Published online Dec 9, 2025. doi: 10.5409/wjcp.v14.i4.109022
Revised: May 31, 2025
Accepted: September 19, 2025
Published online: December 9, 2025
Processing time: 182 Days and 19.7 Hours
Acute respiratory infections (ARI) and diarrhoea are among the leading causes of infant and under-five mortality worldwide. Zinc, the second most abundant trace element in the human body, is widely used in the treatment of both conditions. It mitigates diarrhoea by restoring mucosal integrity and enhancing enterocyte brush border enzyme activity. In ARI, zinc boosts immune function, promotes epithelial regeneration, and inhibits the replication of respiratory viruses.
To assess the effectiveness of prophylactic intermittent zinc supplementation in preventing acute diarrhoea and ARI in infants.
This open-label, randomized controlled trial with a 1:1 allocation ratio was conducted over 15 months (October 2022 to December 2023) at a tertiary care hospital in Eastern India. A total of 320 infants attending the outpatient dep
The mean annual incidence of ARI and diarrhoea was significantly lower in the zinc group than in the control group [ARI: 0.25 ± 0.61 vs 0.92 ± 1.22; mean difference = -0.67 (95%CI: -0.88 to -0.45), P < 0.001, Cohen’s d = -0.69] and [diarrhoea: 1.04 ± 1.30 vs 2.07 ± 2.09; mean difference = -1.03 (95%CI: -1.42 to -0.65), P < 0.001, Cohen's d =
Prophylactic intermittent zinc supplementation administered alongside routine immunization substantially reduces the incidence of ARI and diarrhoea in infants and promotes improved growth. This affordable strategy holds promise for reducing infant morbidity and mortality without increasing healthcare burdens.
Core Tip: This randomized controlled trial evaluated the efficacy of prophylactic intermittent zinc supplementation in reducing acute respiratory infections (ARI) and diarrhoea among infants. The study demonstrated that infants receiving zinc supplementation had a significantly lower incidence of ARI and diarrhoea, as well as greater gains in weight and length, compared to the control group. These findings suggest that intermittent zinc supplementation, administered alongside routine vaccinations, is an effective and low-cost strategy to reduce infant morbidity and mortality from ARI and diarrhoea, and can be feasibly implemented in resource-limited healthcare settings.
- Citation: Kumar CM, Ghorui A, Hamsay K. Efficacy of prophylactic intermittent zinc supplementation for reducing acute respiratory infections and diarrhoea in infants: A randomized controlled trial. World J Clin Pediatr 2025; 14(4): 109022
- URL: https://www.wjgnet.com/2219-2808/full/v14/i4/109022.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i4.109022
Acute respiratory infection (ARI) refers to inflammation affecting any part of the respiratory tract, from the nasal passages to the alveoli[1]. Diarrhoea is defined as a recent change in stool frequency and consistency and may occur with varying degrees of dehydration: None, some, or severe[2]. The severity of diarrhoea is commonly assessed using the Modified Vesikari Score. After iron, zinc is the most abundant trace element found in the human body. It plays essential physiological roles in over 200 enzymatic reactions, including gene transcription, protein synthesis, cell regeneration, and immune modulation. Mild to moderate zinc deficiency is widespread in many low- and middle-income countries, including India, where dietary staples often contain low levels of zinc and high levels of phytates that impair its absorption and utilization[3]. Infants and young children, due to their rapid growth and higher zinc requirements, are particularly vulnerable to deficiency and may benefit from supplementation[1]. In cases of acute diarrhoea, zinc acts through multiple mechanisms: It restores the mucosal barrier, enhances enterocyte brush border enzyme activity, promotes local immune responses, regenerates the intestinal epithelium, and inhibits chloride channel-mediated fluid secretion[4]. In the context of ARI, zinc contributes by bolstering immune defense, supporting epithelial regeneration, and suppressing the replication of respiratory viruses, including respiratory syncytial virus and coronaviruses[5-7]. Globally, ARI and diarrhoea are leading contributors to infant and under-five mortality. In India, the prevalence of diarrhoea and pneumonia among children under five years is 7.3% and 2.8%, respectively. Bihar reports the highest rates, with 13.7% of children affected by diarrhoea and 3.5% by pneumonia[8]. A national survey conducted from 2016 to 2018 reported zinc deficiency in 18.9% of preschool-aged children in India[9]. Zinc supplementation offers a safe, cost-effective, and efficient means of managing both diarrhoea and ARI in children. Accordingly, the World Health Organization (WHO) and the United Nations Children's Fund (UNICEF) recommend zinc as part of diarrhoea management in children[10,11]. Although evidence supports its benefit in ARI treatment, zinc supplementation has not yet been included in WHO's formal recommendations for respiratory infections[12].
This study aimed to evaluate the effect of prophylactic intermittent zinc supplementation on the incidence of acute diarrhoea and ARI, as well as its impact on growth outcomes in infants attending vaccination clinics in a tertiary care setting.
