Published online Dec 9, 2025. doi: 10.5409/wjcp.v14.i4.111069
Revised: July 10, 2025
Accepted: September 23, 2025
Published online: December 9, 2025
Processing time: 131 Days and 7.1 Hours
Children with juvenile idiopathic arthritis (JIA) and inflammatory bowel disease (IBD) face an elevated risk of severe infection owing to their diseases and the immunosuppressive treatment for disease control.
To compare scheduled vaccination coverage and the levels of post-vaccine antibodies against measles, mumps, rubella (MMR) and hepatitis B in pediatric patients with IBD and JIA.
A comparative cohort study included 97 patients with IBD and 170 patients with JIA. Vaccination history was obtained from medical records, while post-vaccination immunity was assessed prospectively by measuring specific IgG antibody titers using enzyme-linked immunosorbent assays (Vector-Best JSC, Russia; IBL International, Germany) during routine visits between January 2022 and April 2023.
A complete two-dose MMR course had been administered to 66.3% of IBD patients and 55.9% of JIA patients (P = 0.121). By contrast, the three-dose hepatitis B schedule was completed in 74.2 % of IBD and 100 % of JIA patients (P < 0.001). Protective level of anti-vaccine antibodies against measles (47.7% vs 57.7%; P = 0.168); mumps (75.3% vs 80.0%; P = 0.366); rubella (74.4% vs 98.2%; P < 0.0001); and hepatitis B (44.8% vs 50.0%; P = 0.514) were detected in IBD and JIA patients, respectively.
Patients with IBD and JIA demonstrated different vaccination coverage patterns and levels of anti-vaccine antibodies. Routine baseline serology and timely booster vaccination should be implemented for all pediatric patients receiving chronic immunosuppression.
Core Tip: Vaccination is the primary strategy for preventing infections in children. Patients with juvenile idiopathic arthritis (JIA) and inflammatory bowel disease (IBD) have reduced immunity and increased susceptibility to infections. The immunogenicity of vaccines in children with IBD and JIA remains a topic of controversy. The study compared vaccination coverage and antibody levels against measles, mumps, rubella, and hepatitis B in children with IBD and JIA. Patients with IBD demonstrated lower rates of seropositivity, particularly for rubella and hepatitis B. In contrast, patients with JIA tend to maintain a more stable response to vaccination.
- Citation: Makarova E, Goleva O, Gabrusskaya T, Lubimova N, Ulanova N, Volkova N, Shilova E, Revnova M, Kharit S, Kostik M. Vaccine coverage, antibodies against measles, mumps, rubella, hepatitis B in inflammatory bowel disease and juvenile idiopathic arthritis children. World J Clin Pediatr 2025; 14(4): 111069
- URL: https://www.wjgnet.com/2219-2808/full/v14/i4/111069.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i4.111069
Vaccination is the principal strategy for preventing vaccine-preventable infections in childhood. Its implementation becomes markedly more complex when long-term immunosuppression is required, as in juvenile idiopathic arthritis (JIA) and inflammatory bowel disease (IBD).
Immunosuppressive therapy increases susceptibility to common pathogens and heightens the risk of severe outcomes; it may also attenuate vaccine immunogenicity[1]. Treatments such as tumor necrosis factor (TNF) inhibitors, high-dose glucocorticoids, and immunomodulators are effective in suppressing disease activity. However, they may impair immune function, thereby increasing susceptibility to vaccine-preventable infections[2].
Despite both diseases being immune-compromised, there are differences in the unique immunopathogenesis and treatment approaches between JIA and IBD, with a higher prevalence of biologics in pediatric rheumatology practice and more frequent use of corticosteroids and cytostatics in IBD patients. Also, IBD patients often have additional factors influenced by anti-vaccine antibodies, such as malnutrition, diarrhea, hepatic disease, and GI surgery.
The European Crohn's and Colitis Organisation (ECCO) and the European League Against Rheumatism therefore recommend accelerated and, where feasible, complete schedules of inactivated and live vaccines before treatment initiation or, when necessary, during ongoing therapy[3,4]. Despite these recommendations, vaccine uptake remains suboptimal—particularly among those treated with biologic agents—due to concerns about vaccine safety, potential disease flares, and reduced immunogenicity[5,6].
While numerous studies have confirmed the safety of vaccines in pediatric patients with immune-mediated conditions, including no significant increase in disease flares following immunization[3,4], the degree of vaccine-induced antibodies in this population remains a matter of debate. Some studies report adequate antibody responses, whereas others suggest diminished seroconversion rates, reduced antibody persistence, and lower efficacy in the context of ongoing immunosuppression[2,7,8].
