Published online Nov 9, 2021. doi: 10.5492/wjccm.v10.i6.323
Peer-review started: March 8, 2021
First decision: May 13, 2021
Revised: May 24, 2021
Accepted: August 23, 2021
Article in press: August 23, 2021
Published online: November 9, 2021
Processing time: 241 Days and 12.9 Hours
Coronavirus disease 2019 (COVID-19) related acute respiratory distress syndrome (ARDS) is a severe complication of infection with severe acute respiratory syndrome coronavirus 2, and the primary cause of death in the current pandemic. Critically ill patients often undergo extracorporeal membrane oxygenation (ECMO) therapy as the last resort over an extended period. ECMO therapy requires sedation of the patient, which is usually achieved by intravenous administration of sedatives. The shortage of intravenous sedative drugs due to the ongoing pandemic, and attempts to improve treatment outcome for COVID-19 patients, drove the application of inhaled sedation as a promising alternative for sedation during ECMO therapy. Administration of volatile anesthetics requires an appropriate delivery. Commercially available ones are the anesthetic gas reflection systems AnaConDa® and MIRUSTM, and each should be combined with a gas scavenging system. In this review, we describe respiratory management in COVID-19 patients and the procedures for inhaled sedation during ECMO therapy of COVID-19 related ARDS. We focus particularly on the technical details of administration of volatile anesthetics. Furthermore, we describe the advantages of inhaled sedation and volatile anesthetics, and we discuss the limitations as well as the requirements for safe application in the clinical setting.
Core Tip: This article summarizes the use of inhaled sedation for extracorporeal membrane oxygenation in patients suffering from coronavirus disease 2019 (COVID-19) related acute respiratory distress syndrome, including a description of respiratory management, the technical aspects, and requirements for delivery of volatile anesthetics. The article closes with important future considerations for inhaled sedation in critically ill COVID-19 patients undergoing extracorporeal membrane oxygenation therapy.
- Citation: Bellgardt M, Özcelik D, Breuer-Kaiser AFC, Steinfort C, Breuer TGK, Weber TP, Herzog-Niescery J. Extracorporeal membrane oxygenation and inhaled sedation in coronavirus disease 2019-related acute respiratory distress syndrome. World J Crit Care Med 2021; 10(6): 323-333
- URL: https://www.wjgnet.com/2220-3141/full/v10/i6/323.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v10.i6.323
The ongoing pandemic is caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) that triggers a variety of symptoms in the human host. One major complication of infection with SARS-CoV-2 is the acute respiratory distress syndrome (ARDS). Coronavirus disease 2019 (COVID-19) related ARDS is a severe condition associated with high mortality and is the primary cause of death among COVID-19 patients. Treatment of this condition is mainly supportive and requires considerable resources, but effective coordination enables the health care system to cope with the influx of critically ill patients[1].
If respiratory failure occurs in COVID-19 patients despite all efforts, extracorporeal membrane oxygenation (ECMO) treatment over an extended period is the last remaining therapeutic option[2]. Since the outcome of this treatment is poor, better prevention and treatment are urgently needed.
ECMO therapy requires sedation of the patient, often via high doses of intravenous sedatives such as midazolam, ketamine, or propofol in combination with an opioid and neuromuscular blocking agent. The ongoing pandemic is exhausting supplies of these drugs, so alternative approaches have to be considered[3]. One practical alternative approach is inhaled sedation with volatile anesthetics, such as isoflurane, sevoflurane, or desflurane[4,5].
Beside the low costs, volatile anesthetics are associated with faster onset and offset of sedation and thus allow efficient control of administration. Application of these drugs does not rely on electronic infusion pumps, which have become scarce during the pandemic. In addition, volatile anesthetics cause fewer hallucinations and lower opioid needs than intravenous anesthetics. Moreover, a recent study suggests that inhaled sedation could be associated with a better outcome than intravenous sedation[6]. In particular, sevoflurane yielded superior outcomes than other anesthetics[7,8]. Nonetheless, the application of inhaled sedation faces limitations. Most critical care units lack proper delivery and gas scavenging systems for limiting pollution with volatile anesthetics[9]. Further, health care professionals require special training to administer appropriately the anesthetics and to recognize contraindications, such as malignant hyperthermia.
In this review, we summarize the requirements for inhaled sedation in COVID-19 patients under ECMO therapy, and we highlight the technical aspects of administration of volatile anesthetics.
