©The Author(s) 2025.
World J Crit Care Med. Dec 9, 2025; 14(4): 103782
Published online Dec 9, 2025. doi: 10.5492/wjccm.v14.i4.103782
Published online Dec 9, 2025. doi: 10.5492/wjccm.v14.i4.103782
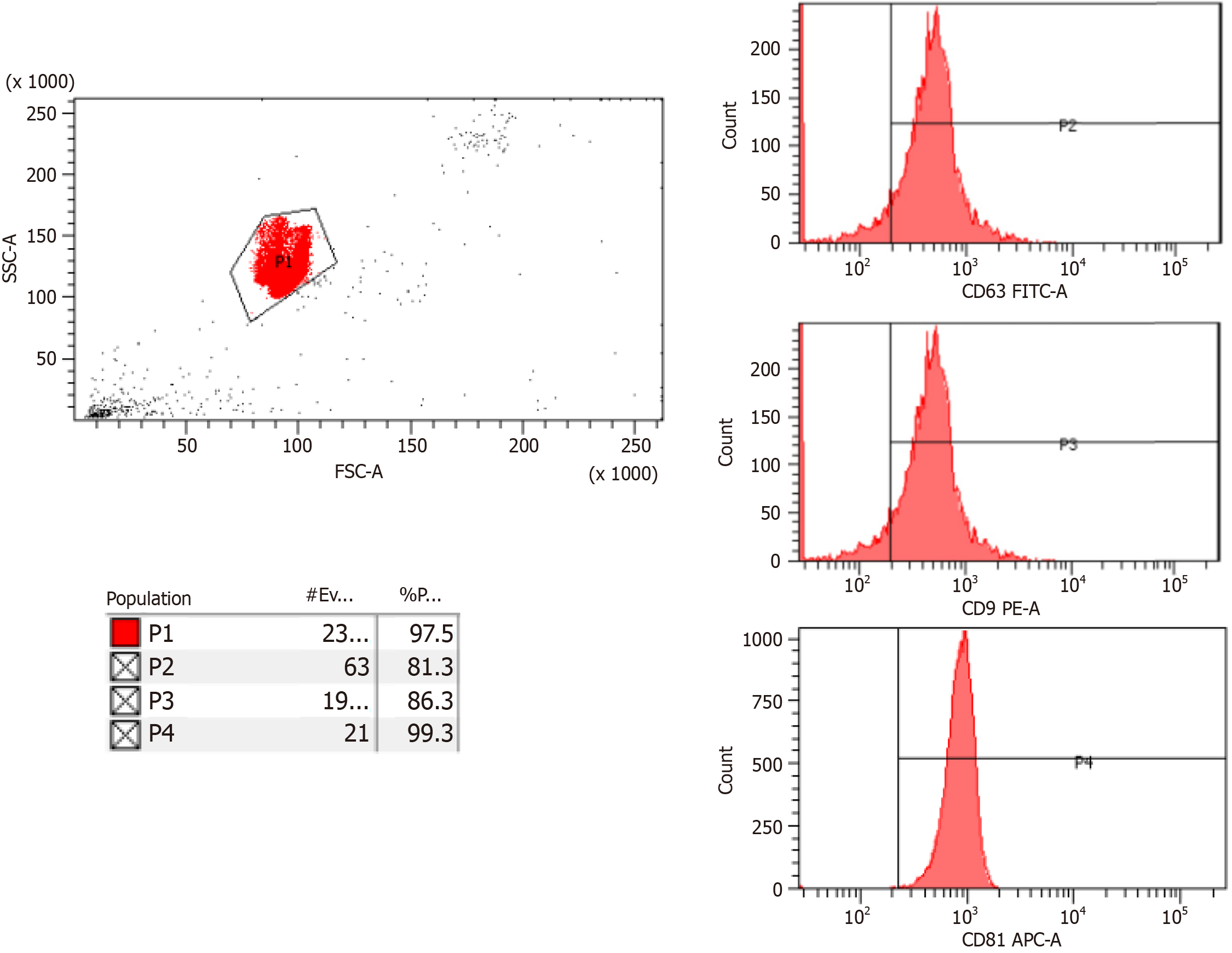
Figure 1 Flow cytometry analysis of exosomes.
