Published online Dec 9, 2025. doi: 10.5409/wjcp.v14.i4.111441
Revised: July 24, 2025
Accepted: August 27, 2025
Published online: December 9, 2025
Processing time: 119 Days and 11.3 Hours
Visual impairment during early childhood can hinder motor, language, and social development, yet data on its developmental impact across common pediatric oc
To investigate the developmental impact of low vision and blindness on children under six with common ocular diseases.
This retrospective study reviewed records of new patients under six with visual impairment at Siriraj Hospital’s low vision rehabilitation center (January 2017-October 2022). We collected ocular, systemic, and developmental data; recorded visual acuity in the better-seeing eye after refractive correction; and assessed developmental domains with the Denver II. Univariable and multi
A total of 161 pediatric patients (mean age 24.9 ± 18.9 months) were enrolled and evaluated based on their ability to fix on and follow an object or light source. Some were further assessed using the Allen picture chart and all had visual acuity worse than 1.07 ± 0.58 LogMAR, and 83.2% were identified as having global developmental delay (GDD). The three most common ocular causes were cortical visual impairment (CVI), optic neuropathy/atrophy, and optic nerve hypoplasia. Extremely poor visual acuity (inability to fixate and follow) was significantly associated with GDD [adjusted odds ratio (AOR) 41.0] and delays in all developmental domains: Gross motor (AOR 10.0), fine motor (AOR 12.8), language (AOR 5.3), and personal-social skills (AOR 13.4) (P ≤ 0.002). Multiple disabilities, most often visual impairment with cerebral palsy, were also significantly associated with gross motor delays (AOR 7.7) and fine motor delays (AOR 4.0) (P < 0.05). CVI was also related to delays in language and personal-social skills (AOR 9.1 each) (P < 0.05).
This study underscores the developmental issues in children with visual impairment, especially those with poorer acuity, CVI, and multiple disabilities. Significant delays were observed in all domains, including GDD. A timely referral to specialists is strongly recommended.
Core Tip: In this study of 161 children under 6 years, 83.2% of visually impaired patients were identified as having global developmental delay, a proportion markedly higher than reported in prior studies. Children unable to fixate and follow objects had 41-fold increased odds of global developmental delay, with significant delays across all domains—gross motor, fine motor, language, and personalsocial skills. Cortical visual impairment and the presence of multiple disabilities emerged as key risk factors. These findings highlight the high prevalence and significant associations of developmental delay in young children with visual impairment and underscore the importance of timely, multidisciplinary intervention.
- Citation: Wannapaschaiyong P, Chotikavanich S, Sutchritpongsa S, Rojmahamonkol P, Penphattarakul A, Saksiriwutto P, Eiamsamarng A, Setthawong S, Phongsuphan T, Jaruniphakul P, Yingyong R, Sarinak N, Eksupapan E, Sagan S, Onlamul P. Impact of low vision and blindness on characteristics of developmental delay in children younger than 6 years. World J Clin Pediatr 2025; 14(4): 111441
- URL: https://www.wjgnet.com/2219-2808/full/v14/i4/111441.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i4.111441
Sensory experiences play a crucial role in the developmental process during early childhood, particularly in children under six years of age[1]. If, during this early stage, abnormal sensory experiences are detected, especially those involving vision or hearing loss, they may lead to delays in various aspects of development, including gross and fine motor, language, and personal and social skills[2]. An increasing prevalence of visual impairment in early childhood further amplifies the impact of this issue[3,4]. However, reports on the detailed developmental characteristics of children with vision loss in real-world clinical settings remain limited.
To our knowledge, there were only a few previous reports from the 1990s. McConachie et al[5] showed that language skills of severely visually impaired and blind children tend to develop later than those of sighted children, while Janson[6] revealed that behavioral and emotional issues, including autism, have been identified in children with developmental anomalies associated with retinopathy of prematurity. However, these studies did not assess other developmental aspects, such as motor or problem-solving skills.
Other publications examining the impact of visual loss on development have focused only on relatively rare conditions that cause visual impairment in children. These include Usher syndrome, CHARGE syndrome[7], and retinoblastoma[8], all of which have been associated with developmental delays, particularly in motor skills. However, more common causes such as cortical visual impairment (CVI), optic nerve hypoplasia, and other congenital ocular abnormalities have not been reported[9-11].
This study aims to thoroughly investigate the impact of visual impairment in early childhood, covering all common ocular diseases associated with low vision and blindness and their effects on all aspects of development. Furthermore, the assessment emphasizes the use of standard clinical tools and evaluations by ophthalmologists and pediatricians within a multidisciplinary service. All ocular and systemic factors contributing to these impacts were also evaluated.
