Published online Dec 9, 2025. doi: 10.5409/wjcp.v14.i4.109476
Revised: June 3, 2025
Accepted: August 26, 2025
Published online: December 9, 2025
Processing time: 172 Days and 6.9 Hours
Gestational diabetes mellitus (GDM) is a metabolic condition caused by chronic insulin resistance during pregnancy, affecting millions of women globally and causing significant health concerns. Its consequences are far-reaching, associated with poor feto-maternal outcomes. GDM has serious implications on metabolic health in both mother and child. Early diagnosis and management of GDM are crucial to prevent related consequences. Traditional diagnostic and predictive biomarkers for GDM, including oral glucose tolerance test, adiponectin, resistin, etc., have limitations. Recent advances in research have identified novel bioma
Core Tip: Gestational diabetes mellitus (GDM) is a prevalent metabolic condition caused by chronic insulin resistance during pregnancy, affecting millions of women worldwide. It leads to poor maternal and neonatal outcomes. Traditional diagnostic and predictive biomarkers have limitations. Recent research has identified novel biomarkers, such as microRNAs, cell-free DNA, exosomes, glycolytic intermediates, inflammatory and metabolic biomarkers, amino acids, lipids, and gut microbiota metabolites, which offer promising alternatives for early diagnosis and prediction. These biomarkers may pave the way for personalized medicine, improving the prediction of GDM and related pediatric outcomes.
- Citation: Gautam T, Shamsad A, Singh R, Banerjee M. Emerging biomarkers for gestational diabetes mellitus and related pediatric outcomes. World J Clin Pediatr 2025; 14(4): 109476
- URL: https://www.wjgnet.com/2219-2808/full/v14/i4/109476.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i4.109476
Gestational diabetes mellitus (GDM), characterized by hyperglycemia that emerges or is first identified during pregnancy, is more prevalent alongside the onset of type 2 diabetes mellitus (T2DM) and obesity. GDM is a prevailing condition of pregnancy, affecting around 14% of women worldwide, with variations in frequency according to geo
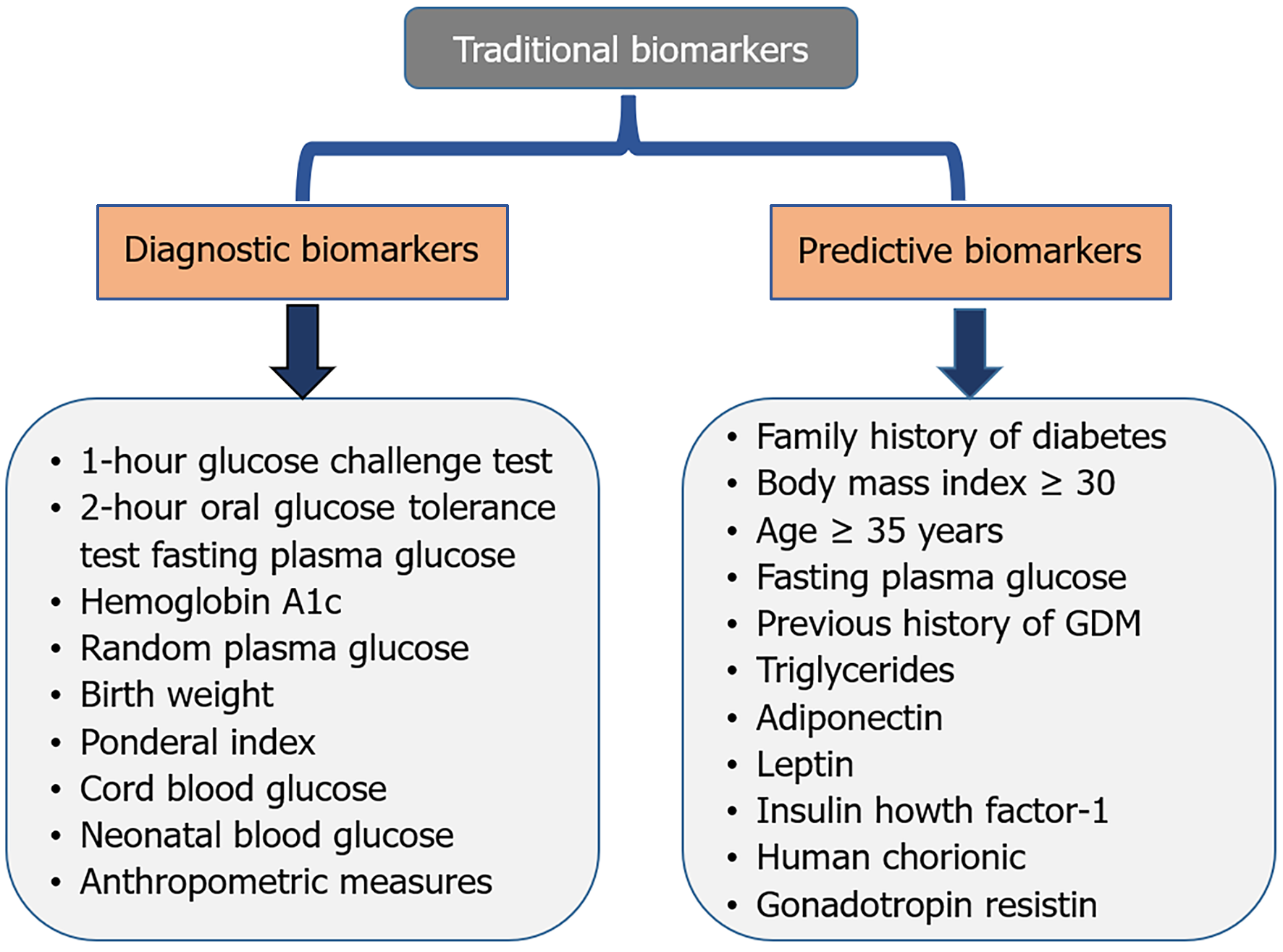
Traditional diagnostic and predictive biomarkers for GDM include glucose challenge test (GCT), fasting plasma glucose (FPG), hemoglobin A1c (HbA1c), oral glucose tolerance test (OGTT), family history, previous history of GDM, body mass index (BMI), age, human chorionic gonadotropin (hCG), insulin growth factor-1 (IGF-1), adiponectin, leptin, resistin, etc. (Figure 1)[8,9].
Diagnostic biomarkers for GDM help to identify pregnant women at risk. Precise diagnosis facilitates immediate intervention for managing GDM, hence lowering the risk of problems for both the mother and the infant[10]. Common diagnostic biomarkers include GCT, FPG, OGTT, HbA1c, and random plasma glucose for the diagnosis of GDM (Table 1)[4,11-24].
| Biomarker | Description | Diagnostic criteria | Sensitivity | Specificity | Notes | Ref. |
| Traditional biomarkers for GDM diagnosis | ||||||
| 1-hour glucose challenge test | Measures glucose concentrations 1-hour post-glucose administration | ≥ 140 mg/dL (7.8 mmol/L) | 70%-90% | 70%-80% | May require additional testing for diagnosis | Hosier et al[11], Ayesha et al[12], Zhao et al[13], Moon and Jang[14] |
| 2-hour oral glucose tolerance test | Measures glucose concentrations 2-hour post-glucose administration | ≥ 153 mg/dL (8.5 mmol/L) | 80%-90% | 90%-95% | Considered a gold standard for GDM diagnosis | Gautam et al[4], Jamieson et al[15], Prior et al[16], Madugalle et al[17] |
| Fasting plasma glucose | Measures glucose levels after an overnight fast | ≥ 92 mg/dL (5.1 mmol/L) | 70%-80% | 80%-90% | May miss cases of GDM with normal fasting glucose | Ayesha et al[12], Beunen et al[18], Hasan et al[19] |
| Hemoglobin A1c | Evaluate mean glucose concentrations throughout the preceding 2-3 months | ≥ 6.5% (48 mmol/moL) | 40%-60% | 80%-90% | May not be sensitive enough for GDM diagnosis | Valadan et al[20], Xiang et al[21], Tripathy et al[22] |
| Random plasma glucose | Monitors glucose levels at any time | ≥ 200 mg/dL (11.1 mmol/L) with symptoms | Variable | Variable | Not recommended as a standalone diagnostic test | Jamieson et al[15], Huhn et al[23], Shaarbaf Eidgahi et al[24] |
Diagnostic biomarkers are useful to find out the GDM-associated pediatric outcomes by helping to identify the risk to children. Precise diagnosis facilitates immediate intervention for managing and lowering the risk of problems for both the mother and the infant. Common diagnostic biomarkers[4,25,26] include birth weight, ponderal index, cord blood glucose, neonatal blood glucose, and anthropometric measures are biomarkers for the diagnosis of pediatric outcomes of GDM (Table 2)[23,27-38].
| Biomarker | Pediatric outcome | Description | Diagnostic criteria | Sensitivity | Specificity | Notes | Ref. |
| Traditional biomarkers for pediatric outcomes in GDM | |||||||
| Birth weight | Macrosomia | Measures fetal growth | > 4000 g | 70%-80% | 80%-90% | Linked with an increased risk of obesity and metabolic diseases | Huhn et al[23], Zheng et al[27], Hu[28], Bernea et al[29] |
| Ponderal index | Fetal growth restriction | Measures fetal growth | < 2.2 or > 2.8 | 60%-70% | 70%-80% | May indicate fetal growth restriction or macrosomia | Powel et al[30], Teshome et al[31], Mirabelli et al[32] |
| Cord blood glucose | Neonatal hypoglycemia | Measures glucose levels at birth | < 40 mg/dL (2.2 mmol/L) | 80%-90% | 90%-95% | Associated with increased risk of neonatal hypoglycemia | Wang et al[33], Shao et al[34] |
| Neonatal blood glucose | Neonatal hypoglycemia | Measures glucose levels after birth | < 40 mg/dL (2.2 mmol/L) | 80%-90% | 90%-95% | May require monitoring and treatment | Kariniemi et al[35], García-Moreno et al[36] |
| Anthropometric measures | Childhood obesity | Measures body mass index | > 95th percentile | 70%-80% | 80%-90% | Linked with an increased risk of obesity and metabolic diseases | Huang et al[37], Castaneda et al[38] |
The development of new biomarkers can predict pediatric outcomes in GDM. Biomarkers can help tailor treatment strategies to individual patients and improve pediatric outcomes. Long-term follow-up investigations are necessary to elucidate the correlation between GDM and pediatric outcomes.
Predictive biomarkers for GDM and pediatric outcomes can help identify high-risk pregnancies and improve feto-maternal outcomes[39]. Predictive Biomarkers for GDM include age, family history of diabetes, previous history of GDM, BMI, FPG, triglyceride, adiponectin, leptin, resistin, hCG, and IGF-1 (Table 3)[26,31,40-61].
| Biomarker | Description | Predictive value | Sensitivity | Specificity | Notes | Ref. |
| Family history of diabetes | Diabetes prevalence among first-degree relatives | High risk | 50%-70% | 70%-80% | Important risk factor for GDM | Monod et al[40], Basil et al[41] |
| Body mass index ≥ 30 | Obesity | Moderate risk | 50%-70% | 70%-80% | Increased risk of GDM with obesity | Teshome et al[31], Chen et al[42], Antoniou et al[43] |
| Age ≥ 35 years | Advanced maternal age | Moderate risk | 40%-60% | 60%-70% | Increased risk of GDM with advancing age | Deng et al[44], Guarga Montori et al[45], Machado-Gédéon et al[46] |
| Previous history of GDM | Previous diagnosis of GDM in a prior pregnancy | High risk | 70%-90% | 80%-90% | Strong predictor of GDM in subsequent pregnancies | Liang et al[47], Kouhkan et al[48] |
| Fasting plasma glucose in early pregnancy | Measures glucose levels in early pregnancy | High risk | 70%-80% | 80%-90% | May predict GDM development | Wang et al[49], Tong et al[50] |
| Triglycerides | Measures triglyceride levels | Moderate risk | 50%-60% | 70%-80% | May predict GDM development | Liang et al[47], Shi et al[51] |
| Adiponectin | Measures adiponectin levels, an adipokine involved in glucose regulation | Moderate risk | 60%-70% | 70%-80% | May predict GDM development | Mihai et al[52], Muntean et al[53], Moyce Gruber et al[54], Moyce Gruber et al[55] |
| Leptin | Measures leptin levels, an adipokine involved in energy balance | Moderate risk | 50%-60% | 70%-80% | May predict GDM development | Chico-Barba et al[56] |
| HCG | Measures elevated HCG levels | Moderate risk | 50%-60% | 70%-80% | May predict GDM development | Mandić-Marković et al[26], Kantomaa et al[57] |
| IGF-1 | Measures IGF-1 levels, which may be associated with insulin resistance | Moderate risk | 50%-60% | 70%-80% | May predict GDM development | Tumminia et al[58], Alekseenkova et al[59] |
| Resistin | Measures resistin levels, which may be associated with insulin resistance | Moderate risk | 50%-60% | 70%-80% | May predict GDM development | Ferdousi et al[60], Saucedo et al[61] |
Fetal ultrasound and maternal glucose levels are valuable tools and important biomarkers for monitoring fetal development and predicting pediatric outcomes[5,39]. Fetal ultrasound enables early detection of potential issues, allowing for timely interventions and informing personalized care plans for pregnant women and their babies[62]. Whereas, maternal glucose levels may impact long-term pediatric outcomes, such as obesity and metabolic disorders[63]. Regular monitoring of maternal glucose levels is crucial for managing diabetes during pregnancy and inform personalized care plans that can reduce the risk of adverse pediatric outcomes (Table 4)[5,29,39,64-67].
| Predictive biomarkers for pediatric outcomes of GDM | Ref. | |
| Fetal ultrasound | Valuable tool for monitoring the fetal development and pediatric outcomes. Detect growth restriction, growth patterns, congenital anomalies, and fetal macrosomia. Predict neurodevelopmental outcomes, such as cerebral palsy. Enables early detection of potential issues. Timely interventions and informing personalized care plans | Rathnayake et al[5], Parsaei et al[39], David et al[64], Sodje[65], Debbink et al[66] |
| Maternal glucose level | High level can lead to fetal macrosomia, increasing the risk of birth injuries and complications. Impact long-term pediatric outcomes, such as obesity and metabolic disorders. Regular monitoring is crucial for managing GDM and inform personalized care plans for pregnant women | Rathnayake et al[5], Bernea et al[29], Parsaei et al[39], Ornoy et al[67] |
Predictive biomarkers can identify high-risk pregnancies early, enable timely intervention to manage GDM and improve pediatric outcomes, and reduce the risk of complications for both mother and baby.