FITC-A: Fluorescein isothiocyanate-area; PE-A: Phycoerythrin-area; APC-A: Allophycocyanin-area.
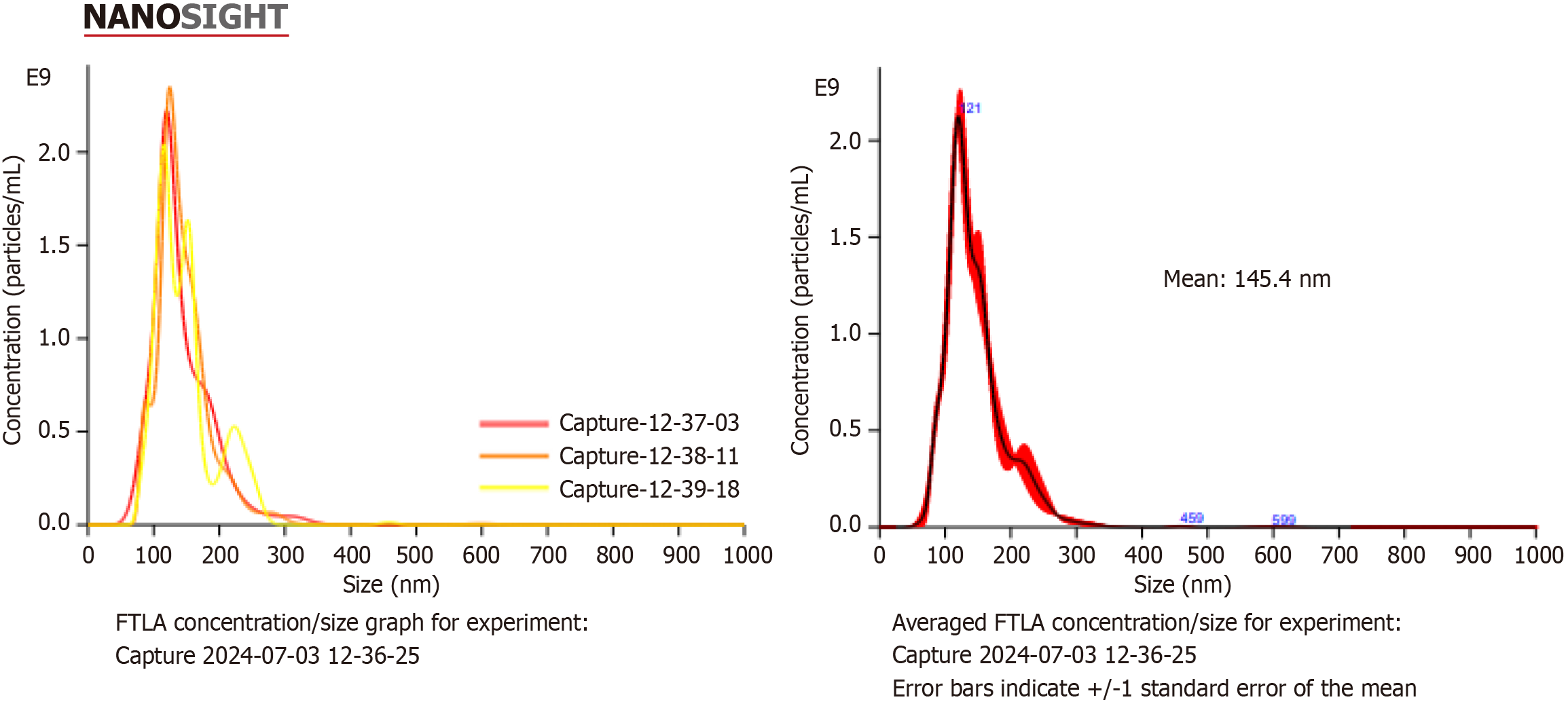
Figure 2 Nanoparticle tracking analysis of exosomes.
FTLA: Fitted track length average.
