Published online Nov 9, 2021. doi: 10.5492/wjccm.v10.i6.310
Peer-review started: April 29, 2021
First decision: June 17, 2021
Revised: June 28, 2021
Accepted: September 30, 2021
Article in press: September 30, 2021
Published online: November 9, 2021
Processing time: 190 Days and 3.3 Hours
Accurate assessment of the hemodynamic status is vital for appropriate management of patients with critical illness. As such, there has been a constant quest for reliable and non-invasive bedside tools to assess and monitor circulatory status in order to ensure end-organ perfusion. In the recent past, point of care ultrasonography (POCUS) has emerged as a valuable adjunct to physical examination in various specialties, which basically is a clinician-performed bedside ultrasound to answer focused questions. POCUS allows visualization of the internal anatomy and flow dynamics in real time, guiding apt interventions. While both arterial (forward flow) and venous (organ outflow or afterload) limbs of hemodynamic circuit are important for tissue perfusion, the venous side remains relatively under-explored. With recent data underscoring the deleterious consequences of iatrogenic volume overload, objective evaluation of venous congestion is gaining attention. Bedside Doppler ultrasound serves this purpose and aids in diagnosing and monitoring the congestion/venous blood flow pattern. In this article, we summarize the rationale for integrating this technology into routine care of patients with volume-related disorders, discuss the normal and abnormal waveforms, limitations, and future directions.
Core Tip: Point-of-care Doppler ultrasonography is emerging as a valuable bedside diagnostic tool for the assessment of venous congestion. Doppler interrogation of the abdominal veins such as the hepatic, portal, renal parenchymal veins in addition to inferior vena cava ultrasound provides useful insights into a patient’s hemodynamics, when interpreted in conjunction with other sonographic parameters such as the cardiac pump function, lung ultrasound and conventional clinical assessment.
- Citation: Galindo P, Gasca C, Argaiz ER, Koratala A. Point of care venous Doppler ultrasound: Exploring the missing piece of bedside hemodynamic assessment. World J Crit Care Med 2021; 10(6): 310-322
- URL: https://www.wjgnet.com/2220-3141/full/v10/i6/310.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v10.i6.310
Objective assessment of hemodynamic status is fundamental to guide resuscitative efforts in a critically ill patient[1]. Among the myriad of methods used at the bedside, only a few have stood the test of time. Capillary refill time and passive leg raise with non-invasive cardiac output (CO) monitoring can be counted amongst these as both strategies have been shown to improve relevant patient outcomes in controlled trials[2,3]. The success of these assessments seems to rely on avoiding unnecessary fluid loading thus mitigating fluid overload, which has been increasingly linked to adverse patient outcomes[4].
Inevitably, intensive care unit physicians will encounter over-resuscitated patients as well as those presenting with pre-existing volume overload[5]. While avoiding further fluid loading is important, efforts to actively decrease extracellular volume (de-resuscitation) have been shown to lead to potentially beneficial clinical outcomes[6]. De-resuscitation is especially relevant for patients presenting with or developing heart failure during the course of their critical illness as volume overload in this patient population results in increasing severity of venous congestion[7]. Increased left-sided filling pressures facilitate lung congestion and lead to worsening respiratory status[8]. Less appreciated however, are the consequences of systemic venous congestion secondary to increased right-sided filling pressures. Increased right atrial pressure (RAP) can be transmitted backwards across the venous tree and lead to congestive organ dysfunction[9]. This can manifest as elevated bilirubin from congestive hepatopathy[10], delirium from congestive encephalopathy[11], acute oliguric kidney injury from ‘intra-capsular tamponade’[7], and gut edema resulting in increased endotoxemia[12,13].
The degree of congestive organ dysfunction is not only a function of absolute RAP, but also depends on the degree of transmission of such pressure to the peripheral organs. Increased RAP becomes initially attenuated along the venous vascular tree as a consequence of venous distensibility[14]. However, progressive increases in venous volume will eventually result in maximally stretched venous walls reaching the flat part of the venous compliance curve; At this point, pressure transmission will be greatly enhanced leading to peripheral organ congestion. Because of this, assessing congestion at the level of the organs can provide valuable information regarding the mechanisms of organ dysfunction[15]. Given venous congestion results in altered patterns of organ venous flow, Doppler point-of-care ultrasonography (POCUS) allows quantification of these alterations at the bedside[16].
Sonographic assessment of the collapsibility/distensibility of inferior vena cava (IVC) to predict volume responsiveness has several caveats and, in our opinion, should not be used for such purpose[17]. However, a plethoric (> 20 mm) non collapsible IVC is not normal and will only be seen in patients with pathological venous congestion[18]. Evaluation of the IVC using POCUS is a well-accepted surrogate of venous congestion as it mainly reflects RAP; However, many factors influence IVC size and collapsibility such as respiratory effort in spontaneously breathing patients[19] and the presence of intra-abdominal hypertension[20]. Another problem is inherent to the conventional long axis view of interrogation; Given the IVC is a three-dimensional structure with elliptical shape, evaluation of diameters in both long and short axes has been shown to be a better estimate of central venous pressure (CVP)[21].
Although a plethoric non-collapsible IVC establishes the presence of venous congestion, this information alone is not always adequate to guide management for two important reasons: Firstly, obstructive pathologies acutely leading to venous congestion need immediate resolution by specific interventions that have nothing to do with extracellular volume (cardiac tamponade, tension pneumothorax, massive pulmonary embolism). In these cases, focused cardiac ultrasound is necessary to establish diagnosis and management[22]. The second reason is that certain cardiac pathologies (chronic severe pulmonary hypertension, right ventricular failure, severe valvulopathies, restrictive cardiomyopathy or constrictive pericarditis) require elevated RAP in order to maintain CO; as such, excessive volume removal targeting a normal IVC diameter and collapsibility is not in the best interest of these patients[23]. However, progressive volume overload beyond what is needed to maintain CO will lead to an excessive increase in RAP, which can be transmitted to peripheral organs resulting in their dysfunction[7]. Thus, in this particular setting, evaluating pressure transmission using Doppler ultrasonography is a valuable non-invasive adjunct to overall clinical assessment.
To assess the venous system with Doppler ultrasound, it is important to understand that flow pattern is the main variable being measured. Flow is generated by a pressure differential between two points, given a relatively constant vessel diameter, this pressure differential will determine flow velocity. Equilibration of pressures will cause flow to cease. When assessing flow with pulsed wave Doppler ultrasound, the direction is represented by positive or negative deflections from the baseline, while speed will be represented by the deflection amplitude. If the flow moves away from the transducer, the image will show a negative deflection (analogous to ‘blue’ on color Doppler). A positive deflection will be seen if flow is directed towards the transducer (analogous to ‘red’ on color Doppler)[24]. The normal venous flow patterns are determined by the changes in RAP throughout the cardiac cycle and modified by venous compliance and distance from the heart[25]. Therefore, the flow patterns will be different depending on the site being evaluated. Normal waveforms can be pulsatile with discernable flow corresponding to the phases of cardiac cycle as in the case of hepatic vein (HV), or continuous as with portal and intra-renal veins. Moreover, respirophasic changes in amplitude can be demonstrated reflecting the increased venous return during inspiration in spontaneously breathing patients.