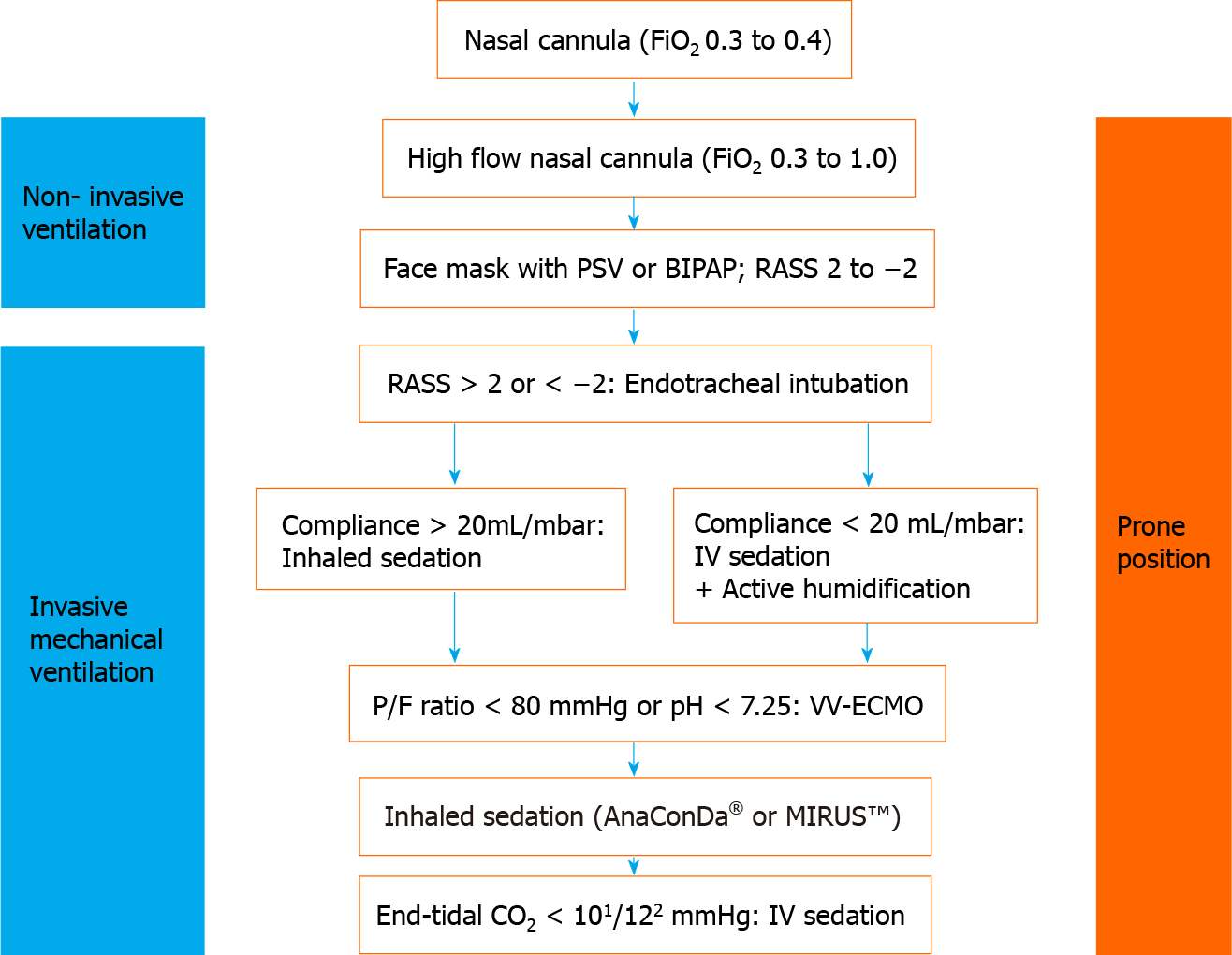
Continuous monitoring of oxygen saturation in the patient is necessary, since a drop in saturation indicates a severe progression of COVID-19. If oxygen levels fall, respiratory management is required, but spontaneous breathing should be maintained as long as possible and reasonable. A number of approaches to support spontaneous breathing is available and has been comprehensively summarized elsewhere[10]. A schematic overview of the strategy for respiratory management in COVID-19 patients is presented in Figure 1.
The use of nasal cannula is the first method of choice; however, the fraction of inspired oxygen (FiO2) is limited to 0.3 to 0.4. If insufficient, high flow nasal cannula produces a high flow and continuous positive airway pressure, capable of achieving higher FiO2. This can be further supported by shifting the patient in a prone position[11]. The last resort of noninvasive intervention for respiratory management is the use of bilevel positive airway pressure and pressure support ventilation. These measures are capable of providing high FiO2 and can be combined with placing the patient in prone position for further support. It should be mentioned that these measures require high quality masks for respiration to prevent pressure injuries on the skin or the nose of the patient.
Severe hypoxia, which is associated with COVID-19 related ARDS, impairs consciousness, vigilance, or compliance. For instance, impaired compliance of the patient can hinder the use of facial masks, leading to a dramatic drop in oxygen saturation. Consequently, severe hypoxia requires invasive measures, e.g., endo
If the partial pressure of oxygen/FiO2 ratio drops below 150 mmHg, the patient should be placed in prone position for more than 12 h[12]. Individual measures can be taken to manage severe hypoxia, such as application of inhaled nitric oxide, muscle relaxants, or recruitment maneuver. If respiratory function remains poor (i.e. lower than 20 mL/mbar, partial pressure of oxygen/FiO2 ratio less than 80 mmHg, or pH less than 7.25) despite prone positioning, veno-venous ECMO is the last resort to save the life of the patient[13,14].
Deploying an ECMO system can only be considered if all other approaches are unsuccessful and if there are no contraindications[15]. ECMO therapy can cause adverse events and suboptimal responses, in particular in COVID-19 patients who are predisposed to bleeding and thrombotic complications[16]. Such events could suggest withdrawal of ECMO therapy. Furthermore, a recent study reported an in-hospital mortality of 37.4% for patients with severe COVID-19 related ARDS 90 d after the initiation of ECMO therapy[17]. This highlights the importance of an open discussion with the patient and relatives at an early stage in order to clarify treatment goals, expectations, and possible outcomes as well as to obtain consent from the patient regarding continuation or discontinuation of therapy[18].
During ECMO therapy, patients with ARDS in prone position should be kept in at least light sedation, corresponding to a Richmond Agitation-Sedation Scale of ≥ 2[12]. Sedation is associated with side effects such as delirium, respiratory depression, and immunosuppression. Further, deep sedation is a risk factor for COVID-19 patients and is associated with poorer outcome. Thus, sedation must be monitored carefully. Processed electroencephalogram monitoring is a very useful approach to assess anesthesia and to recognize burst suppression. In case of inhaled sedation, measur
The prerequisite for using the Anaesthetic Conserving Device (ACD) AnaConDa® (Sedana Medical AB, Danderyd, Sweden) or the MIRUSTM system (TIM, Koblenz, Germany) depends on several clinical parameters (see Figure 1). If lung compliance is acceptable, and CO2 can be reduced sufficiently, both types of systems are able to maintain spontaneous breathing[19-21]. However, if lung compliance is poor, reduction of dead space and active humidification is necessary, which can be facilitated by inhaled sedation via a circle breathing system[22,23].
The AnaConDa® system is capable of achieving adequate sedation with isoflurane or sevoflurane. In addition to isoflurane and sevoflurane, the MIRUS system can also apply desflurane.
The pathophysiological basis for COVID-19 related ARDS is the altered blood-air barrier. The diffusion distance for adequate gas exchange in the lung alveoli is impaired by inflammation, edema, and accumulated mucus. These impairments severely limit O2 uptake and CO2 release. However, volatile anesthetics are still able to establish an effective concentration in the blood stream under ARDS conditions, provided the necessary concentration gradient is maintained[24,25]. Volatile anesthetics show superior diffusion properties than O2 and CO2, which can be attributed to the lipophilic nature of the anesthetic gas. Only if both the tubus and the bronchial system are completely clogged, intravenous sedation is necessary.