This retrospective review of medical records was conducted at Siriraj Hospital, one of the largest national tertiary referral centers in Bangkok, Thailand. The inclusion criteria for this study included all records of new consecutive pediatric patients under 6 years of age who attended the low vision rehabilitation clinic between January 2017 and October 2022. The patients were referred to the clinic by ophthalmologists after being diagnosed with visual impairment, whether low vision or blindness, mostly caused by irreversible conditions. Therefore, the cases of uncorrected refractive error, which can be corrected with simple glasses, were excluded, as this treatable condition is expected to have a lesser or only temporary impact on developmental issues. Patients with incomplete medical records, especially those without a developmental history, were also excluded.
This study adhered to the principles of the Declaration of Helsinki, and the Institutional Review Board of the Faculty of Medicine, Siriraj Hospital, Mahidol University, approved the study (approval number: Si779/2022).
The data collected included demographic information, such as age at the first developmental assessment, gender, ocular diagnosis, visual acuity, systemic diseases, and risk factors affecting development (prenatal, perinatal, and postnatal). Although only new patients (prior to receiving services) were enrolled, information on any previous developmental stimulation received elsewhere was also documented.
The ocular diagnosis and visual acuity were recorded based on the condition of the better eye after correction for refractive error. Because most of the patients were preverbal or preschool-age children, visual impairment was evaluated by their ability to fix and follow an object or a light source. Visual acuity was then categorized as: No fixation and following, poor/fair fixation and following, or good fixation and following. While fixation and following behaviors were used as clinical indicators of visual function for most patients, these are subjective and lack the precision of standardized, quantitative methods. More objective tools, such as preferential looking techniques (e.g., Teller acuity cards), were not employed routinely due to time constraints and patient cooperation limitations in the clinic setting.
In verbal and cooperative patients, visual acuity was additionally measured using the Allen picture chart and recorded in logMAR units. Other quantitative vision measurements, including visual evoked potential (VEP), which is time-consuming and requires a separate appointment, were not performed routinely and were not recorded.
Because the clinic is staffed by a multidisciplinary team, including ophthalmologists and pediatricians, the initial developmental assessment using the Denver II instrument was conducted at the time. The Denver II is a developmental screening tool for children from birth to 72 months of age. It was used to assess gross and fine motor skills, language, and personal-social development. The Developmental Quotient (DQ; developmental age/chronological age × 100) was used to display each aspect of development. In this study, a DQ of less than 70 in a given developmental domain was defined as delayed development in that domain. The sensitivity of the test ranges from 0.56 to 0.83, and the specificity ranges from 0.43 to 0.80[12]. Global developmental delay (GDD) was diagnosed when two or more developmental domains were delayed. It should be noted that no additional confirmatory developmental assessments were conducted beyond the Denver II. All determinations of developmental delay in this study were based solely on the results of this screening instrument. Behavioral and mood problems were also noted.
Descriptive statistics were used to summarize patient demographics and characteristics. Categorical data were presented as frequencies and percentages. Factors associated with developmental delay were analyzed using univariable and multivariable logistic regression analyses. All statistical analyses were conducted using IBM SPSS Statistics, version 28 (IBM Corp., Armonk, NY, United States) and MedCalc, version 19.6.4 (MedCalc Software Ltd., Ostend, Belgium). A P < 0.05 was considered statistically significant.
A total of 161 patients were recruited for this study. The mean age was 24.9 ± 18.9 months (57.8% male, 42.2% female). No missing data were observed in the key demographic and perinatal variables used in this analysis. The ocular diagnoses that cause visual impairment are shown in Table 1. The three most common conditions in these young children were CVI (37 patients, 23.0%), optic neuropathy/atrophy (26 patients, 16.1%), and optic nerve hypoplasia (15 patients, 9.3%), followed by retinopathy of prematurity, microphthalmos/anophthalmos, and delayed visual maturation.