Recent data suggest that specific immunosuppressive agents, including TNF inhibitors, high-dose glucocorticoids, and B-cell-depleting therapies, can negatively impact vaccine responses. A 2023 prospective cohort study showed that the effectiveness of the conjugated MenACWY vaccine against meningococcal infection in adolescents with JIA and IBD decreases with the use of anti-TNF therapy. It highlights the potential requirement for booster doses[7]. A systematic review of 31 studies further confirmed that patients on TNF inhibitors have impaired serologic responses to vaccines, such as hepatitis B and influenza[8].
The immunogenicity of the measles, mumps, rubella (MMR), and hepatitis B vaccines in patients with IBD and JIA has been evaluated in separate studies, with conflicting results. A cross-sectional study in IBD patients showed no significant difference in MMR antibody levels compared to healthy peers[9]. In contrast, a larger cohort of 437 IBD patients demonstrated significantly lower seropositivity, particularly among those with ulcerative colitis[10]. These discrepancies suggest variability in long-term vaccine-induced protection and highlight the importance of evaluating booster requirements[11]. A 2021 meta-analysis involving IBD patients found that only 39.7% achieved seroprotective levels following hepatitis B vaccination, with disease activity, older age, and combination immunosuppressive therapy identified as risk factors for poor response[12].
Despite substantial evidence on vaccine immunogenicity in either IBD or JIA cohorts, direct comparative studies between these two populations are lacking. Given differences in disease pathogenesis, immune dysregulation, and treatment strategies, it is plausible that vaccine responses may differ between these patient groups[3]. It is the first study to directly compare the anti-vaccine antibody levels between two diseases with some similarities in pathogenesis and treatment.
This study aims to compare vaccine coverage and post-vaccination antibody levels against MMR, and hepatitis B in children with IBD and JIA and to explore the potential impact of immunosuppressive therapy on serologic responses.
This prospective cohort study included 97 pediatric patients with IBD and 170 pediatric patients with JIA. This cross-sectional analysis was conducted prospectively during routine clinical visits between January 2022 and April 2023. Blood samples for anti-vaccine antibody measurement were collected prospectively during the study period, while vaccination history was retrieved retrospectively from medical records.
Inclusion criteria: Pediatric patients (aged 2–18 years) with a confirmed diagnosis of Crohn's disease, ulcerative colitis, or JIA who provided informed consent to participate. Availability of complete vaccination records and corresponding post-vaccination antibody data for MMR, and hepatitis B.
Exclusion criteria: Patients with missing vaccination data for MMR, or hepatitis B. Receipt of blood transfusions or intravenous immunoglobulin within the preceding six months due to potential interference with antibody titers.
Demographic information: Sex, age at disease onset, and age at study inclusion.
Clinical data: Type of IBD and JIA were assessed, and disease activity was evaluated using the Pediatric Crohn's Disease Activity Index (PCDAI) and the Pediatric Ulcerative Colitis Activity Index (PUCAI) at diagnosis and study inclusion. The IBD course was divided into three categories: Acute (< 6 months since the onset), chronic persistent (no remission episodes with a duration of more than 6 months during the relevant treatment), and chronic relapsed (remission lasting more than 6 months). For JIA: International League of Associations for Rheumatology classification subtype, disease duration, and treatment history.
Vaccination history: The type and timing of vaccinations, total number of doses received for measles, rubella, mumps, and hepatitis B, and compliance with the National Vaccination Schedule[13] were collected from patients who continued with scheduled vaccinations, particularly for MMR and hepatitis B. Additionally, disease course and treatment details were extracted from medical records.
Immunosuppressive therapy: Data on treatment regimens, including TNF-α inhibitors, immunomodulators (aza
Levels of anti-vaccine antibodies: IgG concentrations of anti-vaccine antibodies for measles, rubella, mumps, and hepatitis B were measured using enzyme-linked immunosorbent assay with commercially available kits from Vector-Best JSC (Russia) and IBL (Germany). The antibody levels were analyzed based on calibration curves generated with Dynex Technologies Inc. software (United States). Laboratory technicians performing antibody measurements were blinded to patient diagnosis and treatment group to minimize detection bias.
Protective antibody thresholds were established according to the manufacturer's guidelines: Measles: ≥ 0.18 IU/mL [coefficient of variation (CV): 8%; analytical sensitivity: 0.07–30 IU/mL]; rubella: ≥ 10 IU/mL (CV: 8%; analytical sensitivity: 2 IU/mL); hepatitis B (anti-HBs antibodies): ≥ 10 mIU/mL (CV: 8%; analytical sensitivity: 2 mIU/mL); mumps: Protective IgG level defined as positivity coefficient > 1.0.