This open-label, randomized controlled trial was conducted in the outpatient department of a tertiary care medical college hospital in Eastern India over 15 months (October 2022 to December 2023). Ethical approval was obtained from the Institutional Ethics Committee (Ref No. AIIMS/Pat/IEC/2022/944), and the study was registered with the Clinical Trials Registry of India (Ref No. CTRI/2022/04/053869, dated 08/09/2022). The primary objectives were to evaluate the effect of intermittent prophylactic zinc supplementation on the incidence, duration, and severity of acute diarrhoea and ARI in infants. The secondary objectives were to assess its impact on infant growth, specifically in terms of gains in length and weight.
Term neonates with normal birth weight, attending the routine immunization clinic at AIIMS Patna for their 6- or 10-week vaccinations (as per the National Immunization Schedule), were screened for eligibility. Written informed consent was obtained from parents or guardians after providing a thorough explanation of the study protocol, including potential benefits and risks. Infants were excluded if they had received zinc supplementation within the preceding two weeks, presented with severe malnutrition (weight-for-age or length-for-age < -3 standard deviations), or were suspected of having a malabsorption syndrome.
The expected incidence of ARI was estimated to be 0.93 episodes per child-year, based on data from a community-based cohort study in rural North India by Krishnan et al[13]. Additionally, a Cochrane review by Lassi et al[14] reported that prophylactic zinc supplementation could reduce ARI incidence by 13%. Based on these findings, a sample size of 320 infants (160 per group) was calculated to detect a 13% reduction in incidence with 80% power and a 5% level of sig
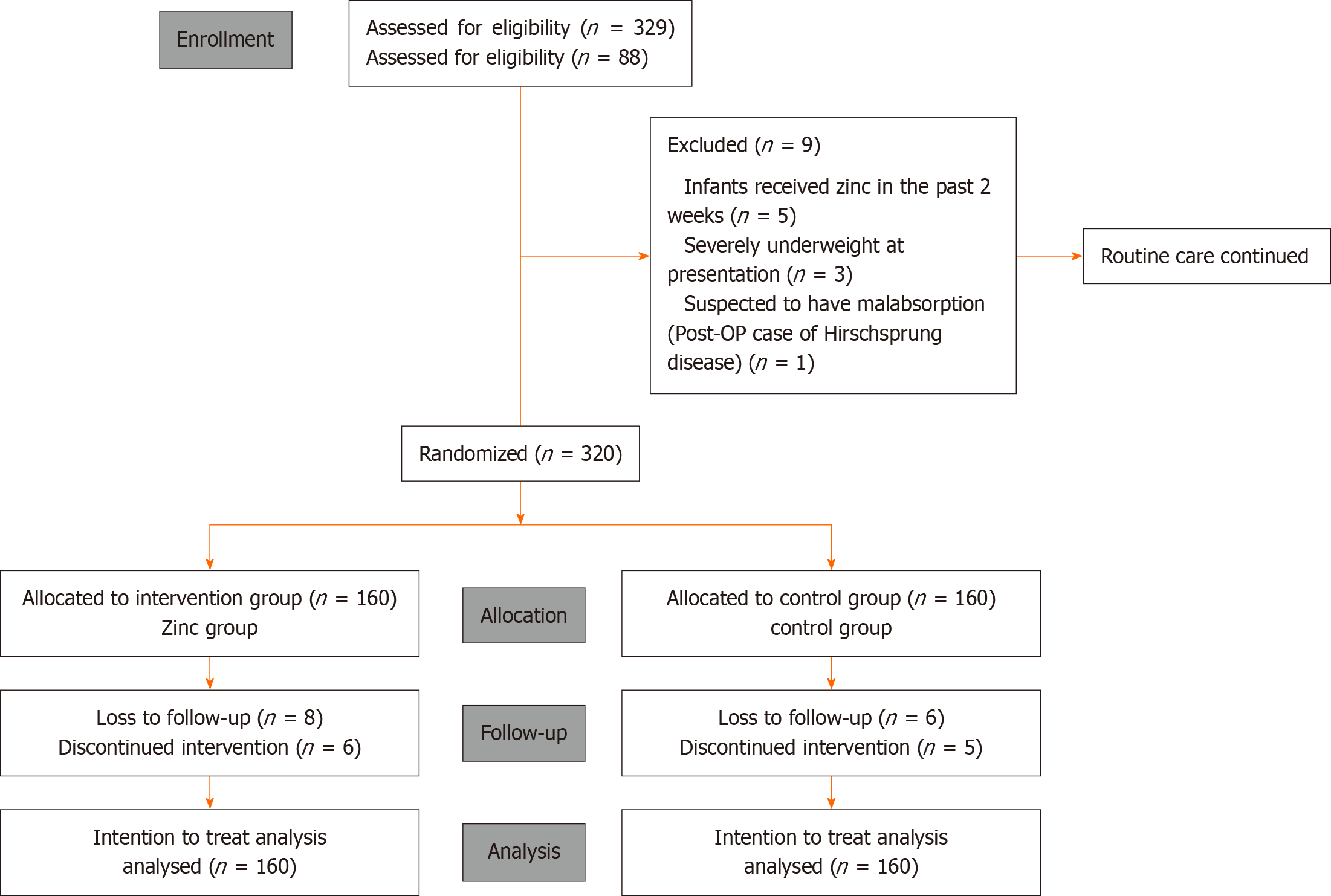
A total of 329 infants were assessed for eligibility. After excluding 9 who met the exclusion criteria, 320 were enrolled in the study. These infants were randomly assigned to the intervention or control group using a computer-generated block randomization sequence with variable block sizes of 4 and 6.