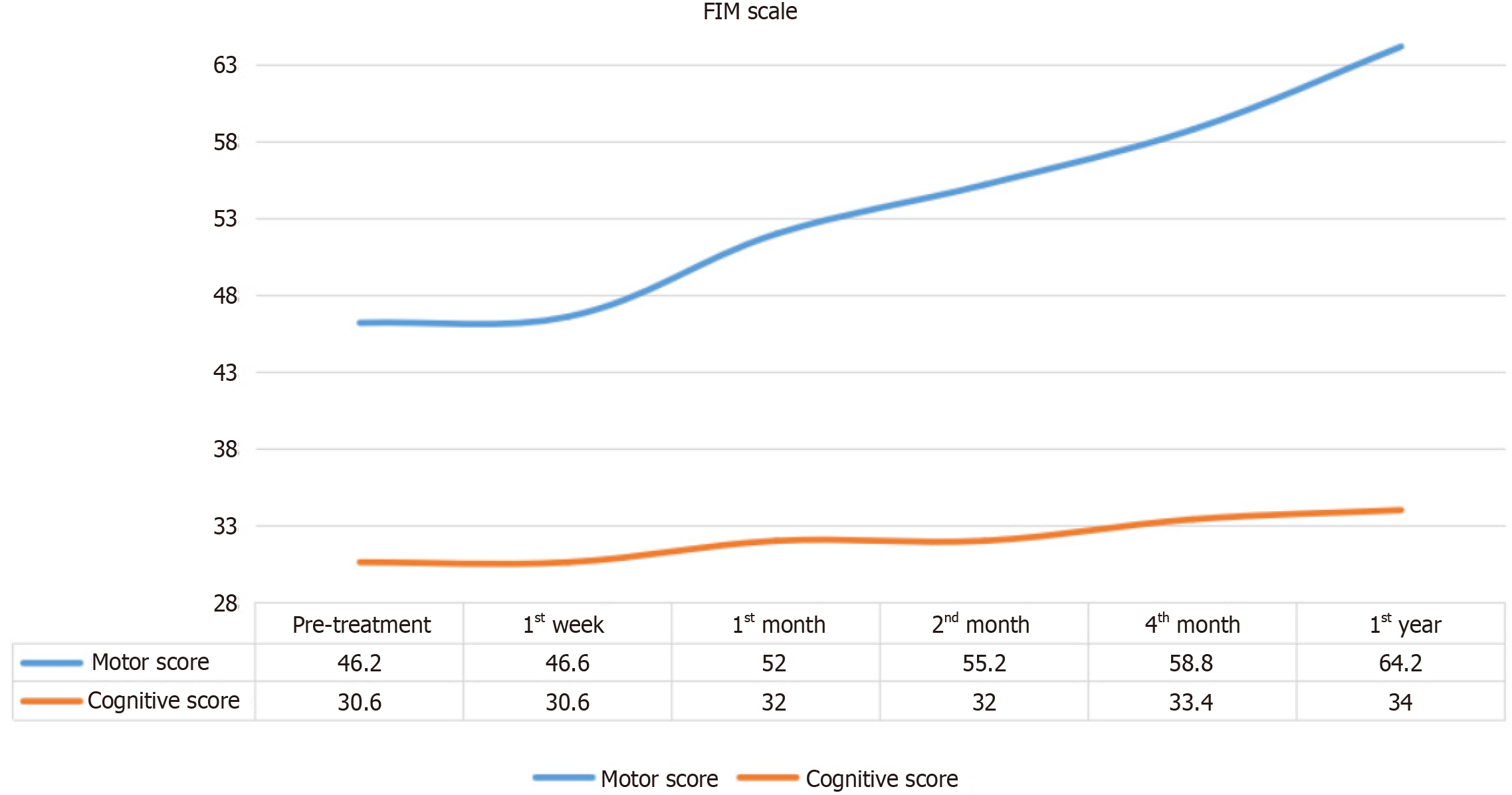
Figure 3
Changes observed in the pre-test and post-test means of the patients' functional independence measure Motor and Cognitive scores.