The study was approved by the Ethics Committee of St. Petersburg State Pediatric Medical University (protocol No. 09/02, dated November 2, 2022). The study adhered to the ethical principles of the Declaration of Helsinki, ensuring the protection of participants’ rights. All data were collected and processed anonymously.
All statistical analyses were performed using Statistica software (version 10.0, StatSoft Corporation, Tulsa, OK, United States). The Kolmogorov-Smirnov test was used to assess the normality of the quantitative data distribution. Missing data were excluded from the analysis. Descriptive statistics were reported as follows: Continuous variables: Median and interquartile range (Q1; Q3). Categorical variables: Absolute values and proportions. Comparative analysis: Pearson's χ² test or Fisher's exact test was used for categorical variables. The Mann-Whitney U test was used to compare continuous variables between two independent groups. A two-tailed P value of < 0.05 was considered statistically significant.
A total of 267 pediatric patients were included in the comparative analysis: 97 with IBD and 170 with JIA.
The JIA group consisted of 170 patients, with a significantly higher proportion of females (67.7%) compared to the IBD group (46.4%, P < 0.001). The median age at study inclusion was lower in JIA patients [11.4 years; interquartile range (IQR): 7.6–14.8] compared to IBD patients (14.0 years; IQR: 11.0–16.0; P = 0.03). The age at disease onset was also significantly lower in the JIA cohort (6.0 years; IQR: 3.7–9.0) compared to the IBD cohort (11.0 years; IQR, 6.0–14.0; P = 0.01). The main JIA categories were systemic arthritis (n = 16, 9.4%), oligoarthritis (n = 73, 42.9%), polyarthritis (n = 61, 35.9%), and enthesitis-related arthritis (n = 20, 11.8%).
Among IBD patients, 73 (75.0%) were diagnosed with Crohn’s disease and 24 (25.0%) with ulcerative colitis. Ex
Disease activity decreased significantly from diagnosis to study inclusion: Median PCDAI score for Crohn's disease decreased from 37.0 (IQR: 25–45) to 15.0 (IQR: 0–30). The median PUCAI score for ulcerative colitis decreased from 35.0 (IQR: 33–45) to 2.5 (IQR: 0–10), indicating effective disease control at the time of inclusion.
Regarding the disease course, 10 patients (10.2%) had an acute course, 29 (29.6%) had chronic, persistent disease, and 58 (59.8%) had a chronic, relapsing course.
Systemic corticosteroids (greater than 1 mg/kg/day of prednisone equivalent) were used in 56 patients (60.1%) for a median duration of 2.0 months (interquartile range, 1.0–3.0 months). Conventional immunosuppressive therapy included azathioprine (65.3%), methotrexate (22.5%), 6-mercaptopurine (10.2%), and tacrolimus (2.0%). Topical anti-inflammatory therapy was used in 39 patients (39.8%).
Among the 76 patients who received at least one biologic agent, infliximab was used in 54 (70.6%), adalimumab in 23 (30.8%), and vedolizumab in 8 (10.3%); several patients received more than one biologic agent during their treatment course. The median duration of TNF-α inhibitor therapy was 3.5 months (interquartile range, IQR: 1.2–22.0). IBD-related surgical intervention was required in 12 patients (12.4%).
Serologic testing was performed while patients were receiving their current immunosuppressive treatment regimens, with no pre-treatment baseline titers available for comparison. The median duration of therapy at the time of antibody measurement was 6 (3; 8) years for measles, mumps, and rubella and 11.0 (6.8; 13.3) for hepatitis B.
At the time of study inclusion, TNF-α inhibitors were more frequently used in IBD patients (51.8%) compared to those with JIA (33.0%, P < 0.0001). A high percentage of patients in both groups had received conventional non-biological immunosuppressive treatment at disease onset, with 83.5% in the IBD group and 90.6% in the JIA group (P = 0.09).