The ECMO system itself can be used for administration of volatile anesthetics[26,27]. This requires installation of a vaporizer into the oxygen tube and connection of a pipe for exhaust gas removal to the outlet and the negative pressure device. The inhaled and exhaled portion of the anesthetic gas must be carefully monitored in order to determine the depth of sedation and to detect possible leakage. Since leakage can easily lead to pollution of the intensive care unit (ICU), a proper scavenging system is crucial. Nonetheless, it must be noted that these scavengers can create high back pressure that increases the risk for gas embolism. Another important technical aspect to take into consideration is the type of membrane oxygenator. Transmembrane passage of the anesthetic gas is facilitated only via hollowfiber membrane oxygenators, which are made of polypropylene. If the oxygenators are made of poly(4methyl
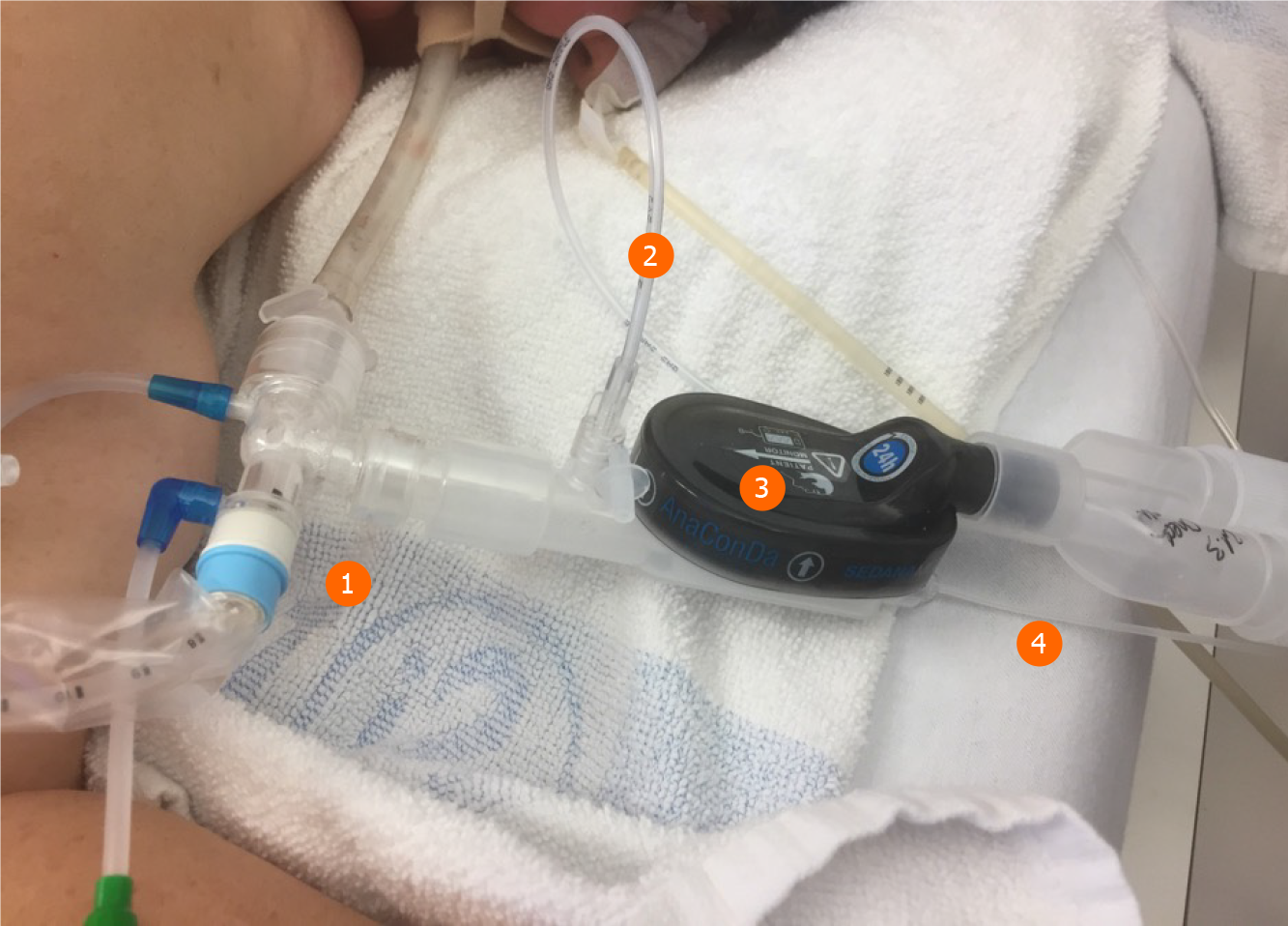
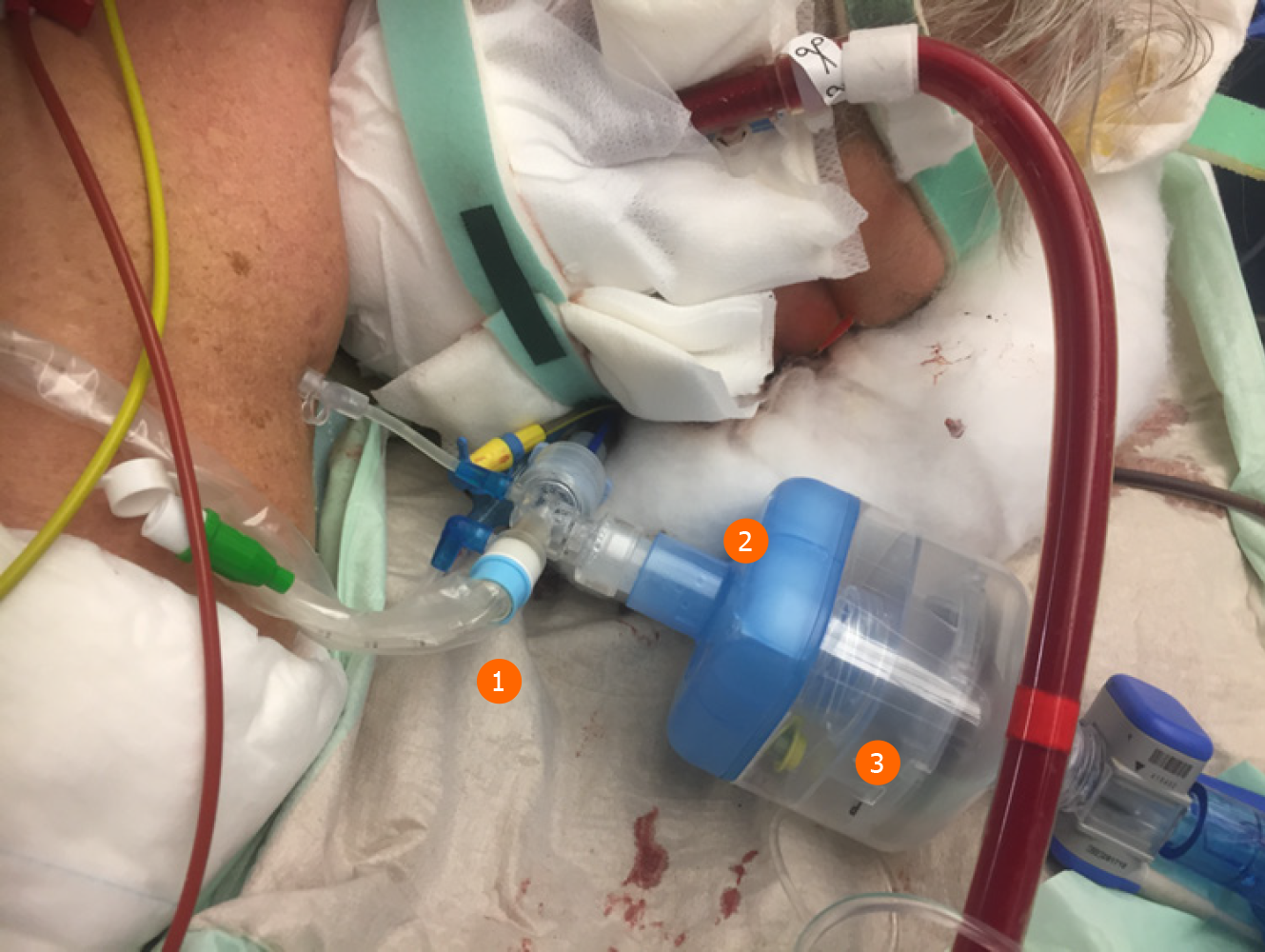
One major anesthetic gas reflection system is the AnaConDa® system, which is commercially available as a larger version, i.e. ACD-100, and a smaller version, i.e. ACD-50 (which is also known as AnaConDa®-S) (Figure 2). The other major gas reflection system is the MIRUSTM system (Figures 3 and 4). Recent studies showed that the AnaConDa® systems can be used successfully for sedation of ARDS patients during ECMO therapy[20,28,29]. Similarly, a study using the MIRUSTM system demonstrated successful application of inhaled sedation for ECMO therapy in patients with COVID-19 related ARDS[30].
