Published online Dec 9, 2025. doi: 10.5409/wjcp.v14.i4.108878
Revised: May 11, 2025
Accepted: August 12, 2025
Published online: December 9, 2025
Processing time: 190 Days and 2.7 Hours
Juvenile arthritis damage index (JADI) is a tool that measures the degree of agg
To evaluate the potential of JADI as a predictor of bDMARD treatment response in JIA patients.
This prospective study included 112 highly active non-systemic JIA biologic-naïve patients with a mean age of 12.2 ± 4.6 years and a median disease duration of 2.5 (interquartile range: 1-5) years. Their clinical and radiological assessment, juvenile arthritis disease activity score 71, JADI-A, and JADI-E, were evaluated twice: Before the biologic initiation (baseline) and 12 months after (end of study). At baseline, 50% had any damage, with 43% with articular damage and 23% with extraarticular damage.
During the study, JADI-A/JADI-E improved (33.9%/9.8%), worsened (8.9%/5.4%), or remained unchanged (57.1%/84.8%). Patients with baseline damage had higher markers of JIA activity: Polyarticular course, earlier onset age, ANA-positivity, and more active joints. Patients without initial structural damage (JADI“-“) were more likely (odds ratio = 3.8, 95% confidence interval: 1.6-9.0, P < 0.004) to achieve a low degree of activity or remission (46.2%), while on biological therapy, their scores were comparable to JADI-positive (18.3%). Pre-biological joint damage according to the JADI-A index (P = 0.003), wrist (P = 0.035), elbow (P = 0.027), cervical spine limitation of motion (P = 0.051), and erosions confirmed by magnetic resonance imaging (P = 0.002), were associated with poor response to biological treatment and follow-up JIA activity.
Baseline structural damage in JIA is associated with diminished bDMARDs efficacy, increased disability, and shorter remission duration. JADI enhances conventional clinical risk stratification by facilitating timely initiation of bDMARDs, adherence to treat-to-target strategy and tailored patient care.
Core Tip: This prospective study of biological-naïve juvenile idiopathic arthritis patients demonstrates that baseline articular/extraarticular damage (assessed via the juvenile arthritis damage index) predicts reduced response to biological disease-modifying antirheumatic drugs. Patients with baseline damage demonstrated higher disease activity (polyarticular course, elevated juvenile arthritis disease activity score 71, magnetic resonance imaging erosions) than those without damage. The observed juvenile arthritis damage index reduction in juvenile idiopathic arthritis patients showed significant associations with baseline joint damage status, treatment stability, and absence of uveitis/osteonecrosis. This index en
- Citation: Kolkhidova ZA, Nikishina IP, Glukhova SI, Melikhov OG, Kostik MM. Juvenile arthritis damage index predicts poor response to biological treatment: A prospective cohort study. World J Clin Pediatr 2025; 14(4): 108878
- URL: https://www.wjgnet.com/2219-2808/full/v14/i4/108878.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i4.108878
Juvenile idiopathic arthritis (JIA) is one of the most common chronic rheumatic diseases in children. It plays a significant role in developing functional disorders of the musculoskeletal system and extraarticular damage to structures that can be both temporary and irreversible[1]. Due to progress in JIA treatment, previously achieving remission or minimal disease activity has become a realistic and important goal of therapy, which is accompanied by a reduction in joint damage and a decrease in the degree of functional disorders[2].
According to the literature, factors influencing the effectiveness of biological therapy in patients with JIA include: The female sex[3,4], systemic JIA category[3,5], later age of disease onset[5-7], previous inefficiency, and the number of disease-modifying antirheumatic drug (DMARD)[5,8], high baseline score according to the questionnaire assessment of the functional status of the Childhood Health Assessment Questionnaire (CHAQ)[3], high disease activity according to the physician’s assessment, absence or short periods of remission in the past[9], the presence of uveitis[5], long disease duration[6], the presence of human leukocyte antigen B27 and enthesitis-related JIA category[10], rheumatoid factor (RF) and antibodies to cyclic citrullinated peptide positivity[3], high baseline level of pro-inflammatory cytokines[11], antirheumatic therapy compliance[8], and hip involvement[4].
One poorly studied but critically important factor determining the effectiveness of biological therapy in achieving remission or low disease activity is the aggressive course of arthritis, accompanied by the development of irreversible joint and extraarticular changes. According to research, early detection and prevention of joint damage are key in op
Disease activity in JIA is routinely monitored using composite measures such as [Juvenile arthritis disease activity score (JADAS)][13], ACRpedi criteria[14], the CHAQ[3], and criteria for Wallace et al’s study[15] inactive disease status. None of these tools provides a universal method for objective assessment of irreversible cumulative joint and organ damage over time, relying instead on subjective physician or patient evaluations. Psychological distress in children with JIA and their parents, along with the increased prevalence of anxiety and depression among parents of JIA patients, may distort the true clinical picture of the disease[16,17].
In 1999, Len et al[18] proposed the pediatric Escola Paulista de Medicina Range of Motion (ROM) Scale to reduce the reliance on subjective assessments and associated bias. This scale measures joint mobility in children with JIA by evaluating ROM. However, it cannot differentiate between ROM limitations caused by active inflammation vs persistent contractures resulting from chronic inflammation and irreversible joint and periarticular soft tissue damage.
The pediatric Escola Paulista de Medicina ROM Scale does not assess ROM in small joints of hands, feet, or temporomandibular joints, which are frequently affected in JIA patients, thus limiting comprehensive functional evaluation[18]. These limitations prompted development of the juvenile arthritis damage index (JADI), a user-friendly instrument specifically designed for evaluating cumulative JIA-related damage[19].
This index comprises both JADI - articular damage (JADI-A) and JADI - extraarticular damage (JADI-E) assessments. The JADI was designed for rapid and convenient calculation based on physical examination and retrospective review of patient medical records. The JADI evaluates damage severity, defined as persistent changes resulting from either previous active disease or treatment-related complications[19].
The articular component (JADI-A) specifically assesses three principal forms of joint damage that persist for ≥ 6 months and are unrelated to current active arthritis: (1) Limited ROM; (2) Ankylosis; and (3) Prior surgical interventions, including joint replacement[19].
The JADI was validated in a cross-sectional study of 158 JIA patients meeting International League of Associations of Rheumatology classification criteria (excluding enthesitis-related arthritis) with ≥ 5 years disease duration. Both JADI-A and CHAQ showed comparable strong correlations with Steinbrocker functional class. However, JADI-A demonstrated: (1) Stronger association with number of joints with limited ROM (ρ = 0.73 vs 0.61) and Poznanski score (ρ = 0.68 vs 0.52); and (2) Weaker correlation with active joint count than CHAQ (ρ = 0.42 vs 0.61).
The extra-articular damage index (JADI-E) significantly correlated with functional class[19]. The JADI has been utilized to identify JIA predictors and establish criteria for biologic therapy initiation. In their landmark study, Russo and Katsicas[20] utilized JADI assessment to demonstrate that cervical spine involvement and corticosteroid use within the first 6 months of disease constitute independent predictors of both global and articular damage in JIA. Key predictors for early biologic therapy identified through JADI evaluation include: (1) Polyarticular JIA subtype; (2) Upper extremity, hip, or ankle joint involvement within 6 months of diagnosis; and (3) > 35% disease activity time during the first year[21,22].