Therapeutic adjustments were as follows: 23.6% of IBD patients on biologics required dose escalation or interval shortening, and 13.2% needed a switch to another agent, compared with 9.4% in the JIA cohort. These figures illustrate the dynamic nature of treatment optimization in both diseases and will be considered when interpreting vaccine-immunity outcomes. Demographic characteristic is in Table 1.
| Parameter | IBD (n = 97) | JIA (n = 170) | P value |
| Sex (females) | 45 (46.4) | 115 (67.7) | < 0.001 |
| Age of the inclusion in the study | 14.0 (11.0-16.2) | 11.4 (7.6-14.8) | 0.03 |
| Age of the disease onset | 11.0 (6.0-14.0) | 6.0 (3.7-9.0) | 0.01 |
| Biological immunosuppressive treatment at study inclusion | 88 (51.8) | 32 (33.0) | < 0.0001 |
| Immunosuppressive therapy at the time of the study | 81 (83.5) | 154 (90.6) | 0.09 |
The proportion of children with protective levels of post-vaccine antibodies differed between the two cohorts (Table 2). Overall, children with IBD demonstrated lower levels of antibodies against all vaccines, with statistically significant gaps observed for mumps and rubella. The information for each vaccine is listed below.
| Parameter | IBD (n = 97) | JIA (n = 170) | P value |
| Antibodies against measles, IgG, GM, (IU/mL) | 0.15 (0.0-0.35) | 0.21 (0.04-0.53) | < 0.001 |
| Complete vaccination against measles for age1 | 62 (63.9) | 95 (55.9) | 0.200 |
| Patients with protective levels of antibodies against measles | 41 (47.7) | 98 (57.7) | 0.016 |
| Antibodies against mumps, IgG, GM, (IU/mL) | 2.8 (0.9-5.0) | 2.9 (1.3-5.3) | 0.570 |
| Complete vaccination against mumps for age1 | 69 (71.1) | 95 (55.8) | 0.014 |
| Patients with protective levels of antibodies against mumps | 73 (75.3) | 96 (56.5) | 0.003 |
| Antibodies against rubella, IgG, GM | 32 (0.0-52.0) | 87.3 (45.3-198.3) | < 0.0001 |
| Complete vaccination against rubella for age1 | 31 (32.0) | 75 (44.1) | 0.051 |
| Patients with protective levels of antibodies against rubella | 64 (74.4) | 168 (98.2) | < 0.0001 |
| Complete vaccination against hepatitis B for age1 | 78 (80.4) | 163 (95.9) | 0.00004 |
| Patients with protective levels of antibodies against hepatitis B | 39 (44.8) | 85 (50.0) | 0.432 |
| Antibodies against hepatitis B, IgG, GM | 0 (0-30) | 9.3 (0.03-41.9) | 0.024 |
| Patients whose anti-vaccine antibody levels was detected before the treatment | 4 (14.3) | ||
| Vaccine-associated adverse events anytime | |||
| No | 85 (88.5) | ||
| Fever | 4 (4.2) | ||
| Injection site reaction | 6 (6.2) | ||
| IBD flare | 1 (1.0) | ||
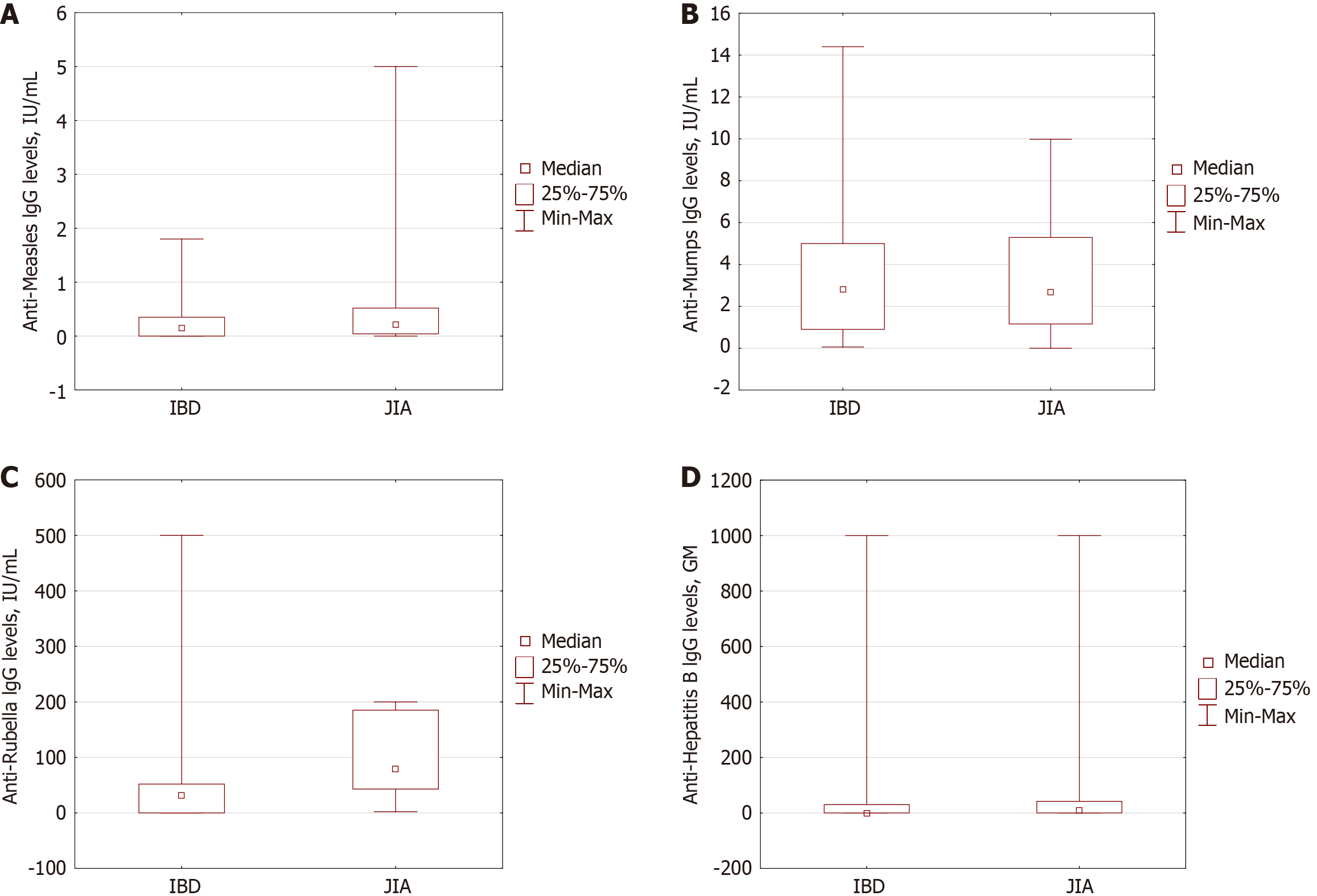