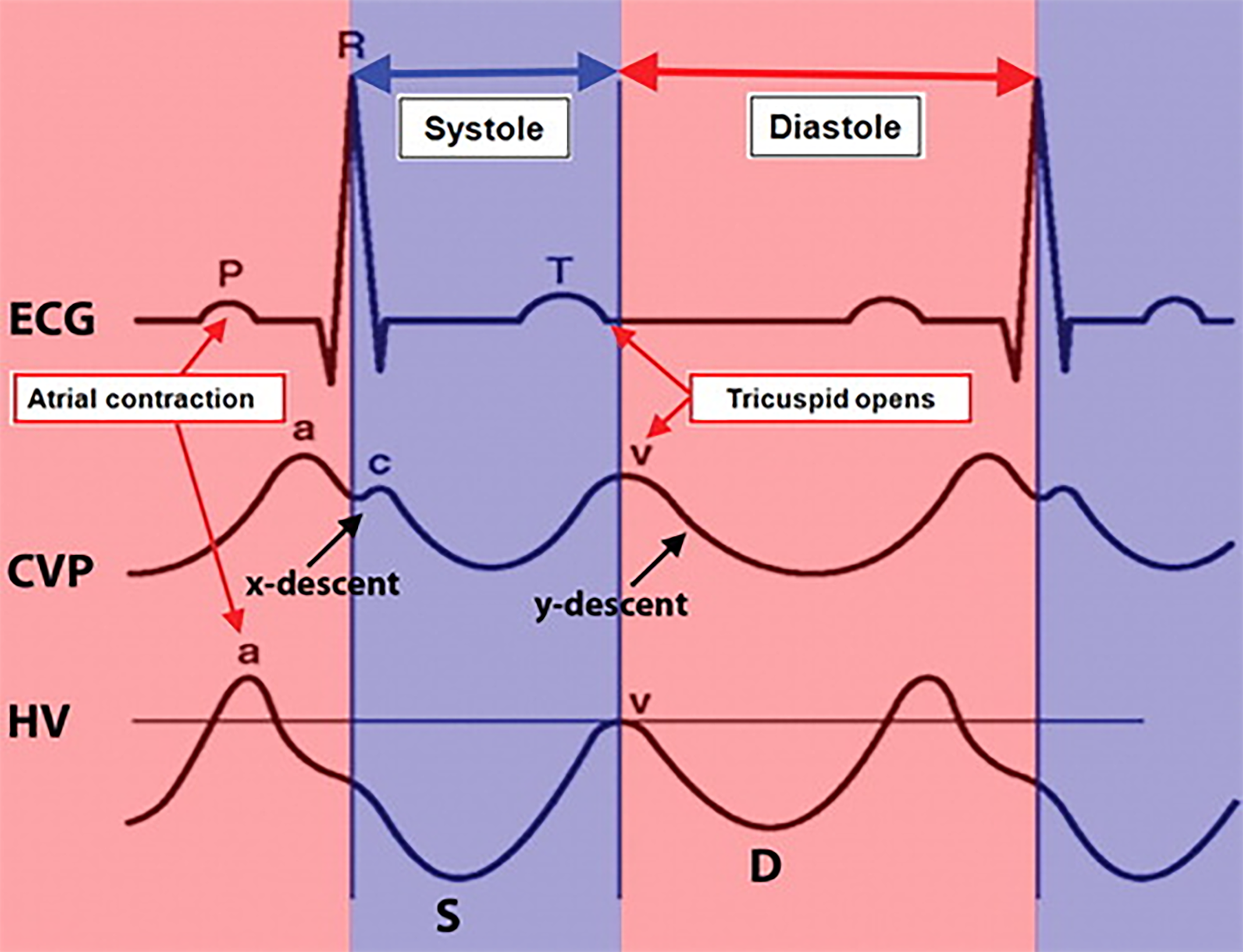
In a normal CVP trace, atrial systolic contraction results in a rise in RAP represented as the A-wave. After the tricuspid valve closes (C-wave), the right atrium relaxes, and the ventricular systole pulls down the annulus towards apex resulting in a fall of RAP represented as the X-descent. The RA filling from the venous system during ventricular systole causes a progressive rise in RAP and forms the V wave. The Y-descent is then caused by tricuspid valve opening.
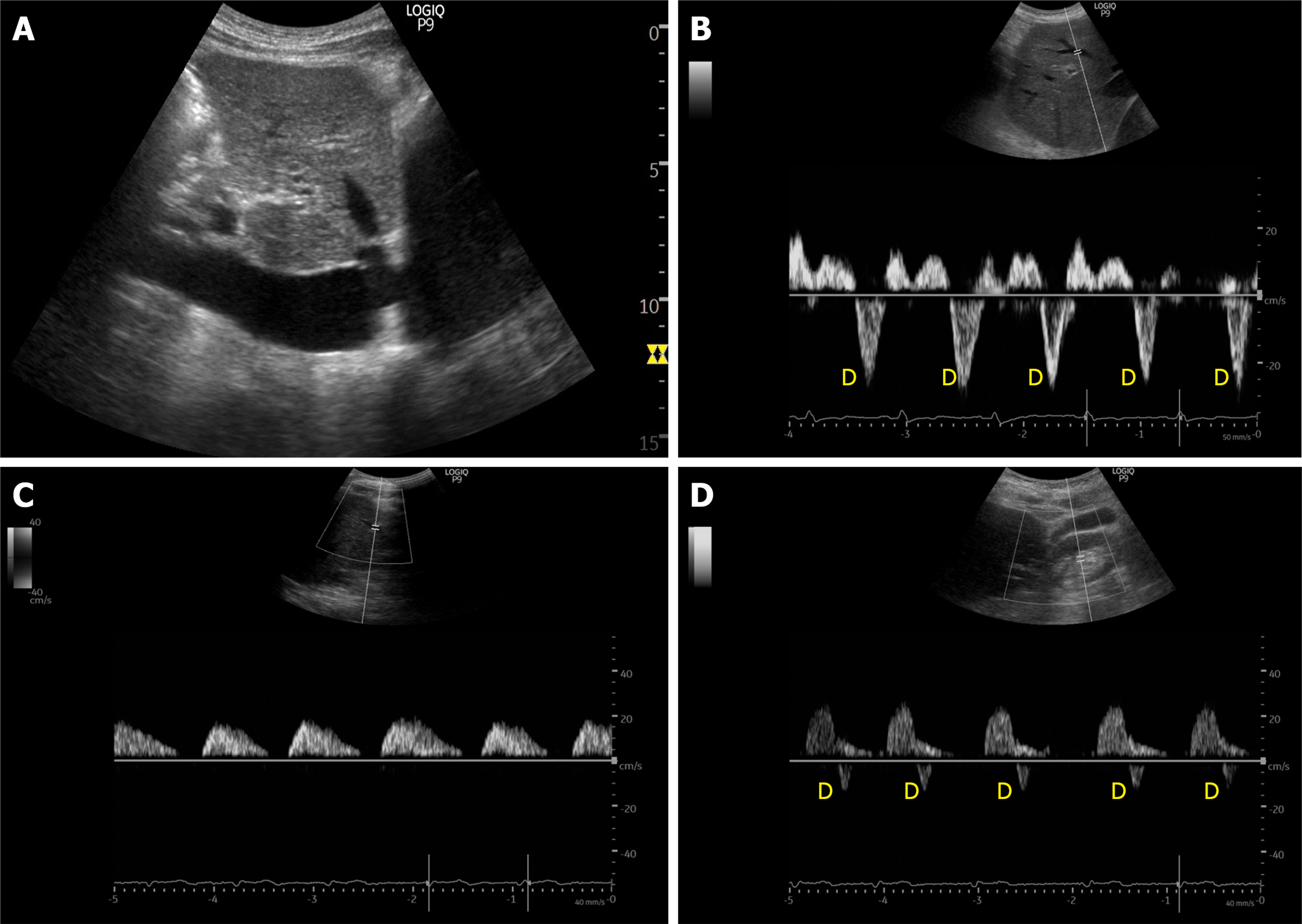
Since HV directly joins the IVC, their flow pattern is a mirror reflection of RAP variations throughout the cardiac cycle. Normal HV flow pattern consists of a positive/retrograde wave (A) that represents atrial systolic contraction analogous to A-wave of the CVP, and two negative/antegrade systolic (S) and diastolic (D) waves that represent the X and Y-descents of the CVP, respectively. Since X-descent is deeper than Y-descent, the HV S-wave usually has a larger amplitude than the D-wave (S > D)[26]. Figure 1 illustrates the normal time-correlated electrocardiographic (ECG) findings, CVP tracing and HV Doppler waveform.