The CO2 signal has no effect on the performance; nonetheless, a CO2 pressure of at least 10 mmHg is an indication for an open and managed airway and is associated with a higher survival rate[31,32]. Consequently, the authors call for a minimum of 10 mmHg as standard for the AnaConDa® system. If this minimum cannot be maintained by adjusting the ventilation, the level of sedation must be monitored carefully and, if necessary, complemented by intravenous sedation of the patient.
By contrast, the MIRUSTM system requires an end-tidal CO2 pressure of at least 15 mmHg. If the end-tidal CO2 pressure drops below 12 mmHg, the MIRUSTM system stops administering the anesthetic, indicated by a red alert. This could result in the inadvertent awakening of the patient, which would then require intravenous sedation to restore anesthesia.
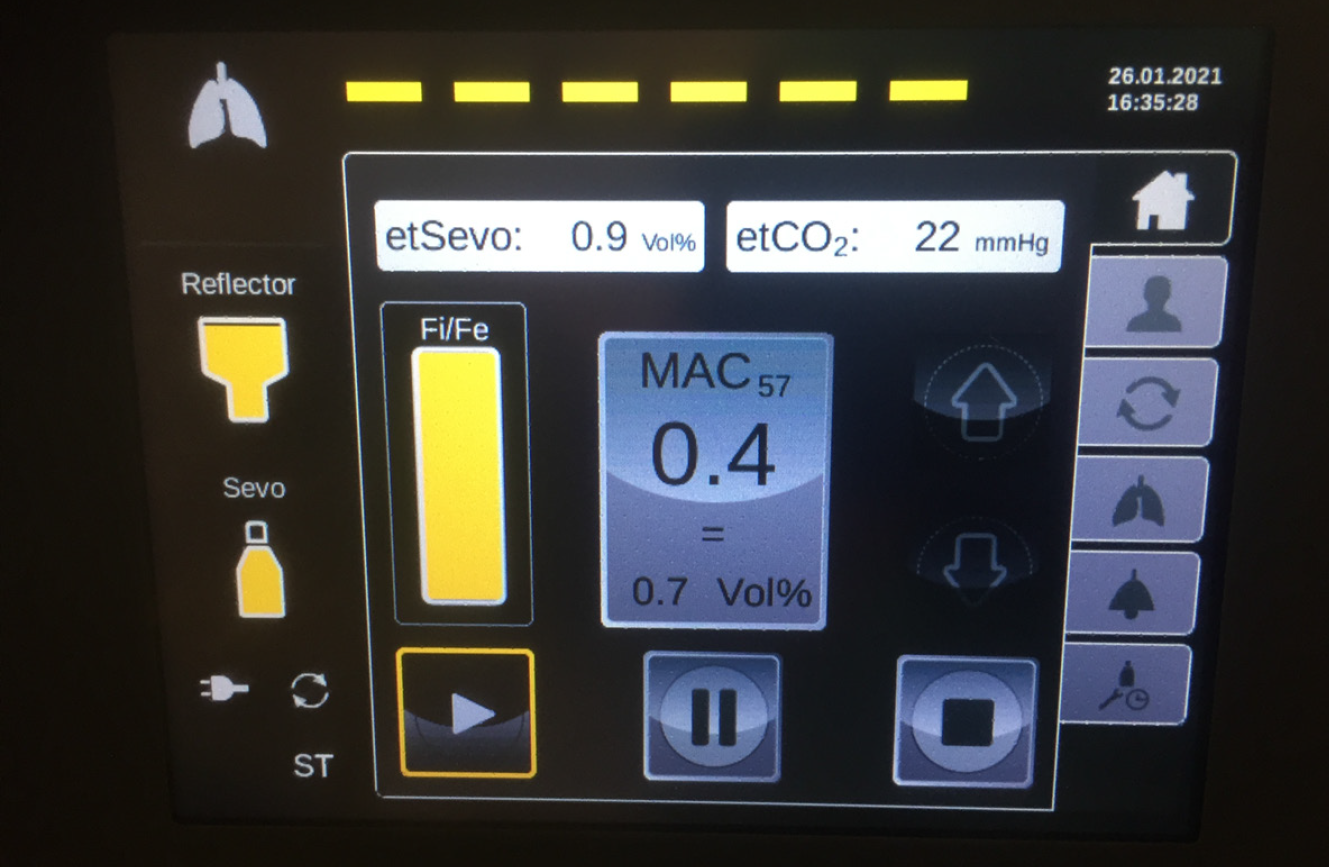
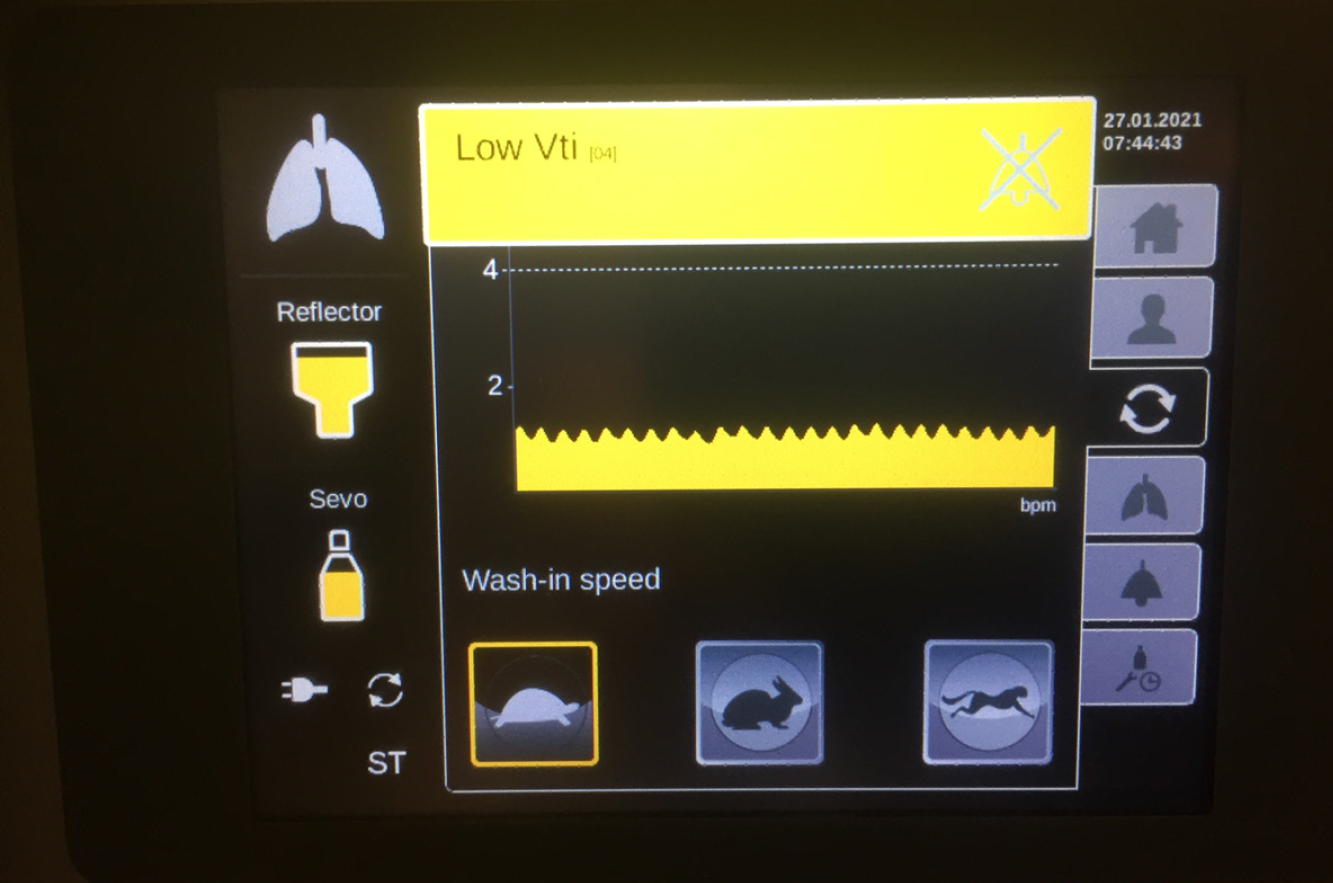
The most recent MIRUSTM systems (starting from version 2.0 onward) indicate a tidal volume of less than 200 mL by a yellow alert (Figure 5). During ECMO therapy, the minimum respiratory minute volume falls frequently below the minimum tidal volume of the MIRUSTM system. The yellow alert can be acknowledged in order to continue administration; however, this procedure is associated with the risk of overdosing with the anesthetic. In case of isoflurane, the MIRUSTM system displays a higher gas concentration under ECMO therapy. Hence, a concentration of more than 2% can be displayed, although it does not correspond to the actual end-tidal values. Because higher effective concentrations are required for the same MAC with sevoflurane and desflurane, this effect is not as pronounced with these gases. Nevertheless, the operator should choose the lowest wash-in speed (i.e. the setting “tortoise”) for all of three anesthetics isoflurane, sevoflurane, and desflurane (Figure 5).
An important consideration for the application of anesthetic gas reflection systems is the volume of a breath that does not participate in gas exchange, i.e. the dead space. The volumetric dead space of the MIRUSTM system is 100 mL[19], whereas the volumetric dead space for the AnaConDa® system ACD-100 is 100 mL and 50 mL for the ACD-50[23]. However, the ECMO system eliminates CO2 effectively. Thus, volumetric and reflective dead space of the anesthetic gas reflection systems are irrelevant for ECMO therapy.
Another alternative device for administration of volatile anesthetics is the circle breathing system. Usage of this system has been reported during the ongoing SARS-CoV-2 pandemic. However, to the best of our knowledge, the deployment of a circle breathing system in conjunction of ECMO therapy in the ICU has not been described in literature.