Despite the similar rate of complete vaccination coverage between two cohorts (63.9% in IBD vs 55.9% in JIA; P = 0.200), JIA patients demonstrated higher geometric mean (GM) IgG levels (0.21 0.04-0.53 IU/mL) compared to IBD (0.15 0.0-0.35 IU/mL; P < 0.001), as well as the part of the patients with protective level (57.7% in JIA and 47.7% in IBD group; P = 0.016).
In contrast, substantial differences were observed regarding the vaccination component against mumps. JIA patients had significantly lower levels of antibodies against vaccines (56.5%) compared to IBD patients (75.3%; P = 0.003), as well as a similar vaccination completeness rate (71.1% vs 55.8%; P = 0.014).
The completeness of vaccination against the rubella was relatively higher in JIA patients (44%) compared to IBD (32%; P = 0.051) with higher IgG GM levels in JIA patients (87.3 IU/mL) compared to IBD (32.0 IU/mL; P < 0.0001) lead to higher proportion of JIA patients with positive antibodies (98.8%) than in IBD (74.4%, P < 0.0001).
Complete vaccination against hepatitis B was reported in practically all JIA patients, and only 80.4% of IBD patients met the age-appropriate immunization (P = 0.00004). The IgG GM levels of antibodies were higher in the JIA cohort (9.3 0.03-41.9 vs 0 0-30 IU/mL; P = 0.024) compared to in IBD patients, but the rate of patients with positive antibodies was equal (50% in JIA and 44.8% in IBD; P = 0.432). The data are presented in Figure 1 and Table 2.
This study presents a comparative analysis of vaccine coverage and post-vaccination immunity in pediatric patients with IBD and JIA, highlighting both shared challenges and disease-specific differences in maintaining protective antibody responses. These results indicate sub-optimal long-term humoral immunity in both diseases, with JIA patients retaining significantly better protection against rubella, while immunity to measles, mumps, and hepatitis B remains insufficient in both cohorts. The marked rubella gap may reflect differences in booster timing, age at diagnosis, or treatment-related modulation of immune memory.
Our data confirm that children with IBD have less complete vaccine-derived immunity than those with JIA, but the magnitude of the gap varies by the vaccine. The most significant and statistically robust difference concerned rubella: Protective IgG was present in 98.8% of JIA patients vs 74.4% of IBD patients (P < 0.0001). For mumps, the proportion of patients with positive antibodies was higher in the IBD cohort, but the difference was modest—75.3% vs 56.5% (P = 0.014). The observed differences could be explained by the younger onset age of JIA patients, a more extended period of exposure between the last specific vaccination and anti-vaccine antibody measurements, and a relatively longer treatment duration.