| Demographic data | Total, n = 161 |
| Diagnosis (eye) | |
| Cortical visual impairment | 37 (23.0) |
| Optic neuropathy/atrophy | 26 (16.1) |
| Optic nerve hypoplasia | 15 (9.3) |
| Retinopathy of prematurity | 12 (7.5) |
| Microphthalmos/anophthalmos | 11 (6.8) |
| Delay in visual maturation | 10 (6.2) |
| Leber’s congenital amaurosis | 6 (3.7) |
| Achromatopsia | 5 (3.1) |
| Foveal hypoplasia | 4 (2.5) |
| Retinal coloboma | 4 (2.5) |
| Congenital cataract | 4 (2.5) |
| Persistent fetal vasculature | 3 (1.9) |
| Congenital glaucoma | 3 (1.9) |
| Others1 | 21 (13.0) |
| Visual acuity (better eye) | |
| Not fix and follow | 73 (45.3) |
| Poor/fair fix and follow | 38 (23.6) |
| Good fix and follow | 50 (31.1) |
| Disability type | |
| Visual impairment only | 100 (62.1) |
| Cerebral palsy with VI (multiple disabilities) | 54 (33.5) |
| Autistic with VI (multiple disabilities) | 1 (0.6) |
| Hearing impairment with VI (multiple disabilities) | 13 (8.1) |
| Prenatal risk | |
| Genetic disorders | 25 (15.5) |
| Cerebral dysgenesis | 54 (33.5) |
| Intrauterine infections | 7 (4.3) |
| Perinatal risk | |
| Preterm, GA < 32 weeks | 17 (10.5) |
| Preterm, GA 32-36 weeks | 24 (14.9) |
| VLBW (< 1500 g) | 16 (9.9) |
| LBW (1500-2500 g) | 31 (19.3) |
| Birth asphyxia | 23 (14.3) |
| Postnatal risk | |
| CNS infection | 3 (1.9) |
| Head trauma | 7 (4.3) |
| History of developmental stimulation elsewhere | |
| No | 129 (80.1) |
| Yes | 32 (19.9) |
At the time of the first developmental assessment, visual acuity could be assessed in all patients using the fix-and-follow method. Most of the patients were unable to fix and follow objects (73 patients, 45.3%), followed by those with good fixation and following (50 patients, 31.1%) and those with poor or fair fixation and following (38 patients, 23.6%). Among them, 42 patients also had visual acuity measured using the Allen picture chart, recorded either at the same time or during the nearest period to the first developmental assessment, with a mean time difference of 25.8 ± 21.1 months. In the group of 'not fix-and-follow', all patients had severe visual impairment and were unable to see the chart at the time, except for two cases who later underwent surgery for congenital cataracts. For the remaining 40 patients, the mean visual acuity in the good fix-and-follow group (7 patients) was 1.07 ± 0.58 LogMAR, and in the poor/fair fix-and-follow group (33 patients), it was 1.95 ± 0.89 LogMAR. A statistically significant difference in mean visual acuity was also observed between these two groups (P = 0.039).
Most of the patients had visual impairment as their only type of disability (62.1%). Among patients with multiple disabilities, cerebral palsy was the most common comorbidity, found in one-third of the patients (33.5%), followed by hearing impairment (8.3%). Regarding the risk of developmental delay, cerebral dysgenesis was the most common prenatal risk, while low birth weight was the most common perinatal risk.
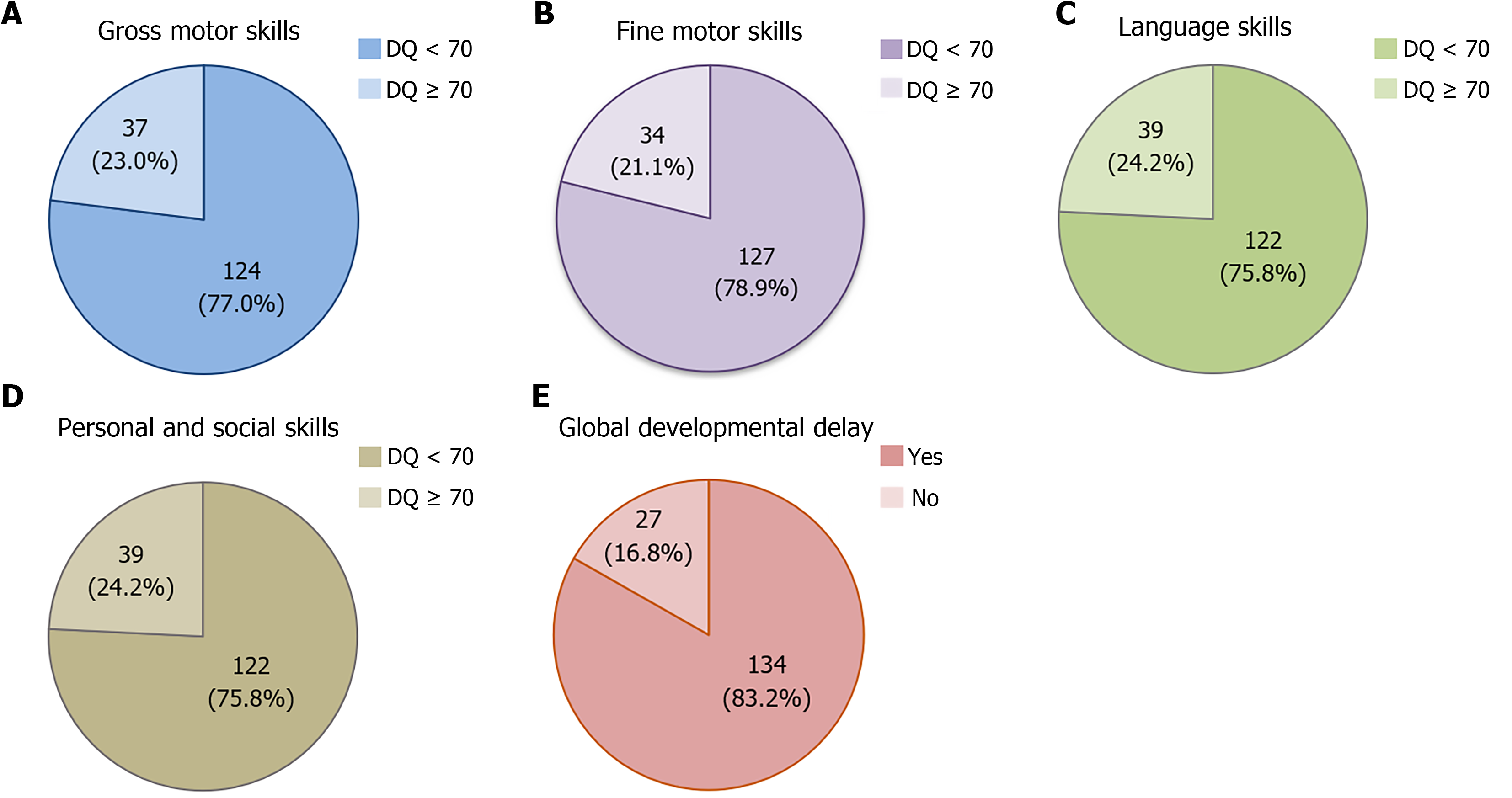
