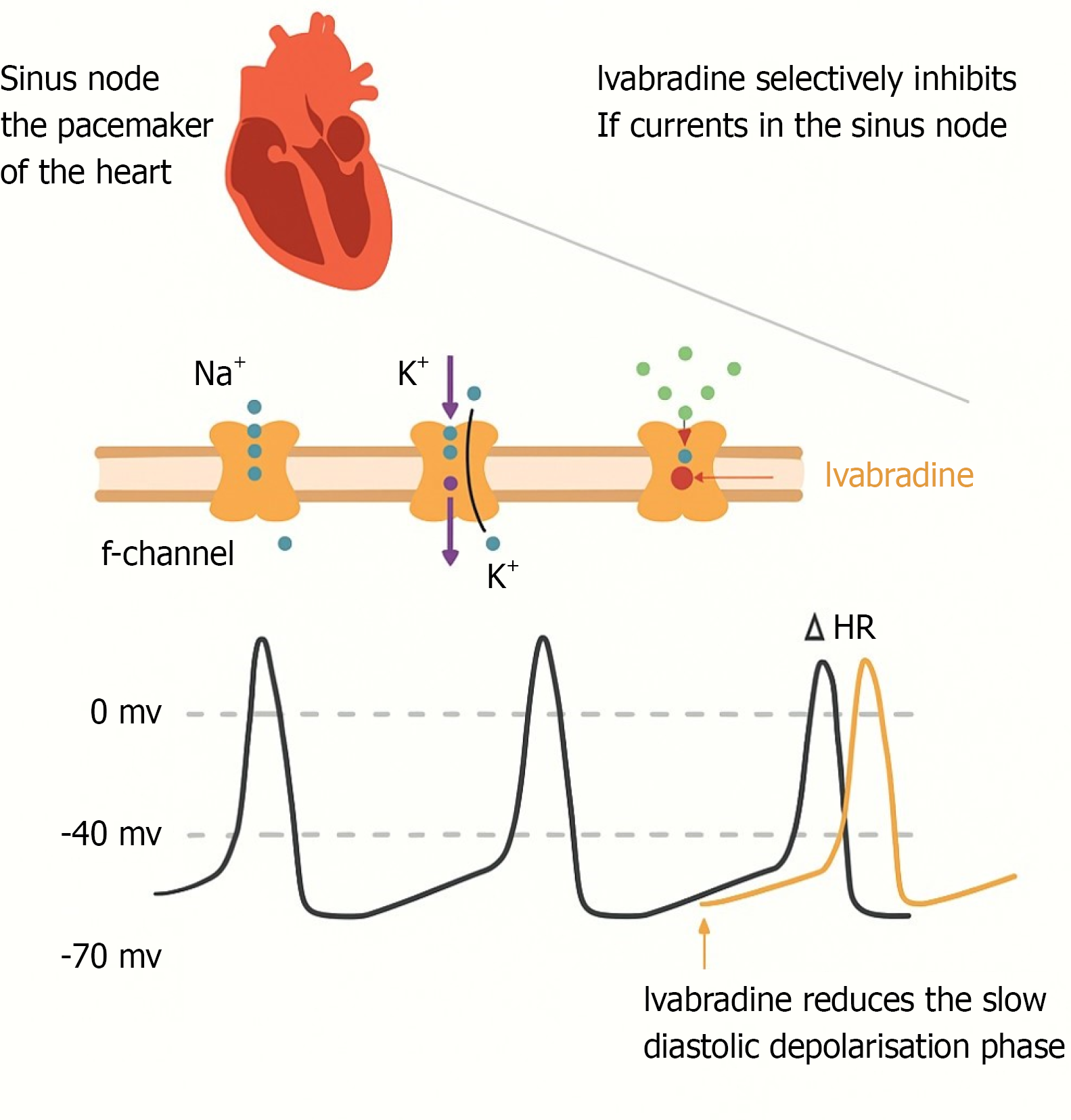
Ivabradine selectively inhibits the funny current, reducing heart rate without affecting myocardial contractility. Initially approved for the treatment of chronic heart failure, recent studies have investigated its efficacy in acute care settings (Figure 1). This review compiles recent studies to evaluate the growing significance of ivabradine in critical care settings.