JADI has proven to be a simple, practical tool for comprehensive assessment of: (1) Chronic articular damage; (2) Extra-articular manifestations; and (3) Long-term inflammatory consequences in JIA. Notably, no prior studies have investigated the relationship between joint damage, as quantified by JADI, and treatment response patterns. This study aimed to evaluate the JADI damage index’s prognostic value in predicting the response to biological therapy among patients diagnosed with JIA.
In the prospective cohort study analysis, 112 biologic-naïve patients with non-systemic JIA categories were included.
(1) Diagnosis of JIA according to the ILAR (International League of Associations for Rheumatology) criteria[23]; (2) Non-systemic JIA categories: Oligoarthritis (persistent and extended), polyarthritis (RF positive and negative), enthesitis-related arthritis, and psoriatic arthritis; (3) Treatment with either non-biologic (nb) or biologic disease-modified antirheumatic drugs (bDMARDs) or their combination; and (4) Age at study inclusion of 2-17 years.
JIA with systemic onset, undifferentiated JIA category.
The following parameters were evaluated in each patient.
Demography: Sex, onset age, disease duration, JIA category.
Clinical assessment: Number of active joints, swollen joints, painful joints, joints with limited ROM, clinical assessment of JIA activity with visual-analogue scale (VAS): Physician’s VAS (MDVAS) and patients/parental VAS, JADAS 71[13]. CHAQ[3,24], assessment of JADI-A and JADI-E[19]. According to the JADI assessment method, the patient was marked as positive if he/she had at least one sign of articular or extraarticular damage[19]. Uveitis was confirmed via split-lamp examination with an ophthalmologist, and the previous records were used if uveitis was detected earlier.
The joint was considered damaged only in the case of persistent limitation ROM unrelated to current active arthritis and present for at least 6 months, despite previous therapy, including exercise and rehabilitation. The damage is often irreversible and cumulative, and therefore, the index value is most often expected to increase or remain stable over time. However, some forms of damage may regress or even disappear over time in children[19].
The articular and extraarticular damage indices JADI-A and JADI-E were measured at the time of each patient examination by the same researcher (Kolkhidova ZA). The methodology for calculating the JADI-A index was based on a routine clinical examination using a goniometer to measure the angles of contractures and calculate the total points according to the table described by the index developers (Supplementary Table 1). This study used the Russian version of the electronic calculator based on the Yandex Forms service (Supplementary material) to simplify the JADI-A calculation.
The JIA activity according to JADAS71 was determined according to previously published cut-offs[13]: (1) Oli
Laboratory assessment of JIA activity: RF, anti-cyclic citrullinated peptide (antibodies to cyclic citrullinated peptide), and antinuclear antibody (ANA). ANA was considered positive if its titer was at least 1/320 in the human epithelial type 2 immune-fluorescent assay.
Imaging assessment: Joint X-ray and magnetic resonance imaging (MRI) were performed according to the attending physician’s opinion. An independent radiologist performed interpretation, and the following patterns were analyzed: Early signs of arthritis (periarticular osteoporosis), presence of bone erosions and ankyloses on X-ray, and bone marrow edema, suggesting osteitis or the presence of erosions on MRI. In cases of multiple patterns, the worst indicator was considered.
Treatment: Previous treatment with nbDMARDs (number of drugs and name of the drugs) and corticosteroid use were evaluated before the baseline and during the study.
Individual case report forms and an electronic database were developed and completed for all patients. The patients were examined at two control points: (1) At the time of the biological treatment initiation (baseline); and (2) 6-12 months after its start (end of study) in real clinical practice. The baseline examination, which included a detailed assessment of clinical and anamnestic parameters, joint status, and determination of the JADI damage index, was directly performed by the same contractor (Kolkhidova ZA).
The attending physician determined the scope and choice of clinical laboratory and instrumental assessments because this study was based on data from real clinical practice and implied the use of a set of routine laboratory and instrumental tests, as well as additional assessments by the decision of the attending physician, which were determined by a specific clinical situation.
The sample size for a studied population with a margin of error of 5% and 95% significance is 87 patients. All statistical calculations were performed with the Statistica 10 software package (Tulsa, OK, United States). For all numerical data, preliminary testing for the normality of the distribution was performed using the Shapiro-Wilk test, and the quantitative data were presented as means with standard deviations or medians with interquartile ranges (IQR) if the distribution was normal or not, respectively. The absolute number (n) and the percentage (%) are reported for categorical variables. Missing data were not included in the analysis. The sets of independent quantitative variables were compared using the Mann-Whitney and χ2 tests used for categorical variables. In cases where the expected frequency was lower than 5, Fisher’s exact test was applied. Wilcoxon matched pairs test was performed for two dependent numerical variables. Sensitivity and specificity were assessed for each of the studied parameters to assess the ability of each feature to distinguish patients with dichotomous characteristics. For quantitative variables, critical values were calculated using the area under the receiver-operating curve with a 95% confidence interval (CI), and the odds ratio was calculated using a 2 × 2 table without considering the time of the event of interest. Independent predictors of damage were identified as the event of interest using binary logistic regression by including quantitative and qualitative indicators related to the dependent variable in the analysis. A prognostic model for the risk of a certain outcome (damage) was constructed using multivariate logistic regression. Independent variables were selected using stepwise direct selection with the Wald statistic as an exclusion criterion. All significant predictors were included in the following multiple regression analysis, excluding the duplicated factors to avoid multicollinearity. Multicollinearity was assessed with correlation analysis and clinically meaningful overlap. Differences were considered statistically significant at P < 0.05.
Written informed consent was obtained according to the Declaration of Helsinki Study, and participation was voluntary. The local Ethics Committee of the V.A. Nasonova Institute of Rheumatology approved the trial protocol, No. 22, dated 2 December 2021.
The cohort (n = 112) had a mean age of 12.6 ± 4.5 years with female predominance (58% vs 42%). Median disease duration was 1.9 years (IQR: 1.0-3.9). All patients exhibited high disease activity [JADAS71: 22 (16.1-27.9)] and received either biologic or synthetic DMARDs.
Biologic/synthetic DMARDs including: Etanercept: 50 (45%); adalimumab: 36 (32%); golimumab: 14 (12%); secukinumab: 3 (3%); and tofacitinib: 9 (8%).
Prior nbDMARD exposure (n = 104, 93%) included: (1) Single nbDMARD (n = 84, 75%): Methotrexate: 81 (72%); and sulfasalazine: 3 (2.7%); (2) Sequential nbDMARD combinations (n = 19, 17%): Methotrexate + sulfasalazine: 14 (12.5%); methotrexate + leflunomide: 2 (2%); methotrexate + cyclosporine: 3 (2.7%); and methotrexate + hydroxychloroquine: 1 (0.9%); and (3) Triple therapy (n = 1, 0.9%): Methotrexate + sulfasalazine + leflunomide.