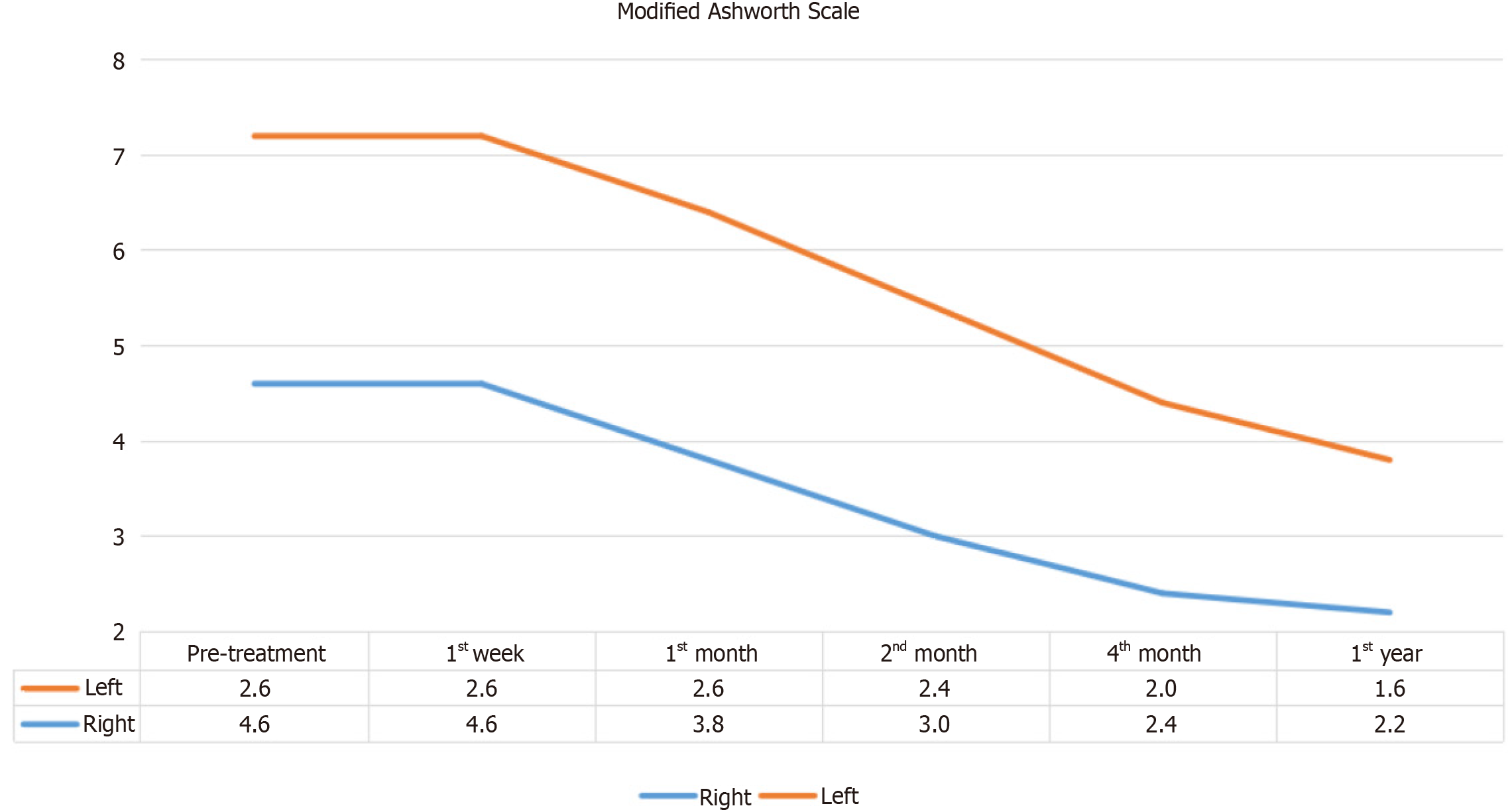
Figure 4
Changes observed in the pre-test and post-test means of the patients' Modified Ashworth Grading Right and Left values.