The funny current (If) is a mixed sodium-potassium inward current contributing to spontaneous depolarisation in sinoatrial node cells. Ivabradine blocks the channel responsible for the cardiac pacemaker current, which regulates heart rate. This results in prolonged diastolic time and reduced heart rate[3]. Ivabradine selectively inhibits the If current, reducing heart rate without adverse inotropic effects. This unique mechanism offers advantages in acute cardiovascular conditions, where tachycardia worsens myocardial oxygen consumption and leads to hemodynamic instability (Figure 2 and Table 1)[4-12].
| Ref. | Study population | Study type No. of patients | Outcome measured | Implications for clinical practice |
| Zheng et al[4], 2024 | Sepsis | Prospective, multicenter, randomised, open-label (n = 172) | Difference in reduction in HR below 95 bpm and the effect of ivabradine on hemodynamics between the standard treatment group and the ivabradine group within the first 96 hours after randomisation | Trial ongoing |
| Colombo et al[5], 2022 | Cardiogenic shock on mechanical circulatory support | Case series (n = 6) | HR, stroke volume, ECMO flow, vasopressor requirements | Significant reduction in HR observed after ivabradine administration. SV improvement allowing the reduction of ECMO flow support and vasopressors administration |
| Datta et al[6], 2021 | Septic shock | RCT (n = 60) | HR, stroke volume, vasopressor dose, survival outcomes | Enteral ivabradine is effective in reducing HR, improving haemodynamic parameters, and cardiac function |
| Nguyen et al[7], 2018 | Low cardiac output syndrome treated by dobutamine after elective coronary artery bypass surgery | Multicenter RCT (n = 19) | HR, cardiac index, continuous CO monitoring | IV ivabradine achieved effective and rapid correction of sinus tachycardia. Simultaneously, stroke volume and systolic blood pressure increased, suggesting a beneficial effect of this treatment on tissue perfusion |
| Nuding et al[8], 2018 | Multiple organ dysfunction syndrome | Single-centre RCT (n = 70) | HR reduction ≥ 10 bpm at 96 hours, hemodynamics, disease severity, vasopressor use, mortality | HR reduction after oral ivabradine did not differ significantly between groups. It did not affect hemodynamics or disease severity |
| Barillà et al[9], 2016 | STelevation myocardial infarction complicated by cardiogenic shock | Single-centre RCT (n = 58) | HR reduction, clinical, and hemodynamic outcomes | Associated with a short-term favourable outcome and can be effectively administered by nasogastric intubation |
| Gallet et al[10], 2014 | Severe systolic dysfunction | RCT (n = 22) | HR, diastolic function, perfusion, cardiac output | Demonstrates the safety and potential benefit of as HR HR-lowering agent |
| De Santis et al[11], 2014 | MODS | Case report (n = 3) | Hemodynamic variables | HR reduction in MODS patients |
| Franke et al[12], 2011 | Acute heart failure due to myocarditis | Case report (n = 2) | Hemodynamic variables | Beneficially influence outcome by allowing optimisation of the patient′s HR |
| Swedberg et al[1], 2010 | Chronic heart failure | Randomised placebo-controlled (n = 6558) | Composite of cardiovascular death or hospital admission for worsening heart failure | HR is reduced with improvement in clinical outcomes |
An elevated heart rate in acute coronary syndrome (ACS) increases myocardial oxygen demand, thereby exacerbating ischemia. Ivabradine's ability to selectively lower heart rate has been evaluated in several trials. Calabrò et al[13] indicated its effectiveness in reducing heart rate and improving left ventricular function, particularly in patients undergoing primary percutaneous coronary intervention. They suggested that ivabradine also aids in left ventricular remodeling post-ST-segment elevation myocardial infarction.