Our pediatric cohort revealed a greater loss of protective titers, specifically protective IgG for measles, which persisted in only 47.7% of children with IBD and 57.7% with JIA (P = 0.016). A prospective United States study by Cleveland et al[14] likewise showed that 13% of 122 adult IBD patients lacked detectable measles antibodies, and a further 3% had equivocal titers. Older age (≥ 50 years) and longer disease duration (≥ 10 years) were independent predictors of waning immunity[14]. Although the absolute proportions differ (adults generally receive an additional MMR booster in adolescence), both datasets converge on the same point: Documentation of a completed MMR schedule does not provide current measles protection once chronic immune dysregulation is present, so IBD patients have a higher risk of inadequate protective titers-particularly for rubella—echoing earlier reports of suboptimal vaccine-induced immunity in pediatric IBD cohorts[15].
Our study confirms that long-term humoral protection against hepatitis B in children receiving chronic immunosuppression is disappointingly low: Only 44.8% of patients with IBD and 50% of those with JIA, maintained anti-HBs levels ≥ 10 mIU/mL, despite documented completion of the primary three-dose schedule in 74.2% and 100% of cases, respectively.
These figures parallel—and only modestly exceed—the single-center experience of Watts et al[16], who found pro
On the other hand, our data sit below the pooled 61% (95%CI: 53–69) seroconversion rate reported in the meta-analysis by Jiang et al[17] for patients revaccinated against hepatitis B virus (HBV). Two factors likely explain this gap. First, Jiang et al's endpoint was the acute antibody response to a (re)-vaccination course, whereas we assessed the persistence of antibodies many years after infancy immunization[17]. Second, all children in our cohort had already received at least one immunosuppressive agent. In contrast, the meta-analysis revealed significantly higher response rates when vaccination was administered before such therapy [relative risk (RR) = 1.33 for immunomodulators and RR = 1.57 for anti-TNF-α agents].
Patients with JIA may have experienced more delays or hesitations related to vaccination after diagnosis. A notable observation was the relation between current TNF-α–inhibitor therapy and serological protection. In the pooled cohort, children receiving a biologic had higher odds of protective IgG against both measles (P = 0.004) and rubella (P = 0.0001), indicating that first-line TNF blockade does not diminish humoral immunity to these antigens and may even help maintain it. No significant association emerged for mumps (P = 0.81) or hepatitis B (P = 0.94), suggesting that determinants of positive titers for these vaccines lie outside simple TNF inhibition—potentially in booster timing, baseline antibody decay or concomitant methotrexate use. It should be noted, however, that children with at least three sequential biologic agents were over-represented in the rubella-non-protected subgroup (18.5% vs 0%; P = 0.002). Thus, while standard TNF-α inhibition does not appear to compromise vaccine-induced immunity[1,3,15], cumulative or intensified biological exposure may eventually erode long-term protection against rubella. Prospective studies that serially measure titers before and after biological switches are needed to clarify this dose-response effect.
Several studies have previously addressed vaccine immunogenicity in pediatric patients with IBD and JIA, yet the results remain conflicting. Some reports suggest that patients on immunosuppressive therapy can still mount adequate immune responses, while others indicate reduced levels of anti-vaccine antibodies compared to those of healthy individuals[10,15,17,20,21].
Our findings underscore the need for enhanced vaccination strategies in pediatric patients with IBD and JIA. Given the lower titers of anti-vaccine antibodies observed in IBD patients, clinicians should consider: Baseline serology and targeted boosters. All children with IBD or JIA should undergo baseline serological testing for the core childhood vaccines—MMR, hepatitis B, and, where available, diphtheria and tetanus—at the time of diagnosis and again before any significant escalation of immunosuppressive therapy. Patients with low anti-vaccine antibodies should prompt immediate booster vaccination, according to ECCO guidelines[1,3].
Because biologics and conventional disease-modifying drugs may accelerate antibody waning, monitoring every 3 to 5 years is advisable, with shorter intervals for those receiving multiple biologic switches.
Embedding vaccination checkpoints into gastroenterology and rheumatology follow-up visits—rather than relying solely on primary-care reminders—would help close the gap between real-life coverage and positive levels of antibodies against vaccines.
Because biologics and conventional disease-modifying drugs may accelerate antibody waning, monitoring every 3 to 5 years is advisable, with shorter intervals recommended for individuals who receive multiple biologic switches.
The primary limitation of this study is the relatively small sample size, which may affect the generalizability of the findings. Additionally, the study population exhibited high heterogeneity in terms of disease characteristics, treatment approaches, and the time elapsed since disease onset and study inclusion. Variations in the age of disease onset contributed to differences in vaccination coverage, with older patients being more likely to have missed scheduled vaccinations, which may have led to lower levels of anti-vaccine antibodies. These factors may have influenced the study outcomes and should be considered when interpreting the results. Differences in variables such as disease severity, time since vaccination, age at diagnosis, type and duration of immunosuppressive therapy, and time of antibody testing relative to treatment and cohort imbalance could skew the results. Although our data demonstrate lower rates of positive antibodies against vaccines in patients under immunosuppressive therapy, causality cannot be established due to the cross-sectional design and absence of pre-treatment antibody measurements. The observed differences may reflect treatment-related effects, underlying immune dysregulation, or a combination of both.