In more distal vascular beds such as intra-renal and femoral veins, the tracing will not directly reflect RAP variations; this is explained by the high compliance of the venous system and the attenuation of RAP variations with increasing distance from the heart. The flow pattern in normal distal veins will be predominantly continuous with no discernible waves, although low amplitude S and D-waves may be seen[27,28]. It is of note that the intra-renal Doppler is usually obtained at the level of interlobar vessels, which pass through the renal parenchyma and hence thought to better reflect organ perfusion compared to the main renal vein. Intra-renal venous trace is often accompanied by arterial trace above the baseline as the Doppler sample volume overlies both interlobar vein and artery, which are much smaller compared to other vessels such as HV.
The portal vein (PV) is part of a distinct venous system that it is isolated from central veins by the hepatic sinusoids and from the arterial system by splanchnic capillaries. Therefore, the Doppler waveform of the normal PV will not reflect RAP variations unlike that of HV and appears as a characteristic positive (flow towards the transducer), continuous (or mildly pulsatile) flow[29].
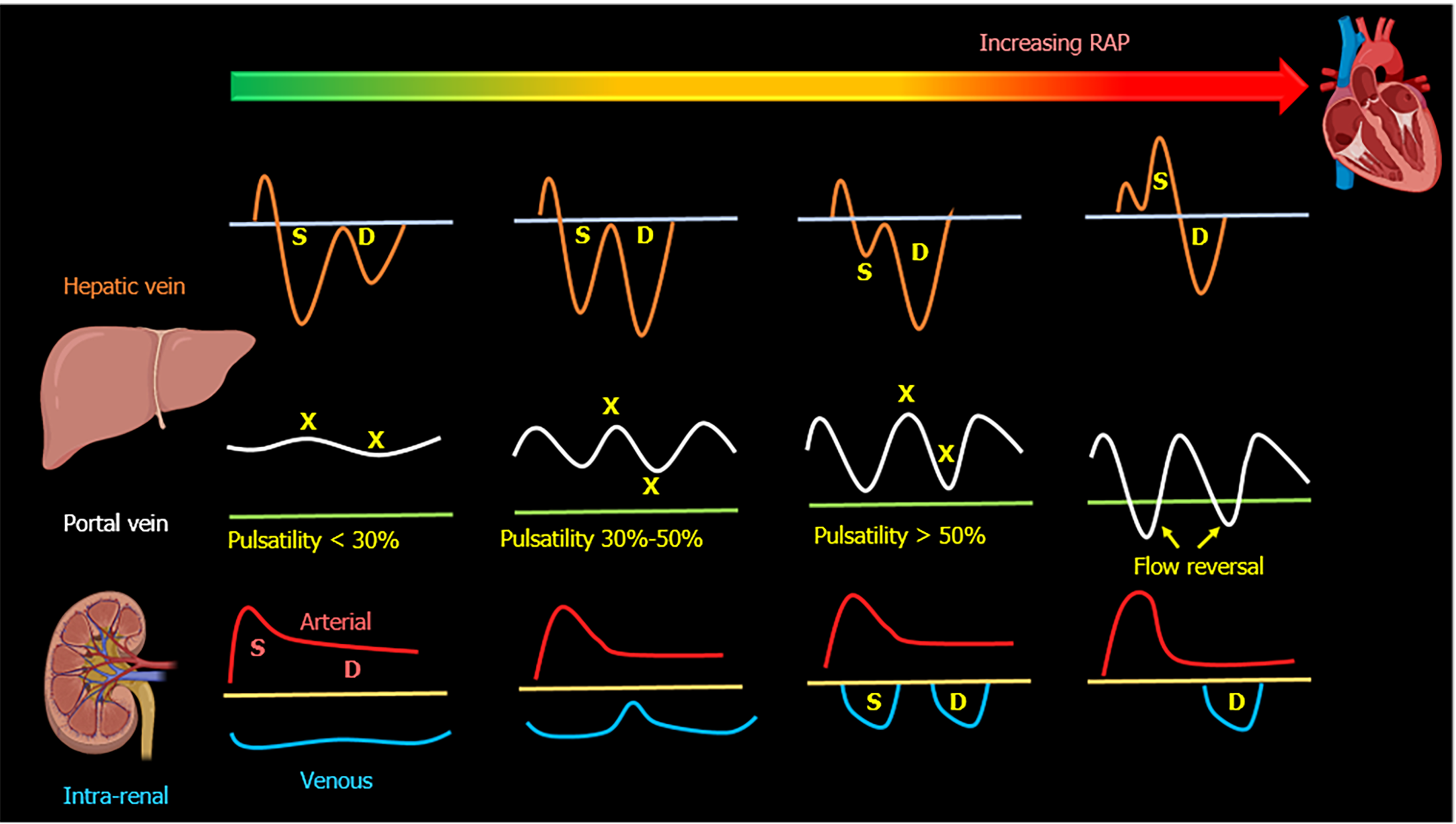
When the RAP increases, the characteristic ascending and descending waves formed within the RA will change. As the RA filling pressure increases, the X-descent decreases in amplitude while the Y-descent amplitude increases. This is due to loss of RA compliance and decreased right ventricular systolic pull of the tricuspid valve annulus. Right ventricular overload will eventually cause tricuspid annular dilation and tricuspid regurgitation, leading to obliteration of the X descent and fusion of C-V waves of the CVP waveform. All of this will be reflected in the HV flow; initially, the amplitude of the S-wave decreases compared to that of D-wave (S < D pattern)[30-32]. With worsening congestion, the S-wave can be obliterated or become reversed/ retrograde if severe tricuspid regurgitation if present[33-35]. HV alterations have been shown to correlate with increased PV pulsatility, abnormal intra-renal venous flow and adverse kidney events including acute kidney injury (AKI) in recent studies[16,36-38].