The allocation sequence was concealed from the researchers by placing the group assignments (zinc or control) into sealed, opaque envelopes, prepared by the Head of the Department of Paediatrics. Upon enrolment, the envelope corresponding to the participant was opened, and the group assignment was disclosed to the research team.
The intervention group received age-appropriate doses of zinc acetate drops [Drop Z&D, 20 mg elemental zinc/mL] for two weeks. Infants under six months of age received 0.5 mL (10 mg) daily, while those over six months received 1 mL (20 mg) daily. Each child received zinc supplementation during the 6/10-week and 10/14-week vaccination visits (10 mg/day for 14 days each), and again during the 9-month vaccination (20 mg/day for 14 days), resulting in a total cumulative dose of 560 mg per child (10 mg × 14 days × 2 + 20 mg × 14 days). The intermittent dosing schedule was selected based on a recent study by Malik et al[15], which demonstrated that two weeks of prophylactic zinc is both effective and safe for preventing ARI. Due to logistical constraints in the preparation, packaging, and distribution of inert drops, the control group did not receive a placebo. Regardless of group allocation, all mothers were counselled using standard protocols on exclusive breastfeeding and safe infant care practices.
Participants were followed up one month after enrolment, coinciding with the next vaccination visit at either 10 or 14 weeks. At this visit, the same intervention protocol was repeated for another two weeks. The final follow-up occurred after six months, during the 9-month vaccination visit. After each dosing phase, weekly telephone calls were made for two weeks to monitor adherence and reinforce compliance. During the subsequent six-month period leading up to the final follow-up, monthly phone calls were conducted to ensure continued engagement and accurate maintenance of the follow-up booklet.
Diarrhoea was defined as a recent change in both the frequency and consistency of stools. Severity was assessed using the Modified Vesikari Score, which incorporates factors such as duration of diarrhoea (in hours), maximum number of diarrhoeal stools in 24 hours, duration of vomiting, maximum vomiting episodes in 24 hours, highest recorded fever, and the need for healthcare visits. ARI was defined as the presence of fever, cough, and rapid breathing (respiratory rate > 50 breaths per minute), with or without chest retraction. The severity of ARI was categorized based on the level of medical intervention required: Mild (managed as an outpatient), moderate (requiring oxygen support or ward admission), and severe (requiring paediatric intensive care unit admission or ventilatory support).
At the time of enrolment, follow-up diary cards were distributed to both groups. Parents were trained to assess the severity of diarrhoea using the Modified Vesikari Score to ensure reliable caregiver reporting. They were also instructed to document any healthcare visits, particularly those related to pneumonia or other illnesses, and to record the treatments received. To promote adherence and accurate reporting, the study team maintained biweekly telephone contact in addition to scheduled in-person follow-ups. Caregiver-reported data were verified through cross-referencing with hospital admission records.
Infant weight was measured at enrolment and during each follow-up visit using the Phoenix® Baby Weighing Scale NBY-20 (Phoenix Medical Systems Pvt. Ltd., India), which offers 10 g accuracy and a maximum capacity of 20 kg. Infant length was measured using the ACZET® Infantometer BS11 (Aczet Private Limited, India), accurate to 0.1 cm. All measurements were performed by a trained nursing officer at the immunization clinic to ensure consistency and relia
During each follow-up visit, diary entries were reviewed, anthropometric assessments were performed, and all data were entered into an online database.
Data analysis followed an intention to treat (ITT) approach and was conducted using JAMOVI (Version 2.3, 2022). Continuous variables were expressed as mean ± SD. Their distribution was assessed using Q-Q plots and the Shapiro-Wilk test. Depending on the distribution, continuous variables were reported as mean ± SD or median with interquartile range (IQR). Intergroup differences for continuous variables were analyzed using an independent Student's t-test or Welch's t-test, while categorical variables were compared using the χ2 test. Time-based differences between groups were analyzed using ANOVA. All statistical analyses were conducted at a 95% confidence level with a significance threshold set at P < 0.05. Cohen's d was also calculated to estimate effect sizes for the intervention.
Caregivers were not involved in the design, recruitment, or conduct of the study. However, some outcome data were derived from follow-up diaries maintained by the parents.
The study was initially designed as a 12-month longitudinal trial, with a sample size calculated based on an expected ARI incidence of 0.93 episodes per child year. However, a dropout rate exceeding 20% was observed, primarily due to an additional follow-up hospital visit at 12 months that was not linked to routine immunization. As a result, this visit was removed from the study protocol. Additionally, the original placebo-controlled design could not be implemented due to logistical challenges in preparing and distributing inert drops. All protocol amendments were reviewed and approved by the Institutional Ethics Committee.
The study initially enrolled 160 participants in each group; however, 146 participants in the intervention group and 149 in the control group completed the final follow-up. The participant flow is illustrated in the CONSORT diagram (Figure 1). The final analysis was performed following the ITT protocol. Additionally, a per-protocol analysis was performed (Supplementary Table 1).
As it was assumed that a single infant could experience multiple episodes of diarrhoea and/or ARI, each episode—rather than each infant—was considered the unit of analysis.