The SARS-CoV-2 pandemic is estimated to be one of the most expensive natural disasters in recorded history[33]. Besides economic repercussions due to the containment measures, adequate treatment of patients causes a substantial financial strain for the global health care systems. ECMO, in particular, is a very expensive procedure, and thus the reduction of associated costs is highly desirable. Treatment of COVID-19 related ARDS requires larger amounts of sedatives than treatment of non-COVID-19 patients. Consequently, treatment of COVID-19 patients, who undergo invasive mechanical ventilation without ECMO therapy, demands a large consum
Besides consumption, a certain amount of anesthetic gas is lost in the delivery system, i.e. at the exhalation outlet of the ventilator or at the oxygenator of the ECMO device. The exhalation outlet has the advantage that the gas flow can conduct viral particles and hence reduces the risk of infection for the health care personnel[34,35]. The oxygenator was used initially for intraoperative delivery of the anesthetic gas but modern reflection systems require containment of volatile anesthetics. For instance, the oxygenator of the Cardiohelp System (Getinge Group, Gothenburg, Sweden) does not leak anesthetic gas, according to the manufacturer and independent researchers[26]. While the loss of anesthetic gas via the oxygenator is theoretically still possible, instruments in the ICUs usually lack such device.
Respiratory management has also to take into account the SARS-CoV-2 infection. Hence, tracheotomy is often avoided if the patient shows a high viral load. Nevertheless, tracheostomy is suggested to improve the outcome of COVID-19 patients, in particular, if the intervention is performed between day 13 and day 17 post intubation[36].
The handling of medical devices and instruments requires strict hygiene measures. Firstly, a closed suction system is mandatory (Figures 2 and 3). Secondly, the tube has to be clamped off prior to disconnect it from heat and moisture exchanger filters.
Here, the MIRUSTM system has an advantage since the heat and moisture exchanger filters are integrated in the device, so that the controller and measuring units remain in a clean and safe distance. By contrast, the AnaConDa® systems measure the concentration of the anesthetics in close proximity to the patient and hence are exposed to a high risk of contamination. However, the water trap at the gas monitor is sealed, which prevents intrusion of viral pathogens.
In order to connect either the MIRUSTM or the AnaConDa® system to the vacuum connection on the ICUs, a suitable gas flow conduction system is required. The CleanAirTM system (TIM, Koblenz, Germany) is recommended for gas flow conduction, because it is independent of the reflector (Figure 6). It operates well under vacuum, is sealed off the environment, and thus eliminates the risk of disseminating viral particles in the air[35].
The use of volatile anesthetics is beneficial for the treatment of patients with SARS-CoV-2 infection, but the exposure of health care professionals to waste anesthetic gas is a concern. Poor air-conditioning in ICUs and inconsistent international limits for anesthetic gas concentrations amplify the problem. The United States National Institute of Occupational Safety and Health defined an exposure limit of 2 ppm for isoflurane, sevoflurane, and desflurane, but other countries use higher exposure limits[35,38]. Most studies on air pollution report gas concentrations of less than 2 ppm while using MIRUSTM or AnaConDa® systems in mechanically ventilated patients; nonetheless, these studies use an air-conditioning system with at least six air exchanges per hour and a scavenging system (e.g., vacuum-based open reservoir gas scavenging systems or adsorbers with activated charcoal)[35,38,39].
To the best of our knowledge, there are no studies focusing on occupational gas exposure by inhalational sedation in patients undergoing ECMO therapy. Nevertheless, Meiser and colleagues observed that gas consumption during isoflurane sedation via AnaConDa® was exceptionally low, and they concluded that the sweep gas of the oxygenator did not contain the volatile anesthetic[20]. Our group measured the air pollution using photoacoustic gas monitoring in a similar setting (single room, isoflurane via AnaConDa®, vacuum-based scavenging system, air-conditioning with 11 air exchanges per hour) and detected concentrations of approximately 0.5 ppm to 2 ppm (unpublished data). Obviously, a proper application of all systems must be ensured as well as “good workplace practice”, including leak testing of the respirator and training of health care professionals. The conformity between the applied systems and respirators is of particular importance, as problems may occur even without active suction.
ECMO therapy is associated with high mortality and hence is deemed as the last resort after all other possible interventions failed. In case of COVID-19 related ARDS, the mortality 90 d post ECMO initiation is very high[17]. Besides the high mortality, ECMO therapy is also associated with a number of long-term effects, which are known from ICU survivors.
Postintensive care syndrome describes the impairments in physical function as well as cognitive and mental health that ICU survivors experience. The aftermaths of influenza A H1N1 or SARS showed that this syndrome can persist for years and hamper recovery[40]. In addition, a substantial portion of ICU survivors suffers from post-traumatic stress disorder. The data on ECMO survivors is sparse, but a limited number of studies demonstrated that these patients show impaired recovery, chronic pain, and mental illness, including post-traumatic stress disorder for up to 3 years after hospitalization[41-44]. Only very few studies suggest that ECMO treatment had no effect on ARDS patients after initiation of therapy[45]. Consequently, the authors of this article emphasize that indication for ECMO therapy must considered very carefully.
The application of inhaled sedation for ECMO treatment has a number of advantages. For instance, the supply of volatile anesthetics is currently not limited, in contrast to intravenous anesthetics. Volatile anesthetics are also less hallucinogenic, and patients require less opiates during inhaled sedation than during intravenous sedation. If applied properly, volatile anesthetics allow easier control of the depth of sedation of the patient, even if gas exchange is severely limited by COVID-19 related ARDS. Furthermore, the volumetric and reflective dead space of the delivery systems as well as CO2 retention are negligible for ECMO therapy. So far, few studies reported the successful application of isoflurane and sevoflurane for ECMO therapy[6-8]. The application of volatile anesthetics depends on adequate delivery and gas scavenging systems, which are not established in all ICUs[9]; however, the lack of electronic infusion pumps for intravenous sedatives due to the SARS-CoV-2 pandemic could be an incentive to equip ICUs with such hardware.
Currently, the application of volatile anesthetics for inhaled sedation during ECMO treatment is still not widely established. Consequently, the health care personnel lacks the adequate training for application of this procedure as well as for recognizing contraindications of which malignant hyperthermia is the most notable one.