Traditional biomarkers have certain limitations due to their limited sensitivity, specificity, and effectiveness of interventions, late diagnosis, and may not detect all cases of GDM. There is a need for additional biomarkers that can detect GDM earlier and more accurately. Research is ongoing to develop new biomarkers that may improve diagnostic accuracy and predictive value.
GDM results in an elevated risk of maternal cardiovascular disease (CVD) and T2DM, along with macrosomia and complications during delivery for the newborns[67,68]. The newborn has a prolonged risk of obesity, T2DM, and metabolic disorders[4,69,70]. Premature delivery, macrosomia, neonatal hypoglycemia, and shoulder dystocia are many short-term effects for the infant that may arise from GDM (Table 5)[4,67,69-78]. Oral therapies for GDM management, like glyburide and metformin, have potential; nonetheless, concerns persist over their long-term safety for both the mother and the child[4]. New oral drugs and innovative biomarkers for the early diagnosis and prediction of GDM may be beneficial in mitigating its effects and occurrence. By highlighting gaps in the research, it recommends for further investigations and a multidisciplinary approach, finally aiming to improve the treatment strategies, early diagnosis, predictive approaches, and care for women with GDM.
| Short-term outcomes | Ref. | |
| Macrosomia | Excessive birth weight, which can increase the risk of complications during delivery | Gautam et al[4], Ornoy et al[67], Chen et al[71] |
| Neonatal hypoglycemia | Low blood sugar in newborns, which can be a complication of GDM | Gautam et al[4], Nakshine and Jogdand[69], Corcillo et al[72] |
| Respiratory distress syndrome | Difficulty breathing in newborns, which can be associated with GDM | Gautam et al[4], Chulkov et al[73], Cahen-Peretz et al[74] |
| Birth injuries | Elevated risk of birth injuries, including shoulder dystocia, linked to macrosomia | Gautam et al[4], Nakshine and Jogdand[69], Chen et al[71] |
| Long-term outcomes | ||
| Obesity | Children of mothers with GDM are at an increased risk of having obese in later life | Gautam et al[4], Ornoy et al[67], Nakshine and Jogdand[69], Semnani-Azad et al[70], Mantzorou et al[75] |
| T2DM | Children of mothers with GDM are at an elevated risk of getting T2DM in later life | Gautam et al[4], Nakshine and Jogdand[69], Semnani-Azad et al[70], Corcillo et al[72] |
| Metabolic syndrome | Children of mothers with GDM may have an elevated risk of developing metabolic syndrome, a group of disorders that enhance the risk of cardiovascular disease, cerebrovascular accidents, and T2DM | Gautam et al[4], Nakshine and Jogdand[69], Semnani-Azad et al[70], Corcillo et al[72], Pathirana et al[76] |
| Neurodevelopmental outcomes | Certain studies indicate that children delivered to mothers with GDM may have an elevated risk of neurodevelopmental delays or abnormalities | Ornoy et al[67], Hirata et al[77], Kim et al[78] |
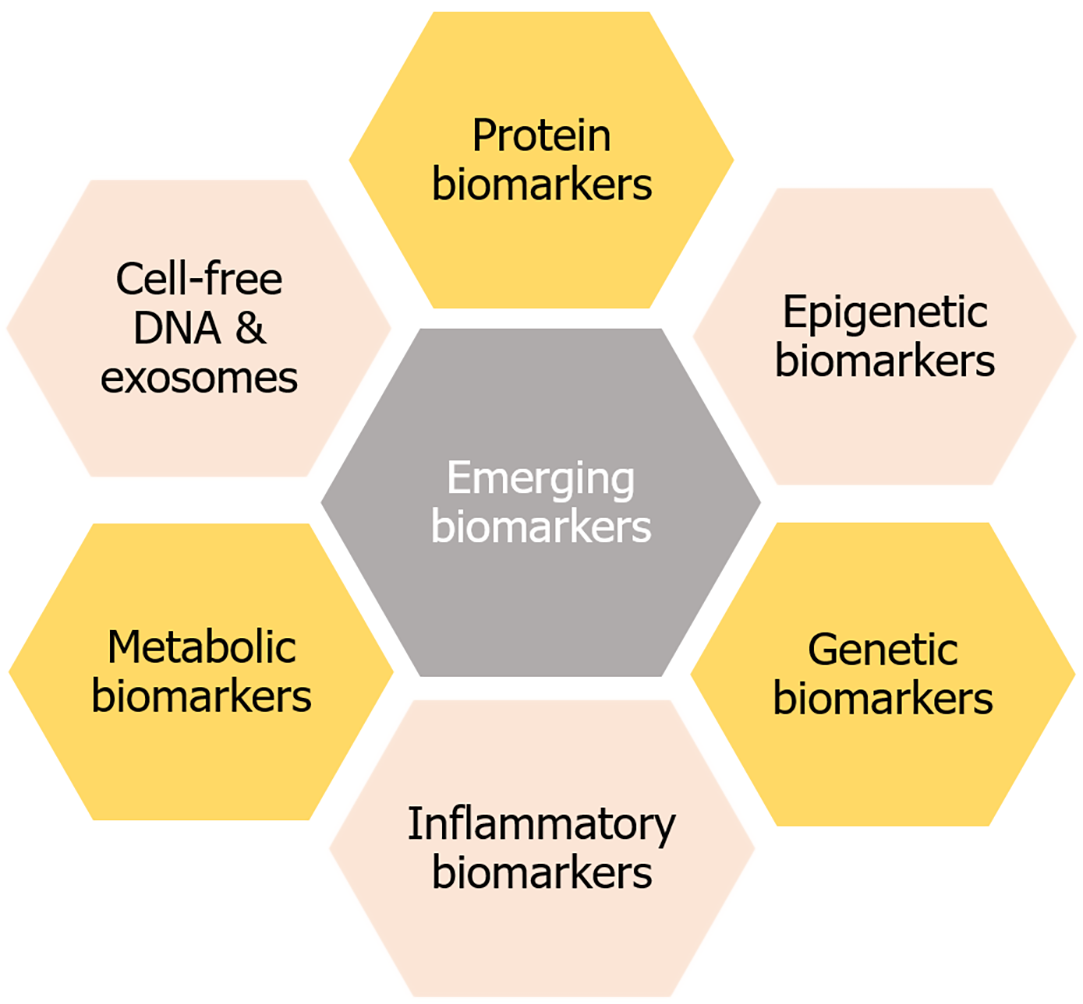
Emerging biomarkers for GDM are being researched to improve diagnosis and prediction. Here are some potential biomarkers that enable early intervention and better outcomes for mothers and babies by improving the diagnosis and prediction strategies. However, more research is needed to validate their effectiveness and practicality in clinical settings (Figure 2).
Epigenetic biomarkers play a crucial role in understanding the pathophysiology of GDM[3]. Epigenetic biomarkers are involved in glucose regulation, insulin signaling, cholesterol efflux, regulating the cell cycle, and energy metabolism[79]. They can aid in early detection, prediction of GDM risk and complications, enable timely interventions, and develop personalized treatment strategies to facilitate preventive measures. Epigenetic biomarkers include DNA methylation, miRNA, and histone modification. Among them, miRNA could be a promising emerging biomarker[80]. Emerging microRNAs (miRNAs) as biomarkers for GDM are being researched for their potential in early detection and diagnosis. miRNAs play a crucial role in regulating gene expression, and their dysregulation has been linked to various diseases[81,82], including GDM[3,63]. The relationship between miRNA biomarkers and GDM is still being researched to investigate the underlying mechanisms and to refine their application in GDM diagnosis and treatment. Further studies are needed to fully understand their potential and effectiveness in diverse populations (Table 6)[83-104].
| MicroRNAs | Expression level | Potential role | Ref. |
| MiR-16 | Upregulated | Involved in insulin resistance and glucose metabolism | Hocaoglu et al[83], Alimoradi et al[84], Sørensen et al[85] |
| MiR-29a | Upregulated | Involved in glucose metabolism and insulin signaling | Hocaoglu et al[83], Li et al[86], Dalgaard et al[87] |
| MiR-335 | Upregulated | Regulating insulin resistance and pancreatic islet β-cell secretion | Gezginci-Oktayoglu et al[88], Li et al[89] |
| MiR-132 | Upregulated | Associated with insulin resistance and GDM | Carr et al[90], Sałówka and Martinez-Sanchez[91] |
| MiR-222 | Need for further research | Contributing to estrogen-induced insulin resistance | He et al[92], Valerio et al[93] |
| MiR-17 | Upregulated | Involved in insulin signaling pathways | Ejaz et al[94], Jiang et al[95] |
| MiR-19a | Upregulated | Enhance β-cell function by targeting suppressor of cytokine signaling 3, contribute to pancreatic β-cell dysfunction and insulin resistance | Holvoet[96], Du et al[97] |
| MiR-19b | Upregulated | Involved in insulin signaling pathways | Chao et al[98], He et al[99] |
| MiR-20a | Upregulated | Involved in insulin signaling pathways | He et al[92], da Silva et al[100] |
| MiR-223 | Downregulated | Associated with insulin sensitivity and GDM | He et al[92], da Silva et al[100], Masete et al[101] |
| MiR-330-3p | Upregulated | Potential biomarker for GDM diagnosis | He et al[92], da Silva et al[100] |
| MiR-144 | Downregulated | Associated with insulin sensitivity and GDM | Juchnicka et al[102], Zhang et al[103] |
| MiR-195 | Upregulated | Associated with insulin resistance and GDM | He et al[92], da Silva et al[100], Masete et al[101] |
| MiR-21 | Upregulated | Potential biomarker for GDM prediction | Silva et al[100], Kunysz et al[104] |
Genetic biomarkers are characteristics derived from DNA or RNA that reflect normal or abnormal biological processes, including disease conditions. Genetic biomarkers include single-nucleotide polymorphisms (SNPs), copy number variations (CNVs), restriction fragment length polymorphisms, microsatellites, and mutations. They identify, diagnose, and monitor illnesses, predict therapeutic responses, and comprehend disease aetiology[105]. Among them, SNPs could be a promising genetic biomarker for the prediction and diagnosis of GDM and associated pediatric outcomes (Table 7)[106-119].
| Gene | Variant/mutation | Potential role | Ref. |
| TCF7 L2 | Rs7903146 (C/T) | Associated with GDM risk and insulin secretion | Shalabi et al[106], Fang et al[107] |
| KCNQ1 | Rs2237892 (C/T) | Regulating insulin secretion and glucose metabolism | Ortega-Contreras et al[108], Alshammary et al[109] |
| CDKAL1 | Rs7754840 (C/G) | Associated with GDM risk and insulin secretion | Mahdizade et al[110], Wang et al[111] |
| HHEX | Rs1111875 (C/T) | Associated with GDM risk and pancreatic function | Zeng et al[112], Xie et al[113] |
| SLC30A8 | Rs13266634 (C/T) | Regulating zinc transport and insulin secretion | Xie et al[113], Zeng et al[114] |
| GCK | Rs1799884 (A/T) | Regulating glucose sensing and insulin secretion | Hu et al[115], Popova et al[116] |
| MTNR1B | Rs10830963 (G/C) | Associated with GDM risk and insulin secretion | Chen et al[117], Bai et al[118] |
| PPARγ | Rs1801282 (C/G) | Regulating glucose metabolism, insulin sensitivity, and adipogenesis | Chen et al[117], Wu et al[119] |
Inflammatory biomarkers represent inflammation, the body's innate reaction to an injury or disease. These indicators may consist of proteins, enzymes, or other chemicals released into the blood circulation or tissues during the inflammatory process[120]. Inflammatory biomarkers can help to identify the presence and severity of inflammation in various diseases, including infections, autoimmune conditions, and even some cancers[121]. Inflammatory biomarkers may alter insulin signalling pathways, resulting in insulin resistance and elevated blood glucose levels, which are fundamental characteristics of GDM[3,4,63]. They may be used to monitor disease progression, assess therapy efficacy, and anticipate the outcomes of certain disorders. They serve as an essential tool in research to elucidate the fundamental causes of disease and to develop novel therapeutics (Table 8)[21,61,122-130].
| Biomarker | Description | Expression level | Potential role | Ref. |
| IL-6 | Pro-inflammatory cytokine | Upregulated | Associated with insulin resistance and GDM | Srivastava et al[122], Hosseini et al[123], Tutar et al[124] |
| IL-1β | Pro-inflammatory cytokine | Upregulated | Involved in inflammation and GDM | Zgutka et al[125], Yousif et al[126] |
| IL-8 | Pro-inflammatory cytokine | Upregulated | Associated with inflammation and GDM | Vilotić et al[127] |
| CRP | Acute-phase protein | Upregulated | Exacerbating insulin resistance and glucose metabolism dysregulation | Quansah et al[128], Chakraborty et al[129] |
| Tumor necrosis factor-alpha | Pro-inflammatory cytokine | Upregulated | Involved in inflammation and GDM | Saucedo et al[61], Hosseini et al[123] |
| High-sensitivity CRP | Sensitive marker of inflammation | Upregulated | Potential biomarker for GDM prediction | Xiang et al[21], Tao et al[130] |
Amino acid metabolism (tryptophan, phenylalanine, histidine, and branched-chain amino acids), fatty acid (omega-6-fatty acids) and glycerolipid metabolism, and Inositol phosphate metabolism are the metabolic pathways that can play a role in insulin resistance[131–133]. Other metabolites, such as bile acids, steroids, acylcarnitine, and N-Acetylproline, may also be associated with GDM (Table 9)[61,132,134-161]. Emerging metabolic biomarkers for GDM are being researched for their potential in early diagnosis and prediction, improving patient outcomes. Metabolic biomarkers can help tailor treatment strategies in individual patients.