The demographic profiles of the two study groups were comparable, with no statistically significant differences observed in baseline characteristics, including age, weight, length at enrolment, exclusive breastfeeding status, socioeconomic status, and maternal education level, as shown in Table 1.
| Characteristic | Intervention group (Zinc group), n = 160 | Control group, n = 160 | P value |
| Mean age, month; mean ± SD | 2.2 (0.7) | 2.3 (0.6) | 0.40 |
| Weight, gram; mean ± SD | 4140 (136) | 4137 (122) | 0.83 |
| Length, cm; mean ± SD | 56.3 (1.7) | 56.5 (1.1) | 0.08 |
| Male:Female ratio | 0.75 | 0.74 | 0.97 |
| Lower & upper lower SES | 78/160 (49) | 82/160 (51) | 0.66 |
| Lower middle, upper middle & upper SES | 82/160 (51) | 78/160 (49) | 0.66 |
| Exclusive breast feeding | 114/160 (71) | 119/160 (74) | 0.53 |
| Maternal education level | 0.41 | ||
| None or below primary | 115/160 (72) | 109/160 (68) | |
| Primary | 36/160 (22) | 39/160 (24) | |
| Secondary or above | 9/160 (6) | 12/160 (8) | |
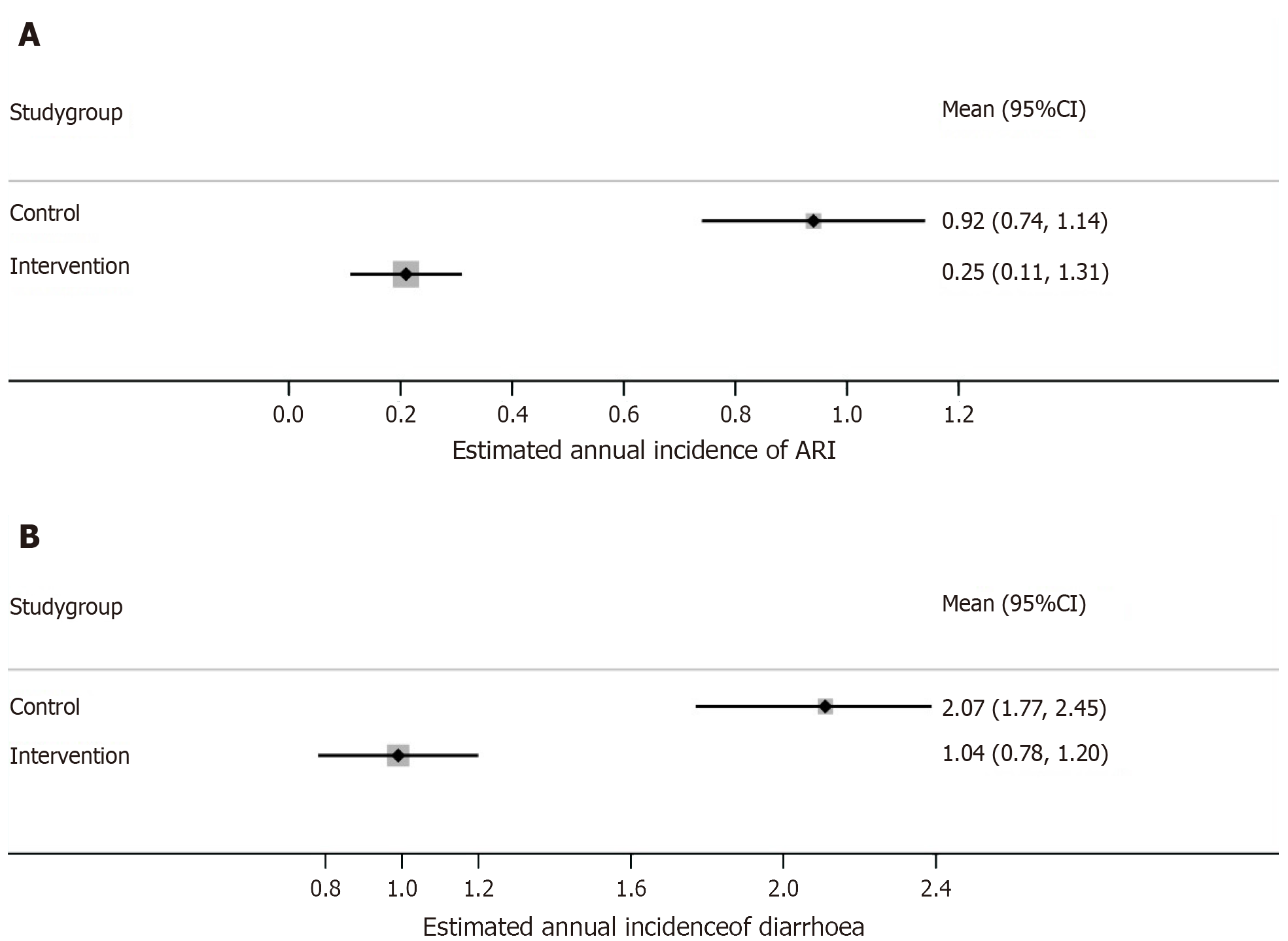
Descriptive statistics: Out of the total 93 episodes of ARI, 20 episodes occurred in the zinc group and 73 in the control group, at the end of 6 months of follow-up, accounting for an estimated mean annual incidence of 0.25 and 0.92 per child year, respectively.