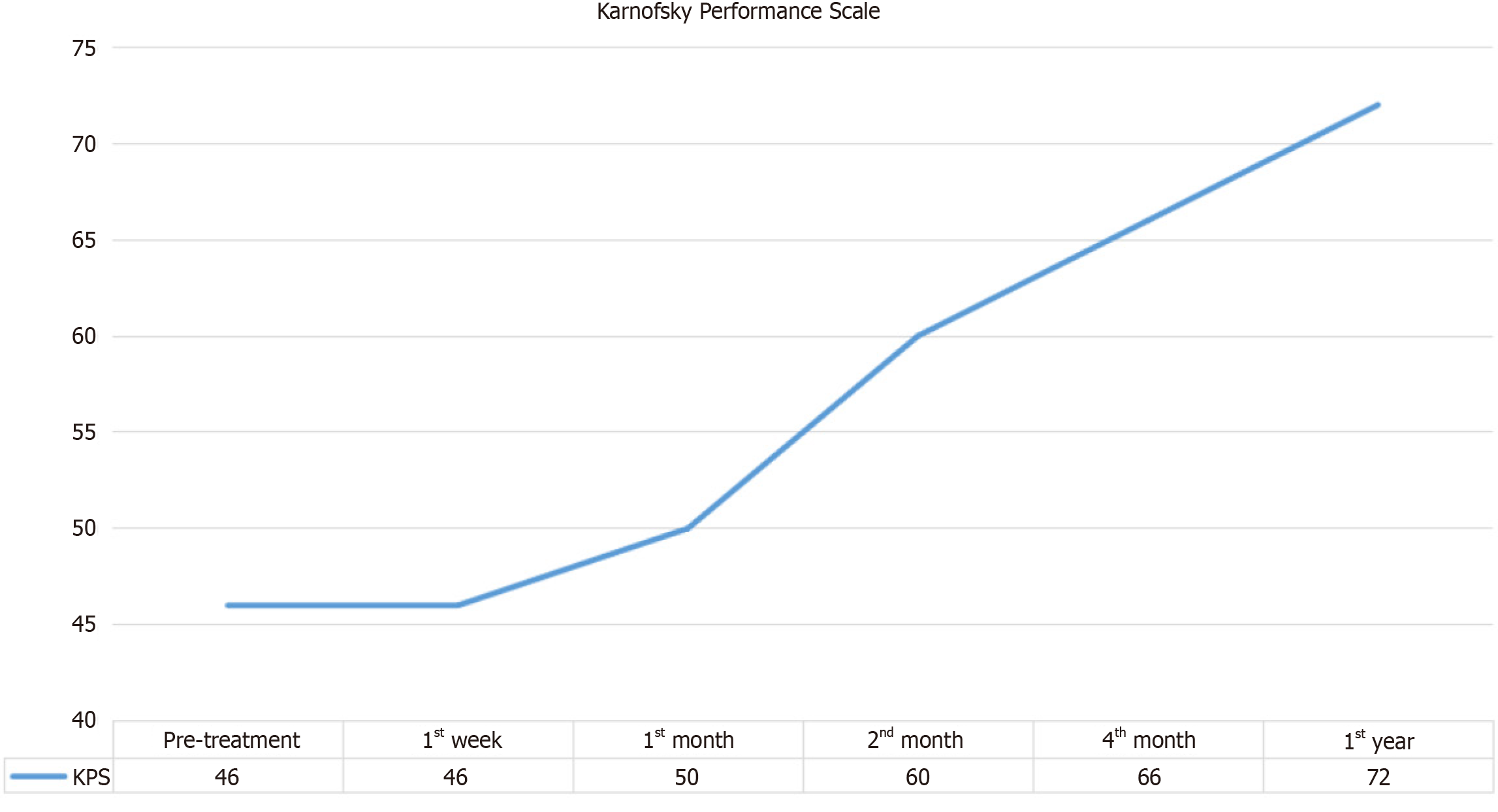
Figure 5
Changes observed in the pre-test and post-test means of the Karnofsky Performance Scale values of the patients.
- Citation: Kabatas S, Civelek E, Savrunlu EC, Kaplan N, Akkoc T, Küçükçakır N, Bozkurt M, Karaöz E. Efficacy and safety of exosomes from Wharton’s Jelly-derived mesenchymal stem cells in traumatic brain injury. World J Crit Care Med 2025; 14(4): 103782
- URL: https://www.wjgnet.com/2220-3141/full/v14/i4/103782.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v14.i4.103782