| Biomarker | Amino acid/metabolic pathway | Potential role | Ref. |
| Metabolic biomarkers | |||
| Amino acid metabolites | |||
| Branched-chain amino acids | Leucine, isoleucine, valine | Associated with insulin resistance and GDM | Li et al[134], Ademolu[135] |
| Tryptophan | Tryptophan | Involved in various metabolic processes and altered tryptophan metabolism have been linked to insulin resistance and GDM | Zhou et al[136], Özdemir et al[137] |
| Phenylalanine | Phenylalanine | Elevated phenylalanine levels have been linked to insulin resistance and GDM | Yang et al[138] |
| Histidine | Histidine | Modified histidine concentrations have been associated with insulin resistance and GDM | Zhou et al[136], Yang et al[138] |
| Glutamic acid | Glutamic acid | Potential biomarker for GDM diagnosis | Yang et al[138], Kong et al[139] |
| N-Acetylproline | Proline | Altered N-Acetylproline levels have been linked to insulin resistance and GDM | Aleidi et al[140] |
| Alanine | Alanine | Potential biomarker for GDM diagnosis | Zhou et al[141], Spanou et al[142] |
| Tyrosine | Tyrosine | Potential biomarker for GDM prediction | Yang et al[138], Spanou et al[142] |
| Arginine | Arginine | Employed in glucose metabolism and GDM | Spanou et al[142], Zhan et al[143] |
| Glycine | Glycine | Associated with insulin sensitivity and GDM | Yang et al[138], Zhou et al[141], Spanou et al[142] |
| Lipid metabolites | |||
| Triacylglycerols | Insulin resistance-related lipid metabolites | Potential biomarkers for GDM prediction | Zhang et al[144], Balachandiran et al[145] |
| Inositol phosphate | Insulin resistance and glucose metabolism | Insulin resistance and glucose intolerance in GDM may be exacerbated by altered IP3 signaling | Pillai et al[146], Mazzera et al[147] |
| Glycerolipid | GDM and insulin resistance may exacerbate by elevated triacylglycerols | Predictive biomarker for GDM risk | Zhan et al[143], Zhang et al[144] |
| Omega-6-fatty acid | Changes in amino acid levels might be a factor in GDM and insulin resistance | Predictive biomarker for GDM risk | Egalini et al[132], Hosseinkhani et al[148] |
| Phosphatidylcholines | Phospholipids involved in glucose metabolism | Potential biomarkers for GDM diagnosis | Zhou et al[141], Wang et al[149] |
| Sphingomyelins | Sphingolipids associated with insulin resistance | Potential biomarkers for GDM prediction | Fakhr et al[150], Pinto et al[151] |
| Lysophosphatidylcholines | Phospholipid metabolites associated with GDM | Potential biomarkers for GDM diagnosis | Zhou et al[141], Zhan et al[143], Hung et al[152] |
| Ceramides | Sphingolipids associated with insulin resistance | Potential biomarkers for GDM prediction | Mustaniemi et al[153], Lantzanaki et al[154] |
| Free fatty acids | Lipid metabolites associated with insulin resistance | Potential biomarkers for GDM prediction | Kong et al[139], Hosseinkhani et al[148] |
| Acylcarnitines | Fatty acid metabolites associated with insulin resistance | Potential biomarkers for GDM prediction | Zhan et al[143], Pinto et al[151] |
| Glycolytic intermediates | |||
| 1,5-Anhydroglucitol | Glycolysis | Marker of glycemic control and GDM diagnosis | Xu et al[155], Lin et al[156] |
| Pyruvate | Glycolysis | Potential biomarker for GDM diagnosis | Zhou et al[141], Bhushan et al[157] |
| Glyceraldehyde-3-phosphate | Glycolysis | Potential biomarker for GDM prediction | Saucedo et al[61] |
| Lactate | Glycolysis | Associated with insulin resistance and GDM | Zhou et al[141] |
| Fructose-1,6-bisphosphate | Glycolysis | Potential biomarker for GDM prediction | Wei et al[158], Wang et al[159] |
| Glucose-6-phosphate | Glycolysis | Potential biomarker for GDM diagnosis | Wei et al[158] |
| Phosphoenolpyruvate | Glycolysis | Potential biomarker for GDM prediction | Lai et al[160], Xu et al[161] |
Cell-free DNA (cfDNA) refers to DNA that is freely circulating in the bloodstream or other bodily fluids, rather than being contained within cells[162]. The cfDNA typically consists of short fragments of DNA, often around 160-180 base pairs in length. The cfDNA can originate from various sources, including apoptotic cells, necrotic cells, and actively released DNA[163]. The cfDNA is relatively stable in circulation, allowing for its detection and analysis. The cfDNA can be used for non-invasive prenatal testing, such as screening for fetal aneuploidies[164]. The cfDNA can be used to detect genetic or epigenetic changes associated with various diseases. The cfDNA is emerging as a potential biomarker for GDM. The cfDNA can be isolated from maternal blood or other bodily fluids and may enable early detection of GDM. The cfDNA can provide information on specific genetic or epigenetic changes associated with GDM[165]. Changes in cfDNA fragment size and concentration may also be indicative of GDM.
Exosomes carry specific proteins, lipids, and nucleic acids that can reflect the biological state of the mother and fetus[166]. Exosomes can be isolated from maternal blood or other bodily fluids, making them a potential non-invasive biomarker. Exosomes may enable early detection of GDM[167]. Exosomes are emerging as potential biomarkers for GDM. Specific miRNAs carried by exosomes may be associated with GDM[168]. Exosomal proteins, such as insulin signaling proteins, may be altered in GDM. Exosomes may also carry other molecules, such as lipids and metabolites that can serve as biomarkers[166,169]. Exosomal biomarkers may improve the diagnosis and prediction of GDM and help tailor treatment strategies for individual patients. Exosomes may also be used to monitor disease progression and treatment response.
Protein biomarkers are protein molecules that serve as indicators of specific processes or conditions inside an organism, their altered levels play a crucial role in the pathophysiology of various diseases such as cancer, CVDs, and other me
| Biomarker | Protein function | Association with GDM | Potential role | Clinical utility | Advantages | Ref. |
| Fetuin-A | Insulin resistance | Elevated levels of fetuin-A have been associated with GDM | Fetuin-A may play a role in insulin resistance and glucose metabolism during pregnancy | Fetuin-A may be used to identify women at high risk of developing GDM, allowing for early intervention and prevention strategies | Fetuin-A is a relatively stable protein that can be measured in maternal serum or plasma, making it a potentially useful biomarker for GDM | Bogdanet et al[6], Ruszała et al[7], Wu et al[170], Cai et al[171] |
| IGFBP-1 | Glucose metabolism | Decreased levels of IGFBP-1 have been associated with GDM | IGFBP-1 may play a role in glucose metabolism and insulin sensitivity during pregnancy | IGFBP-1 may be used to identify women at high risk of developing GDM, allowing for early intervention and prevention strategies | IGFBP-1 is a relatively stable protein that can be measured in maternal serum or plasma, making it a potentially useful biomarker for GDM | Alekseenkova et al[59], Hivert et al[172], Hong et al[173], Martín-Estal et al[174] |
| SHBG | Sex hormone transport | Low levels of SHBG have been associated with GDM | Associated with insulin resistance and GDM | SHBG may be used to identify women at high risk of developing GDM, allowing for early intervention and prevention strategies | SHBG is a relatively stable protein that can be measured in maternal serum or plasma, making it a potentially useful biomarker for GDM | Bruno et al[175], Sharmin et al[176], Liu et al[177] |
| RBP4 | Retinol transport | Elevated levels of RBP4 have been associated with GDM | RBP4 may play a role in insulin resistance and glucose metabolism during pregnancy | RBP4 may be used to identify women at high risk of developing GDM, allowing for early intervention and prevention strategies | RBP4 is a relatively stable protein that can be measured in maternal serum or plasma, making it a potentially useful biomarker for GDM | Kučerová et al[178], Leca et al[179], Mousavi et al[180] |
| Afamin | Vitamin E transport | Elevated levels of Afamin have been associated with GDM | Afamin may play a role in glucose metabolism and insulin sensitivity during pregnancy | Afamin may be used to identify women at high risk of developing GDM, allowing for early intervention and prevention strategies | Afamin is a relatively stable protein that can be measured in maternal serum or plasma, making it a potentially useful biomarker for GDM | Eroğlu et al[181], Atakul et al[182], Wang et al[183] |
| Fibronectin | Cell adhesion and migration | Altered levels of fibronectin have been associated with GDM | Fibronectin may play a role in placental development and function, and its dysregulation may contribute to GDM | Fibronectin may be used to identify women at high risk of developing GDM, allowing for early intervention and prevention strategies | Fibronectin is a relatively stable protein that can be measured in maternal serum or plasma, making it a potentially useful biomarker for GDM | Ruszała et al[7], Karoutsos et al[184] |
| Betatrophin | Glucose and lipid metabolism | Altered levels of Betatropin have been associated with GDM | Betatropin may play a role in regulating glucose metabolism and insulin sensitivity during pregnancy | Betatropin may be used to identify women at high risk of developing GDM, allowing for early intervention and prevention strategies | Betatropin is a relatively novel biomarker that may provide new insights into GDM pathophysiology | Xu et al[185], Guo et al[186], Kirlangic et al[187], Melekoglu and Celik[188] |
| PAPP-A | Regulating placental function and fetal growth | Altered levels of PAPP-A have been associated with GDM | PAPP-A may play a role in regulating insulin-like growth factor bioavailability and glucose metabolism during pregnancy | PAPP-A may be used to identify women at high risk of developing GDM, allowing for early intervention and prevention strategies | PAPP-A is a well-established biomarker for placental function and may provide insights into GDM pathophysiology | Conover and Oxvig[189], Yanachkova et al[190] |
| SFlt-1 | Regulating angiogenesis and vascular function | Altered levels of sFlt-1 have been associated with GDM | SFlt-1 may play a role in regulating angiogenesis and vascular function in GDM | SFlt-1 may be used to identify women at high risk of developing GDM, allowing for early intervention and prevention strategies | SFlt-1 is a well-established biomarker for preeclampsia and may provide insights into GDM pathophysiology | Joshi et al[191], Liao et al[192], Gul Kara et al[193] |
| PlGF | Promoting angiogenesis, regulating placental development and enhancing vascular function | Altered levels of PlGF have been associated with GDM | PlGF may play a role in regulating angiogenesis and placental function in GDM | PlGF may be used to identify women at high risk of developing GDM, allowing for early intervention and prevention strategies | PlGF is a well-established biomarker for placental function and may provide insights into GDM pathophysiology | Yanachkova et al[190], Bolatai et al[194] |
| Apo | Lipid metabolism | Altered levels of Apo (e.g., ApoA1, ApoB) have been associated with GDM | Apo may play a role in regulating lipid metabolism and insulin sensitivity in GDM | Apo may be used to identify women at high risk of developing GDM, allowing for early intervention and prevention strategies | Apo are well-established biomarkers for cardiovascular disease and may provide insights into GDM pathophysiology | Balachandiran et al[145], Bernea et al[169] |
Further research is needed to validate the utility of emerging proteomic biomarkers for GDM. The development of point-of-care tests for proteomic biomarkers may improve accessibility and convenience. Combining proteomic biomarkers with clinical risk factors may improve predictive value.
The gut microbiota-gut-brain axis is a bidirectional communication pathway that plays a crucial role in glucose metabolism and insulin sensitivity, influencing GDM development. Gut microbiota produces metabolites that signal to the brain, and neural pathways facilitate bidirectional communication between the gut and brain. Neurotransmitters and hormones involved in glucose homeostasis, regulated by gut microbes. Gut dysbiosis plays a major role in the development of insulin resistance, exacerbating the inflammation and oxidative stress, which leads to GDM. Alterations in the gut microbiota play a significant role in the development of GDM. Key microbial metabolites such as butyrate (a short-chain fatty acid) and mevalonate may act as intermediaries linking gut dysbiosis to impaired glucose metabolism and hormonal imbalance during pregnancy. Reduced butyrate levels are associated with inflammation and insulin resistance, while altered mevalonate metabolism may affect the synthesis of pregnancy-related hormones. Additionally, increased levels of lipopolysaccharides derived from gut bacteria can trigger systemic inflammation, further contributing to insulin resistance.
These findings suggest that microbial metabolites could serve as early biomarkers and therapeutic targets for the prevention and management of GDM[195,196]. Importantly, GDM-induced dysbiosis not only affects maternal health but also alters the fetal gut microbial environment, potentially impacting fetal neurodevelopment. These findings underscore the gut microbiota as a central player in GDM pathophysiology and support the potential of microbial metabolites as early biomarkers and intervention targets to improve outcomes for both mother and child[197,198]. Emerging research indicates that GDM disrupts maternal metabolic health and adversely affects both maternal and fetal microbiomes, leading to gut dysbiosis. This microbial imbalance has been associated with an increased risk of neurodevelopmental disorders in offspring, including attention-deficit/hyperactivity disorder, schizophrenia, intellectual disabilities, anxiety, and depression. The underlying mechanisms involve changes in microbial composition and function, which may impact fetal brain development through the microbiota–gut–brain axis[197]. Gut microbiota profiles and exploring the potential of gut microbiota modulation may improve GDM management and outcomes and serve as emerging potential biomarkers for GDM risk assessment.