Corticosteroid use: (1) Historical: 17 (15%); and (2) Baseline: 4 patients - 1.25 mg/day (n = 3) and 2.5 mg/day (n = 1).
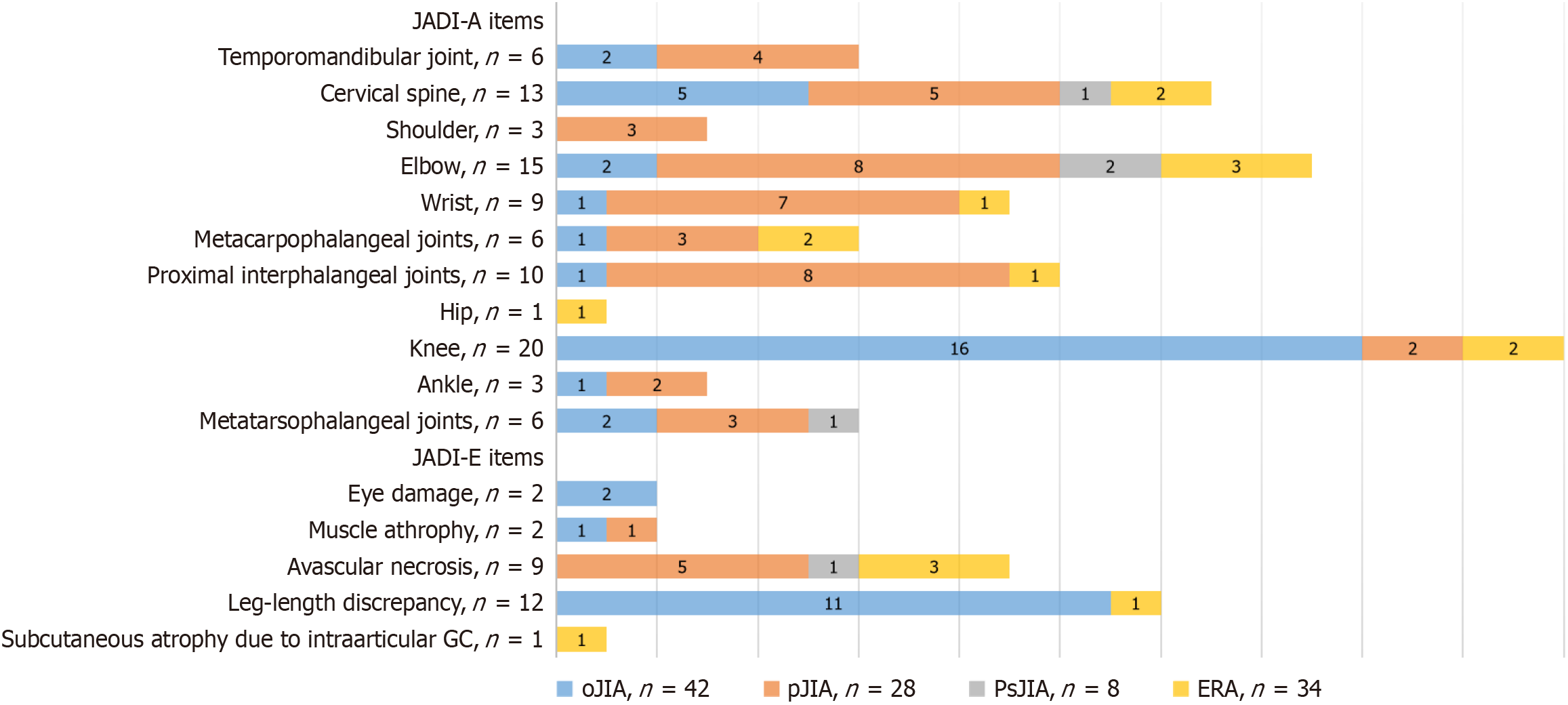
The JADI-positive group comprised 60 patients (53.5%), with: (1) 52 (46%) showing articular damage (JADI-A+); (2) 25 (22%) having extra-articular damage (JADI-E+); and (3) 17 (15%) demonstrating both damage types. Damage severity (median scores) were JADI-A: 2 (IQR: 1-4) and JADI-E: 0 (IQR: 0-1). Distribution of joint damage in JADI-positive patients is presented at Table 1.
| Parameter | Se | Sp | OR (95%CI) | RR (95%CI) | P value |
| Polyarthricular JIA category | 35.0 | 86.5 | 3.5 (1.3-9.0) | 1.6 (1.2-2.2) | 0.017 |
| ANA positivity | 45.0 | 75.0 | 2.5 (1.1-5.5) | 1.5 (1.1-2.1) | 0.027 |
| Arthritis of ≥ 5 joints at onset | 25.0 | 90.4 | 3.1 (1.1-9.3) | 1.5 (1.1-2.1) | 0.034 |
| Active joints > 8 at the study inclusion | 55.0 | 76.9 | 4.1 (1.8-9.3) | 1.8 (1.3-2.6) | < 0.001 |
| Parental/patient VAS > 6 cm | 53.3 | 71.2 | 2.8 (1.3-6.2) | 3.2 (1.6-6.2) | 0.009 |
| MDVAS > 6 cm | 88.3 | 50.0 | 7.6 (2.9-19.7) | 3.2 (1.6-6.2) | < 0.001 |
| JADAS71 > 17.7 points | 81.7 | 48.1 | 4.1 (1.8-9.7) | 2.1 (1.3-3.6) | 0.001 |
| CHAQ > 0 points | 68.3 | 59.6 | 3.2 (1.5-6.9) | 1.7 (1.2-2.6) | 0.003 |
| Ankylosis, confirmed by X-ray | 29.8 | 97.9 | 19.5 (2.4-155.8) | 2.2 (1.7-3.0) | 0.008 |
| MRI-confirmed erosions | 49.0 | 90.9 | 12.5 (3.8-40.9) | 2.2 (1.6-3.1) | < 0.001 |
Positive extra-articular manifestations were observed in 25 patients (22%): (1) Leg length discrepancy (> 1 cm): 12 (11%); and (2) Imaging-confirmed osteonecrosis: 9 (8%). No cases of growth retardation, pubertal delay, osteoporosis, fractures, diabetes mellitus, or morphologically confirmed amyloidosis were documented. The pattern of JADI-assessed damage across JIA categories is presented in Figure 1.
Univariate analysis demonstrated the following significant predictors of any structural damage included: (1) Disease characteristics: Polyarticular JIA category; ANA positivity; and baseline arthritis affecting ≥ 5 joints; (2) Disease activity markers: > 8 active joints at enrollment; Parent/patient VAS > 6 cm; MDVAS > 6 cm; JADAS71 score > 17.7; and CHAQ score > 0; and (3) Structural findings: Radiographically confirmed ankylosis; MRI-documented erosions.