Figure 1 shows the impact of visual impairment on developmental characteristics. Most of the pediatric patients (83.2%) in this study had GDD. Furthermore, when considering individual developmental domains, the majority of the patients showed delays in all areas, including gross motor (77.0%), fine motor (78.9%), language (75.8%), and personal-social skills (75.8%).
The results of the univariate logistic regression analysis for potential factors related to GDD and each developmental domain, with a significance level of P < 0.2, are shown in Tables 2, 3, and 4. Significant related factors include a diagnosis of CVI, impaired visual acuity, multiple disabilities (visual impairment accompanied by cerebral palsy, hearing im
| Factors | Global developmental delay | |||||
| Univariable analysis | Multivariable analysis | |||||
| Yes, n = 134 | No, n = 27 | OR (95%CI) | P valuea | AOR (95%CI) | P valueb | |
| Eye disease | ||||||
| Cortical visual impairment | 36 (26.9) | 1 (3.7) | 9.6 (1.3-73.0) | 0.01 | 4.0 (0.5-35.3) | 0.212 |
| Visual acuity | ||||||
| Not fix and follow | 72 (53.7) | 1 (3.7) | 52.1 (6.7-405.8) | < 0.001 | 41.0 (5.2-324.8) | < 0.001b |
| Poor/fair fix and follow | 33 (24.6) | 5 (18.5) | 4.8 (1.6-14.3) | 0.005 | 3.9 (1.3-12.3) | < 0.018b |
| Good fix and follow | 29 (21.6) | 21 (77.8) | Reference | Reference | ||
| Multiple disabilities | 58 (43.3) | 3 (11.1) | 6.1 (1.8-21.3) | 0.002 | 3.8 (1.0-14.8) | 0.55 |
| Hx previous stimulation1 | 32 (23.9) | 0 | 0.006 | |||
| Factors | Gross motor | Fine motor | ||||||||||
| DQ | Univariable analysis | Multivariable analysis | DQ | Univariable analysis | Multivariable analysis | |||||||
| < 70, n = 124 | ≥ 70, n = 37 | OR (95%CI) | P valuea | AOR (95%CI) | P valueb | < 70, n = 127 | ≥ 70, n = 34 | OR (95%CI) | P valuea | AOR (95%CI) | P valueb | |
| Eye disease | ||||||||||||
| Cortical visual impairment | 37 (29.8) | 0 | - | < 0.001 | - | - | 35 (27.6) | 2 (5.9) | 6.1 (1.4-26.8) | 0.010 | 2.6 (0.5-13.0) | 0.251 |
| Retinopathy of prematurity | 7 (5.4) | 6 (15.8) | 0.3 (0.1-0.9) | 0.031 | 0.2 (0.0-2.1) | 0.176 | - | - | - | - | - | - |
| Visual acuity | ||||||||||||
| Not fix and follow | 67 (54.0) | 6 (16.2) | 8.8 (3.2-24.) | < 0.001 | 10.0 (3.0-33.0) | < 0.001b | 69 (54.3) | 4 (11.8) | 15.9 (5.0-50.3) | < 0.001 | 12.8 (3.8-42.0) | < 0.001b |
| Poor/fair fix and follow | 29 (23.4) | 9 (24.3) | 2.5 (1.0-6.4) | 0.051 | 2.7 (0.8-8.9) | 0.101 | 32 (25.2) | 6 (17.6) | 4.9 (1.8-13.8) | < 0.001 | 4.3 (1.4-12.8) | < 0.001b |
| Good fix and follow | 28 (22.6) | 22 (59.5) | Reference | Reference | 26 (20.5) | 24 (70.6) | Reference | Reference | ||||
| Multiple disabilities | 58 (46.8) | 3 (8.1) | 10.0 (2.9-34.1) | < 0.001 | 7.7 (2.0-29.7) | 0.003b | 57 (44.9) | 4 (11.8) | 6.2 (2.0-18.4) | < 0.001 | 4.0 (1.9-13.2) | 0.026b |
| Prenatal risk | ||||||||||||
| Cerebral dysgenesis | 49 (39.5) | 5 (13.5) | 4.2 (1.5-11.5) | 0.005 | 3.1 (1.0-9.7) | 0.056 | 47 (37.0) | 7 (20.6) | 2.3 (0.9-5.6) | 0.101 | 1.9 (0.7-5.4) | 0.229 |