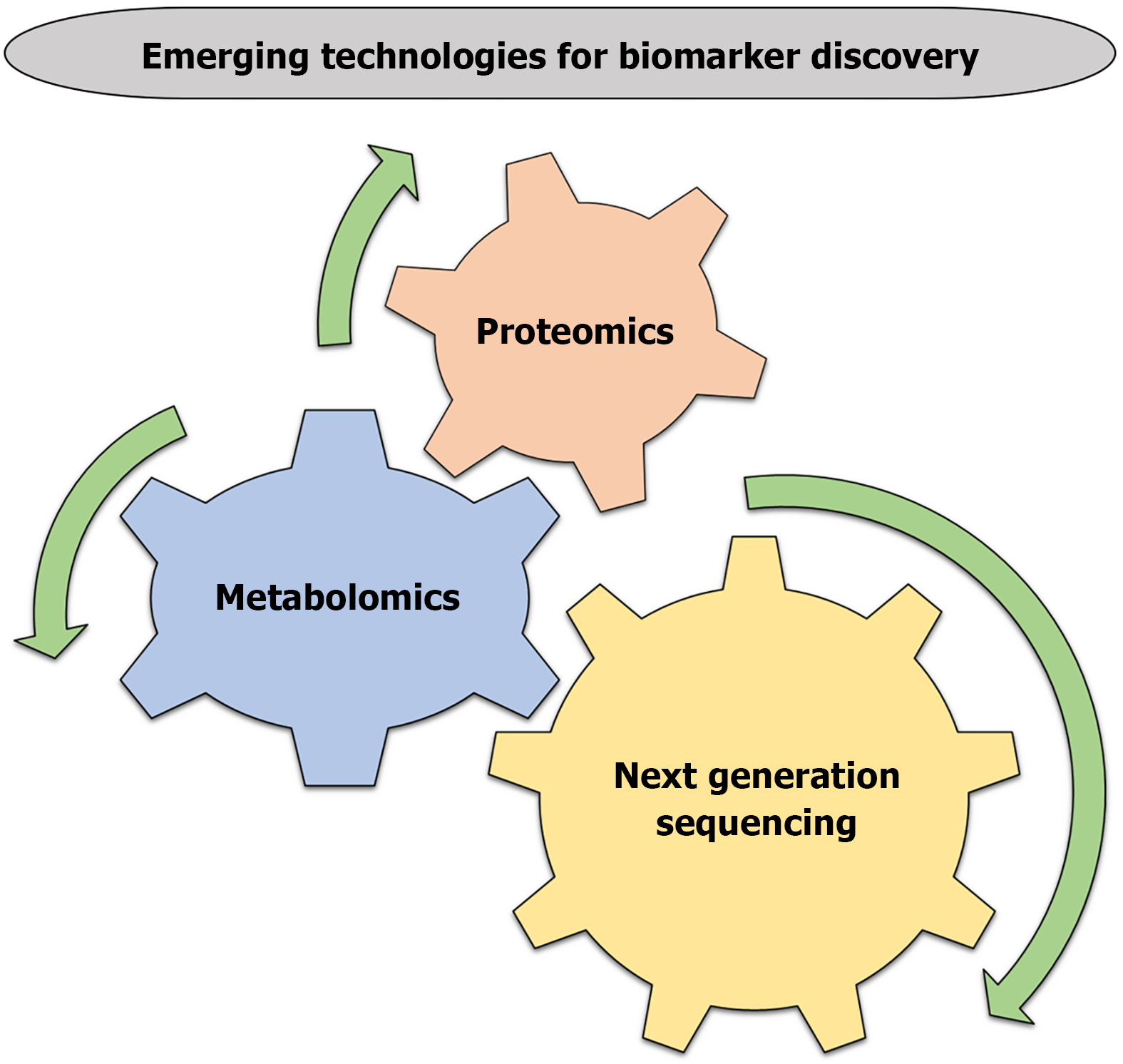
Next-generation sequencing (NGS) is a powerful tool for discovering novel biomarkers for GDM. NGS allows for the analysis of entire genomes, transcriptomes, and epigenomes, providing a comprehensive understanding of the genetic and molecular mechanisms underlying GDM[199,200]. Whole-genome sequencing, whole-exome sequencing, RNA sequencing, and epigenome sequencing are fundamental approaches for NGS[201,202]. NGS can identify genetic variants such as SNPs[203] and CNVs[204], genes that are differentially expressed, discover novel transcripts, such as long non-coding RNAs and circular RNAs, and epigenetic biomarkers, such as DNA methylation and histone modification, providing insights into the molecular mechanisms underlying the disease[205-207]. NGS may enable early detection, personalized medicine for GDM women. NGS is still being researched for discovering the biomarkers, and more studies are needed to fully understand their potential (Figure 3).
Metabolomics, the study of small molecules in biological systems, is a promising approach for discovering biomarkers for GDM. By analyzing metabolic profiles, researchers can identify potential biomarkers that may aid in the early detection, diagnosis, and treatment of GDM[208]. Targeted and untargeted metabolomics approaches are involved; targeted metabolomics mainly focuses on specific metabolites or pathways, such as glucose, amino acids, or lipids. Whereas, un
Proteomics, the study of proteins and their functions, is a promising approach for discovering biomarkers for GDM. Mass spectrometry, two-dimensional gel electrophoresis, and protein microarrays are tools which involved in proteomics approaches[213,214]. Proteomics may enable early identification of GDM risk, allowing for timely interventions and helping to tailor treatment strategies to individual patients[215]. The relationship between proteomics and GDM is still being researched, and more studies are needed to fully understand their potential.
Emerging biomarkers for GDM must undergo validation to ensure their accuracy, reliability, and therapeutic value[62,216]. For the validation, those steps are followed by the researchers: (1) Discovery phase (various omics approaches, such as genomics, proteomics, and metabolomics are used to identify the potential biomarkers); (2) Verification phase (identified biomarkers validate in a separate cohort of patients to confirm their association with GDM); (3) Validation phase (performance of the biomarkers evaluate in a larger, more diverse population to assess their sensitivity, specificity, and predictive value); and (4) Clinical validation (clinical utility of the biomarkers assess in real-world settings, including their impact on patient outcomes and healthcare costs)[217].
Validation criteria for novel biomarkers are important, and are as follows[217,218]: (1) Sensitivity (ability of the biomarker to detect GDM women); (2) Specificity (ability of the biomarker to exclude women without the GDM); (3) Positive predictive value (proportion of patients with a positive biomarker result who have GDM); (4) Negative pre
Collaborative research is essential to validate emerging biomarkers for GDM. Standardization of analytical methods and criteria for biomarker validation will facilitate comparison of results across studies.
Development of biomarker panels for GDM involves combining multiple biomarkers to improve diagnostic accuracy and predictive value. Biomarker panels can improve the sensitivity and specificity of GDM diagnosis, provide a more accurate prediction of GDM risk and outcomes, and help to expand the personalized medicine approaches[5,24]. Metabolic, inflammatory, proteomic, and genetic/epigenetic biomarker panels are important and involved in GDM early prediction and diagnosis strategies[219,220]. Discovery, verification, panel development, and clinical validation are the key steps for the development of biomarker panels[221,222]. Collaboration among researchers, standardization of methods, and integration with clinical risk factors are the major topics that need further research.
Personalized medicine for GDM entails customizing treatment plans for each patient according to their distinct traits. Emerging diagnostic and predictive biomarkers play a crucial role in this approach[215,223]. Biomarkers and epigenetic changes reflecting insulin resistance, chronic inflammation, and altered placental function highlight the complex interplay between genetic and environmental factors[224]. Emerging diagnostic and predictive biomarkers help to identify women at high risk for GDM. Biomarkers may complement existing clinical risk factors to identify women at high risk of developing GDM and may help to distinguish women who may benefit from targeted strategies to reduce GDM development[225]. Personalized medicine approaches may lead to better outcomes for mothers and offspring by tailoring treatment to individual needs[4].
GDM is a complex condition that requires early detection and prediction to prevent adverse outcomes for both mother and fetus. This review has identified and described emerging biomarkers that could potentially replace the traditional biomarkers and be used to both predict and diagnose GDM. Various emerging biomarkers, including metabolic, protein, genetic, epigenetic, inflammatory biomarkers, and gut microbiota metabolites, hold promise for improving GDM diagnosis and prediction. These biomarkers may enable healthcare providers to identify high-risk patients, monitor disease progression, and develop personalized treatment plans. Further research is needed to validate these biomarkers and translate them into clinical practice, ultimately improving outcomes for women with GDM.
The authors are thankful to the Department of Biotechnology, New Delhi, and Maulana Azad National Fellowship, University Grants Commission, New Delhi, respectively, for fellowships. They are also grateful to the Department of Biotechnology, Department of Science and Technology, Indian Council of Medical Research, New Delhi, and Centre of Excellence, Higher Education, Government of Uttar Pradesh, Molecular and Human Genetics Laboratory, Department of Zoology, University of Lucknow, India.
| 1. | Sweeting A, Hannah W, Backman H, Catalano P, Feghali M, Herman WH, Hivert MF, Immanuel J, Meek C, Oppermann ML, Nolan CJ, Ram U, Schmidt MI, Simmons D, Chivese T, Benhalima K. Epidemiology and management of gestational diabetes. Lancet. 2024;404:175-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 206] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 2. | Shamsad A, Gautam T, Singh R, Banerjee M. Association of mRNA expression and polymorphism of antioxidant glutathione-S-transferase (GSTM1 and GSTT1) genes with the risk of Gestational Diabetes Mellitus (GDM). Gene. 2024;928:148746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Shamsad A, Gautam T, Singh R, Banerjee M. Genetic and epigenetic alterations associated with gestational diabetes mellitus and adverse neonatal outcomes. World J Clin Pediatr. 2025;14:99231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Gautam T, Shamsad A, Singh R, Raza Baqri SS, Banerjee M. Pharmacological therapy for Gestational Diabetes Mellitus: A comprehensive overview. Obes Med. 2025;54:100587. [DOI] [Full Text] |
| 5. | Rathnayake H, Han L, da Silva Costa F, Paganoti C, Dyer B, Kundur A, Singh I, Holland OJ. Advancement in predictive biomarkers for gestational diabetes mellitus diagnosis and related outcomes: a scoping review. BMJ Open. 2024;14:e089937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Bogdanet D, Reddin C, Murphy D, Doheny HC, Halperin JA, Dunne F, O'Shea PM. Emerging Protein Biomarkers for the Diagnosis or Prediction of Gestational Diabetes-A Scoping Review. J Clin Med. 2021;10:1533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Ruszała M, Pilszyk A, Niebrzydowska M, Kimber-Trojnar Ż, Trojnar M, Leszczyńska-Gorzelak B. Novel Biomolecules in the Pathogenesis of Gestational Diabetes Mellitus 2.0. Int J Mol Sci. 2022;23:4364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Inthavong S, Jatavan P, Tongsong T. Predictive Utility of Biochemical Markers for the Diagnosis and Prognosis of Gestational Diabetes Mellitus. Int J Mol Sci. 2024;25:11666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 9. | Thakur A, Agrawal S, Chakole S, Wandile B. A Critical Review of Diagnostic Strategies and Maternal Offspring Complications in Gestational Diabetes Mellitus. Cureus. 2023;15:e51016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Fan YT, Wang XH, Wang Q, Luo XT, Cao J. Perspective on the nursing management for gestational diabetes mellitus: A perspective. Medicine (Baltimore). 2025;104:e41862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Hosier H, Lundsberg LS, Culhane J, Partridge C, Son M. Association between isolated abnormal 1-hour glucose challenge test and adverse pregnancy outcomes: a retrospective review from an urban tertiary care center in the United States. BMC Pregnancy Childbirth. 2025;25:145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Ayesha K, Ajmal A, Akram A, Sadaf J, Usman F, Hafeez S. Diagnostic Accuracy of Fasting Blood Sugar and Oral Glucose Challenge Test for Gestational Diabetes Mellitus. Pak J Health Sci. 2025. [DOI] [Full Text] |
| 13. | Zhao G, Murphy KE, Berger H, Shah BR, Halperin I, Barrett J, Melamed N. The screening performance of glucose challenge test for gestational diabetes in twin pregnancies: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2022;35:7590-7600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Moon JH, Jang HC. Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications. Diabetes Metab J. 2022;46:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 208] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 15. | Jamieson EL, Dimeski G, Flatman R, Hickman PE, Ross Dallas Jones G, V Marley J, David McIntyre H, McNeil AR, Nolan CJ, Potter JM, Sweeting A, Ward P, Williams P, Rita Horvath A. Oral glucose tolerance test to diagnose gestational diabetes mellitus: Impact of variations in specimen handling. Clin Biochem. 2023;115:33-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Prior AK, Dolin CD, Bender W, Durnwald CP, Hamm RF. Effects of Implementing a Routine Postpartum Fasting Blood Glucose on the Completion of the Gold Standard 2-Hour Oral Glucose Tolerance Test in Gestational Diabetics. Am J Perinatol. 2024;41:2284-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Madugalle MEMYDB, Rathnayake RMC, Rajayohan T, Kotigala DS, Ruwanpathirana SA. Analysing the Diagnostic Potency of Oral Glucose Tolerance Test at 20 Weeks of Gestation in High-Risk Pregnancies. Open J Obstet Gynecol. 2024;14:1873-1895. [DOI] [Full Text] |
| 18. | Beunen K, Neys A, Van Crombrugge P, Moyson C, Verhaeghe J, Vandeginste S, Verlaenen H, Vercammen C, Maes T, Dufraimont E, Roggen N, De Block C, Jacquemyn Y, Mekahli F, De Clippel K, Van Den Bruel A, Loccufier A, Laenen A, Devlieger R, Mathieu C, Benhalima K. Fasting plasma glucose level to guide the need for an OGTT to screen for gestational diabetes mellitus. Acta Diabetol. 2022;59:381-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Hasan MR, Sultana N, Panthi S, Hasan M, Jahan S, Aktar Y, Hasanat MA. Fasting Plasma Glucose as a Primary Screening Test for the Diagnosis of Gestational Diabetes mellitus. JMCWH. 2025;21:43-51. [DOI] [Full Text] |
| 20. | Valadan M, Bahramnezhad Z, Golshahi F, Feizabad E. The role of first-trimester HbA1c in the early detection of gestational diabetes. BMC Pregnancy Childbirth. 2022;22:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Xiang LL, Chen C, Wang QY, Zhu YT, Chen YJ, Zeng Y. Impact of inflammatory factors, hemoglobin A1c, and platelet parameters in gestational diabetes mellitus. Arch Gynecol Obstet. 2023;307:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Tripathy S, Murugesan A, Natarajan K, Ramraj B, Mohapatra S. Early screening biomarker HbA1c and Hematocrit for gestational diabetes mellitus. Clin Epidemiol Glob Health. 2022;13:100945. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Huhn EA, Göbl CS, Fischer T, Todesco Bernasconi M, Kreft M, Kunze M, Vogt DR, Dölzlmüller E, Jaksch-Bogensperger H, Heldstab S, Eppel W, Husslein P, Ochsenbein Kölble N, Richter A, Bäz E, Winzeler B, Hoesli I. Sensitivity, specificity, and diagnostic accuracy of WHO 2013 criteria for diagnosis of gestational diabetes mellitus in low risk early pregnancies: international, prospective, multicentre cohort study. BMJ Med. 2023;2:e000330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 24. | Shaarbaf Eidgahi E, Nasiri M, Kariman N, Safavi Ardebili N, Salehi M, Kazemi M, Zayeri F. Diagnostic accuracy of first and early second trimester multiple biomarkers for prediction of gestational diabetes mellitus: a multivariate longitudinal approach. BMC Pregnancy Childbirth. 2022;22:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Ortiz-Martínez M, González-González M, Martagón AJ, Hlavinka V, Willson RC, Rito-Palomares M. Recent Developments in Biomarkers for Diagnosis and Screening of Type 2 Diabetes Mellitus. Curr Diab Rep. 2022;22:95-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 26. | Mandić-Marković V, Dobrijević Z, Robajac D, Miljuš G, Šunderić M, Penezić A, Nedić O, Ardalić D, Miković Ž, Radojičić O, Mandić M, Mitrović J. Biochemical Markers in the Prediction of Pregnancy Outcome in Gestational Diabetes Mellitus. Medicina (Kaunas). 