Despite apparently satisfactory documentation of primary immunization, real levels of anti-vaccine antibodies were found to be markedly lower. The most pronounced immunity gap occurred in IBD children for rubella and hepatitis B. Adherence to the national immunization schedule alone does not provide adequate immunological protection in children receiving chronic immunosuppression.
To minimize the "immunity window", the following measures should include baseline and periodic serological monitoring—prior to treatment initiation and every 3–5 years (or when therapy is escalated)—followed by targeted revaccination, accelerated booster administration of MMR and HBV immediately after diagnosis, especially in early-onset IBD and before initiation of combination immunosuppression and collaboration between specialists and primary physicians, including systematic reminders and incorporation of antibody checks into routine visits. Implementing regular seromonitoring and timely booster immunizations should become a cornerstone of strategies to prevent vaccine-preventable infections in this population.
| 1. | Jansen MHA, Rondaan C, Legger GE, Minden K, Uziel Y, Toplak N, Maritsi D, van den Berg L, Berbers GAM, Bruijning P, Egert Y, Normand C, Bijl M, Foster HE, Koné-Paut I, Wouters C, Ravelli A, Elkayam O, Wulffraat NM, Heijstek MW. EULAR/PRES recommendations for vaccination of paediatric patients with autoimmune inflammatory rheumatic diseases: update 2021. Ann Rheum Dis. 2023;82:35-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (1)] |
| 2. | Gertosio C, Licari A, De Silvestri A, Rebuffi C, Chiappini E, Marseglia GL. Efficacy, immunogenicity, and safety of available vaccines in children on biologics: A systematic review and meta-analysis. Vaccine. 2022;40:2679-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 3. | Kucharzik T, Ellul P, Greuter T, Rahier JF, Verstockt B, Abreu C, Albuquerque A, Allocca M, Esteve M, Farraye FA, Gordon H, Karmiris K, Kopylov U, Kirchgesner J, MacMahon E, Magro F, Maaser C, de Ridder L, Taxonera C, Toruner M, Tremblay L, Scharl M, Viget N, Zabana Y, Vavricka S. ECCO Guidelines on the Prevention, Diagnosis, and Management of Infections in Inflammatory Bowel Disease. J Crohns Colitis. 2021;15:879-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 315] [Article Influence: 63.0] [Reference Citation Analysis (32)] |
| 4. | Rondaan C, Furer V, Heijstek MW, Agmon-Levin N, Bijl M, Breedveld FC, D'Amelio R, Dougados M, Kapetanovic MC, van Laar JM, Ladefoged de Thurah A, Landewé R, Molto A, Müller-Ladner U, Schreiber K, Smolar L, Walker J, Warnatz K, Wulffraat NM, van Assen S, Elkayam O. Efficacy, immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases: a systematic literature review for the 2019 update of EULAR recommendations. RMD Open. 2019;5:e001035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 5. | Açarı C, Ünsal E. Current information about vaccination practice in pediatric rheumatic diseases and recommendations for future applications. Turk J Pediatr. 2017;59:357-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Coenen S, Weyts E, Jorissen C, De Munter P, Noman M, Ballet V, Vermeire S, Van Assche G, Ferrante M. Effects of Education and Information on Vaccination Behavior in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017;23:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Ohm M, van Straalen JW, Zijlstra M, de Joode-Smink G, Sellies AJ, Swart JF, Vastert SJ, van Montfrans JM, Bartels M, van Royen-Kerkhof A, Wildenbeest JG, Lindemans CA, Wolters VM, Wennink RAW, de Boer JH, Knol MJ, Heijstek MW, Sanders EAM, Verduyn-Lunel FM, Berbers GAM, Wulffraat NM, Jansen MHA. Meningococcal ACWY conjugate vaccine immunogenicity and safety in adolescents with juvenile idiopathic arthritis and inflammatory bowel disease: A prospective observational cohort study. Vaccine. 