Sensitivity, specificity, odds ratios, and relative risk calculations are presented in Table 2. Multivariate regression analysis (r2 = 0.33; P = 4 × 10-8) identified three independent predictors (Table 3): (1) > 8 active joints at enrollment; (2) MDVAS > 6 cm; and (3) MRI-confirmed erosions.
| Predictor | Β | SE | P value |
| Intercept | 0.108 | 0.084 | 0.200 |
| Active joints > 8 at the study inclusion | 0.191 | 0.093 | 0.044 |
| MDVAS > 6 cm | 0.318 | 0.102 | 0.003 |
| MRI-confirmed erosions | 0.355 | 0.097 | < 0.001 |
| Parameter | JADI (+ ve), n = 60 | JADI (- ve), n = 52 | P value |
| Age at study inclusion, years, IQR | 11.6 (7.5-15.4) | 13.1 (9-16.1) | 0.244 |
| Sex, females | 36 (60.0) | 29 (55.7) | 0.651 |
| JIA category, according to the ILAR[3] | |||
| Oligoarthritis | 23 (38.0) | 19 (36.5) | 0.845 |
| Polyarthritis | 21 (35.0) | 7 (13.5) | 0.017 |
| Psoriatic arthritis | 4 (7.0) | 4 (8.0) | 0.834 |
| Enthesitis-related arthritis | 12 (20.0) | 22 (42.0) | 0.019 |
| JIA onset age, years, IQR | 8.7 (3-12) | 10.1 (6.9-13.1) | 0.079 |
| Diagnosis delay, moths, IQR | 3.9 (1-12) | 4 (1.5-10) | 0.560 |
| JIA duration, years, IQR | 2.3 (0.8-4.5) | 1.6 (0.9-3.2) | 0.360 |
| Time before non-biologic DMARDs, months, IQR | 5 (2-14) | 6 (2.2-23) | 0.491 |
| ANA positivity (> 1:320) | 27 (45.0) | 13 (25.0) | 0.027 |
| RF-positivity | 6 (10.0) | 2 (3.8) | 0.207 |
| ACCP-positivity | 6 (10.0) | 1 (1.9) | 0.078 |
| Enthesitis | 6 (10.0) | 14 (26.9) | 0.019 |
| Uveitis | 6 (10.0) | 4 (7.7) | 0.669 |
| Arthritis of ≥ 5 joints at onset | 15 (25.0) | 5 (9.6) | 0.034 |
| Number of past nbDMARDs | |||
| No | 3 (5.0) | 5 (9.6) | 0.345 |
| One | 47 (78.3) | 37 (71.2) | 0.512 |
| Two | 10 (16.7) | 9 (17.3) | 0.872 |
| Three | 0 (0) | 1 (1.9) | 0.943 |
| No episodes of nbDMARDs therapy interruption | 45 (75.0) | 41 (78.8) | 0.631 |
| Past corticosteroid treatment | 11 (18.3) | 6 (11.5) | 0.314 |
| Past corticosteroid treatment duration | - | - | 0.622 |
| < 3 months | 8 (13.3) | 5 (9.6) | - |
| ≥ 3 months | 3 (5.0) | 1 (1.9) | - |
| Corticosteroid treatment duration, IQR | 1 (0.3-6) | 1.8 (0.2-2.6) | 0.802 |
| Remission duration ≥ 6 months in the past | 5 (8.3) | 5 (9.6) | 0.812 |
| Active joints, IQR | 9 (6-17.5) | 6 (4-8) | < 0.001 |
| Swollen joints, IQR | 8 (6-15) | 5 (3-7.5) | < 0.001 |
| Painful joints, IQR | 8 (5.5-15) | 6 (3.5-8) | 0.001 |
| Joints with limited ROM, IQR | 3 (2-4.5) | 2 (1.5-2) | < 0.001 |
| Parental/patient VAS (cm), IQR | 7 (5-7) | 2 (1.5-2) | 0.045 |
| MDVAS (cm), IQR | 7 (7-8) | 6 (4-8) | < 0.001 |
| ESR (mm/hour), IQR | 10 (5-24) | 11 (5.5-19) | 0.896 |
| С-reactive protein (mg/L), IQR | 1.8 (0.3-10.5) | 1.5 (0.2-5.6) | 0.512 |
| JADAS71, IQR | 23.5 (20-34.2) | 19.1 (15-25) | < 0.001 |
| CHAQ, IQR | 0.4 (0-0.8) | 0 (0-0.3) | 0.003 |
| X-ray changes | 47/60 (78.3) | 32/52 (61.5) | - |
| Early signs | 25/47 (53.2) | 29/47 (90.6) | 0.002 |
| Erosions | 8/47 (17.0) | 2/47 (6.3) | 0.286 |
| Ankylosis | 14/47 (29.8) | 1/47 (3.1) | 0.008 |
| MRI-changes | 51/60 (85.0) | 44/52 (84,6) | < 0.001 |
| Bone marrow edema | 26/51 (51.0) | 40/44 (90.9) | - |
| Erosions | 25/51 (49.0) | 4/44 (9.1) | - |
The JADI-positive group exhibited significantly different baseline characteristics compared to patients without damage. JIA polyarticular category, the initial involvement of five or more joints, irrespective of JIA category, ANA positivity, more active, painful, swollen joints and joints with limited ROM, higher parental VAS and MDVAS, higher CHAQ and JADAS71 scores, radiological findings were typical for the JADI positive group. The detailed characteristics are in Table 4.