| Perinatal risk | ||||||||||||
| GA < 32 weeks | 10 (8.1) | 7 (18.9) | 0.4 (0.1-1.1) | 0.071 | 0.9 (0.1-7.6) | 0.892 | - | - | - | - | - | - |
| GA 32-36 weeks | 19 (15.3) | 5 (13.5) | 1.0 (0.3-2.9) | 1.000 | 0.7 (0.2-2.8) | 0.597 | - | - | - | - | - | - |
| GA ≥ 37 weeks | 95 (76.6) | 25 (67.6) | Reference | Reference | - | - | - | - | - | - | ||
| Hx previous stimulation1 | 31 (25.0) | 1 (2.7) | 12.0 (1.6-91.2) | 0.004 | 6.9 (0.7-64.6) | 0.089 | 32 (25.2) | 0 | - | 0.001 | - | - |
| Factors | Language | Personal and social | ||||||||||
| DQ | Univariable analysis | Multivariable analysis | DQ | Univariable analysis | Multivariable analysis | |||||||
| < 70, n = 122 | ≥ 70, n = 39 | OR (95%CI) | P valuea | AOR (95%CI) | P valueb | < 70, n = 122 | ≥ 70, n = 39 | OR (95%CI) | P valuea | AOR (95%CI) | P valueb | |
| Eye disease | ||||||||||||
| Cortical visual impairment | 36 (29.5) | 1 (2.6) | 15.9 (2.1-120.3) | < 0.001 | 9.1 (1.1-75.4) | 0.040b | 36 (29.5) | 1 (2.6) | 15.9 (2.1-120.3) | < 0.001 | 9.1 (1.1-75.9) | 0.041b |
| Optic nerve hypoplasia | 14 (11.5) | 1 (2.6) | 4.9 (0.6-38.7) | 0.120 | 6.0 (0.5-66.3) | 0.145 | - | - | - | - | - | - |
| Retinopathy of prematurity | 7 (5.7) | 5 (12.8) | 0.4 (0.1-1.4) | 0.165 | 0.4 (0.1-1.5) | 0.155 | - | - | - | - | - | - |
| Delay visual maturation | 10 (8.2) | 0 | - | 0.120 | - | - | 10 (8.2) | 0 | - | 0.120 | - | - |
| Visual acuity | ||||||||||||
| Not fix and follow | 65 (53.3) | 8 (20.5) | 6.9 (2.8-17.4) | < 0.001 | 5.3 (1.8-15.3) | 0.002b | 69 (56.6) | 4 (10.3) | 18.7 (5.9-59.1) | < 0.001 | 13.4 (4.0-44.7) | < 0.001b |
| Poor/fair fix and follow | 30 (24.6) | 8 (20.5) | 3.2 (1.2-8.3) | < 0.001 | 3.1 (0.9-10.0) | 0.062 | 29 (23.8) | 9 (23.1) | 3.5 (1.4-8.9) | 0.009 | 2.5 (0.9-7.0) | 0.074 |
| Good fix and follow | 27 (22.1) | 23 (59.0) | Reference | Reference | 24 (19.7) | 26 (66.7) | Reference | Reference | ||||
| Multiple disabilities | 55 (45.1) | 6 (15.4) | 4.5 (7.8-11.6) | 0.001 | 2.8 (0.9-8.5) | 0.076 | 55 (45.1) | 6 (15.4) | 4.5 (1.8-11.6) | 0.001 | 2.4 (0.8-7.1) | 0.108 |
| Prenatal risk | ||||||||||||
| Cerebral dysgenesis | 46 (37.7) | 8 (20.5) | 2.3 (1.0-5.5) | 0.053 | 1.0 (0.3-3.5) | 0.943 | - | - | - | - | - | - |
| Intrauterine infections | 3 (2.5) | 4 (10.3) | 0.2 (0.5-1.0) | 0.059 | 0.2 (0.0-1.6) | 0.134 | - | - | - | - | - | - |
| Perinatal risk | ||||||||||||
| Birth asphyxia | - | - | - | - | - | - | 21 (17.2) | 2 (5.1) | 3.8 (0.9-17.2) | 0.069 | 1.9 (0.4-10.5) | 0.446 |
| Postnatal risk | ||||||||||||
| Head trauma | - | - | - | - | - | - | 7 (5.7) | 0 | - | 0.197 | - | - |
| Hx previous stimulation1 | 31 (25.4) | 1 (2.6) | 12.9 (1.7-98.3) | 0.002 | 7.3 (0.9-60.4) | 0.064 | 30 (24.6) | 2 (5.1) | 6.0 (1.4-26.5) | 0.010 | 4.2 (0.8-20.9) | 0.083 |