The main alteration in the PV waveform is progressive increase in pulsatility with elevated RAP. This can be quantified by the pulsatility fraction [(Vmax-Vmin/Vmax) × 100]; a pulsatility fraction ≥ 30% is considered mild elevation while ≥ 50% is considered severe. Further increases in RAP may lead to flow reversal (below the baseline) during systole[39-42]. The physiological explanation of pulsatility is the reduction of flow velocity during systole secondary to retrogradely transmitted waves from the right atrium during this phase of the cardiac cycle.
Most Clinical studies evaluating PV Doppler have been performed in the context of decompensated heart failure and cardiac surgery. PV pulsatility has been correlated with elevated RAP, clinical features of congestion[40], pulmonary wedge pressure, pulmonary artery resistance, right ventricular end diastolic pressure[39], left and right ventricle size[41], mean pulmonary artery pressure and peripheral vascular resistance[42]. Similar to HV, the recent focus has been to study the impact of PV pulsatility on clinical outcomes. In patients with decompensated heart failure, increased PV pulsatility was associated with worse clinical outcomes if present at discharge and predicted response to diuresis at admission[43,44]. In cardiac surgery patients, PV pulsatility was associated with congestive encephalopathy and delirium[11], AKI[45] and right ventricular dysfunction[46].
The intra-renal venous Doppler (IRVD) pattern is continuous, sometimes with a brief interruption during atrial systole. This pattern becomes biphasic as RAP increases and two distinct waves (S and D) can be observed. These waves are analogous to the normal hepatic waveform and represent increased pressure transmission from the heart to the interlobar renal veins[27]. With worsening congestion (intracapsular tamponade) the S-wave can either become reversed or disappear (obscured in the arterial trace). Though venous impedance index [(maximum flow velocity−minimum diastolic flow velocity)/maximum flow velocity] is frequently reported in studies to quantify renal venous pulsatility, pattern recognition described above is simpler. Moreover, when the waveform is discontinuous, the impedance index becomes 1 as the minimum velocity is zero and does not differentiate between biphasic and monophasic patterns. In this regard, renal venous stasis index (RVSI) proposed by Husain-Syed et al[47] better reflects the full continuum of renal congestion. It indicates the proportion of the cardiac cycle during which there is no venous outflow and is calculated as: Cardiac cycle time−venous flow time/cardiac cycle time. Therefore, monophasic pattern has a higher RVSI than biphasic pattern.
Multiple studies have shown that IRVD alterations are not merely a reflection of increased RAP, but also strong predictors of adverse clinical outcomes in patients with compensated[27] and decompensated heart failure[48], those undergoing cardiac surgery[45], patients with pulmonary hypertension and right heart failure[47].
In cardiac surgery patients, altered intra-renal Doppler pattern was shown to be a strong predictor of AKI. However, this was not replicated in less selected populations of critically ill patients[38,49]. Given the multitude of etiologies of AKI in addition to venous congestion (such as tubular injury) in such patients, this lack of association is not surprising. A visual summary of normal and altered venous flow patterns in the above-described veins is shown in Figures 2-4.
As opposed to intra-renal and PV, the common femoral vein is directly connected to the IVC facilitating the quick transmission of pressure waves as RAP increases. Flow in the normal femoral vein is relatively continuous with respiratory variability although a low amplitude positive/retrograde wave (A-wave) and anterograde S and D-waves maybe appreciated depending on the angle of insonation[28]. With elevations in RAP, the retrograde A-wave increases in amplitude or it will fuse with a reversed S-wave if severe tricuspid regurgitation is present. A retrograde wave velocity of ≥ 10 cm/s is considered abnormal and indicative of high RAP[50].
Few studies have integrated femoral vein Doppler flow into diagnostic algorithm; in a recent study including 47 patients with pulmonary thromboembolism, changes in the pulsatility pattern of the femoral vein were seen in all patients with right ventricular dysfunction[51]. Recently, it has been proposed as a quick way to diagnose right ventricular dysfunction in patients undergoing cardiac surgery[28]. Indeed, it is an attractive option in the emergency settings, where femoral vein is often sonographically assessed for central venous catheter placement. Table 1 summarizes the advantages and limitations of the Doppler evaluation of above-discussed vessels.
| Advantages | Limitations | |
| Hepatic vein | Easy to acquire images from the same window used to assess IVC. | Prone to erroneous interpretation without simultaneous EKG tracing. |
| Influenced by arrhythmias (e.g., S-wave can be smaller in atrial fibrillation), right ventricular systolic dysfunction. | ||
| May never normalize in chronic pulmonary hypertension, structural tricuspid regurgitation irrespective of fluid status. | ||
| Portal vein | Easy to assess without EKG. | Not reliable in cirrhosis. |
| Reliably changes with decongestive therapy - can monitor response to diuresis/ultrafiltration in real time. | Can be pulsatile in young, thin individuals without raised RAP. | |
| Tends to improve with decongestion, if not normalize even in chronic pulmonary hypertension. | ||
| Renal parenchymal vein | Simultaneous arterial tracing functions as a built-in EKG. | Difficult to obtain optimal images. |
| Not studied in chronic kidney disease/patients with structural renal abnormalities. | ||
| Interstitial edema may hamper improvement with decongestive therapy in real time (improves but lags behind decongestion). | ||
| May never normalize in chronic pulmonary hypertension, structural tricuspid regurgitation irrespective of fluid status. | ||
| Femoral vein | Technically easier to acquire images of the vein. | Susceptible to excessive transducer pressure. |
| Dependent on correct Doppler angle if measuring absolute velocities (pattern evaluation is less angle dependent). |
Doppler evaluation of venous congestion does not come caveat-free; first of all, the evaluation is operator dependent, meaning that the experience of the observer effects the image acquisition and interpretation. It is not unexpected because Doppler ultrasonography requires a higher skill level than for basic greyscale POCUS applications. Interobserver agreement has been reported mainly with experienced operators. For the HV, the kappa index was 0.95[52]; for the intra-renal venous Doppler and PV, the interobserver agreement was 87% and 95% respectively[45]; and for the femoral vein Doppler, the reproducibility of readings was 80%-98%[50]. Secondly, clinicians must be aware of the false negative and false positive findings that can interfere with interpretation. The HV Doppler should be accompanied by a simultaneous ECG as much as possible; otherwise, the observer can incorrectly identify A-wave as a retrograde S-wave and vice versa. Similarly, S and D waves can be confused for one another. Notably, pulsatile PV flow can be found in young healthy individuals with low body mass index, without elevations in RAP[53]. On the other hand, reduced PV pulsatility despite elevated RAP has been reported in patients with parenchymal liver disease[54-56]. Intra-renal venous Doppler is technically challenging to obtain and more time consuming; it can also be altered by obstructive urological pathologies[57]. Doppler interrogation of the femoral vein may be altered by application of excessive transducer pressure. Due to these limitations, isolated interpretation of individual waveforms may lead to incorrect conclusions. Therefore, assessing IVC and multiple venous sites including HV, PV, IRVD in an organized stepwise manner could enhance diagnostic performance. Corroborating this notion, a recent study employing a protocolized venous Doppler examination termed “VExUS” (venous excess ultrasound score) has shown greater specificity for organ injury than any individual assessments[16].