| Parameter | Improved JADI, n = 41 | Non-improved JADI, n = 19 | P value |
| Presence of the JADI–A at the baseline | 38 (92.7) | 14 (73.7) | 0.044 |
| Baseline JADI-A, IQR | 2 (1-5) | 1 (0-2) | 0.014 |
| Patients with uveitis | 2 (4.9) | 4 (21.1) | 0.05 |
| Treatment with nbDMARDs at the EOS | 40 (97.6) | 14 (73.7) | 0.004 |
| Stable nbDMARD treatment during the study | 39 (95.1) | 12 (63.2) | 0.001 |
| Stable bDMARD treatment during the study | 36 (87.8) | 12 (63.2) | 0.03 |
| Bone osteonecrosis (JADI-E) | 3 (7.3) | 6 (31.6) | 0.014 |
| Eye damage (JADI-E) | 0 (0) | 2 (10.5) | 0.034 |
| MRI-confirmed bone erosions, n/n performed (%) | 14/37 (37.8) | 11/15 (73.3) | 0.021 |
| bDMARD treatment: | 0.297 | ||
| Etanercept | 19 (46.3) | 8 (42.1) | |
| Golimumab | 2 (4.9) | 3 (15.8) | |
| Adalimumab | 14 (34.1) | 8 (42.1) | |
| Tofacitinib | 4 (9.8) | 0 (0) | |
| Secukinumab | 2 (4.9) | 0 (0) | |
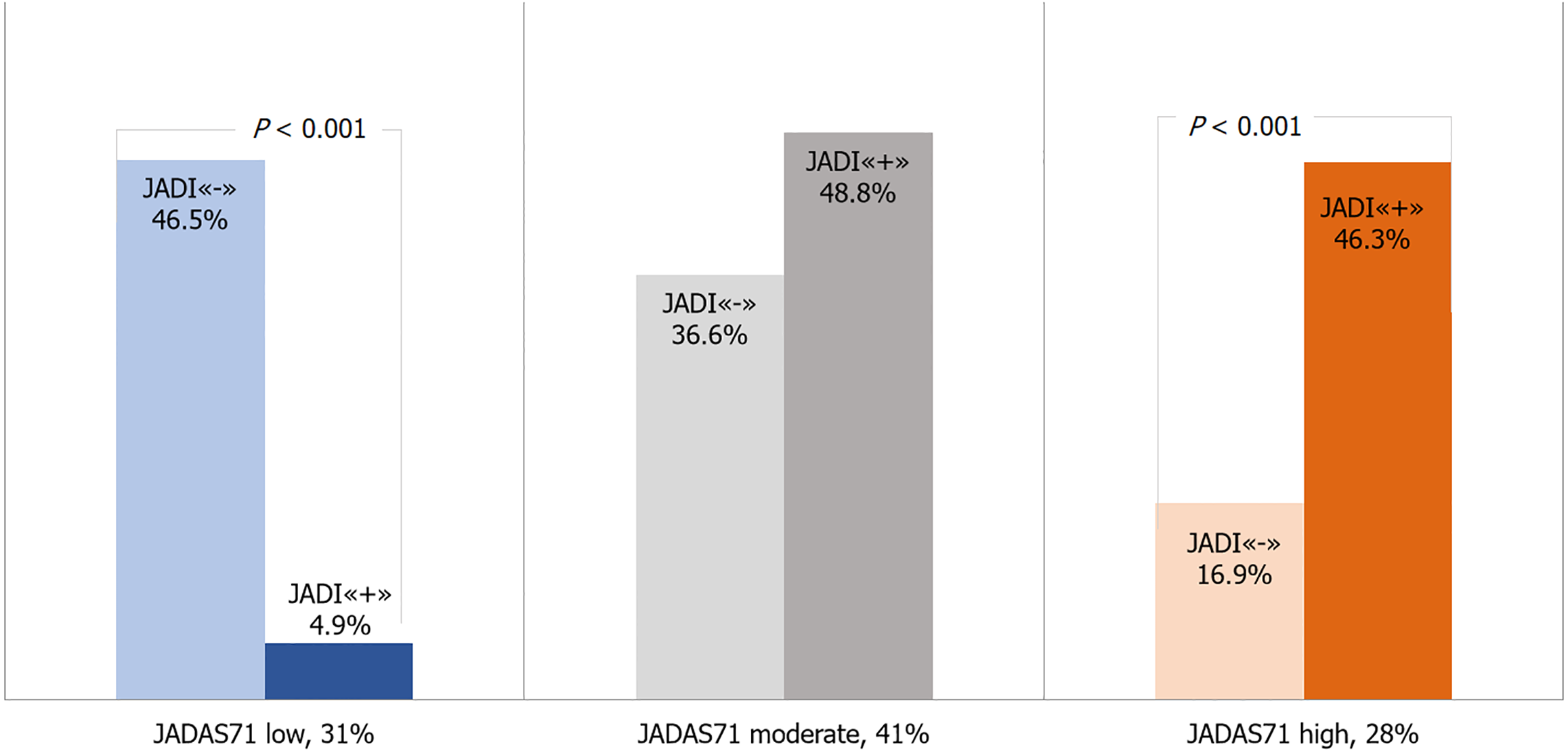
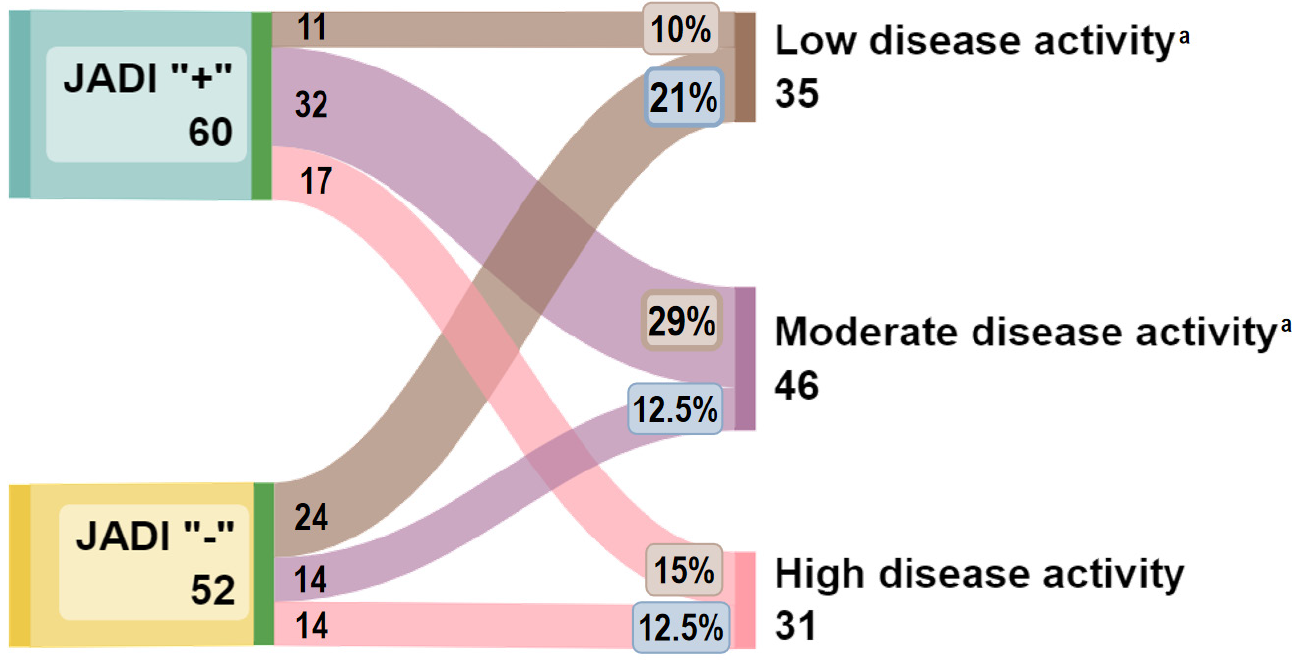
At the time of the follow-up examination, the clinical activity indicators were significantly lower than before the initiation of biological drugs: The median of the JADAS71 index decreased from 22 (16.1-27.9) to 5 (3-9.5), P < 0.001. Low JIA activity, according to the JADAS71 index, was achieved in 35 (31%) patients, moderate activity in 46 (41%) patients, and high activity in 31 (28%) children the degree of JIA activity.