Those potential variables with a P value less than 0.2 in the univariate logistic regression analysis were further examined using multivariable logistic regression (Tables 2, 3, and 4). As a result, impaired visual acuity was significantly associated with GDD, with an adjusted odds ratio (AOR) of 41.0 (95%CI: 5.2–324.8; P < 0.001) in the not fix-and-follow group and an AOR of 3.9 (95%CI: 1.3–12.3; P = 0.018) in the poor/fair fix-and-follow group. When each developmental domain was considered, impaired visual acuity in the not fix-and-follow group was significantly associated with delays in all domains including gross motor skills (AOR 10.0; 95%CI: 3.0–33.0; P < 0.001) and fine motor skills (AOR 12.8; 95%CI: 3.8–42.0; P < 0.001), language (AOR 5.3; 95%CI: 1.8–15.3; P = 0.002), and personal-social skills (AOR 13.4; 95%CI: 4.0–44.7; P < 0.001). However, in the poor/fair fix-and-follow group, a significant association was observed only in fine motor skills (AOR 4.3; 95%CI: 1.4–12.8; P < 0.001).
The other two statistically significant factors were multiple disabilities, which were associated with delays in the domains of gross motor skills (AOR 7.7; 95%CI: 2.0–29.7; P = 0.003) and fine motor skills (AOR 4.0; 95%CI: 1.9–13.2; P = 0.026), and a diagnosis of CVI, which was associated with delays in the domains of language (AOR 9.1; 95%CI: 1.1–75.4; P = 0.040) and personal-social skills (AOR 9.1; 95%CI: 1.1–75.9; P = 0.041).
Although the study aimed to eliminate the confounding effect of previous developmental stimulation by including only new cases, some patients (19.9%) had a history of previous intervention elsewhere. This factor was not significant in the multivariable analysis.
This comprehensive review of 161 pediatric patients with low vision and blindness under 6 years of age, a critical period for developmental growth, is the largest published sample size to date[1,5,13,14]. The findings revealed significant impairments in all domains of developmental delay and identified contributing factors.
The most common causes of visual impairment in this study were CVI and optic neuropathy/atrophy (Table 1). These findings are consistent with previous publications that examined children with developmental delay and investigated the prevalence of ocular manifestations, including a Danish national survey[15] involving 97 visually impaired children and a study from India[16] involving 51 visually impaired children.
The severity of visual impairment in this study was primarily classified using the office-based fix-and-follow method. Although this assessment was not quantitative and did not provide a cutoff criterion for visual impairment worse than 6/18 (20/70) or 0.5 LogMAR[17], all patients in this study were considered to have low vision or blindness based on ocular pathology. Furthermore, in one-fourth of the patients for whom visual acuity could be measured at verbal preschool age, those in the good fix-and-follow and poor/fair fix-and-follow groups had mean visual acuity levels of 1.07 ± 0.58 LogMAR and 1.95 ± 0.89 LogMAR, respectively. Notably, these levels of visual acuity remained within the low vision range, even after adjusting for age-related improvement[18], which averaged only 0.1 LogMAR (one line) over a mean period of 25.8 months.