2024;60:1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 27. | Zheng W, Huang W, Liu C, Yan Q, Zhang L, Tian Z, Yuan X, Li G. Weight gain after diagnosis of gestational diabetes mellitus and its association with adverse pregnancy outcomes: a cohort study. BMC Pregnancy Childbirth. 2021;21:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Hu G. Are insulin sensitivity and β-cell function associated with adverse pregnancy outcomes among women with gestational diabetes? Chin Med J (Engl). 2022;135:2521-2524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Bernea EG, Uyy E, Mihai DA, Ceausu I, Ionescu-Tirgoviste C, Suica VI, Ivan L, Antohe F. New born macrosomia in gestational diabetes mellitus. Exp Ther Med. 2022;24:710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 30. | Powel JE, Zantow EW, Bialko MF, Farley LG, Lawlor ML, Mullan SJ, Vricella LK, Tomlinson TM. Predictive index for adverse perinatal outcome in pregnancies complicated by fetal growth restriction. Ultrasound Obstet Gynecol. 2023;61:367-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Teshome AA, Li Q, Garoma W, Chen X, Wu M, Zhang Y, Zhang X, Lin L, Wang H, Yang X, Hao L, Sun G, Han W, Chen X, Xiong G, Yang N. Gestational diabetes mellitus, pre-pregnancy body mass index and gestational weight gain predicts fetal growth and neonatal outcomes. Clin Nutr ESPEN. 2021;42:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Mirabelli M, Chiefari E, Tocci V, Greco E, Foti D, Brunetti A. Gestational diabetes: Implications for fetal growth, intervention timing, and treatment options. Curr Opin Pharmacol. 2021;60:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 33. | Wang Y, Liu H, Zhang L, Wang X, Wang M, Chen Z, Zhang F. Umbilical artery cord blood glucose predicted hypoglycemia in gestational diabetes mellitus and other at-risk newborns. BMC Endocr Disord. 2023;23:277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | Shao H, Lan Y, Qian Y, Chen R, Peng L, Hua Y, Wang X. Effect of later cord clamping on umbilical cord blood gas in term neonates of diabetic mothers: a randomized clinical trial. BMC Pediatr. 2022;22:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 35. | Kariniemi K, Vääräsmäki M, Männistö T, Mustaniemi S, Kajantie E, Eteläinen S, Keikkala E; Finnish Gestational Diabetes [FinnGeDi] study group. Neonatal outcomes according to different glucose threshold values in gestational diabetes: a register-based study. BMC Pregnancy Childbirth. 2024;24:271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | García-Moreno RM, Benítez-Valderrama P, Barquiel B, González Pérez-de-Villar N, Hillman N, Lora Pablos D, Herranz L. Efficacy of continuous glucose monitoring on maternal and neonatal outcomes in gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials. Diabet Med. 2022;39:e14703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 37. | Huang Y, Zhang L, Ainiwan D, Alifu X, Cheng H, Qiu Y, Zhou H, Liu H, Yu Y. Breastfeeding, Gestational Diabetes Mellitus, Size at Birth and Overweight/Obesity in Early Childhood. Nutrients. 2024;16:1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 38. | Castaneda C, Marsden K, Maxwell T, Ten Eyck P, Kuwaye D, Kenne KA, Merryman AS, Steffen HA, Swartz SR, Merrill AE, Krasowski MD, Jackson JB, Rysavy MB. Prevalence of maternal obesity at delivery and association with maternal and neonatal outcomes. J Matern Fetal Neonatal Med. 2022;35:8544-8551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Parsaei M, Dashtkoohi M, Noorafrooz M, Haddadi M, Sepidarkish M, Mardi-Mamaghani A, Esmaeili M, Shafaatdoost M, Shizarpour A, Moini A, Pirjani R, Hantoushzadeh S. Prediction of gestational diabetes mellitus using early-pregnancy data: a secondary analysis from a prospective cohort study in Iran. BMC Pregnancy Childbirth. 2024;24:849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Monod C, Kotzaeridi G, Linder T, Eppel D, Rosicky I, Filippi V, Tura A, Hösli I, Göbl CS. Prevalence of gestational diabetes mellitus in women with a family history of type 2 diabetes in first- and second-degree relatives. Acta Diabetol. 2023;60:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 41. | Basil B, Mba IN, Myke-Mbata BK, Adebisi SA, Oghagbon EK. A first trimester prediction model and nomogram for gestational diabetes mellitus based on maternal clinical risk factors in a resource-poor setting. BMC Pregnancy Childbirth. 2024;24:346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Chen Y, Wan K, Gong Y, Zhang X, Liang Y, Wang X, Feng P, He F, Zhou R, Yang D, Jia H, Cheng G, Shimokawa T. Assessing the relationship between pregravid body mass index and risk of adverse maternal pregnancy and neonatal outcomes: prospective data in Southwest China. Sci Rep. 2021;11:7591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 43. | Antoniou MC, Quansah DY, Mühlberg S, Gilbert L, Arhab A, Schenk S, Lacroix A, Stuijfzand B, Horsch A, Puder JJ. Maternal and fetal predictors of anthropometry in the first year of life in offspring of women with GDM. Front Endocrinol (Lausanne). 2023;14:1144195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 44. | Deng L, Ning B, Yang H. Association between gestational diabetes mellitus and adverse obstetric outcomes among women with advanced maternal age: A retrospective cohort study. Medicine (Baltimore). 2022;101:e30588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 45. | Guarga Montori M, Álvarez Martínez A, Luna Álvarez C, Abadía Cuchí N, Mateo Alcalá P, Ruiz-Martínez S. Advanced maternal age and adverse pregnancy outcomes: A cohort study. Taiwan J Obstet Gynecol. 2021;60:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 46. | Machado-Gédéon A, Badeghiesh A, Baghlaf H, Dahan MH. Adverse pregnancy, delivery and neonatal outcomes across different advanced maternal ages: A population-based retrospective cohort study. Eur J Obstet Gynecol Reprod Biol X. 2023;17:100180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 47. | Liang JW, Chen MX, Hu XA, Zhou M, Zhang Y, Wang LL. Potential Biomarkers in Early Pregnancy for Predicting Gestational Diabetes Mellitus and Adverse Pregnancy Outcomes. Clin Lab. 2021;67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Kouhkan A, Najafi L, Malek M, Baradaran HR, Hosseini R, Khajavi A, Khamseh ME. Gestational diabetes mellitus: Major risk factors and pregnancy-related outcomes: A cohort study. Int J Reprod Biomed. 2021;19:827-836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Wang C, Wei Y, Yang Y, Su R, Song G, Kong L, Yang H. Evaluation of the value of fasting plasma glucose in the first trimester for the prediction of adverse pregnancy outcomes. Diabetes Res Clin Pract. 2021;174:108736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Tong JN, Wu LL, Chen YX, Guan XN, Tian FY, Zhang HF, Liu K, Yin AQ, Wu XX, Prof JN. Fasting plasma glucose in the first trimester is related to gestational diabetes mellitus and adverse pregnancy outcomes. Endocrine. 2022;75:70-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 51. | Shi P, Tang J, Yin X. Association between second- and third-trimester maternal lipid profiles and adverse perinatal outcomes among women with GDM and non-GDM: a retrospective cohort study. BMC Pregnancy Childbirth. 2023;23:318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 52. | Mihai M, Vladut S, Sonia-Teodora L, Laura Mihaela S, Victoria N, Irina Elena M, Claudiu M. Correlation between Overweight, Obesity, Gestational Diabetes Mellitus, Adipokines (Adipolin and Adiponectin), and Adverse Pregnancy Outcomes: A Pilot Study. Medicina (Kaunas). 2024;60:1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 53. | Muntean M, Săsăran V, Luca ST, Suciu LM, Nyulas V, Mărginean C. Serum Levels of Adipolin and Adiponectin and Their Correlation with Perinatal Outcomes in Gestational Diabetes Mellitus. J Clin Med. 2024;13:4082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 54. | Moyce Gruber BL, Dolinsky VW. The Role of Adiponectin during Pregnancy and Gestational Diabetes. Life (Basel). 2023;13:301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 55. | Moyce Gruber BL, Cole LK, Xiang B, Fonseca MA, Klein J, Hatch GM, Doucette CA, Dolinsky VW. Adiponectin deficiency induces hepatic steatosis during pregnancy and gestational diabetes in mice. Diabetologia. 2022;65:733-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Chico-Barba G, Sámano R, Martínez-Rojano H, Morales-Hernández RM, Barrientos-Galeana E, Luna-Hidalgo A, Kaufer-Horwitz M, Obrador GT, Villa-Romero AR. Total Gestational Weight Gain Is Explained by Leptin and Body Fat, Regardless of Pre-Pregnancy Body Mass Index and Other Adipokines, in Mexican Adolescents. Nutrients. 2024;16:2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 57. | Kantomaa T, Vääräsmäki M, Gissler M, Ryynänen M, Nevalainen J. First trimester maternal serum PAPP-A and free β-hCG levels and risk of SGA or LGA in women with and without GDM. BMC Pregnancy Childbirth. 2024;24:580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 58. | Tumminia A, Scalisi NM, Milluzzo A, Ettore G, Vigneri R, Sciacca L. Maternal Diabetes Impairs Insulin and IGF-1 Receptor Expression and Signaling in Human Placenta. Front Endocrinol (Lausanne). 2021;12:621680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Alekseenkova EN, Babakov VN, Selkov SA, Di Renzo GC, Kogan IY, Kapustin RV. Maternal insulin-like growth factors and insulin-like growth factor-binding proteins for macrosomia prediction in diabetic and nondiabetic pregnancy: A prospective study. Int J Gynaecol Obstet. 2023;162:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 60. | Ferdousi T, Tofail T, Jahan S, Shil KK, Mahrukh H, Hasanat MA. Serum resistin increases in gestational diabetes but does not differ among various trimesters. Heliyon. 2025;11:e41085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 61. | Saucedo R, Valencia J, Moreno-González LE, Peña-Cano MI, Aranda-Martínez A, García Y, Díaz-Velázquez MF, Hernández-Valencia M. Maternal serum adipokines and inflammatory markers at late gestation and newborn weight in mothers with and without gestational diabetes mellitus. Ginekol Pol. 2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Estrela D, Santos RF, Masserdotti A, Silini A, Parolini O, Pinto IM, Cruz A. Molecular Biomarkers for Timely and Personalized Prediction of Maternal-Fetal Health Risk. Biomolecules. 2025;15:312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 63. | Shamsad A, Kushwah AS, Singh R, Banerjee M. Pharmaco-epi-genetic and patho-physiology of gestational diabetes mellitus (GDM): An overview. Health Sci Rev. 2023;7:100086. [DOI] [Full Text] |
| 64. | David AL, Spencer RN. Clinical Assessment of Fetal Well-Being and Fetal Safety Indicators. J Clin Pharmacol. 2022;62 Suppl 1:S67-S78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 65. | Sodje JDK. Fetal Growth Abnormalities: Intrauterine Growth Restriction and Macrosomia. In: Okonofua F, Balogun JA, Odunsi K, Chilaka VN, editors. Contemporary Obstetrics and Gynecology for Developing Countries. Berlin: Springer, 2021. [DOI] [Full Text] |
| 66. | Debbink MP, Son SL, Woodward PJ, Kennedy AM. Sonographic Assessment of Fetal Growth Abnormalities. Radiographics. 2021;41:268-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Ornoy A, Becker M, Weinstein-Fudim L, Ergaz Z. Diabetes during Pregnancy: A Maternal Disease Complicating the Course of Pregnancy with Long-Term Deleterious Effects on the Offspring. A Clinical Review. Int J Mol Sci. 2021;22:2965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 182] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 68. | Espinoza C, Fuenzalida B, Leiva A. Increased Fetal Cardiovascular Disease Risk: Potential Synergy Between Gestational Diabetes Mellitus and Maternal Hypercholesterolemia. Curr Vasc Pharmacol. 2021;19:601-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Nakshine VS, Jogdand SD. A Comprehensive Review of Gestational Diabetes Mellitus: Impacts on Maternal Health, Fetal Development, Childhood Outcomes, and Long-Term Treatment Strategies. Cureus. 2023;15:e47500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 70. | Semnani-Azad Z, Gaillard R, Hughes AE, Boyle KE, Tobias DK, Perng W; ADA/EASD PMDI. Predictors and risk factors of short-term and long-term outcomes among women with gestational diabetes mellitus (GDM) and their offspring: Moving toward precision prognosis? medRxiv. 2023;2023.04.14.23288199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Chen S, Persson M, Wang R, Dalman C, Lee BK, Karlsson H, Gardner RM. Random capillary glucose levels throughout pregnancy, obstetric and neonatal outcomes, and long-term neurodevelopmental conditions in children: a group-based trajectory analysis. BMC Med. 2023;21:260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 72. | Corcillo A, Quansah DY, Kosinski C, Benhalima K, Puder JJ. Impact of Risk Factors on Short and Long-Term Maternal and Neonatal Outcomes in Women With Gestational Diabetes Mellitus: A Prospective Longitudinal Cohort Study. Front Endocrinol (Lausanne). 2022;13:866446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 73. | Chulkov VS, Minina EE, Medvedeva LV. Maternal Diabetes, Respiratory and other Disorders in Offspring: Shortterm and Long-term Outcomes. Curr Respir Med Rev. 2023;19:85-92. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 74. | Cahen-Peretz A, Tsaitlin-Mor L, Abu-Ahmad W, Ben-Shushan MT, Levine H, Walfisch A. Long-term respiratory outcomes in early-term born offspring: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2022;4:100570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 75. | Mantzorou M, Papandreou D, Pavlidou E, Papadopoulou SK, Tolia M, Mentzelou M, Poutsidi A, Antasouras G, Vasios GK, Giaginis C. Maternal Gestational Diabetes Is Associated with High Risk of Childhood Overweight and Obesity: A Cross-Sectional Study in Pre-School Children Aged 2-5 Years. Medicina (Kaunas). 2023;59:455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 76. | Pathirana MM, Lassi ZS, Ali A, Arstall MA, Roberts CT, Andraweera PH. Association between metabolic syndrome and gestational diabetes mellitus in women and their children: a systematic review and meta-analysis. Endocrine. 2021;71:310-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 77. | Hirata K, Ueda K, Wada K, Ikehara S, Tanigawa K, Kimura T, Ozono K, Iso H; Japan Environment and Children’s Study Group. Long-term outcomes of children with neonatal transfer: the Japan Environment and Children's Study. Eur J Pediatr. 2022;181:2501-2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 78. | Kim HY, Cho GJ, Ahn KH, Hong SC, Oh MJ, Kim HJ. Short-term neonatal and long-term neurodevelopmental outcome of children born term low birth weight. Sci Rep. 2024;14:2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 79. | Ramos-Lopez O. Epigenetic Biomarkers of Metabolic Responses to Lifestyle Interventions. Nutrients. 2023;15:4251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Valencia-Ortega J, Saucedo R, Sánchez-Rodríguez MA, Cruz-Durán JG, Martínez EGR. Epigenetic Alterations Related to Gestational Diabetes Mellitus. Int J Mol Sci. 2021;22:9462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 81. | Das K, Rao LVM. The Role of microRNAs in Inflammation. Int J Mol Sci. 2022;23:15479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 113] [Reference Citation Analysis (0)] |