Similar to IVC, internal jugular vein (IJV) ultrasound can also be used to estimate RAP non-invasively. In one study, < 17% increase in right IJV cross sectional area with Valsalva maneuver predicted an elevated RAP (≥ 12 mmHg) with 90% sensitivity and 74% specificity[58]. In patients who cannot follow instructions, assessment of IJV diameter at the end of inspiration and expiration can provide a rough idea of CVP. For example, in a study of 34 spontaneously breathing patients, mean IJV diameter was 7 mm in those with CVP < 10 cm H2O [95% confidence interval (CI): 5.7-8.3] vs 12.5 mm (95%CI, 11.2-13.8) in those with CVP of ≥ 10 cm H2O[59]. In intubated patients, it is of limited utility to predict CVP but an IJV distensibility of > 18% prior to volume challenge has shown to predict response to fluids[60]. While IJV ultrasound appears easy to perform, the amount of information it can provide is limited and cannot be used in lieu of VExUS. Moreover, it is subject to erroneous interpretations due to inadvertent application of excess transducer pressure, limited access to the neck because of the presence of central venous catheters, tracheostomy collars, braces etc. On the other hand, superior vena cava ultrasound has been studied in the context of predicting fluid responsiveness and shown to perform better than IVC[61]. However, transesophageal echocardiography is required to reliably access the vessel, which is not routinely performed in all clinical settings.
Venous Doppler ultrasound should not be used to ‘determine’ fluid status or assess fluid responsiveness. This novel bedside tool should be viewed as another piece of information in the global hemodynamic assessment of the critically ill patient in addition to other sonographic and clinical parameters. Since the information it yields might be particularly relevant for patients with oliguric AKI, the following discussion will center on the resuscitative efforts aimed at restoring renal perfusion (renoresuscitation). The first step in evaluating oliguric kidney injury is excluding obstructive pathology by kidney and urinary bladder ultrasound[62]. Also, looking for the cues to intrinsic kidney injury such as acute tubular necrosis or acute interstitial nephritis is of paramount importance as resuscitative efforts are unlikely to restore renal function in this situation[63]. Intrinsic AKI should be suspected when the clinical and laboratory data point to tubular dysfunction (exposure to nephrotoxins, prolonged hypotension, isosthenuria, high fractional excretion of sodium, abundant muddy brown casts on urine microscopy)[64]. A furosemide stress may help assessing renal tubular integrity as well as bears prognostic significance[63]. While acute glomerulonephritis is uncommon in patients with hospital-acquired AKI, finding of dysmorphic red blood cells on urine sediment examination should prompt nephrology consultation for investigation of glomerular causes of AKI.
On the other hand, evidence of preserved tubular function should lead to presumptive diagnosis of hemodynamic AKI caused by renal hypoperfusion. Evidence of global hypoperfusion (increased capillary refill time, skin mottling, altered mental status) increases the likelihood that resuscitative interventions could result in improved urine output. It is important to understand that renal perfusion pressure is proportional to the difference between mean arterial blood pressure (MABP) and renal venous pressure, and inversely proportional to renal arteriolar resistance[65]. Traditional resuscitative efforts have focused on increasing MABP (vasopressors) or increasing CO (fluids, inotropes). However, less attention has been paid to renal venous pressure even though this is an equally important determinant of renal perfusion. Measurement of intra-abdominal pressure should be performed if there is a suspicion of abdominal compartment syndrome, particularly in patients with trauma or tense ascites[66]. In addition, Doppler evaluation of venous congestion can point to renal congestion (intra-capsular tamponade) as the cause of renal hypoperfusion by demonstrating the effects of raised RAP on venous outflow[16,67]. This previously missing piece of the hemodynamic puzzle can add valuable information as oliguric AKI in the presence of severe venous congestion will likely worsen with fluid administration but is likely to improve following decongestion[67-69]. Finally, it is also important to recognize that microvascular alterations underly many cases of sepsis associated AKI[70]. These alterations are an important determinant of glomerular hydrostatic pressure regardless of macrohemodynamics and as such, are not likely to improve with conventional resuscitative efforts.
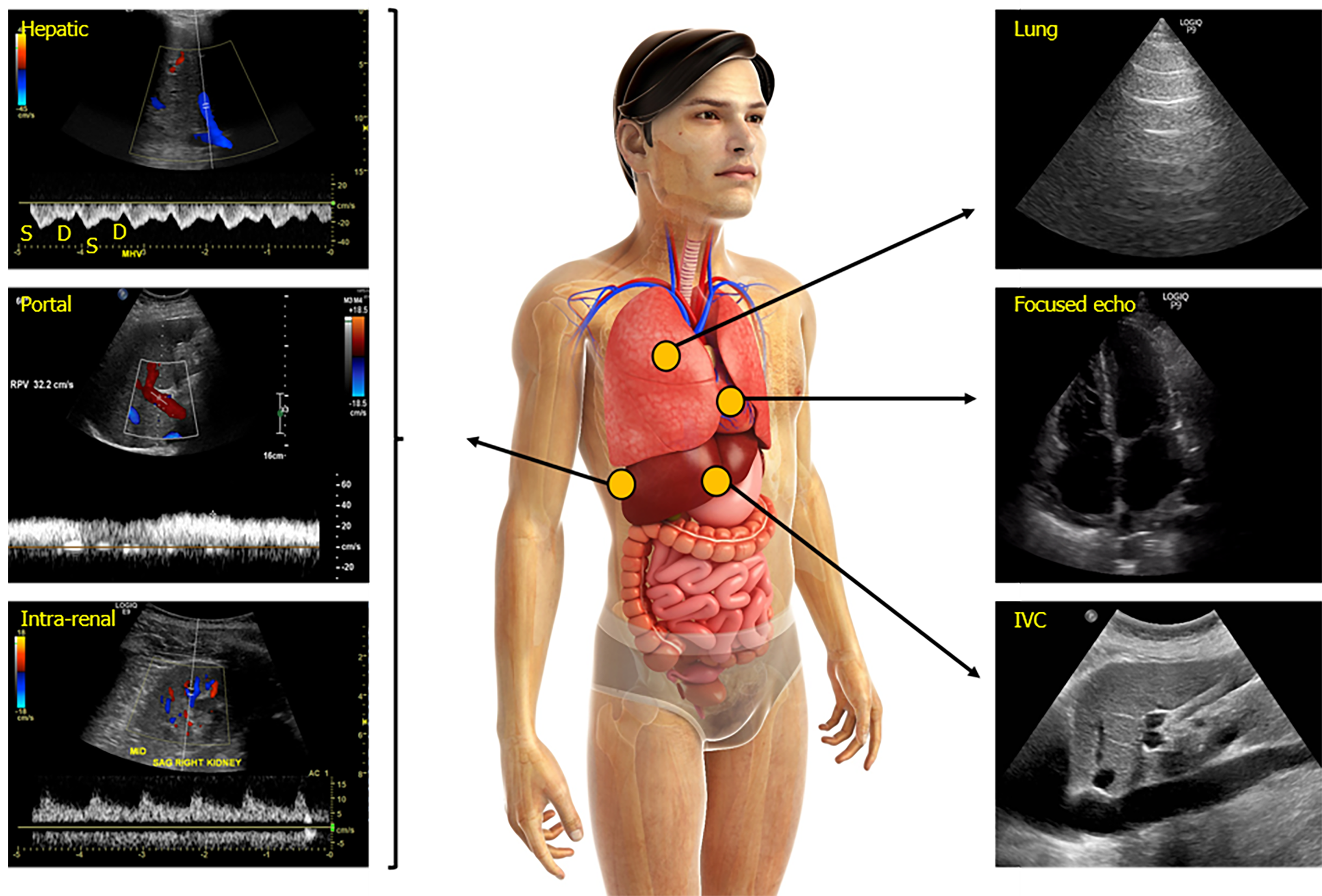
Performing a comprehensive hemodynamic assessment using POCUS in addition to conventional evaluation is vital in the management of critically ill patients as multiple hemodynamic alterations might be present simultaneously (the so-called pump, pipes, leaks strategy)[70]. For example, a septic patient with pre-existing heart failure can display both vasodilation (low peripheral vascular resistance) and severe venous congestion. In this setting, vasopressors and diuretics can be used together to address these alterations. In summary, venous Doppler provides valuable information regarding a patient’s hemodynamic status, when used in combination with multi-organ POCUS as well as clinical and laboratory data.