The impact of visual impairment on development was evident, with GDD identified in the majority of patients (83.2%) based on the Denver II assessment used in this study (Figure 1). These findings contrast with those reported by Dale and Sonksen[14], who found that only 1% of children with low vision had GDD. This discrepancy may be attributed to the small sample size in the earlier study and the use of different developmental assessment tools. Dale and Sonksen[14] study employed the Reynell-Zinkin Scales, which diagnose GDD only when there are delays present in the all three domains: Sensorimotor understanding, verbal comprehension, and expressive language. Consequently, the assessment tools used in their study were more stringent in diagnosing GDD than those used in the present study. This high proportion of GDD was also greater than that reported in a study from Mexico[19], which mainly investigated the causes of visual impairment and also noted the comorbidity of developmental or psychomotor delay in 39.7% of the cases. However, detailed developmental assessments, the evaluation tools used, and missing data were not mentioned in their retrospective chart review.
In addition to GDD, visual impairment was shown to affect each developmental domain individually, including gross and fine motor skills, language, and personal-social skills. Furthermore, these impairments were correlated with the severity of visual impairment, since patients in the not fix-and-follow group demonstrated significantly greater developmental delays, with relatively higher AOR compared to the poor/fair fix-and-follow group, based on multivariable analysis (Tables 2, 3 and 4).
Regarding motor skill development, Radzo Alibegovic[20] reported that visual impairment can hinder motor de
In addition to visual acuity, the presence of multiple disabilities was significantly associated with delays in gross and fine motor skill development, as demonstrated by multivariable analysis (Table 3). The AOR was 7.7 and 4.0, respectively, compared to children with visual impairment only. These findings are consistent with the observation that most cases with multiple disabilities had cerebral palsy, a condition that primarily affects movement, muscle tone, and posture (Table 1).
Furthermore, in addition to visual acuity, multivariable analysis also identified a diagnosis of CVI as significantly associated with delays in language and personal-social skill development. This finding is supported by the study by Hegde et al[16] and the present study, both of which reported that CVI often results from prenatal and perinatal conditions such as cerebral dysgenesis, intrauterine infection, and birth asphyxia (Tables 1 and 4). These conditions typically have direct adverse effects on brain development and contribute to developmental delays. Moreover, when severe brain damage occurs during the antepartum or intrapartum periods, it can result in a marked deterioration of visual acuity and delays in all developmental domains.
These findings provide essential guidance for clinical practice and policy. Children with poor visual fixation or multiple disabilities—particularly cerebral palsy—should be considered at high risk for global and domain-specific developmental delays. Early referral to multidisciplinary services, including physical, occupational, and speech therapy, is strongly recommended to support skill acquisition in motor, communication, and adaptive domains. Furthermore, CVI, shown to be significantly associated with language and personal-social delays, should be regarded as a clinical marker of potential underlying central nervous system pathology. In such cases, neuroimaging and neurologic consultation may be warranted to assess for conditions like periventricular leukomalacia or brain malformations. Integrating developmental surveillance into the routine care of visually impaired children—especially those with CVI or comorbidities—can improve early detection and intervention. These findings suggest that current developmental monitoring guidelines should be expanded to include risk stratification based on visual acuity and neurologic etiology, thereby promoting earlier and more targeted support for this vulnerable population.
This study has some limitations. It is a retrospective review of clinical charts that can result in data loss. In addition, the retrospective chartreview design may introduce selection bias, despite our observation of complete core demographic records. Additionally, the Denver II is only a developmental screening tool. Therefore, future studies should employ standardized developmental assessment instruments and adopt a longitudinal prospective design to yield more accurate results. Furthermore, for visual acuity assessment using the fix-and-follow method, the specific size of the visual target or light stimulus was not recorded and therefore could not be described in this study. We also recognize that the fixandfollow assessment, while pragmatic in our setting, is nonquantitative and lacks standardized stimulus size or luminance. Consequently, future research should adopt more quantitative visual acuity assessment tools, even for preschool-aged children, such as the preferential looking test. Future prospective studies should additionally incorporate objective methods such as Teller acuity cards or VEP to improve measurement precision.
However, a key strength of this study is that it is the first to explore the relationship between low vision and developmental delay in a clinical setting with relatively high reliability. This is because of the assessments conducted by specialized medical professionals, the use of a standardized developmental screening tool, and a sufficiently large sample size that allowed evaluation of all developmental domains and identification of associated factors through multivariate regression analysis to reduce confounding effects and improve the accuracy of the results.