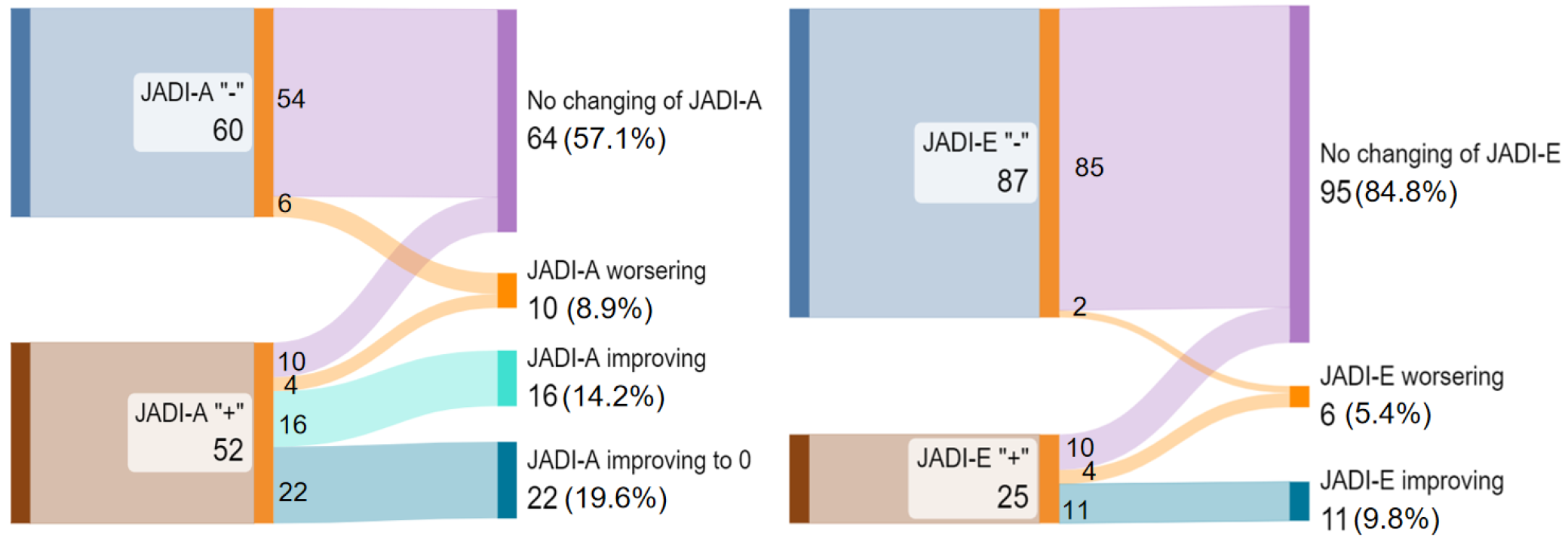
According to Wallace’s criteria, 14 (12.5%) patients reached the inactive stage of the disease. A positive JADI damage index was found on the follow-up examination in 51 (45%) patients. Joint damage JADI-A“+” was recorded in 34 (30%), extraarticular JADI-E“+” in 16 (14%) children, and the presence of both joint and extraarticular damage was noted in 10 (9%) of patients (Figure 2).
The JADI-A index decreased from 2 (1-4.5) in 52 children (46.4%) to 2 (1-3) in 34 children (30.4%), with a statistically significant difference (P < 0.001). The median JADI-E score changed from 1 (1-1) in 25 patients (22.3%) to 1 (1-2) in 16 patients (14.3%), although this difference was not statistically significant (P = 0.421). The JADI-E index increased in four patients was observed following cataract surgery initiated after biologic therapy due to a reduction in JIA activity.
We analyzed factors that potentially influenced the improvement in the JADI damage index (Table 5). Joint contractures at treatment initiation are more amenable to reversal than extraarticular lesions. Specifically, the presence of uveitis with irreversible complications, as well as erosions or osteonecrosis at the initiation of biological treatment, adversely affects the reversibility of damage. Additionally, the stability of both conventional and biological therapies following initiation significantly positively impacts the dynamics of the JADI damage index.
| Parameter | Improved JADI-A, n = 38 | Non-improved JADI-A, n = 14 | P value |
| Baseline JADI-A, IQR | 2 (1-6) | 1.5 (1-2) | 0.096 |
| Patients with uveitis | 2 (5.3) | 2 (14.3) | 0.278 |
| Treatment with nbDMARDs at the EOS | 38 (100) | 9 (64.3) | 0.001 |
| Stable nbDMARD treatment during the study | 37 (97.4) | 8 (57.1) | 0.001 |
| Stable bDMARD treatment during the study | 33 (86.8) | 9 (64.3) | 0.067 |
| Bone osteonecrosis (JADI-E) | 2 (5.3) | 3 (21.4) | 0.079 |
| MRI-confirmed bone erosions | 13/35 (37.1) | 8/12 (66.7) | 0.075 |
| bDMARD treatment | 0.249 | ||
| Etanercept | 17 (44.7) | 5 (35.7) | |
| Golimumab | 2 (5.3) | 3 (21.4) | |
| Adalimumab | 13 (34.2) | 6 (42.8) | |
| Tofacitinib | 4 (10.5) | 0 (0) | |
| Secukinumab | 2 (5.3) | 0 (0) | |
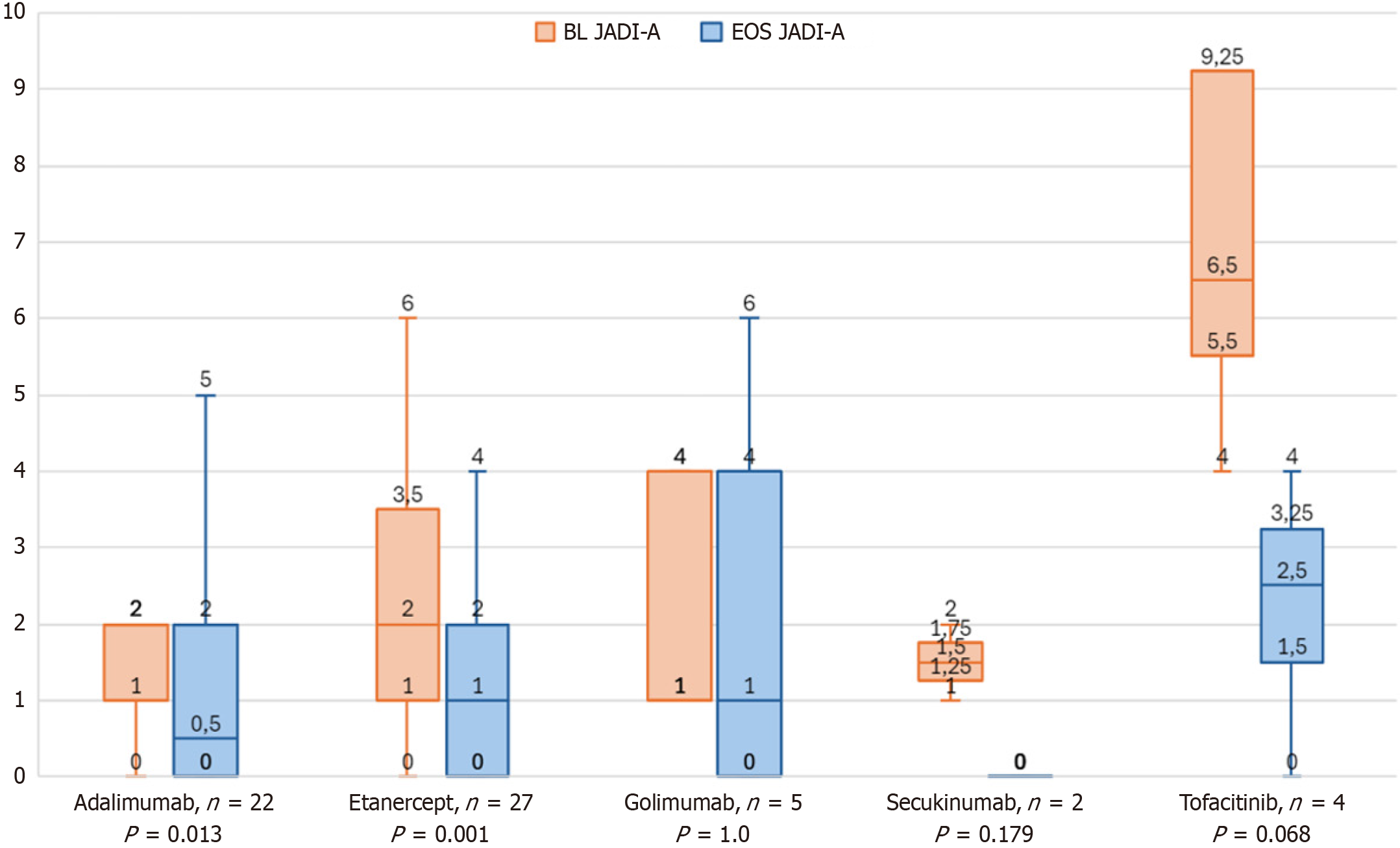
The largest decrease in JADI (Figure 3) was observed in patients treated with tofacitinib (-4.0 points). The number of patients with improved JADI ranged from 100% (tofacitinib and secukinumab) to 70.4% (etanercept), 63.6% (adalimumab), and 40% (golimumab). Post-hoc evaluation revealed significant findings that stable therapy bDMARD
| Predictor | Β | SE | P value |
| Intercept | -0.026 | 0.198 | 0.896 |
| Treatment with nbDMARDs at the EOS | 0.343 | 0.119 | 0.006 |
| Stable bDMARD treatment during the study | 0.249 | 0.119 | 0.042 |
The relationship between the severity of JIA based on the JADAS71 score and the frequency of JADI damage has been identified, allowing the JADI score to predict the future effectiveness of biological treatment (Figure 4). We conducted a comparative analysis of the activity parameters at the follow-up assessment, considering initial damage (JADI-positive) at the time of biological treatment initiation. One year after therapy initiation, patients with initial damage exhibited higher values for the JADAS71 and the CHAQ [median 0 (0-0.4) vs 0 (0-0), P = 0.005]. The VAS scores for patients and their parents/guardians [2.5 (2-4) vs 2 (1-3), P = 0.026] and MDVAS also showed increased values [3 (2-4) vs 1.5 (1-3), P = 0.004]. Additionally, patients with initial damage were less likely to achieve disease remission according to the Wallace criteria
Patients without initial structural damage (JADI “-”) were more likely (OR = 3.8, 95%CI: 1.6-9.0, P < 0.004) to achieve a low degree of activity (n = 24/52; 46.2%) after starting biological therapy, compared with JADI-positive patients (n = 11/60; 18.3%). When treatment was initiated, children with JADI“+” showed a moderate level of disease activity during the follow-up assessment, which was significantly higher compared to JADI“-” (n = 32, 29% vs n = 14, 12.5%, P < 0.05). The high activity degree in patients being on biological therapy did not depend on the initial structural damage (JADI“+” n = 17; 15% vs JADI“-” n = 14; 12.5%) before the start of biological treatment (Figure 5).