Similarly, out of the total 248 episodes of acute diarrhoea, 83 episodes occurred in the zinc group and 165 in the control group, at the end of 6 months of follow-up, accounting for an estimated mean annual incidence of 1.04 and 2.07 per child year, respectively.
Analytical statistics: The estimated mean annual incidence (episodes per child-year) of ARI was significantly less in the zinc group [0.25 ± 0.61 vs 0.92 ± 1.22; mean-difference = -0.67 (95%CI: -0.88 to -0.45), P value < 0.001, Effect size (Cohen's d) = -0.69], with relative risk reduction of 72% (Figure 2A).
There was also a significant reduction in the estimated mean annual incidence (episodes per child-year) of acute diarrhoeal episodes in the intervention group [1.04 ± 1.30 vs 2.07 ± 2.09; mean-difference = -1.03 (95%CI: -1.42 to -0.65), P value < 0.001, effect size (Cohen's d) = -0.59], with relative risk reduction 49% (Figure 2B). Additionally, there was a significant reduction in the estimated mean annual cumulative duration of acute diarrhoeal episodes (days per child-year) in the intervention group [3.12 ± 3.82 vs 7.66 ± 7.67; mean-difference = - 4.54 (95%CI: -5.87 to -3.20), P value < 0.001, Effect size (Cohen's d) = - 0.75].
In biostatistics, the P value indicates the statistical significance of a result, while the effect size reflects its clinical significance. An effect size between 0.2 and 0.5 is considered small, between 0.5 and 0.8 is moderate, and above 0.8 is regarded as large.
Severity-specific outcomes: Furthermore, the estimated mean annual incidence of mild ARI cases was significantly
Similarly, the estimated mean annual incidence of mild diarrhoea cases was significantly (P = 0.006) lower in the zinc supplementation group compared to the control group, with a risk ratio of 0.66 (95%CI: 0.56 to 0.76) and RRR of 34% (95%CI: 24 to 44). Similarly, the estimated mean annual incidence of moderate to severe diarrhoea cases was also significantly (P = 0.002) lower in the zinc group compared to control, with a risk ratio of 0.37 (95%CI: 0.28 to 0.46) and RRR of 63% (95%CI: 54 to 72). These findings indicate that the protective effect of zinc supplementation was more pronounced for moderate to severe diarrhoea cases compared to mild diarrhoea cases.
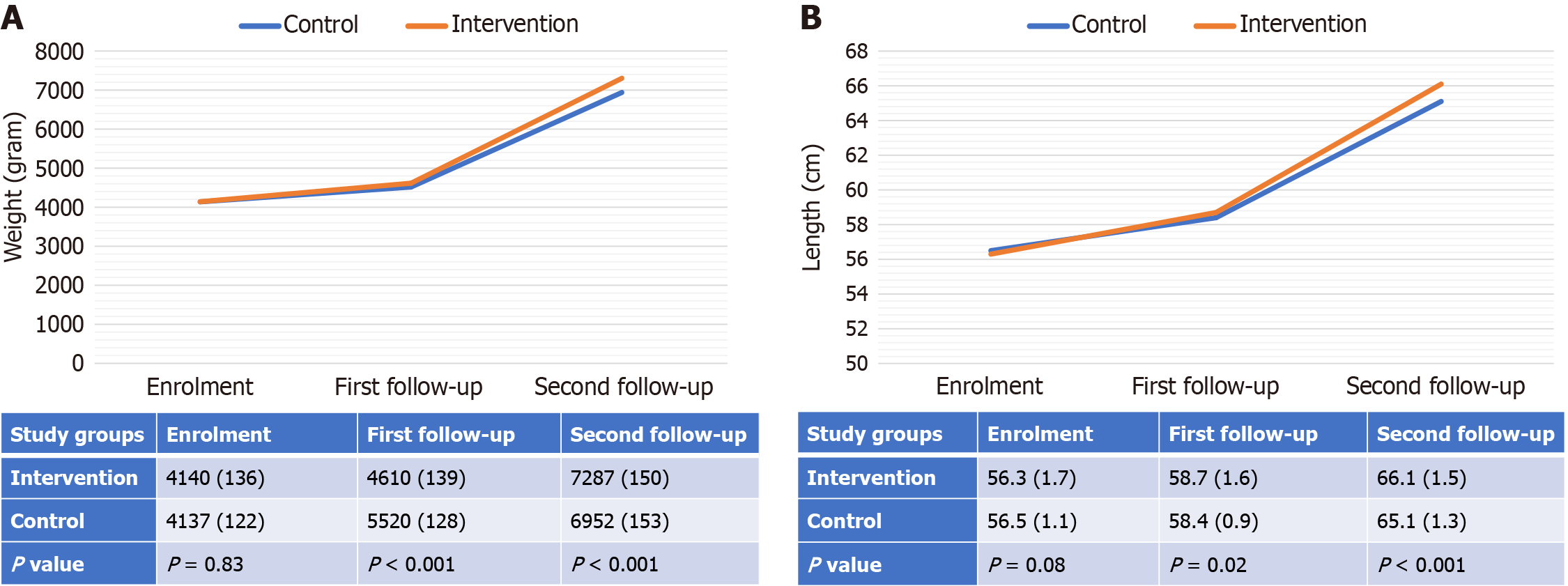
In the zinc group, there was a significantly higher gain in length (cm) over the 6-month follow-up [10 ± 0.6 vs 8.6 ± 0.4; mean-difference = 1.4 (95%CI: 1.3-1.5), P value < 0.001, Effect size (Cohen's d) = 2.74].