Our findings highlight the importance of awareness and recognition of developmental delays in children with visual impairment, particularly those with additional risk factors such as poorer visual acuity, a diagnosis of CVI, or the presence of multiple disabilities. Timely referral and the involvement of a multidisciplinary team, including ophthalmologists and pediatric specialists, are strongly encouraged.
The authors would like to thank Ms. Julaporn Pooliam of the Office for Research and Development for her assistance with the statistical analyses.
| 1. | Reynell J. Developmental patterns of visually handicapped children. Child Care Health Dev. 1978;4:291-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Rainey L, Elsman EBM, van Nispen RMA, van Leeuwen LM, van Rens GHMB. Comprehending the impact of low vision on the lives of children and adolescents: a qualitative approach. Qual Life Res. 2016;25:2633-2643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Varma R, Tarczy-Hornoch K, Jiang X. Visual Impairment in Preschool Children in the United States: Demographic and Geographic Variations From 2015 to 2060. JAMA Ophthalmol. 2017;135:610-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Wang H, Qiu K, Yin S, Du Y, Chen B, Jiang J, Deng D, Zhang M. Prevalence of Visual Impairment in Preschool Children in Southern China. Front Public Health. 2022;10:755407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | McConachie H. Early language development and severe visual impairment. Child Care Health Dev. 1990;16:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Janson U. Normal and deviant behavior in blind children with ROP. Acta Ophthalmol Suppl (1985). 1993;20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Dammeyer J. Development and characteristics of children with Usher syndrome and CHARGE syndrome. Int J Pediatr Otorhinolaryngol. 2012;76:1292-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Ross G, Lipper EG, Abramson D, Preiser L. The development of young children with retinoblastoma. Arch Pediatr Adolesc Med. 2001;155:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Chotikavanich S, Chanvarapha N, Loket S, Yingyong R, Dongngam S, Nujoi W, Sangsre P, Maneephagaphan K, Rungsiri K, Krutthong W. A 5-year retrospective record review of hospital-based low-vision rehabilitation in Thailand. Clin Optom (Auckl). 2018;10:41-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Morelli F, Aprile G, Martolini C, Ballante E, Olivier L, Ercolino E, Perotto E, Signorini S. Visual Function and Neuropsychological Profile in Children with Cerebral Visual Impairment. Children (Basel). 2022;9:921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Garzón-Rodríguez MC, Reyes-Figueredo LS, Velandia-Rodríguez LÁ, Méndez-Ruiz OD, Gómez-Rodríguez MA, Esguerra-Ochoa LT, García-Lozada D. Causes of low vision in children: A systematic review. Arch Soc Esp Oftalmol (Engl Ed). 2023;98:83-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Frankenburg WK, Dodds J, Archer P, Shapiro H, Bresnick B. The Denver II: a major revision and restandardization of the Denver Developmental Screening Test. Pediatrics. 1992;89:91-97. [PubMed] |
| 13. | Tröster H, Brambring M. Early social-emotional development in blind infants. Child Care Health Dev. 1992;18:207-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Dale N, Sonksen P. Developmental outcome, including setback, in young children with severe visual impairment. Dev Med Child Neurol. 2002;44:613-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Nielsen LS, Skov L, Jensen H. Visual dysfunctions and ocular disorders in children with developmental delay. I. prevalence, diagnoses and aetiology of visual impairment. Acta Ophthalmol Scand. 2007;85:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Hegde V, Jain R, Bappal A, Shambhu R. Ocular manifestations in children with developmental delay at a tertiary center in South India. Saudi J Ophthalmol. 2021;35:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Dandona L, Dandona R. Revision of visual impairment definitions in the International Statistical Classification of Diseases. BMC Med. 2006;4:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Hutchinson AK, Morse CL, Hercinovic A, Cruz OA, Sprunger DT, Repka MX, Lambert SR, Wallace DK; American Academy of Ophthalmology Preferred Practice Pattern Pediatric Ophthalmology/Strabismus Panel. Pediatric Eye Evaluations Preferred Practice Pattern. Ophthalmology. 2023;130:P222-P270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 19. | López Ulloa JA, Burn H, Beauregard AM. Causes of Blindness and Visual Impairment in Early Childhood at a Low Vision Service in Mexico City: A 15-year Review. Ophthalmic Epidemiol. 2021;28:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Radzo Alibegovic D. Development of motor function in children with normal vision and children with visual impairments. Hum Res Rehabil. 2023;13:69-76. [DOI] [Full Text] |
| 21. | Peltzer-Karpf A. The dynamic landscape of exceptional language development. Strabismus. 2012;20:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Mosca R, Kritzinger A, van der Linde J. Language and communication development in preschool children with visual impairment: A systematic review. S Afr J Commun Disord. 2015;62:e1-e10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/