Multi-point Doppler evaluation of the venous system allows clinicians to assess the downstream effects of elevated RAP on peripheral organs. This tool should not be used as a marker of fluid status or volume responsiveness but rather as a means to determine if congestion is contributing to organ dysfunction and gauge the response to decongestive therapy. This information should be integrated into a comprehensive hemodynamic evaluation in order to choose the appropriate resuscitative strategy. Future studies should focus on investigating whether incorporating venous Doppler ultrasound in the diagnostic and treatment algorithms translates into better clinical outcomes. Furthermore, as most of the current data are from patients with heart failure, research should be undertaken in other subsets of patients susceptible to fluid overload such as those with liver cirrhosis and chronic kidney disease.
| 1. | Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaeschke R, Mebazaa A, Pinsky MR, Teboul JL, Vincent JL, Rhodes A. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1795-1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 947] [Cited by in RCA: 1125] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 2. | Douglas IS, Alapat PM, Corl KA, Exline MC, Forni LG, Holder AL, Kaufman DA, Khan A, Levy MM, Martin GS, Sahatjian JA, Seeley E, Self WH, Weingarten JA, Williams M, Hansell DM. Fluid Response Evaluation in Sepsis Hypotension and Shock: A Randomized Clinical Trial. Chest. 2020;158:1431-1445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 3. | Hernández G, Ospina-Tascón GA, Damiani LP, Estenssoro E, Dubin A, Hurtado J, Friedman G, Castro R, Alegría L, Teboul JL, Cecconi M, Ferri G, Jibaja M, Pairumani R, Fernández P, Barahona D, Granda-Luna V, Cavalcanti AB, Bakker J; The ANDROMEDA SHOCK Investigators and the Latin America Intensive Care Network (LIVEN), Hernández G, Ospina-Tascón G, Petri Damiani L, Estenssoro E, Dubin A, Hurtado J, Friedman G, Castro R, Alegría L, Teboul JL, Cecconi M, Cecconi M, Ferri G, Jibaja M, Pairumani R, Fernández P, Barahona D, Cavalcanti AB, Bakker J, Hernández G, Alegría L, Ferri G, Rodriguez N, Holger P, Soto N, Pozo M, Bakker J, Cook D, Vincent JL, Rhodes A, Kavanagh BP, Dellinger P, Rietdijk W, Carpio D, Pavéz N, Henriquez E, Bravo S, Valenzuela ED, Vera M, Dreyse J, Oviedo V, Cid MA, Larroulet M, Petruska E, Sarabia C, Gallardo D, Sanchez JE, González H, Arancibia JM, Muñoz A, Ramirez G, Aravena F, Aquevedo A, Zambrano F, Bozinovic M, Valle F, Ramirez M, Rossel V, Muñoz P, Ceballos C, Esveile C, Carmona C, Candia E, Mendoza D, Sanchez A, Ponce D, Ponce D, Lastra J, Nahuelpán B, Fasce F, Luengo C, Medel N, Cortés C, Campassi L, Rubatto P, Horna N, Furche M, Pendino JC, Bettini L, Lovesio C, González MC, Rodruguez J, Canales H, Caminos F, Galletti C, Minoldo E, Aramburu MJ, Olmos D, Nin N, Tenzi J, Quiroga C, Lacuesta P, Gaudín A, Pais R, Silvestre A, Olivera G, Rieppi G, Berrutti D, Ochoa M, Cobos P, Vintimilla F, Ramirez V, Tobar M, García F, Picoita F, Remache N, Granda V, Paredes F, Barzallo E, Garcés P, Guerrero F, Salazar S, Torres G, Tana C, Calahorrano J, Solis F, Torres P, Herrera L, Ornes A, Peréz V, Delgado G, López A, Espinosa E, Moreira J, Salcedo B, Villacres I, Suing J, Lopez M, Gomez L, Toctaquiza G, Cadena Zapata M, Orazabal MA, Pardo Espejo R, Jimenez J, Calderón A, Paredes G, Barberán JL, Moya T, Atehortua H, Sabogal R, Ortiz G, Lara A, Sanchez F, Hernán Portilla A, Dávila H, Mora JA, Calderón LE, Alvarez I, Escobar E, Bejarano A, Bustamante LA, Aldana JL. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA. 2019;321:654-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 567] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 4. | Malbrain MLNG, Langer T, Annane D, Gattinoni L, Elbers P, Hahn RG, De Laet I, Minini A, Wong A, Ince C, Muckart D, Mythen M, Caironi P, Van Regenmortel N. Intravenous fluid therapy in the perioperative and critical care setting: Executive summary of the International Fluid Academy (IFA). Ann Intensive Care. 2020;10:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (1)] |
| 5. | Malbrain MLNG, Van Regenmortel N, Saugel B, De Tavernier B, Van Gaal PJ, Joannes-Boyau O, Teboul JL, Rice TW, Mythen M, Monnet X. Principles of fluid management and stewardship in septic shock: it is time to consider the four D's and the four phases of fluid therapy. Ann Intensive Care. 2018;8:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 406] [Cited by in RCA: 372] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 6. | Bissell BD, Laine ME, Thompson Bastin ML, Flannery AH, Kelly A, Riser J, Neyra JA, Potter J, Morris PE. Impact of protocolized diuresis for de-resuscitation in the intensive care unit. Crit Care. 2020;24:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Rosenkranz S, Howard LS, Gomberg-Maitland M, Hoeper MM. Systemic Consequences of Pulmonary Hypertension and Right-Sided Heart Failure. Circulation. 2020;141:678-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 206] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 8. | Verbrugge FH, Guazzi M, Testani JM, Borlaug BA. Altered Hemodynamics and End-Organ Damage in Heart Failure: Impact on the Lung and Kidney. Circulation. 2020;142:998-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 9. | Verbrugge FH, Dupont M, Steels P, Grieten L, Malbrain M, Tang WH, Mullens W. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol. 2013;62:485-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 10. | Styczynski G, Milewska A, Marczewska M, Sobieraj P, Sobczynska M, Dabrowski M, Kuch-Wocial A, Szmigielski C. Echocardiographic Correlates of Abnormal Liver Tests in Patients with Exacerbation of Chronic Heart Failure. J Am Soc Echocardiogr. 2016;29:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Benkreira A, Beaubien-Souligny W, Mailhot T, Bouabdallaoui N, Robillard P, Desjardins G, Lamarche Y, Cossette S, Denault A. Portal Hypertension Is Associated With Congestive Encephalopathy and Delirium After Cardiac Surgery. Can J Cardiol. 2019;35:1134-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Brunkhorst FM. Endotoxins in chronic heart failure. Lancet. 1999;354:599-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Ikeda Y, Ishii S, Yazaki M, Fujita T, Iida Y, Kaida T, Nabeta T, Nakatani E, Maekawa E, Yanagisawa T, Koitabashi T, Inomata T, Ako J. Portal congestion and intestinal edema in hospitalized patients with heart failure. Heart Vessels. 2018;33:740-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Schroedter WB, White JM, Garcia AR, Ellis ME. Presence of Lower-Extremity Venous Pulsatility is not always the Result of Cardiac Dysfunction. J Vasc Ultrasound. 2018;38:71-75. [DOI] [Full Text] |
| 15. | Tang WH, Kitai T. Intrarenal Venous Flow: A Window Into the Congestive Kidney Failure Phenotype of Heart Failure? JACC Heart Fail. 2016;4:683-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Beaubien-Souligny W, Rola P, Haycock K, Bouchard J, Lamarche Y, Spiegel R, Denault AY. Quantifying systemic congestion with Point-Of-Care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J. 2020;12:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 385] [Article Influence: 64.2] [Reference Citation Analysis (1)] |