There was also a statistically significant higher gain in weight (gram) over the 6 months of follow-up in the zinc group [3150 ± 108 vs 2818 ± 76; mean-difference = 332 (95%CI: 310-352), P value < 0.001, Effect size (Cohen's d) = 3.56].
The mean differences in weight-for-age Z-score (WAZ) and length-for-age Z-score (LAZ) were also significantly higher in the innervation group [mean difference = 0.32 (0.23–0.42), P value < 0.001, effect size (Cohen's d) = 0.76] and [mean difference = 0.14 (0.13–0.15), P value < 0.001, effect size (Cohen's d) = 2.73], respectively (Table 2).
| Outcome | Intervention group (Zinc group), n = 160 | Control group, n = 160 | Mean difference (95%CI) | P value | Effect size (Cohen’s d value) |
| Estimated total annual episodes of ARI per group | 40 | 147 | ---- | ---- | ---- |
| Estimated mean annual incidence of ARI (episodes/child-year); mean ± SD | 0.25 (0.61) | 0.92 (1.22) | -0.67 (-0.88 to -0.45) | < 0.001 | -0.69 |
| Estimated total annual episodes of diarrhoea per group | 166 | 331 | ---- | ---- | ---- |
| Estimated mean annual incidence of acute diarrhoea (episodes/child-year); mean ± SD | 1.04 (1.30) | 2.07 (2.09) | -1.03 (-1.42 to -0.65) | < 0.001 | -0.59 |
| Estimated mean annual duration of diarrhoea (days/child-year) | 3.10 (3.82) | 7.64 (7.67) | -4.54 (-5.87 to -3.20) | < 0.001 | -0.75 |
| Gain in weight over 6 months (gram) | 3150 (108) | 2818 (76) | 332 (310 to 352) | < 0.001 | 3.56 |
| Gain in length over 6 months (cm) | 10 (0.6) | 8.6 (0.4) | 1.4 (1.3 to 1.5) | < 0.001 | 2.74 |
| Estimated mean annual incidence of mild ARI | 0.11 (0.27) | 0.52 (0.68) | RR = 0.21 (0.1 to 0.32), RRR = 79% (68 to 90) | P = 0.037 | 0.69 |
| Estimated mean annual incidence of moderate to severe ARI | 0.14 (0.35) | 0.40 (0.53) | RR = 0.35 (-0.13 to 0.85), RRR = 65% (15 to 113) | P = 0.64 | 0.35 |
| Estimated mean annual incidence of mild diarrhoea | 0.63 (0.78) | 0.95 (0.96) | RR = 0.66 (0. 56 to 0.76), RRR = 34% (24 to 44) | P = 0.006 | 0.61 |
| Estimated mean annual incidence of moderate to severe diarrhoea | 0.41 (0.53) | 1.12 (1.13) | RR = 0.37 (0.28 to 0.46), RRR = 63% (54 to 72) | P = 0.002 | 1.07 |
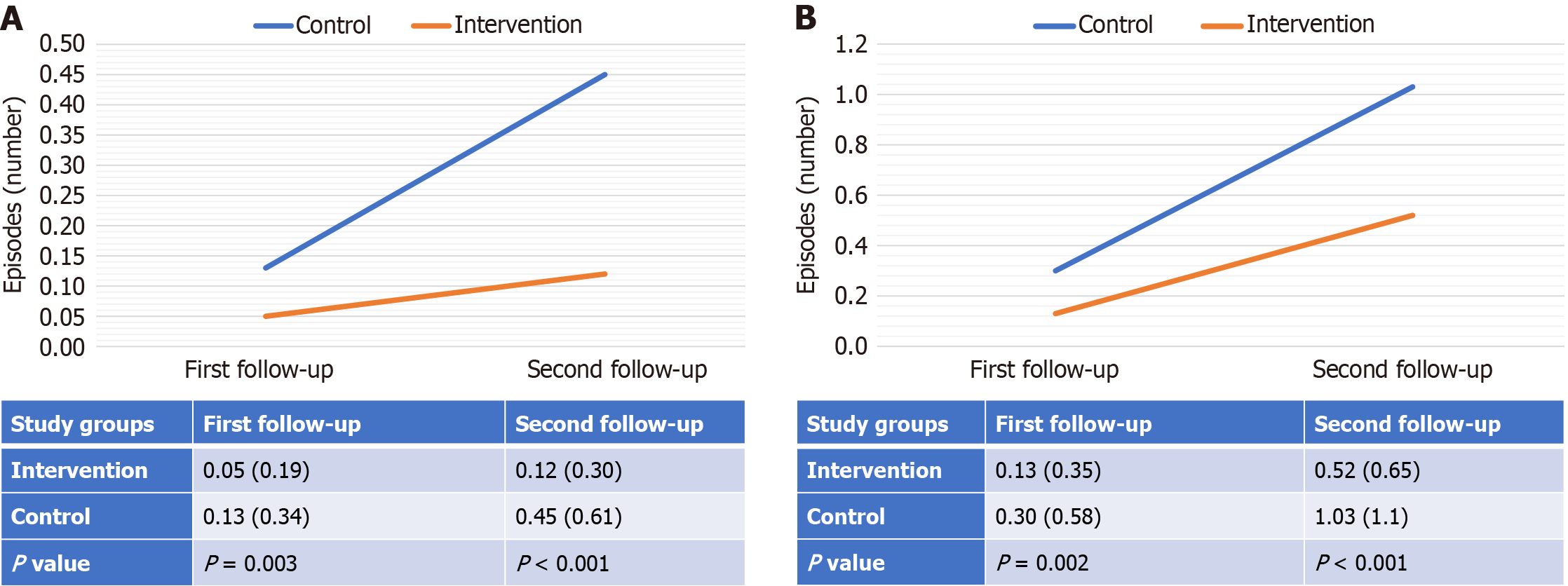
ANOVA test revealed that the intervention group had a statistically significant improvement in cumulative episodes of ARI and acute diarrhoea over time, compared to the control group, at both the 1st and 2nd follow-up(s) (Figure 3).