| 82. | Wu YL, Lin ZJ, Li CC, Lin X, Shan SK, Guo B, Zheng MH, Li F, Yuan LQ, Li ZH. Epigenetic regulation in metabolic diseases: mechanisms and advances in clinical study. Signal Transduct Target Ther. 2023;8:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 251] [Reference Citation Analysis (0)] |
| 83. | Hocaoglu M, Demirer S, Loclar Karaalp I, Kaynak E, Attar E, Turgut A, Karateke A, Komurcu-Bayrak E. Identification of miR-16-5p and miR-155-5p microRNAs differentially expressed in circulating leukocytes of pregnant women with polycystic ovary syndrome and gestational diabetes. Gynecol Endocrinol. 2021;37:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 84. | Alimoradi N, Firouzabadi N, Fatehi R. Metformin and insulin-resistant related diseases: Emphasis on the role of microRNAs. Biomed Pharmacother. 2021;139:111662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 85. | Sørensen AE, van Poppel MNM, Desoye G, Damm P, Simmons D, Jensen DM, Dalgaard LT; The DALI Core Investigator Group. The Predictive Value of miR-16, -29a and -134 for Early Identification of Gestational Diabetes: A Nested Analysis of the DALI Cohort. Cells. 2021;10:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 86. | Li J, Zhang Y, Ye Y, Li D, Liu Y, Lee E, Zhang M, Dai X, Zhang X, Wang S, Zhang J, Jia W, Zen K, Vidal-Puig A, Jiang X, Zhang CY. Pancreatic β cells control glucose homeostasis via the secretion of exosomal miR-29 family. J Extracell Vesicles. 2021;10:e12055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 87. | Dalgaard LT, Sørensen AE, Hardikar AA, Joglekar MV. The microRNA-29 family: role in metabolism and metabolic disease. Am J Physiol Cell Physiol. 2022;323:C367-C377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 88. | Gezginci-Oktayoglu S, Sancar S, Karatug-Kacar A, Bolkent S. Glucotoxicity suppresses function of pancreatic beta and duct cells via miR-335-targeted Runx2 and insulin-mediated mechanism. Protoplasma. 2025;262:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 89. | Li G, Zhang L. miR-335-5p aggravates type 2 diabetes by inhibiting SLC2A4 expression. Biochem Biophys Res Commun. 2021;558:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 90. | Carr ER, Higgins PB, McClenaghan NH, Flatt PR, McCloskey AG. MicroRNA regulation of islet and enteroendocrine peptides: Physiology and therapeutic implications for type 2 diabetes. Peptides. 2024;176:171196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 91. | Sałówka A, Martinez-Sanchez A. Molecular Mechanisms of Nutrient-Mediated Regulation of MicroRNAs in Pancreatic β-cells. Front Endocrinol (Lausanne). 2021;12:704824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 92. | He L, Wang X, Chen X. Unveiling the role of microRNAs in metabolic dysregulation of Gestational Diabetes Mellitus. Reprod Biol. 2024;24:100924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 93. | Valerio J, Barabash A, Garcia de la Torre N, De Miguel P, Melero V, Del Valle L, Moraga I, Familiar C, Durán A, Torrejón MJ, Diaz A, Jiménez I, Matia P, Rubio MA, Calle-Pascual AL. The Relationship between Serum Adipokines, miR-222-3p, miR-103a-3p and Glucose Regulation in Pregnancy and Two to Three Years Post-Delivery in Women with Gestational Diabetes Mellitus Adhering to Mediterranean Diet Recommendations. Nutrients. 2022;14:4712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 94. | Ejaz M, Usman SM, Amir S, Khan MJ. Holistic expression of miR-17-92 cluster in obesity, kidney diseases, cardiovascular diseases, and diabetes. Mol Biol Rep. 2023;50:6913-6925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 95. | Jiang Y, Wei L, Zhang H, Chen Y, Gao P, Zhang J, Zhou X, Zhu S, Du Y, Fang C, Li J, Feng L, He M, Wang S, Yu J. miR-17-5p Promotes Glucose Uptake of HTR8/SVneo Trophoblast Cells by Inhibiting TXNIP/NLRP3 Inflammasome Pathway. Diabetes Metab Syndr Obes. 2022;15:3361-3374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 96. | Holvoet P. Non-coding RNAS Related to Type 2 Diabetes. In: Holvoet P, editor. Non-coding RNAs at the Cross-Road of Cardiometabolic Diseases and Cancer. Berlin: Springer, 2021. [DOI] [Full Text] |
| 97. | Du R, Vidal-puig A, Rodriguez-cuenca S. MiRNAs provide mechanisms for integrated control of endocrine pancreas homeostasis and metabolic disease pathogenesis: a narrative review. ExRNA. 2022;4:24-24. [DOI] [Full Text] |
| 98. | Chao Y, Gu T, Zhang Z, Wu T, Wang J, Bi Y. The role of miRNAs carried by extracellular vesicles in type 2 diabetes and its complications. J Diabetes. 2023;15:838-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 99. | He X, Kuang G, Wu Y, Ou C. Emerging roles of exosomal miRNAs in diabetes mellitus. Clin Transl Med. 2021;11:e468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 100. | da Silva PHCM, Santos KF, da Silva L, da Costa CCP, Santos RDS, Reis AADS. MicroRNAs Associated with the Pathophysiological Mechanisms of Gestational Diabetes Mellitus: A Systematic Review for Building a Panel of miRNAs. J Pers Med. 2023;13:1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 101. | Masete M, Dias S, Malaza N, Adam S, Pheiffer C. A Big Role for microRNAs in Gestational Diabetes Mellitus. Front Endocrinol (Lausanne). 2022;13:892587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 102. | Juchnicka I, Kuźmicki M, Niemira M, Bielska A, Sidorkiewicz I, Zbucka-Krętowska M, Krętowski AJ, Szamatowicz J. miRNAs as Predictive Factors in Early Diagnosis of Gestational Diabetes Mellitus. Front Endocrinol (Lausanne). 2022;13:839344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 103. | Zhang L, Zhang T, Sun D, Cheng G, Ren H, Hong H, Chen L, Jiao X, Du Y, Zou Y, Wang L. Diagnostic value of dysregulated microribonucleic acids in the placenta and circulating exosomes in gestational diabetes mellitus. J Diabetes Investig. 2021;12:1490-1500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 104. | Kunysz M, Cieśla M, Darmochwał-Kolarz D. Evaluation of miRNA Expression in Patients with Gestational Diabetes Mellitus: Investigating Diagnostic Potential and Clinical Implications. Diabetes Metab Syndr Obes. 2024;17:881-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 105. | Jain KK. Molecular Diagnostics in Personalized Medicine. In: Jain KK, editor. Textbook of Personalized Medicine. Berlin: Springer, 2021. [DOI] [Full Text] |
| 106. | Shalabi TA, Amr KS, Shaker MM. Are single nucleotide polymorphisms rs7903146 and rs12255372 in transcription factor 7-like 2 gene associated with an increased risk for gestational diabetes mellitus in Egyptian women? J Genet Eng Biotechnol. 2021;19:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 107. | Fang C, Wu S, Zhang J, Tian Q, Zhang Z, Wu L. Impaired glucolipid metabolism in gestational diabetes mellitus with T variation of TCF7L2 rs7903146: A case–control study. Int J Diabetes Dev Ctries. 2024;44:182-189. [DOI] [Full Text] |
| 108. | Ortega-Contreras B, Armella A, Appel J, Mennickent D, Araya J, González M, Castro E, Obregón AM, Lamperti L, Gutiérrez J, Guzmán-Gutiérrez E. Pathophysiological Role of Genetic Factors Associated With Gestational Diabetes Mellitus. Front Physiol. 2022;13:769924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 109. | Alshammary AF, Al-Hakeem MM, Ali Khan I. Saudi Community-Based Screening Study on Genetic Variants in β-Cell Dysfunction and Its Role in Women with Gestational Diabetes Mellitus. Genes (Basel). 2023;14:924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 110. | Mahdizade AH, Bahreiny SS, Bastani M, Dabbagh MR, Aghaei M, Ali Malayeri F, Yousefifard A, Taghizadeh E. The influence of CDKAL1 (rs7754840) gene polymorphism on susceptibility to gestational diabetes mellitus in pregnant women: a systematic review and meta-analysis. Int J Diabetes Dev Ctries. 2024;44:3-12. [DOI] [Full Text] |
| 111. | Wang H, Li J, Liu J, Leng J, Li W, Yu Z, Tam CHT, Hu G, Ma RCW, Fang Z, Wang Y, Yang X. Interactions of CDKAL1 rs7747752 polymorphism and serum levels of L-carnitine and choline are related to increased risk of gestational diabetes mellitus. Genes Nutr. 2022;17:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 112. | Zeng Q, Liu J, Liu X, Liu N, Wu W, Watson RG, Zou D, Wei Y, Guo R. Association between genetic polymorphisms and gestational diabetes mellitus susceptibility in a Chinese population. Front Endocrinol (Lausanne). 2024;15:1397423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 113. | Xie W, Zhang L, Wang J, Wang Y. Association of HHEX and SLC30A8 Gene Polymorphisms with Gestational Diabetes Mellitus Susceptibility: A Meta-analysis. Biochem Genet. 2023;61:2203-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 114. | Zeng Q, Tan B, Han F, Huang X, Huang J, Wei Y, Guo R. Association of solute carrier family 30 A8 zinc transporter gene variations with gestational diabetes mellitus risk in a Chinese population. Front Endocrinol (Lausanne). 2023;14:1159714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 115. | Hu Y, Wang A, Yi K. GCK rs1799884 Polymorphism and Gestational Diabetes Mellitus: A System Review and Meta-Analysis. Clin Exp Obstet Gynecol. 2024;51. [DOI] [Full Text] |
| 116. | Popova PV, Klyushina AA, Vasilyeva LB, Tkachuk AS, Vasukova EA, Anopova AD, Pustozerov EA, Gorelova IV, Kravchuk EN, Li O, Pervunina TM, Kostareva AA, Grineva EN. Association of Common Genetic Risk Variants With Gestational Diabetes Mellitus and Their Role in GDM Prediction. Front Endocrinol (Lausanne). 2021;12:628582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 117. | Chen F, Fei X, Li M, Zhang Z, Zhu W, Zhang M, Chen X, Xu J, Zhang M, Shen Y, Du J. Associations of the MTNR1B rs10830963 and PPARG rs1801282 variants with gestational diabetes mellitus: A meta-analysis. Int J Diabetes Dev Ctries. 2023;43:1029-1042. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 118. | Bai Y, Tang L, Li L, Li L. The roles of ADIPOQ rs266729 and MTNR1B rs10830963 polymorphisms in patients with gestational diabetes mellitus: A meta-analysis. Gene. 2020;730:144302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 119. | Wu L, Song Y, Zhang Y, Liang B, Deng Y, Tang T, Ye YC, Hou HY, Wang CC. Novel Genetic Variants of PPARγ2 Promoter in Gestational Diabetes Mellitus and its Molecular Regulation in Adipogenesis. Front Endocrinol (Lausanne). 2020;11:499788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 120. | Chopra D, Shukla S, Rana P, Kamar MD, Gaur P, Bala M, Pathaniya D. Overview of Inflammation. In: Tripathi A, Dwivedi A, Gupta S, Poojan S, editors. Inflammation Resolution and Chronic Diseases. Berlin: Springer, 2024. [DOI] [Full Text] |
| 121. | Karra P, Winn M, Pauleck S, Bulsiewicz-Jacobsen A, Peterson L, Coletta A, Doherty J, Ulrich CM, Summers SA, Gunter M, Hardikar S, Playdon MC. Metabolic dysfunction and obesity-related cancer: Beyond obesity and metabolic syndrome. Obesity (Silver Spring). 2022;30:1323-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 122. | Srivastava N, Singh K, Singh N, Mahdi AA. Association between serum interleukin-6, leptin and insulin in gestational diabetes mellitus - a cross- sectional study. J Diabetes Metab Disord. 2023;22:639-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 123. | Hosseini E, Mokhtari Z, Salehi Abargouei A, Mishra GD, Amani R. Maternal circulating leptin, tumor necrosis factor-alpha, and interleukine-6 in association with gestational diabetes mellitus: a systematic review and meta-analysis. Gynecol Endocrinol. 2023;39:2183049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (1)] |