2023;41:3782-3789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Altunöz ME, Senateş E, Yeşil A, Calhan T, Ovünç AO. Patients with inflammatory bowel disease have a lower response rate to HBV vaccination compared to controls. Dig Dis Sci. 2012;57:1039-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 9. | Caldera F, Misch EA, Saha S, Wald A, Zhang Y, Hubers J, Megna B, Ley D, Reichelderfer M, Hayney MS. Immunosuppression Does Not Affect Antibody Concentrations to Measles, Mumps, and Rubella in Patients with Inflammatory Bowel Disease. Dig Dis Sci. 2019;64:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Shiga H, Takahashi T, Shiraki M, Kojima Y, Tsuji T, Takagi S, Hiramoto K, Yokoyama N, Sugimura M, Iwabuchi M, Endo K, Onodera M, Sato Y, Shimodaira Y, Nomura E, Kikuchi T, Chiba H, Oomori S, Kudo H, Kumada K, Nagaie S, Ogishima S, Nagami F, Shimoyama Y, Moroi R, Kuroha M, Kakuta Y, Ishige T, Kinouchi Y, Masamune A. Reduced antiviral seropositivity among patients with inflammatory bowel disease treated with immunosuppressive agents. Scand J Gastroenterol. 2023;58:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Kochhar GS, Mohan BP, Khan SR, Chandan S, Kassab LL, Ponnada S, Desai A, Caldera F, Dulai PS, Farraye FA. Hepatitis-B Vaccine Response in Inflammatory Bowel Disease Patients: A Systematic Review and Meta-analysis. Inflamm Bowel Dis. 2021;27:1610-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Cekic C, Aslan F, Kirci A, Gümüs ZZ, Arabul M, Yüksel ES, Vatansever S, Yurtsever SG, Alper E, Ünsal B. Evaluation of factors associated with response to hepatitis B vaccination in patients with inflammatory bowel disease. Medicine (Baltimore). 2015;94:e940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Namazova-baranova LS, Fedoseenko MV, Baranov AA. New Horizons of National Immunization Calendar. Vopr Sovr Pediatr. 2019;18:13-30. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Cleveland NK, Rodriquez D, Wichman A, Pan I, Melmed GY, Rubin DT. Many Inflammatory Bowel Disease Patients Are Not Immune to Measles or Pertussis. Dig Dis Sci. 2016;61:2972-2976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 15. | Benchimol EI, Tse F, Carroll MW, deBruyn JC, McNeil SA, Pham-Huy A, Seow CH, Barrett LL, Bessissow T, Carman N, Melmed GY, Vanderkooi OG, Marshall JK, Jones JL. Canadian Association of Gastroenterology Clinical Practice Guideline for Immunizations in Patients With Inflammatory Bowel Disease (IBD)-Part 1: Live Vaccines. Gastroenterology. 2021;161:669-680.e0. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Watts A, Bennett WE, Molleston JP, Gupta SK, Croffie JM, Waseem S, McFerron BA, Steiner SJ, Kumar S, Vanderpool CP, Hon EC, Bozic MA, Subbarao GC, Pfefferkorn MD. Incidence of Low Seroimmunity to Hepatitis B Virus in Children With Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr. 2017;65:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Jiang HY, Wang SY, Deng M, Li YC, Ling ZX, Shao L, Ruan B. Immune response to hepatitis B vaccination among people with inflammatory bowel diseases: A systematic review and meta-analysis. Vaccine. 2017;35:2633-2641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Pratt PK Jr, David N, Weber HC, Little FF, Kourkoumpetis T, Patts GJ, Weinberg J, Farraye FA. Antibody Response to Hepatitis B Virus Vaccine is Impaired in Patients With Inflammatory Bowel Disease on Infliximab Therapy. Inflamm Bowel Dis. 2018;24:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Jansen MH, Rondaan C, Legger G, Minden K, Uziel Y, Toplak N, Maritsi D, van den Berg M, Berbers G, Bruijning P, Egert Y, Normand C, Bijl M, Foster H, Kone-Paut I, Wouters C, Ravelli A, Elkayam O, Wulffraat NM, Heijstek MW. Efficacy, Immunogenicity and Safety of Vaccination in Pediatric Patients With Autoimmune Inflammatory Rheumatic Diseases (pedAIIRD): A Systematic Literature Review for the 2021 Update of the EULAR/PRES Recommendations. Front Pediatr. 2022;10:910026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Welzel T, Kuemmerle-Deschner J, Sluka C, Carlomagno R, Cannizzaro Schneider E, Kaiser D, Hofer M, Hentgen V, Woerner A. Vaccination completeness in children with rheumatic diseases: A longitudinal, observational multicenter cohort study in Switzerland. Front Pediatr. 2022;10:993811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Groot N, Heijstek MW, Wulffraat NM. Vaccinations in paediatric rheumatology: an update on current developments. Curr Rheumatol Rep. 2015;17:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/