In COVID-19 related ARDS, inhaled sedation demonstrated many advantages, including spontaneous breathing and deep sedation in prone position. Inhaled sedation also allows safe monitoring of sedation depth via measurement of the anesthetic gas. In addition, veno-venous ECMO avoids problems concerning dead space and CO2 increase, as sometimes seen during inhaled sedation via AnaConDa® or MIRUSTM. Further, inhaled sedation allows administration of isoflurane, which shows favorable properties, especially in light of the shortage of intravenous sedatives. This procedure, however, requires preparation and training. Hence, medical professionals should use the time of moderate occupancy rates in the ICUs accordingly.
| 1. | Liu Y, Li J, Feng Y. Critical care response to a hospital outbreak of the 2019-nCoV infection in Shenzhen, China. Crit Care. 2020;24:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 2. | Mang S, Kalenka A, Broman LM, Supady A, Swol J, Danziger G, Becker A, Hörsch SI, Mertke T, Kaiser R, Bracht H, Zotzmann V, Seiler F, Bals R, Taccone FS, Moerer O, Lorusso R, Bělohlávek J, Muellenbach RM, Lepper PM; COVEC-Study Group. Extracorporeal life support in COVID-19-related acute respiratory distress syndrome: A EuroELSO international survey. Artif Organs. 2021;45:495-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Ammar MA, Sacha GL, Welch SC, Bass SN, Kane-Gill SL, Duggal A, Ammar AA. Sedation, Analgesia, and Paralysis in COVID-19 Patients in the Setting of Drug Shortages. J Intensive Care Med. 2021;36:157-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Orser BA, Wang DS, Lu WY. Sedating ventilated COVID-19 patients with inhalational anesthetic drugs. EBioMedicine. 2020;55:102770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Suleiman A, Qaswal AB, Alnouti M, Yousef Md, Suleiman B, Jarbeh ME, Alshawabkeh G, Bsisu I, Santarisi A, Ababneh M. Sedating Mechanically Ventilated COVID-19 Patients with Volatile Anesthetics: Insights on the Last-Minute Potential Weapons. Sci Pharm. 2021;89. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Bellgardt M, Bomberg H, Herzog-Niescery J, Dasch B, Vogelsang H, Weber TP, Steinfort C, Uhl W, Wagenpfeil S, Volk T, Meiser A. Survival after long-term isoflurane sedation as opposed to intravenous sedation in critically ill surgical patients: Retrospective analysis. Eur J Anaesthesiol. 2016;33:6-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Jabaudon M, Boucher P, Imhoff E, Chabanne R, Faure JS, Roszyk L, Thibault S, Blondonnet R, Clairefond G, Guérin R, Perbet S, Cayot S, Godet T, Pereira B, Sapin V, Bazin JE, Futier E, Constantin JM. Sevoflurane for Sedation in Acute Respiratory Distress Syndrome. A Randomized Controlled Pilot Study. Am J Respir Crit Care Med. 2017;195:792-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 8. | Nieuwenhuijs-Moeke GJ, Jainandunsing JS, Struys MMRF. Sevoflurane, a sigh of relief in COVID-19? Br J Anaesth. 2020;125:118-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Jerath A, Parotto M, Wasowicz M, Ferguson ND. Volatile Anesthetics. Is a New Player Emerging in Critical Care Sedation? Am J Respir Crit Care Med. 2016;193:1202-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Kichloo A, Kumar A, Amir R, Aljadah M, Farooqi N, Albosta M, Singh J, Jamal S, El-Amir Z, Kichloo A, Lone N. Utilization of extracorporeal membrane oxygenation during the COVID-19 pandemic. World J Crit Care Med. 2021;10:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (2)] |
| 11. | Bamford P, Bentley A, Dean J, Whitmore D, Wilson-Baig N. ICS guidance for prone positioning of the conscious COVID patient. Int Care Soc 2020. [cited 8 March 2021]. Available from: https://emcrit.org/wp-content/uploads/2020/04/2020-04-12-Guidance-for-conscious-proning.pdf. |
| 12. | Deutsche Gesellschaft für Anästhesiologie & Intensivmedizin. S3-Leitlinie Invasive Beatmung und Einsatz extrakorporaler Verfahren bei akuter respiratorischer Insuffizienz. 2017. AWMF Leitlinien-Register Nr 001/021. [cited 12 August 2021]. Available from: https://www.awmf.org/leitlinien/detail/ll/001-021.html. |
| 13. | Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis. 2015;7:E166-E176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 184] [Reference Citation Analysis (0)] |
| 14. | Biancari F, Mariscalco G, Dalén M, Settembre N, Welp H, Perrotti A, Wiebe K, Leo E, Loforte A, Chocron S, Pacini D, Juvonen T, Broman LM, Perna DD, Yusuff H, Harvey C, Mongardon N, Maureira JP, Levy B, Falk L, Ruggieri VG, Zipfel S, Folliguet T, Fiore A. Six-Month Survival After Extracorporeal Membrane Oxygenation for Severe COVID-19. J Cardiothorac Vasc Anesth. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Murugappan KR, Walsh DP, Mittel A, Sontag D, Shaefi S. Veno-venous extracorporeal membrane oxygenation allocation in the COVID-19 pandemic. J Crit Care. 2021;61:221-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1779] [Cited by in RCA: 1730] [Article Influence: 288.3] [Reference Citation Analysis (0)] |
| 17. | Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, Bartlett RH, Tonna JE, Hyslop R, Fanning JJ, Rycus PT, Hyer SJ, Anders MM, Agerstrand CL, Hryniewicz K, Diaz R, Lorusso R, Combes A, Brodie D; Extracorporeal Life Support Organization. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 669] [Cited by in RCA: 666] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 18. | Young MJ, Brown SE, Truog RD, Halpern SD. Rationing in the intensive care unit: to disclose or disguise? Crit Care Med. 2012;40:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Bellgardt M, Drees D, Vinnikov V, Procopiuc L, Meiser A, Bomberg H, Gude P, Vogelsang H, Weber TP, Herzog-Niescery J. Use of the MIRUS™ system for general anaesthesia during surgery: a comparison of isoflurane, sevoflurane and desflurane. J Clin Monit Comput. 2018;32:623-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Meiser A, Bomberg H, Lepper PM, Trudzinski FC, Volk T, Groesdonk HV. Inhaled Sedation in Patients With Acute Respiratory Distress Syndrome Undergoing Extracorporeal Membrane Oxygenation. Anesth Analg. 2017;125:1235-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Meiser A, Groesdonk HV, Bonnekessel S, Volk T, Bomberg H. Inhalation Sedation in Subjects With ARDS Undergoing Continuous Lateral Rotational Therapy. Respir Care. 2018;63:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Tempia A, Olivei MC, Calza E, Lambert H, Scotti L, Orlando E, Livigni S, Guglielmotti E. The anesthetic conserving device compared with conventional circle system used under different flow conditions for inhaled anesthesia. Anesth Analg. 