A comparative analysis of patients with a low degree of activity and patients with a moderate/high degree of activity according to the JADAS71 index was conducted to find predictors of refractory to targeted therapy. A moderate-high degree of activity (JADAS71) at the follow-up assessment was significantly associated with the following indicators at the time of biological treatment initiation: Joint damage according to the JADI-A index (n = 9; 26% vs n = 43; 56%, P = 0.003), wrist contracture (n = 0; 0% vs 9; 12%, P = 0.035), elbow joints (n = 1; 3% vs n = 14; 18%, P = 0.027), cervical spine limitation of motion (n = 1; 3% vs n = 12; 16%, P = 0.051), and erosions confirmed by MRI (n = 2; 7% vs n = 27; 39%, P = 0.002).
Many studies have been conducted globally to predict the response to biological therapy in JIA[25-34]. However, the role of damage indices in this context has been inadequately explored despite compelling evidence suggesting their pivotal significance. These indices are crucial, as they reflect the long-term outcomes of therapeutic interventions[6,13,21,22,35,36]. We assumed that the damage index is the most significant indicator for assessing long-term effectiveness of therapy, integrating all aspects of its effects. At the same time, the number of papers devoted to this problem is extremely limited[6,29,37,38], and most of them are of a one-step, cross-sectional design, rarely dividing patients into those who had no experience with biological therapy and those who had already undergone such treatment. The distinctive feature of our research is the establishment of a prospective follow-up cohort, wherein the alterations in the JADI index were systematically observed during the administration of biological therapy. Additionally, we assessed JADI’s contribution to predicting this therapeutic intervention’s efficacy.
Among 112 patients who did not receive biological therapy, 53.5% had long-term changes manifested by a positive JADI index. JADI-A“+” was diagnosed in 46% of the participants, JADI-E“+” in 22%, and a combined (JADI-A and JADI-E) was detected in 15% of the patients. Similar rates of irreversible changes among children before the biological therapy have been described in studies by Italian and Indian colleagues: JADI-A“+” was registered in 32.4% and 60.7%, and JADI-E“+” - in 26.1% and 39.3%, respectively[39-41]. However, these studies were conducted 16 years earlier, in 2008, when the use of biological drugs had just appeared and was not generally available for children with JIA. The median duration of arthritis in these studies was 6.7 (5.4-9.3) and 5 (1-20) years, while in our cohort, this indicator was significantly lower - 1.9 (1.0-3.9) years. In more modern studies performed in Thailand in 2023[42] and India in 2018[43], the prevalence of JADI-A“+” among patients was 36.6% and 30.6%, and JADI-E“+” was 22.2% and 24%, respectively. At the same time, the median duration of the disease in these studies was comparable to that in our study: 2.7 (0.9-4.9) and 2 (0.6-5.0) years.
In our study, we focused on a group of patients with polyarticular JIA, among whom 75% had a relationship between joint damage and the maximum JADI score of 5 points (2-14). In most cases, the damage index for polyarticular JIA included contractures of the joints of the upper extremities: Elbow (28.5%), proximal interphalangeal (28.5%), and wrist (25%) joints. Such localization of lesions is consistent with the research data of Solari and colleagues, as well as Sarma and coauthors: Involvement of the wrist joints ranged from 23% to 60%, proximal interphalangeal joints - from 24% to 40%, elbow joints - from 22% to 38% in RF-positive and RF-negative poliarticular JIA categories[40,41]. Routine markers of current disease activity, encompassing the number of active joints, the number of joints with limited function, the painful and swollen joints count, the visual analog scale assessments by both the physician and the patient/parent, the JADAS index, and the CHAQ questionnaire, demonstrated a statistically significant association with the presence of joint damage in patients, consistent with the findings of previous studies[27,38,40].