Sinus tachycardia worsens symptoms and prognosis in patients with acute decompensated heart failure. Beta-blockers are effective but poorly tolerated in patients with hypotension. Ivabradine provides an alternative by selectively con
Sepsis-induced tachycardia contributes to hemodynamic instability and worsens outcomes. Ivabradine’s selective heart rate reduction has been explored in septic patients. A prospective trial demonstrated improved hemodynamic para
A prospective study compared ivabradine to beta-blockers in patients with ACS, observing both to be equally effective in reducing heart rate. However, ivabradine was better tolerated in patients with contraindications to beta blockers[15]. Unlike beta-blockers, ivabradine does not affect blood pressure or contractility, making it suitable for specific patient populations.
Ivabradine has a favourable safety profile, characterised by mild, reversible side effects. The most common adverse effects include bradycardia (often dose-dependent) and visual disturbances (transient effects due to retinal If channel inhibition). Importantly, ivabradine lacks the negative inotropic and vasodilatory properties of beta-blockers, making it a safer alternative in patients with hypotension or left ventricular dysfunction (Table 2)[16].
| Variable | Ivabradine | Beta-blockers |
| Mechanism | Blocks If channels in SA node (reduces HR only) | Block β1/β2-adrenergic receptors (reduces HR + BP) |
| Common side effects | Luminous phenomena (visual brightness). Bradycardia. Headache. Rare: Atrial fibrillation | Fatigue. Cold. hands/feet. Bronchospasm (worsens COPD/asthma) Erectile dysfunction. Sleep disturbances. Depression |
| Metabolic effects | Neutral (no impact on glucose/Lipids) | May worsen: Insulin resistance. Triglycerides (↑). HDL (↓) |
| Contraindications | HR < 60 bpm. Acute heart failure. Pacemaker-dependent | Asthma/COPD. Severe bradycardia. Heart block (2nd/3rd degree) |
| Advantages | Pure HR control. Safe in lung diseases. No sexual dysfunction. No metabolic interference | Broader benefits (angina, HTN, post-MI). Lower cost |
| Uses | COPD/asthma patients. Diabetes patients. HR reduction without BP effects | Hypertension. Post-heart attack. Arrhythmias |
Despite encouraging findings supporting ivabradine's role in acute care, several challenges persist, including the reliance on small sample sizes in clinical trials, which may limit the generalizability of results, the relatively short follow-up durations that restrict assessments of long-term effectiveness and safety, and the lack of comprehensive long-term efficacy data in critically ill patients. Future large-scale, multicenter, randomized controlled trials are needed to determine the definitive role of ivabradine in acute and critical care settings.
Ivabradine represents a novel approach to heart rate modulation in the acute care setting. Its selective inhibition of the If provides a heart rate reduction strategy without compromising myocardial contractility, differentiating it from beta-blockers. While current evidence suggests potential benefits in heart rate control without adverse hemodynamic effects, robust data from diverse patient populations are lacking. Future large-scale studies should aim to assess long-term outcomes, optimal dosing strategies, and potential interactions with other critical care medications to support the broader use of ivabradine in intensive care settings.