Similar improving trends were also observed for the gain in length and weight over time across the groups (Figure 4).
During the follow-up period, a few minor side effects were reported, including vomiting and constipation. However, there were no deaths reported, either due to the study drug or due to complications of the underlying diseases. These findings indicate that the treatment approach did not introduce any significant safety concerns during the course of the study.
The results of our study demonstrated a statistically significant reduction in the incidence of ARI by 72% and acute diarrhoea by 49% over a six-month follow-up period. These findings are consistent with existing evidence supporting the prophylactic efficacy of zinc. For ARI, Malik et al[15] reported a 62% reduction in incidence following two weeks of prophylactic zinc supplementation over five months of follow-up. Similarly, Osendarp et al[16] observed a 70% reduction in ARI episodes among healthy infants with low baseline serum zinc levels who received zinc prophylaxis. A Cochrane review by Lassi et al[14] concluded that zinc supplementation reduced ARI incidence and prevalence by 13% and 41%, respectively. However, some studies in healthy infants have shown no or non-significant reductions in ARI episodes, suggesting that the benefits of zinc may be more pronounced in zinc-deficient populations[17-20]. Despite this supporting evidence, the WHO has yet to recommend zinc supplementation for either the prevention or treatment of ARI[12].
About diarrhoea, a Cochrane review by Lazzerini et al[4] confirmed zinc's therapeutic role in reducing the duration and severity of diarrhoeal episodes, particularly in children from regions with high rates of zinc deficiency or malnutrition. The effect was more substantial among children aged six months or older, while evidence for younger infants remains limited. WHO and UNICEF advise zinc supplementation for the management of acute diarrhoea and to reduce the risk of recurrence over the next 2-3 months[10,11]. However, despite its therapeutic benefits, no official recommendation exists for its prophylactic use. Martinez-Estevez et al[21] demonstrated that daily prophylactic zinc supplementation for 12 months significantly reduced diarrhoea incidence, with a reported incidence rate ratio of 1.73 in the non-supplemented group. Additionally, a Cochrane review by Lassi et al[22] evaluating zinc supplementation in infants under six months found significant improvements in growth after six months, with standardized mean differences in WAZ and LAZ Z-scores of 0.16 (0.03-0.29) and 0.06 (0.07-0.19), respectively. Similarly, our study showed significantly higher gains in both WAZ and LAZ in the intervention group, at 0.32 (0.23-0.42) and 0.14 (0.13-0.15), respectively, over six months.
A notable limitation of our study was the absence of serum zinc measurements, which would have enabled direct assessment of zinc deficiency and its correction. Nevertheless, a national survey conducted between 2016 and 2018 reported a zinc deficiency prevalence of 18.9% among preschool-aged children in India[9], suggesting that the population studied was likely to be zinc-deficient. Another limitation was the open-label design, which may have introduced reporting bias for subjective outcomes. Caregivers aware of group allocation may have over- or under-reported sym
Despite these limitations, our findings are consistent with the broader body of evidence supporting the use of prophylactic zinc supplementation. The results suggest that intermittent zinc supplementation may be an effective strategy to reduce the burden of acute diarrhoea, ARI episodes, and associated growth delays, particularly in settings where zinc deficiency is prevalent, such as in India. However, as this trial did not include a placebo control, the findings are susceptible to potential bias. Therefore, further well-designed studies incorporating placebo controls are warranted to minimize such bias.
In our study, prophylactic intermittent zinc supplementation administered alongside routine vaccinations was shown to effectively prevent ARI and acute diarrhoea—two leading causes of mortality among infants and children under five worldwide. Additionally, this intervention was associated with improved growth, highlighting its significant public health implications, particularly in low-resource settings where zinc deficiency and related health issues are prevalent. Integrating prophylactic intermittent zinc supplementation into existing vaccination programs could serve as a cost-effective strategy to reduce morbidity and mortality associated with ARI, acute diarrhoea, and malnutrition in developing countries such as India. However, further research is needed before widespread implementation to determine optimal dosing regimens, assess long-term safety, and evaluate potential interactions with other micronutrients.