| 17. | Via G, Tavazzi G, Price S. Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: a physiologically based point of view. Intensive Care Med. 2016;42:1164-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 18. | Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66:493-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 741] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 19. | Simonson JS, Schiller NB. Sonospirometry: a new method for noninvasive estimation of mean right atrial pressure based on two-dimensional echographic measurements of the inferior vena cava during measured inspiration. J Am Coll Cardiol. 1988;11:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 153] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Bauman Z, Coba V, Gassner M, Amponsah D, Gallien J, Blyden D, Killu K. Inferior vena cava collapsibility loses correlation with internal jugular vein collapsibility during increased thoracic or intra-abdominal pressure. J Ultrasound. 2015;18:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Huguet R, Fard D, d'Humieres T, Brault-Meslin O, Faivre L, Nahory L, Dubois-Randé JL, Ternacle J, Oliver L, Lim P. Three-Dimensional Inferior Vena Cava for Assessing Central Venous Pressure in Patients with Cardiogenic Shock. J Am Soc Echocardiogr. 2018;31:1034-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Labovitz AJ, Noble VE, Bierig M, Goldstein SA, Jones R, Kort S, Porter TR, Spencer KT, Tayal VS, Wei K. Focused cardiac ultrasound in the emergent setting: a consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010;23:1225-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 536] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 23. | Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G; European Society of Cardiology; European Society of Intensive Care Medicine. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail. 2010;12:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 513] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 24. | Merritt CR. Doppler US: the basics. Radiographics. 1991;11:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Reynolds T, Appleton CP. Doppler flow velocity patterns of the superior vena cava, inferior vena cava, hepatic vein, coronary sinus, and atrial septal defect: a guide for the echocardiographer. J Am Soc Echocardiogr. 1991;4:503-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | McNaughton DA, Abu-Yousef MM. Doppler US of the liver made simple. Radiographics. 2011;31:161-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Iida N, Seo Y, Sai S, Machino-Ohtsuka T, Yamamoto M, Ishizu T, Kawakami Y, Aonuma K. Clinical Implications of Intrarenal Hemodynamic Evaluation by Doppler Ultrasonography in Heart Failure. JACC Heart Fail. 2016;4:674-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 230] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 28. | Denault AY, Aldred MP, Hammoud A, Zeng YH, Beaubien-Souligny W, Couture EJ, Jarry S, Gebhard CE, Langevin S, Lamarche Y, Robillard P. Doppler Interrogation of the Femoral Vein in the Critically Ill Patient: The Fastest Potential Acoustic Window to Diagnose Right Ventricular Dysfunction? Crit Care Explor. 2020;2:e0209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Abu-Yousef MM. Normal and respiratory variations of the hepatic and portal venous duplex Doppler waveforms with simultaneous electrocardiographic correlation. J Ultrasound Med. 1992;11:263-268. [PubMed] [DOI] [Full Text] |
| 30. | Nagueh SF, Kopelen HA, Zoghbi WA. Relation of mean right atrial pressure to echocardiographic and Doppler parameters of right atrial and right ventricular function. Circulation. 1996;93:1160-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 190] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Mattioli AV, Castelli A, Mattioli G. Relationship between mean right atrial pressure and Doppler parameters in patients with right ventricular infarction. Clin Cardiol. 2000;23:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Ghio S, Recusani F, Sebastiani R, Klersy C, Raineri C, Campana C, Lanzarini L, Gavazzi A, Tavazzi L. Doppler velocimetry in superior vena cava provides useful information on the right circulatory function in patients with congestive heart failure. Echocardiography. 2001;18:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Ommen SR, Nishimura RA, Hurrell DG, Klarich KW. Assessment of right atrial pressure with 2-dimensional and Doppler echocardiography: a simultaneous catheterization and echocardiographic study. Mayo Clin Proc. 2000;75:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 152] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Pennestrí F, Loperfido F, Salvatori MP, Mongiardo R, Ferrazza A, Guccione P, Manzoli U. Assessment of tricuspid regurgitation by pulsed Doppler ultrasonography of the hepatic veins. Am J Cardiol. 1984;54:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Sakai K, Nakamura K, Satomi G, Kondo M, Hirosawa K. Evaluation of tricuspid regurgitation by blood flow pattern in the hepatic vein using pulsed Doppler technique. Am Heart J. 1984;108:516-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 45] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Koratala A, Sturgill D. Point-of-care venous Doppler ultrasound in the management of heart failure and hyponatremia. Clin Nephrol. 2021;96:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Eljaiek R, Cavayas YA, Rodrigue E, Desjardins G, Lamarche Y, Toupin F, Denault AY, Beaubien-Souligny W. High postoperative portal venous flow pulsatility indicates right ventricular dysfunction and predicts complications in cardiac surgery patients. Br J Anaesth. 2019;122:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 38. | Spiegel R, Teeter W, Sullivan S, Tupchong K, Mohammed N, Sutherland M, Leibner E, Rola P, Galvagno SM Jr, Murthi SB. The use of venous Doppler to predict adverse kidney events in a general ICU cohort. Crit Care. 2020;24:615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 39. | Hu JT, Yang SS, Lai YC, Shih CY, Chang CW. Percentage of peak-to-peak pulsatility of portal blood flow can predict right-sided congestive heart failure. World J Gastroenterol. 2003;9:1828-1831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 40. | Rengo C, Brevetti G, Sorrentino G, D'Amato T, Imparato M, Vitale DF, Acanfora D, Rengo F. Portal vein pulsatility ratio provides a measure of right heart function in chronic heart failure. Ultrasound Med Biol. 1998;24:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Catalano D, Caruso G, DiFazzio S, Carpinteri G, Scalisi N, Trovato GM. Portal vein pulsatility ratio and heart failure. J Clin Ultrasound. 1998;26:27-31. [PubMed] [DOI] [Full Text] |
| 42. | Goncalvesova E, Lesny P, Luknar M, Solik P, Varga I. Changes of portal flow in heart failure patients with liver congestion. Bratisl Lek Listy. 2010;111:635-639. [PubMed] |