| 124. | Tutar D, Çintesun FNİ, Günenç O, Çetinkaya ÇD. The association of interleukin-6, interleukin-27, and body roundness index with gestational diabetes mellitus. J Obstet Gynaecol. 2022;42:1728-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 125. | Zgutka K, Tkacz M, Tomasiak P, Piotrowska K, Ustianowski P, Pawlik A, Tarnowski M. Gestational Diabetes Mellitus-Induced Inflammation in the Placenta via IL-1β and Toll-like Receptor Pathways. Int J Mol Sci. 2024;25:11409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 126. | Yousif AF, Al-salih RMH, Al-naser AH. Association between Gestational Diabetes and Proinflammatory Cytokine (IL-1β). Indian J Forensic Med Toxicol. 2021;15:160-169. [DOI] [Full Text] |
| 127. | Vilotić A, Nacka-Aleksić M, Pirković A, Bojić-Trbojević Ž, Dekanski D, Jovanović Krivokuća M. IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies. Int J Mol Sci. 2022;23:14574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 134] [Reference Citation Analysis (0)] |
| 128. | Quansah DY, Horsch A, Gilbert L, Donath MY, Puder JJ; MySweetheart research group. C-reactive protein during pregnancy and in the early postpartum predicts adverse metabolic health outcomes at 1 year postpartum in women with gestational diabetes. Cardiovasc Diabetol. 2023;22:291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 129. | Chakraborty M, Sil AK, Chakraborty S. Assessment of Serum Ferritin, CRP and Insulin Levels in First Trimester of Pregnancy as a Predictive Biomarker of Gestational Diabetes Mellitus: A Longitudinal Study. Int J Clin Diagn Res. 2022. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 130. | Tao J, Huang Y, Li Y, Dai W. Platelet-to-lymphocyte ratio and serum hsCRP levels in third trimester and adverse pregnancy outcomes in women with gestational diabetes mellitus. Sci Rep. 2023;13:20963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 131. | Arjmand B, Ebrahimi Fana S, Ghasemi E, Kazemi A, Ghodssi-Ghassemabadi R, Dehghanbanadaki H, Najjar N, Kakaii A, Forouzanfar K, Nasli-Esfahani E, Farzadfar F, Larijani B, Razi F. Metabolic signatures of insulin resistance in non-diabetic individuals. BMC Endocr Disord. 2022;22:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 132. | Egalini F, Guardamagna O, Gaggero G, Varaldo E, Giannone B, Beccuti G, Benso A, Broglio F. The Effects of Omega 3 and Omega 6 Fatty Acids on Glucose Metabolism: An Updated Review. Nutrients. 2023;15:2672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 133. | Wei R, Ning R, Han C, Wei S, Teng Y, Li L, Liu H, Hu S, Kang B, Xu H. Lipidomics analysis reveals new insights into the goose fatty liver formation. Poult Sci. 2023;102:102428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 134. | Li N, Li J, Wang H, Liu J, Li W, Yang K, Huo X, Leng J, Yu Z, Hu G, Fang Z, Yang X. Branched-Chain Amino Acids and Their Interactions With Lipid Metabolites for Increased Risk of Gestational Diabetes. J Clin Endocrinol Metab. 2022;107:e3058-e3065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 135. | Ademolu AB. Branched Chain Amino Acids and Gestational Diabetes Mellitus. J Clin Endocrinol Metab. 2022;107:e4322-e4323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 136. | Zhou Y, Zhao R, Lyu Y, Shi H, Ye W, Tan Y, Li R, Xu Y. Serum and Amniotic Fluid Metabolic Profile Changes in Response to Gestational Diabetes Mellitus and the Association with Maternal-Fetal Outcomes. Nutrients. 2021;13:3644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 137. | Özdemir M, Abusoglu S, Baldane S, Kıraç CO, Unlu A, Onmaz DE, Çelik M, Abusoglu G. Analyzing serum tryptophan metabolites in patients with gestational diabetes mellitus. Rev Rom Med Lab. 2023;31:251-262. [DOI] [Full Text] |
| 138. | Yang J, Wu J, Tekola-Ayele F, Li LJ, Bremer AA, Lu R, Rahman ML, Weir NL, Pang WW, Chen Z, Tsai MY, Zhang C. Plasma Amino Acids in Early Pregnancy and Midpregnancy and Their Interplay With Phospholipid Fatty Acids in Association With the Risk of Gestational Diabetes Mellitus: Results From a Longitudinal Prospective Cohort. Diabetes Care. 2023;46:722-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 139. | Kong X, Zhu Q, Dong Y, Li Y, Liu J, Yan Q, Huang M, Niu Y. Analysis of serum fatty acid, amino acid, and organic acid profiles in gestational hypertension and gestational diabetes mellitus via targeted metabolomics. Front Nutr. 2022;9:974902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 140. | Aleidi SM, Al Fahmawi H, AlMalki RH, Al Mogren M, Alwahsh M, Mujammami M, Costanzo M, Abdel Rahman A. Untargeted metabolomics profiling of gestational diabetes mellitus: insights into early diagnosis and metabolic pathway alterations. Front Mol Biosci. 2024;11:1485587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 141. | Zhou J, Yu J, Ren J, Ren Y, Zeng Y, Wu Y, Zhang Q, Xiao X. Association of maternal blood metabolomics and gestational diabetes mellitus risk: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2025;26:205-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 142. | Spanou L, Dimou A, Kostara CE, Bairaktari E, Anastasiou E, Tsimihodimos V. A Study of the Metabolic Pathways Affected by Gestational Diabetes Mellitus: Comparison with Type 2 Diabetes. Diagnostics (Basel). 2022;12:2881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 143. | Zhan Y, Wang J, He X, Huang M, Yang X, He L, Qiu Y, Lou Y. Plasma metabolites, especially lipid metabolites, are altered in pregnant women with gestational diabetes mellitus. Clin Chim Acta. 2021;517:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 144. | Zhang Z, Zhou Z, Li H. The role of lipid dysregulation in gestational diabetes mellitus: Early prediction and postpartum prognosis. J Diabetes Investig. 2024;15:15-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 145. | Balachandiran M, Bobby Z, Dorairajan G, Jacob SE, Gladwin V, Vinayagam V, Packirisamy RM. Placental Accumulation of Triacylglycerols in Gestational Diabetes Mellitus and Its Association with Altered Fetal Growth are Related to the Differential Expressions of Proteins of Lipid Metabolism. Exp Clin Endocrinol Diabetes. 2021;129:803-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 146. | Pillai RA, Islam MO, Selvam P, Sharma N, Chu AHY, Watkins OC, Godfrey KM, Lewis RM, Chan SY. Placental Inositol Reduced in Gestational Diabetes as Glucose Alters Inositol Transporters and IMPA1 Enzyme Expression. J Clin Endocrinol Metab. 2021;106:e875-e890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 147. | Mazzera I, Graziano A, Vizzielli G, Driul L. The role of inositols during pregnancies complicated by gestational diabetes mellitus: a narrative review. Gynecol Endocrinol. 2024;40:2411727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 148. | Hosseinkhani S, Dehghanbanadaki H, Aazami H, Pasalar P, Asadi M, Razi F. Association of circulating omega 3, 6 and 9 fatty acids with gestational diabetes mellitus: a systematic review. BMC Endocr Disord. 2021;21:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 149. | Wang Y, Huang Y, Wu P, Ye Y, Sun F, Yang X, Lu Q, Yuan J, Liu Y, Zeng H, Song X, Yan S, Qi X, Yang CX, Lv C, Wu JHY, Liu G, Pan XF, Chen D, Pan A. Plasma lipidomics in early pregnancy and risk of gestational diabetes mellitus: a prospective nested case-control study in Chinese women. Am J Clin Nutr. 2021;114:1763-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 150. | Fakhr Y, Brindley DN, Hemmings DG. Physiological and pathological functions of sphingolipids in pregnancy. Cell Signal. 2021;85:110041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 151. | Pinto GDA, Murgia A, Lai C, Ferreira CS, Goes VA, Guimarães DAB, Ranquine LG, Reis DL, Struchiner CJ, Griffin JL, Burton GJ, Torres AG, El-Bacha T. Sphingolipids and acylcarnitines are altered in placentas from women with gestational diabetes mellitus. Br J Nutr. 2023;130:921-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 152. | Hung SC, Chan TF, Chan HC, Wu CY, Chan ML, Jhuang JY, Tan JQ, Mei JB, Law SH, Ponnusamy VK, Chan HC, Ke LY. Lysophosphatidylcholine Impairs the Mitochondria Homeostasis Leading to Trophoblast Dysfunction in Gestational Diabetes Mellitus. Antioxidants (Basel). 2024;13:1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 153. | Mustaniemi S, Keikkala E, Kajantie E, Nurhonen M, Jylhä A, Morin-Papunen L, Öhman H, Männistö T, Laivuori H, Eriksson JG, Laaksonen R, Vääräsmäki M; FinnGeDi Study Group. Serum ceramides in early pregnancy as predictors of gestational diabetes. Sci Rep. 2023;13:13274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 154. | Lantzanaki M, Veneti S, Mintziori G, Begou O, Pappas PD, Gika H, Goulis DG, Bili H, Taousani E, Vavilis D. Plasma Ceramide Concentrations in Full-Term Pregnancies Complicated with Gestational Diabetes Mellitus: A Case-Control Study. Metabolites. 2022;12:1123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 155. | Xu H, Chen R, Hou X, Li N, Han Y, Ji S. The clinical potential of 1,5-anhydroglucitol as biomarker in diabetes mellitus. Front Endocrinol (Lausanne). 2024;15:1471577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 156. | Lin Y, Wu J, Zhu Y, Hinkle SN, Rawal S, Liang L, Weir NL, Tsai MY, Zhang C. A longitudinal study of plasma acylcarnitines throughout pregnancy and associations with risk of gestational diabetes mellitus. Clin Nutr. 2021;40:4863-4870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 157. | Bhushan R, Trivedi R, Raj R, Rani A, Rai S, Tripathi A, Rawat SG, Kumar A, Kumar D, Dubey PK. Integration of transcriptomics and metabolomics data revealed role of insulin resistant SNW1 gene in the pathophysiology of gestational diabetes. Sci Rep. 2025;15:4159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 158. | Wei X, Wei S, Chen M, Tan Y, Yang Z, Feng W, Yang G, Han Z, Luo X. Subcutaneous adipose tissue compensates for the perturbations in circulating one-carbon metabolism in women with gestational diabetes. Acta Diabetol. 2025;62:1313-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 159. | Wang S, Ning J, Huai J, Yang H. Hyperglycemia in Pregnancy-Associated Oxidative Stress Augments Altered Placental Glucose Transporter 1 Trafficking via AMPKα/p38MAPK Signaling Cascade. Int J Mol Sci. 2022;23:8572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 160. | Lai M, Li J, Yang J, Zhang Q, Gong Y, Ma Y, Fang F, Li N, Zhai Y, Shen T, Peng Y, Liu J, Wang Y. Metabolomic profiling reveals decreased serum cysteine levels during gestational diabetes mellitus progression. J Mol Cell Biol. 2024;16:mjae010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 161. | Xu Z, Fang J, Wang Y, Wu X, Liu L, Cui X, Zhong H, Zhong T. Targeted metabolomics reveals novel biomarkers for gestational diabetes mellitus. Diabetes Obes Metab. 2025;27:2163-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 162. | Bronkhorst AJ, Ungerer V, Diehl F, Anker P, Dor Y, Fleischhacker M, Gahan PB, Hui L, Holdenrieder S, Thierry AR. Towards systematic nomenclature for cell-free DNA. Hum Genet. 2021;140:565-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 163. | Hu Z, Chen H, Long Y, Li P, Gu Y. The main sources of circulating cell-free DNA: Apoptosis, necrosis and active secretion. Crit Rev Oncol Hematol. 2021;157:103166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 164. | Rather RA. Fetal Origin Circulating Cell-Free Nucleic Acids in Maternal Circulation and Their Clinical Importance. In: Rather RA, Saha SC, editors. Non-invasive Prenatal Screening (NIPS) in Clinical Practice. Berlin: Springer, 2024. [DOI] [Full Text] |
| 165. | Tang Z, Wang S, Li X, Hu C, Zhai Q, Wang J, Ye Q, Liu J, Zhang G, Guo Y, Su F, Liu H, Guan L, Jiang C, Chen J, Li M, Ren F, Zhang Y, Huang M, Li L, Zhang H, Hou G, Jin X, Chen F, Zhu H, Li L, Zeng J, Xiao H, Zhou A, Feng L, Gao Y, Liu G. Longitudinal integrative cell-free DNA analysis in gestational diabetes mellitus. Cell Rep Med. 2024;5:101660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 166. | Ghafourian M, Mahdavi R, Akbari Jonoush Z, Sadeghi M, Ghadiri N, Farzaneh M, Mousavi Salehi A. The implications of exosomes in pregnancy: emerging as new diagnostic markers and therapeutics targets. Cell Commun Signal. 2022;20:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 167. | Sun Y, Tao Q, Wu X, Zhang L, Liu Q, Wang L. The Utility of Exosomes in Diagnosis and Therapy of Diabetes Mellitus and Associated Complications. Front Endocrinol (Lausanne). 2021;12:756581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 168. | Ye Z, Wang S, Huang X, Chen P, Deng L, Li S, Lin S, Wang Z, Liu B. Plasma Exosomal miRNAs Associated With Metabolism as Early Predictor of Gestational Diabetes Mellitus. Diabetes. 2022;71:2272-2283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 169. | Bernea EG, Suica VI, Uyy E, Cerveanu-Hogas A, Boteanu RM, Ivan L, Ceausu I, Mihai DA, Ionescu-Tîrgoviște C, Antohe F. Exosome Proteomics Reveals the Deregulation of Coagulation, Complement and Lipid Metabolism Proteins in Gestational Diabetes Mellitus. Molecules. 2022;27:5502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 170. | Wu P, Wang Y, Ye Y, Yang X, Lu Q, Yuan J, Zha L, Liu Y, Song X, Yan S, Wen Y, Qi X, Yang CX, Wang Y, Liu G, Lv C, Pan XF, Pan A. Serum Fetuin-A and Risk of Gestational Diabetes Mellitus: An Observational Study and Mendelian Randomization Analysis. J Clin Endocrinol Metab. 2022;107:e3841-e3849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 171. | Cai Z, Yang Y, Zhang J. Hepatokine levels during the first or early second trimester of pregnancy and the subsequent risk of gestational diabetes mellitus: a systematic review and meta-analysis. Biomarkers. 2021;26:517-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 172. | Hivert MF, White F, Allard C, James K, Majid S, Aguet F, Ardlie KG, Florez JC, Edlow AG, Bouchard L, Jacques PÉ, Karumanchi SA, Powe CE. Placental IGFBP1 levels during early pregnancy and the risk of insulin resistance and gestational diabetes. Nat Med. 2024;30:1689-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 173. | Hong J, Kumar S. Circulating biomarkers associated with placental dysfunction and their utility for predicting fetal growth restriction. Clin Sci (Lond). 2023;137:579-595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 174. | Martín-Estal I, Castorena-Torres F. Gestational Diabetes Mellitus and Energy-Dense Diet: What Is the Role of the Insulin/IGF Axis? Front Endocrinol (Lausanne). 2022;13:916042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 175. | Bruno B, Blessing MK, Izuchukwu MN, Terry GT, Faeren D. Sex hormone-binding globulin is a valuable diagnostic indicator of gestational diabetes mellitus. Ghana Med J. 2024;58:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 176. | Sharmin A, Khan MRH, Jahan J, Md Shameem -, Afruza S, Keya SL. Association of Serum Sex-Hormone-Binding Globulin in Pregnant Women with Gestational Diabetes Mellitus. TAJ J Teach Assoc. 2021;34:80-85. [DOI] [Full Text] |