We are grateful to the Department of Paediatrics of the All India Institute of Medical Sciences, Patna for their assistance throughout the study. We are sincerely thankful to all the parents of our patients for their support and cooperation.
| 1. | Park K. Epidemiology of Communicable Diseases. In: Park K. Park's Textbook of Preventive and Social Medicine. 25th ed. Jabalpur: M/S Banarsidas Bhanot Pub, 2019: 180-187. |
| 2. | Srivastava A, Jagadisan B, Yachha SK. Disorders of Gastrointestinal System and Liver. In: Paul VK, Bagga A. Ghai Essential Pediatrics. 10th ed. New Delhi: CBS Publishers & Distributors Pvt, 2023: 309-314. |
| 3. | Black RE. Zinc deficiency, infectious disease and mortality in the developing world. J Nutr. 2003;133:1485S-1489S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 133] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Lazzerini M, Wanzira H. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev. 2016;12:CD005436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Sakulchit T, Goldman RD. Zinc supplementation for pediatric pneumonia. Can Fam Physician. 2017;63:763-765. [PubMed] |
| 6. | Suara RO, Crowe JE Jr. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob Agents Chemother. 2004;48:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6:e1001176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 513] [Cited by in RCA: 591] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 8. | National Family Health Survey [Internet]. [cited 24 March 2022]. Available from: http://rchiips.org/nfhs/index.shtml. |
| 9. | Gupta S, Brazier AKM, Lowe NM. Zinc deficiency in low- and middle-income countries: prevalence and approaches for mitigation. J Hum Nutr Diet. 2020;33:624-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 10. | World Health Organization. Zinc supplementation in the management of diarrhoea [Internet]. [cited 24 March 2022]. Available from: https://www.who.int/tools/elena/interventions/zinc-diarrhoea. |
| 11. | UNICEF Supply Division. UNICEF works to ensure reliable supply of co-packed oral rehydration salts and zinc for emergencies and programmes around the world [Internet]. [cited 24 March 2022]. Available from: https://www.unicef.org/supply/stories/oral-rehydration-salts-and-zinc-co-packaging-offers-simple-solution-save-children-diarrhoea. |
| 12. | World Health Organization. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months [Internet]. [cited 24 March 2022]. Available from: https://www.who.int/tools/elena/review-summaries/zinc-pneumonia-children--zinc-supplementation-for-the-prevention-of-pneumonia-in-children-aged-2-months-to-59-months. |
| 13. | Krishnan A, Kumar R, Broor S, Gopal G, Saha S, Amarchand R, Choudekar A, Purkayastha DR, Whitaker B, Pandey B, Narayan VV, Kabra SK, Sreenivas V, Widdowson MA, Lindstrom S, Lafond KE, Jain S. Epidemiology of viral acute lower respiratory infections in a community-based cohort of rural north Indian children. J Glob Health. 2019;9:010433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Lassi ZS, Moin A, Bhutta ZA. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 2016;12:CD005978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Malik A, Taneja DK, Devasenapathy N, Rajeshwari K. Zinc supplementation for prevention of acute respiratory infections in infants: a randomized controlled trial. Indian Pediatr. 2014;51:780-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Osendarp SJ, Santosham M, Black RE, Wahed MA, van Raaij JM, Fuchs GJ. Effect of zinc supplementation between 1 and 6 mo of life on growth and morbidity of Bangladeshi infants in urban slums. Am J Clin Nutr. 2002;76:1401-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Rosado JL, López P, Muñoz E, Martinez H, Allen LH. Zinc supplementation reduced morbidity, but neither zinc nor iron supplementation affected growth or body composition of Mexican preschoolers. Am J Clin Nutr. 1997;65:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 145] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Ruel MT, Rivera JA, Santizo MC, Lönnerdal B, Brown KH. Impact of zinc supplementation on morbidity from diarrhea and respiratory infections among rural Guatemalan children. Pediatrics. 1997;99:808-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 115] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Long KZ, Montoya Y, Hertzmark E, Santos JI, Rosado JL. A double-blind, randomized, clinical trial of the effect of vitamin A and zinc supplementation on diarrheal disease and respiratory tract infections in children in Mexico City, Mexico. Am J Clin Nutr. 2006;83:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Heinig MJ, Brown KH, Lönnerdal B, Dewey KG. Zinc supplementation does not affect growth, morbidity, or motor development of US term breastfed infants at 4-10 mo of age. Am J Clin Nutr. 2006;84:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 21. | Martinez-Estevez NS, Alvarez-Guevara AN, Rodriguez-Martinez CE. Effects of zinc supplementation in the prevention of respiratory tract infections and diarrheal disease in Colombian children: A 12-month randomised controlled trial. Allergol Immunopathol (Madr). 2016;44:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 22. | Lassi ZS, Kurji J, Oliveira CS, Moin A, Bhutta ZA. Zinc supplementation for the promotion of growth and prevention of infections in infants less than six months of age. Cochrane Database Syst Rev. 2020;4:CD010205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/