| 43. | Bouabdallaoui N, Beaubien-Souligny W, Denault AY, Rouleau JL. Impacts of right ventricular function and venous congestion on renal response during depletion in acute heart failure. ESC Heart Fail. 2020;7:1723-1734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Bouabdallaoui N, Beaubien-Souligny W, Oussaïd E, Henri C, Racine N, Denault AY, Rouleau JL. Assessing Splanchnic Compartment Using Portal Venous Doppler and Impact of Adding It to the EVEREST Score for Risk Assessment in Heart Failure. CJC Open. 2020;2:311-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Beaubien-Souligny W, Benkreira A, Robillard P, Bouabdallaoui N, Chassé M, Desjardins G, Lamarche Y, White M, Bouchard J, Denault A. Alterations in Portal Vein Flow and Intrarenal Venous Flow Are Associated With Acute Kidney Injury After Cardiac Surgery: A Prospective Observational Cohort Study. J Am Heart Assoc. 2018;7:e009961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 176] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 46. | Singh NG, Kumar KN, Nagaraja PS, Manjunatha N. Portal venous pulsatility fraction, a novel transesophageal echocardiographic marker for right ventricular dysfunction in cardiac surgical patients. Ann Card Anaesth. 2020;23:39-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Husain-Syed F, Birk HW, Ronco C, Schörmann T, Tello K, Richter MJ, Wilhelm J, Sommer N, Steyerberg E, Bauer P, Walmrath HD, Seeger W, McCullough PA, Gall H, Ghofrani HA. Doppler-Derived Renal Venous Stasis Index in the Prognosis of Right Heart Failure. J Am Heart Assoc. 2019;8:e013584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 48. | Yoshihisa A, Watanabe K, Sato Y, Ishibashi S, Matsuda M, Yamadera Y, Ichijo Y, Yokokawa T, Misaka T, Oikawa M, Kobayashi A, Takeishi Y. Intrarenal Doppler ultrasonography reflects hemodynamics and predicts prognosis in patients with heart failure. Sci Rep. 2020;10:22257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Wiersema R, Kaufmann T, van der Veen HN, de Haas RJ, Franssen CFM, Koeze J, van der Horst ICC, Keus F; SICS Study Group. Diagnostic accuracy of arterial and venous renal Doppler assessment for acute kidney injury in critically ill patients: A prospective study. J Crit Care. 2020;59:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | Abu-Yousef MM, Kakish ME, Mufid M. Pulsatile venous Doppler flow in lower limbs: highly indicative of elevated right atrium pressure. AJR Am J Roentgenol. 1996;167:977-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Taute BM, Schmidt H, Bach AG, Fischer R, Tran CLH, Amoury M, Melnyk H. Spectral Doppler Waveform Analysis of Common Femoral Veins for the Detection of Right Ventricular Dysfunction in Acute Pulmonary Embolism. J Cardiovasc Dis Diagn. 2015;3: 1. [DOI] [Full Text] |
| 52. | Carricart M, Denault AY, Couture P, Limoges P, Babin D, Levesque S, Fortier A, Pellerin M, Tardif JC, Buithieu J. Incidence and significance of abnormal hepatic venous Doppler flow velocities before cardiac surgery. J Cardiothorac Vasc Anesth. 2005;19:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Gallix BP, Taourel P, Dauzat M, Bruel JM, Lafortune M. Flow pulsatility in the portal venous system: a study of Doppler sonography in healthy adults. AJR Am J Roentgenol. 1997;169:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Loperfido F, Lombardo A, Amico CM, Vigna C, Testa M, Rossi E, Costalunga A, Pennestri F, Biasucci LM. Doppler analysis of portal vein flow in tricuspid regurgitation. J Heart Valve Dis. 1993;2:174-182. [PubMed] |
| 55. | Barakat M. Portal vein pulsatility and spectral width changes in patients with portal hypertension: relation to the severity of liver disease. Br J Radiol. 2002;75:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Balci A, Karazincir S, Sumbas H, Oter Y, Egilmez E, Inandi T. Effects of diffuse fatty infiltration of the liver on portal vein flow hemodynamics. J Clin Ultrasound. 2008;36:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Bateman GA, Cuganesan R. Renal vein Doppler sonography of obstructive uropathy. AJR Am J Roentgenol. 2002;178:921-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Simon MA, Kliner DE, Girod JP, Moguillansky D, Villanueva FS, Pacella JJ. Detection of elevated right atrial pressure using a simple bedside ultrasound measure. Am Heart J. 2010;159:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 59. | Donahue SP, Wood JP, Patel BM, Quinn JV. Correlation of sonographic measurements of the internal jugular vein with central venous pressure. Am J Emerg Med. 2009;27:851-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 60. | Guarracino F, Ferro B, Forfori F, Bertini P, Magliacano L, Pinsky MR. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit Care. 2014;18:647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 61. | Upadhyay V, Malviya D, Nath SS, Tripathi M, Jha A. Comparison of Superior Vena Cava and Inferior Vena Cava Diameter Changes by Echocardiography in Predicting Fluid Responsiveness in Mechanically Ventilated Patients. Anesth Essays Res. 2020;14:441-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 62. | Koratala A, Bhattacharya D, Kazory A. Point of care renal ultrasonography for the busy nephrologist: A pictorial review. World J Nephrol. 2019;8:44-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (19)] |
| 63. | Chawla LS, Davison DL, Brasha-Mitchell E, Koyner JL, Arthur JM, Shaw AD, Tumlin JA, Trevino SA, Kimmel PL, Seneff MG. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care. 2013;17:R207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 246] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 64. | Varghese V, Rivera MS, Alalwan AA, Alghamdi AM, Gonzalez ME, Velez JCQ. Diagnostic Utility of Serial Microscopic Examination of the Urinary Sediment in Acute Kidney Injury. Kidney360. 2021;2:182-191. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Ronco C, Bellomo R. Prevention of acute renal failure in the critically ill. Nephron Clin Pract. 2003;93:C13-C20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Mohmand H, Goldfarb S. Renal dysfunction associated with intra-abdominal hypertension and the abdominal compartment syndrome. J Am Soc Nephrol. 2011;22:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 67. | Argaiz ER, Rola P, Gamba G. Dynamic Changes in Portal Vein Flow during Decongestion in Patients with Heart Failure and Cardio-Renal Syndrome: A POCUS Case Series. Cardiorenal Med. 2021;11:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 68. | Singh S, Koratala A. Utility of Doppler ultrasound derived hepatic and portal venous waveforms in the management of heart failure exacerbation. Clin Case Rep. 2020;8:1489-1493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 69. | Peerapornratana S, Manrique-Caballero CL, Gómez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96:1083-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 1024] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 70. | Koratala A, Kazory A. Point of Care Ultrasonography for Objective Assessment of Heart Failure: Integration of Cardiac, Vascular, and Extravascular Determinants of Volume Status. Cardiorenal Med. 2021;11:5-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Qian XQ, Verde F S-Editor: Liu M L-Editor: A P-Editor: Liu M