| 1. | Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1747] [Cited by in RCA: 1867] [Article Influence: 116.7] [Reference Citation Analysis (0)] |
| 2. | Borer JS, Fox K, Jaillon P, Lerebours G; Ivabradine Investigators Group. Anti-anginal and anti-ischemic effects of ivabradine, an I(f) inhibitor, in stable angina: a randomized, double-blind, multicenter, placebo-controlled trial. Circulation. 2003;107:817-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 323] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 3. | Pavasini R, Camici PG, Crea F, Danchin N, Fox K, Manolis AJ, Marzilli M, Rosano GMC, Lopez-Sendon JL, Pinto F, Balla C, Ferrari R. Anti-anginal drugs: Systematic review and clinical implications. Int J Cardiol. 2019;283:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Zheng J, Wen D, Pan Z, Chen X, Kong T, Wen Q, Zhou H, Chen W, Zhang Z. Effect of heart rate control with ivabradine on hemodynamics in patients with sepsis: study protocol for a prospective, multicenter, randomized controlled trial. Trials. 2024;25:710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Colombo CNJ, Dammassa V, Klersy C, Camporotondo R, Pellegrini C, Mojoli F, Tavazzi G. Heart rate control and haemodynamic improvement with ivabradine in cardiogenic shock patient on mechanical circulatory support. Eur Heart J Acute Cardiovasc Care. 2022;11:916-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 6. | Datta PK, Rewari V, Ramachandran R, Singh PM, Ray BR, Aravindan A, Seth S, Parakh N, Trikha A. Effectiveness of enteral ivabradine for heart rate control in septic shock: A randomised controlled trial. Anaesth Intensive Care. 2021;49:366-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Nguyen LS, Squara P, Amour J, Carbognani D, Bouabdallah K, Thierry S, Apert-Verneuil C, Moyne A, Cholley B. Intravenous ivabradine versus placebo in patients with low cardiac output syndrome treated by dobutamine after elective coronary artery bypass surgery: a phase 2 exploratory randomized controlled trial. Crit Care. 2018;22:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Nuding S, Schröder J, Presek P, Wienke A, Müller-Werdan U, Ebelt H, Werdan K. Reducing Elevated Heart Rates in Patients with Multiple Organ Dysfunction Syndrome with The If (Funny Channel Current) Inhibitor Ivabradine. Shock. 2018;49:402-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Barillà F, Pannarale G, Torromeo C, Paravati V, Acconcia MC, Tanzilli G, Mangieri E, Dominici T, Martino F, Pannitteri G, Gaudio C. Ivabradine in Patients with ST-Elevation Myocardial Infarction Complicated by Cardiogenic Shock: A Preliminary Randomized Prospective Study. Clin Drug Investig. 2016;36:849-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Gallet R, Ternacle J, Damy T, Guendouz S, Bremont C, Seemann A, Gueret P, Dubois-Rande JL, Lim P. Hemodynamic effects of Ivabradine in addition to dobutamine in patients with severe systolic dysfunction. Int J Cardiol. 2014;176:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | De Santis V, Frati G, Greco E, Tritapepe L. Ivabradine: a preliminary observation for a new terapeutic role in patients with multiple organ dysfunction syndrome. Clin Res Cardiol. 2014;103:831-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Franke J, Schmahl D, Lehrke S, Pribe R, Bekeredjian R, Doesch AO, Ehlermann P, Schnabel P, Katus HA, Zugck C. Adjuvant Use of Ivabradine in Acute Heart Failure due to Myocarditis. Case Rep Med. 2011;2011:203690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Calabrò LA, Pasetto M, Scolletta S, Annoni F, Demailly Z, Halenarova K, Donadello K, Taccone FS. Ivabradine use in critical care: a systematic review and metanalysis of cardiogenic and septic shock patients. BMC Anesthesiol. 2025;25:276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Imamura T. Clinical Implications of Ivabradine in the Contemporary Era. Medicina (Kaunas). 2024;60:303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Lekkala HK. A prospective considerations and comparing efficacy between ivabradine vs beta blockers in south India acute coronary syndrome patients. Asian J Pharm Clin Res. 2019;12:193-195. [DOI] [Full Text] |
| 16. | Guo X, Yang W, Cui Y, Guo R, Zhu Y, Liu T, Chen K, Liu C. Long-term safety and efficacy of ivabradine after direct percutaneous coronary intervention in patients with acute myocardial infarction complicated by heart failure: a single-center retrospective study. BMC Cardiovasc Disord. 2025;25:422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/