2003;96:1056-1061, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Farrell R, Oomen G, Carey P. A technical review of the history, development and performance of the anaesthetic conserving device "AnaConDa" for delivering volatile anaesthetic in intensive and post-operative critical care. J Clin Monit Comput. 2018;32:595-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Meyer H. Zur Theorie der Alkoholnarkose. Archiv für Experimentelle Pathologie und Pharmakologie. 1899;42:109-18. [RCA] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 600] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Thunberg T. Ernest Overton. Skandinavisches Archiv Für Physiologie. 1934;70: 1-9.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Philipp A, Wiesenack C, Behr R, Schmid FX, Birnbaum DE. High risk of intraoperative awareness during cardiopulmonary bypass with isoflurane administration via diffusion membrane oxygenators. Perfusion. 2002;17:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Nigro Neto C, De Simone F, Cassara L, Dos Santos Silva CG, Marãnhao Cardoso TA, Carco F, Zangrillo A, Landoni G. Tricks, tips, and literature review on the adapted vaporize system to deliver volatile agents during cardiopulmonary bypass. Ann Card Anaesth. 2016;19:240-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Scherer C, Kupka D, Stocker TJ, Joskowiak D, Scheuplein H, Schönegger CM, Born F, Stremmel C, Lüsebrink E, Stark K, Orban M, Petzold T, Peterss S, Hausleiter J, Hagl C, Massberg S. Isoflurane Sedation in Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation Treatment for Cardiogenic Shock-An Observational Propensity-Matched Study. Crit Care Explor. 2020;2:e0086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Rand A, Zahn PK, Schildhauer TA, Waydhas C, Hamsen U. Inhalative sedation with small tidal volumes under venovenous ECMO. J Artif Organs. 2018;21:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Flinspach AN, Zacharowski K, Ioanna D, Adam EH. Volatile Isoflurane in Critically Ill Coronavirus Disease 2019 Patients-A Case Series and Systematic Review. Crit Care Explor. 2020;2:e0256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Sandroni C, De Santis P, D'Arrigo S. Capnography during cardiac arrest. Resuscitation. 2018;132:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 32. | Siobal MS. Monitoring Exhaled Carbon Dioxide. Respir Care. 2016;61:1397-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Cutler DM, Summers LH. The COVID-19 Pandemic and the $16 Trillion Virus. JAMA. 2020;324:1495-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 403] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 34. | Herzog-Niescery J, Seipp HM, Weber TP, Bellgardt M. Inhaled anesthetic agent sedation in the ICU and trace gas concentrations: a review. J Clin Monit Comput. 2018;32:667-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Herzog-Niescery J, Vogelsang H, Gude P, Seipp HM, Uhl W, Weber TP, Bellgardt M. Environmental safety: Air pollution while using MIRUS™ for short-term sedation in the ICU. Acta Anaesthesiol Scand. 2019;63:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Wiesenack C, Wiesner G, Keyl C, Gruber M, Philipp A, Ritzka M, Prasser C, Taeger K. In vivo uptake and elimination of isoflurane by different membrane oxygenators during cardiopulmonary bypass. Anesthesiology. 2002;97:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Takhar A, Surda P, Ahmad I, Amin N, Arora A, Camporota L, Denniston P, El-Boghdadly K, Kvassay M, Macekova D, Munk M, Ranford D, Rabcan J, Tornari C, Wyncoll D, Zaitseva E, Hart N, Tricklebank S. Timing of Tracheostomy for Prolonged Respiratory Wean in Critically Ill Coronavirus Disease 2019 Patients: A Machine Learning Approach. Crit Care Explor. 2020;2:e0279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | National Institute of Occupational Safety and Health. NIOSH Pocket Guide to Chemical Hazards. Washington, DC, USA: Government Printing Office. 1994. [cited 12 August 2021]. Available from: https://www.cdc.gov/niosh/npg/default.html. |
| 39. | González-Rodríguez R, Muñoz Martínez A, Galan Serrano J, Moral García MV. Health worker exposure risk during inhalation sedation with sevoflurane using the (AnaConDa®) anaesthetic conserving device. Rev Esp Anestesiol Reanim. 2014;61:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Sackey PV, Martling CR, Granath F, Radell PJ. Prolonged isoflurane sedation of intensive care unit patients with the Anesthetic Conserving Device. Crit Care Med. 2004;32:2241-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 41. | Mayer KP, Jolley SE, Etchill EW, Fakhri S, Hoffman J, Sevin CM, Zwischenberger JB, Rove JY; the Outcomes and Recovery After COVID-19 Leading to ECMO (ORACLE) Group. Long-term recovery of survivors of coronavirus disease (COVID-19) treated with extracorporeal membrane oxygenation: The next imperative. JTCVS Open. 2021;5:163-168.. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 42. | Harley O, Reynolds C, Nair P, Buscher H. Long-Term Survival, Posttraumatic Stress, and Quality of Life post Extracorporeal Membrane Oxygenation. ASAIO J. 2020;66:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Stoll C, Haller M, Briegel J, Meier M, Manert W, Hummel T, Heyduck M, Lenhart A, Polasek J, Bullinger M, Schelling G. [Health-related quality of life. Long-term survival in patients with ARDS following extracorporeal membrane oxygenation (ECMO)]. Anaesthesist. 1998;47:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Roll MA, Kuys S, Walsh JR, Tronstad O, Ziegenfuss MD, Mullany DV. Long-Term Survival and Health-Related Quality of Life in Adults After Extra Corporeal Membrane Oxygenation. Heart Lung Circ. 2019;28:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Mikkelsen ME, Shull WH, Biester RC, Taichman DB, Lynch S, Demissie E, Hansen-Flaschen J, Christie JD. Cognitive, mood and quality of life impairments in a select population of ARDS survivors. Respirology. 2009;14:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Atogebania JW, Deng L, Zhu C S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wu RR