| 177. | Liu W, Huang Z, Tang S, Zhang Z, Yu Q, He J. Changes of Serum Sex Hormone-Binding Globulin, Homocysteine, and Hypersensitive CRP Levels during Pregnancy and Their Relationship with Gestational Diabetes Mellitus. Gynecol Obstet Invest. 2021;86:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 178. | Kučerová V, Karásek D, Krystyník O, Štefaničková L, Němeček V, Friedecký D. Adipokine Levels of RBP4, Resistin and Nesfatin-1 in Women Diagnosed With Gestational Diabetes. Physiol Res. 2024;73:1037-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 179. | Leca BM, Kite C, Lagojda L, Davasgaium A, Dallaway A, Chatha KK, Randeva HS, Kyrou I. Retinol-binding protein 4 (RBP4) circulating levels and gestational diabetes mellitus: a systematic review and meta-analysis. Front Public Health. 2024;12:1348970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 180. | Mousavi SN, Bahramfard T, Rad EY, Hosseinikia M, Saboori S. Association of Leptin and Retinol Binding Protein 4 with the Risk of Gestational Diabetes: A Systematic Review and Meta-Analysis of Observational Studies. Indian J Endocrinol Metab. 2023;27:96-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 181. | Eroğlu H, Örgül G, Tonyalı NV, Biriken D, Polat N, Yücel A, Yazihan N, Şahin D. The Role of Afamin and Other Trace Elements in the Prediction of GDM: a Tertiary Center Experience. Biol Trace Elem Res. 2021;199:4418-4422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 182. | Atakul N, Atamer Y, Selek Ş, Kılıç BS, Unal F. Novel metabolic marker Afamin: A predictive factor for Large-for-Gestational-Age (LGA) fetus estimation in pregnancies with gestational diabetes mellitus? J Gynecol Obstet Hum Reprod. 2021;50:102201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 183. | Wang X, Zheng X, Yan J, Xu R, Xu M, Zheng L, Xu L, Lin Z. The Clinical Values of Afamin, Triglyceride and PLR in Predicting Risk of Gestational Diabetes During Early Pregnancy. Front Endocrinol (Lausanne). 2021;12:723650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 184. | Karoutsos D, Fasoulakis Z, Michala S, Stefanidis K, Antsaklis P. Glycosylated Fibronectin as a wide-spectrum biomarker in pregnancy. HJOG. 2023;22:49-55. [DOI] [Full Text] |
| 185. | Xu F, Wang N, Li G, Tian D, Shi X. ANGPTL8/Betatrophin Improves Glucose Tolerance in Older Mice and Metabolomic Analysis Reveals Its Role in Insulin Resistance in HepG2 Cells. Diabetes Metab Syndr Obes. 2021;14:4209-4221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 186. | Guo Q, Cao S, Wang X. Betatrophin and Insulin Resistance. Metabolites. 2022;12:925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 187. | Kirlangic MM, Eraslan Sahin M, Sahin E, Madendag Y, Col Madendag I, Akdemir E, Vural Yalman M. First-trimester maternal serum betatrophin levels are decreased in pregnancies complicated by gestational diabetes mellitus. Placenta. 2022;124:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 188. | Melekoglu R, Celik E. Serum Betatrophin: What It Shows and How It Alters in Gestational Diabetes Mellitus. In: Patel VB, Preedy VR, editors. Biomarkers in Disease: Methods, Discoveries and Applications. Berlin: Springer, 2023. [DOI] [Full Text] |
| 189. | Conover CA, Oxvig C. The Pregnancy-Associated Plasma Protein-A (PAPP-A) Story. Endocr Rev. 2023;44:1012-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 190. | Yanachkova V, Staynova R, Stankova T, Kamenov Z. Placental Growth Factor and Pregnancy-Associated Plasma Protein-A as Potential Early Predictors of Gestational Diabetes Mellitus. Medicina (Kaunas). 2023;59:398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 191. | Joshi NP, Madiwale SD, Sundrani DP, Joshi SR. Fatty acids, inflammation and angiogenesis in women with gestational diabetes mellitus. Biochimie. 2023;212:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 192. | Liao L, Zhao X, Zhou M, Deng Y, Li Y, Peng C. sFlt-1: A Double Regulator in Angiogenesis-related Diseases. Curr Pharm Des. 2021;27:4160-4170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 193. | Gul Kara SM, Alkan Bulbul G, Kirtis E, Kandemir H, Ozen Kuçukcetin I, Ozdem S, Doğan NU, Sanhal CY. Maternal and cord serum levels of sFlt-1 and PlGF in pregnancies complicated by gestational diabetes mellitus: a prospective cohort study. J Matern Fetal Neonatal Med. 2025;38:2491454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 194. | Bolatai A, He Y, Wu N. Vascular endothelial growth factor and its receptors regulation in gestational diabetes mellitus and eclampsia. J Transl Med. 2022;20:400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 195. | Lyu X, Wang S, Zhong J, Cai L, Zheng Y, Zhou Y, Zhou Y, Chen Q, Li Q. Gut microbiome interacts with pregnancy hormone metabolites in gestational diabetes mellitus. Front Microbiol. 2023;14:1175065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 196. | Su Z, Liu L, Zhang J, Guo J, Wang G, Zeng X. A scientometric visualization analysis of the gut microbiota and gestational diabetes mellitus. Front Microbiol. 2025;16:1485560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 197. | Biete M, Vasudevan S. Gestational diabetes mellitus: Impacts on fetal neurodevelopment, gut dysbiosis, and the promise of precision medicine. Front Mol Biosci. 2024;11:1420664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 198. | Ren Y, Hao L, Liu J, Wang P, Ding Q, Chen C, Song Y. Alterations in the Gut Microbiota in Pregnant Women with Pregestational Type 2 Diabetes Mellitus. mSystems. 2023;8:e0114622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 199. | Shashikadze B, Flenkenthaler F, Stöckl JB, Valla L, Renner S, Kemter E, Wolf E, Fröhlich T. Developmental Effects of (Pre-)Gestational Diabetes on Offspring: Systematic Screening Using Omics Approaches. Genes (Basel). 2021;12:1991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 200. | Abdi G, Tarighat MA, Jain M, Tendulkar R, Tendulkar M, Barwant M. Revolutionizing Genomics: Exploring the Potential of Next-Generation Sequencing. In: Singh V, Kumar A, editors. Advances in Bioinformatics. Berlin: Springer, 2024. [DOI] [Full Text] |
| 201. | Ashique S, Islam A, Kaur Sandhu N, Sharma B, Pathak R, Sharma H. Next-Generation Whole-Exome Pattern: Advanced Methods and Clinical Significance. Curr Gene Ther. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 202. | Satam H, Joshi K, Mangrolia U, Waghoo S, Zaidi G, Rawool S, Thakare RP, Banday S, Mishra AK, Das G, Malonia SK. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology (Basel). 2023;12:997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 511] [Article Influence: 170.3] [Reference Citation Analysis (0)] |
| 203. | I Udoh L, Peggy Obaseojei W, Uzoebo C. Single Nucleotide Polymorphisms: A Modern Tool to Screen Plants for Desirable Traits. In: Abdurakhmonov IY, editor. Plant Breeding-Current and Future Views. London: IntechOpen, 2021. [DOI] [Full Text] |
| 204. | Tyagi N, Roy S, Vengadesan K, Gupta D. Multi-omics approach for identifying CNV-associated lncRNA signatures with prognostic value in prostate cancer. Noncoding RNA Res. 2024;9:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 205. | Kazimierczyk M, Wrzesinski J. Long Non-Coding RNA Epigenetics. Int J Mol Sci. 2021;22:6166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 206. | Abu Samra N, Jelinek HF, Alsafar H, Asghar F, Seoud M, Hussein SM, Mubarak HM, Anwar S, Memon M, Afify N, Manzoor R, Al-Homedi Z, Osman W. Genomics and Epigenomics of Gestational Diabetes Mellitus: Understanding the Molecular Pathways of the Disease Pathogenesis. Int J Mol Sci. 2022;23:3514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 207. | Alur V, Vastrad B, Raju V, Vastrad C, Kotturshetti S. Bioinformatics analysis of next generation sequencing data to diagnose crucial and novel genes in gestational diabetes mellitus. Discov Endocrinol Metab. 2025;1:3. [DOI] [Full Text] |
| 208. | Alesi S, Ghelani D, Rassie K, Mousa A. Metabolomic Biomarkers in Gestational Diabetes Mellitus: A Review of the Evidence. Int J Mol Sci. 2021;22:5512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 209. | Burzynska-Pedziwiatr I, Dudzik D, Sansone A, Malachowska B, Zieleniak A, Zurawska-Klis M, Ferreri C, Chatgilialoglu C, Cypryk K, Wozniak LA, Markuszewski MJ, Bukowiecka-Matusiak M. Targeted and untargeted metabolomic approach for GDM diagnosis. Front Mol Biosci. 2022;9:997436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 210. | Rahman ML, Feng YA, Fiehn O, Albert PS, Tsai MY, Zhu Y, Wang X, Tekola-Ayele F, Liang L, Zhang C. Plasma lipidomics profile in pregnancy and gestational diabetes risk: a prospective study in a multiracial/ethnic cohort. BMJ Open Diabetes Res Care. 2021;9:e001551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 211. | Fan HM, Mitchell AL, Williamson C. ENDOCRINOLOGY IN PREGNANCY: Metabolic impact of bile acids in gestation. Eur J Endocrinol. 2021;184:R69-R83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 212. | Hill M, Pařízek A, Šimják P, Koucký M, Anderlová K, Krejčí H, Vejražková D, Ondřejíková L, Černý A, Kancheva R. Steroids, steroid associated substances and gestational diabetes mellitus. Physiol Res. 2021;70:S617-S634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 213. | Roverso M, Dogra R, Visentin S, Pettenuzzo S, Cappellin L, Pastore P, Bogialli S. Mass spectrometry-based "omics" technologies for the study of gestational diabetes and the discovery of new biomarkers. Mass Spectrom Rev. 2023;42:1424-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 214. | Lapolla A, Traldi P. Proteomic Approaches in the Study of Placenta of Pregnancy Complicated by Gestational Diabetes Mellitus. Biomedicines. 2022;10:2272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 215. | Addissouky T, Ali M, El Sayed IET, Wang Y. Revolutionary Innovations in Diabetes Research: From Biomarkers to Genomic Medicine. IJDO. 2023;. [DOI] [Full Text] |
| 216. | Tian S, Liu M, Han S, Wu H, Qin R, Ma K, Liu L, Zhao H, Li Y. Novel first-trimester serum biomarkers for early prediction of gestational diabetes mellitus. Nutr Diabetes. 2025;15:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 217. | Ahmad A, Imran M, Ahsan H. Biomarkers as Biomedical Bioindicators: Approaches and Techniques for the Detection, Analysis, and Validation of Novel Biomarkers of Diseases. Pharmaceutics. 2023;15:1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 127] [Reference Citation Analysis (0)] |
| 218. | Meek CL, Tundidor D, Feig DS, Yamamoto JM, Scott EM, Ma DD, Halperin JA, Murphy HR, Corcoy R; CONCEPTT Collaborative Group. Novel Biochemical Markers of Glycemia to Predict Pregnancy Outcomes in Women With Type 1 Diabetes. Diabetes Care. 2021;44:681-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 219. | Pramodkumar TA, Hannah W, Anjana RM, Ram U, Tiwaskar M, Gokulakrishnan K, Popova PV, Mohan V. Biomarkers of Gestational Diabetes Mellitus: Mechanisms, Advances, and Clinical Utility. J Assoc Physicians India. 2025;73:56-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 220. | Grazia Dalfrà M, Burlina S, Lapolla A. Epigenetic: New Insight in Gestational Diabetes Mellitus. In: Radenkovic M, editor. Gestational Diabetes Mellitus-New Developments. London: IntechOpen, 2022. [DOI] [Full Text] |
| 221. | Paver EC, Morey AL. Biomarkers and biomarker validation: a pathologist's guide to getting it right. Pathology. 2024;56:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 222. | Smith BJ, Silva-Costa LC, Martins-de-Souza D. Human disease biomarker panels through systems biology. Biophys Rev. 2021;13:1179-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 223. | Sugandh F, Chandio M, Raveena F, Kumar L, Karishma F, Khuwaja S, Memon UA, Bai K, Kashif M, Varrassi G, Khatri M, Kumar S. Advances in the Management of Diabetes Mellitus: A Focus on Personalized Medicine. Cureus. 2023;15:e43697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 90] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 224. | Gasmi A, Noor S, Menzel A, Doşa A, Pivina L, Bjørklund G. Obesity and Insulin Resistance: Associations with Chronic Inflammation, Genetic and Epigenetic Factors. Curr Med Chem. 2021;28:800-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 225. | Zhou Y, Gan G. Impact of Personalized Education and Supervision on Pregnancy Outcomes in Women with Gestational Diabetes Mellitus. Clin Exp Obstet Gynecol. 2023;50. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/