To confirm the validity of the JADI index in evaluating therapy effectiveness, we conducted a comparative analysis between the JADAS71 activity index and the JADI damage index. At the follow-up observation point, a significant correlation was found between these indicators, and a statistically significant change in the damage index during therapy was confirmed.
The prospective study by Susic et al[44] (n = 87, 2-year follow-up) remains the only published work examining JADI progression under biologic therapy (etanercept initiation in 50.6% cohort). The findings revealed paradoxical trends: The proportion of patients with articular damage increased from 36.8% to 41.4%, extraarticular - from 26.4% to 39.1% (P < 0.01). The mean values of JADI-A increased from 3.00 ± 6.51 to 3.62 ± 7.33 (P < 0.001), JADI-E - from 0.59 ± 1.29 to 0.84 ± 1.44 (P < 0.001). The JADI-A raise correlated with radiological progression, disease duration, and glucocorticoid therapy duration. However, the study’s limitations (small sample, lack of control) did not allow us to identify factors influencing the progression of JIA[40,41].
Our study has established the positive dynamics of JADI-A during biological therapy. The prevalence of joint damage decreased from 46.4% to 30.4%, and the median JADI-A decreased from 2 (1-4.5) to 2 (1-3) (P < 0.001). Extraarticular damage at the follow-up assessment was noted in a smaller number of children: 22.3% and 14.3%, respectively. However, the median JADI-E increased from 1 (1-1) to 1 (1-2) (P = 0.421). Considering the potential for reduction of the JADI Index due to the reversibility of contractures, it is advisable to utilize the joint component of the index (JADI-A) to evaluate the dynamic progression of JIA, as opposed to the extraarticular component (JADI-E), which primarily encompasses irreversible changes[19]. The findings of our study with the literature data support the rationale for employing the JADI-A index to assess disease progression and the efficacy of therapeutic interventions.
Despite the significant results obtained, the present study has several limitations to consider when interpreting the data and planning further work. It is an uncontrolled single-center study with real-world data.
Limited sample size and its structure: One of the main limitations of our study is the sample size and the small number of clinical variants of JIA. Although enough patients were included in the analysis, a larger sample could have improved statistical power and provided more reliable results when analyzing subgroups.
Single-center study: Patients undergoing inpatient treatment at one federal center (V.A. Nasonova Institute of Rheumatology) were included; outpatient patients and children observed at their residences by regional rheumatologists were not included. Differences in treatment methods, available resources, and patient populations may affect the generalization of our findings.
Unbalanced sample: The predominance of patients with high disease activity at the start of biological therapy may create a bias in assessing response predictors because initially, more severe cases have less reserve to achieve remission. The article’s authors could not influence the timing of the diagnosis, the appointment of therapy with non-biological DMARDs, or the timing of treatment at the research organization. These factors could have influenced the damage index’s presence and appearance.
Short-term follow-up: The effectiveness of biologic therapy was evaluated after 6-12 months, which is insufficient to analyze long-term outcomes such as damage progression or sustained response to therapy and limits understanding of predicting the course of JIA.
The lack of randomization and a control group of patients on biological therapy or those who do not need it limits the possibility of establishing causal relationships between the JADI index and the response to biological therapy.
Methodological limitations of assessment tools: The JADI index evaluates clinically apparent lesions but does not consider early predictors of progression (for example, subclinical inflammation according to MRI data), leading to an underestimation of the role of preclinical changes.
Statistical limitations: Analyzing multiple predictors (for example, JADI-A“+”, JIA duration > 3 years) without correction for multiple comparisons increases the risk of false positive associations. Insufficient detail of the interaction between risk factors (for example, the collinearity of JADI-A and erosions on MRI) can distort the contribution of individual variables to the model.
The abovementioned factors make it difficult to extrapolate the results to a broader population of patients with various forms and degrees of disease severity.
Our findings demonstrate that baseline structural damage assessed by JADI serves as an independent predictor of diminished therapeutic response to bDMARDs, greater functional disability progression and reduced remission rates in JIA. JADI as a risk stratification tool enables timely treatment intensification, including earlier bDMARD initiation in high-risk patients, personalized monitoring protocols when combined with ILAR category, MRI erosion status, and JADAS71 trajectories. Incorporating JADI into standard monitoring improves predictive accuracy beyond conventional tools. The JADI index enhances clinical decision-making through earlier intervention before irreversible damage occurs, objective tracking of structural outcomes and family-centered counseling about long-term risks. This evidence supports JADI’s role as a complementary tool in a modern JIA management paradigm.
| 1. | Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 983] [Cited by in RCA: 1102] [Article Influence: 58.0] [Reference Citation Analysis (1)] |
| 2. | Magnani A, Pistorio A, Magni-Manzoni S, Falcone A, Lombardini G, Bandeira M, Rossi F, Sala I, Martini A, Ravelli A. Achievement of a state of inactive disease at least once in the first 5 years predicts better outcome of patients with polyarticular juvenile idiopathic arthritis. J Rheumatol. 2009;36:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Otten MH, Prince FH, Armbrust W, ten Cate R, Hoppenreijs EP, Twilt M, Koopman-Keemink Y, Gorter SL, Dolman KM, Swart JF, van den Berg JM, Wulffraat NM, van Rossum MA, van Suijlekom-Smit LW. Factors associated with treatment response to etanercept in juvenile idiopathic arthritis. JAMA. 2011;306:2340-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Flatø B, Lien G, Smerdel A, Vinje O, Dale K, Johnston V, Sørskaar D, Moum T, Ploski R, Førre Ø. Prognostic factors in juvenile rheumatoid arthritis: a case-control study revealing early predictors and outcome after 14.9 years. J Rheumatol. 2003;30:386-393. [PubMed] |
| 5. | Southwood TR, Foster HE, Davidson JE, Hyrich KL, Cotter CB, Wedderburn LR, Hull RG, Venning HE, Rahman JK, Cummins CL; British Society for Adolescent and Paediatric Rheumatology Biologics and New Drugs Register. Duration of etanercept treatment and reasons for discontinuation in a cohort of juvenile idiopathic arthritis patients. Rheumatology (Oxford). 2011;50:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Giancane G, Muratore V, Marzetti V, Quilis N, Benavente BS, Bagnasco F, Alongi A, Civino A, Quartulli L, Consolaro A, Ravelli A. Disease activity and damage in juvenile idiopathic arthritis: methotrexate era versus biologic era. Arthritis Res Ther. 2019;21:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Ringold S, Angeles-Han ST, Beukelman T, Lovell D, Cuello CA, Becker ML, Colbert RA, Feldman BM, Ferguson PJ, Gewanter H, Guzman J, Horonjeff J, Nigrovic PA, Ombrello MJ, Passo MH, Stoll ML, Rabinovich CE, Schneider R, Halyabar O, Hays K, Shah AA, Sullivan N, Szymanski AM, Turgunbaev M, Turner A, Reston J. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Non-Systemic Polyarthritis, Sacroiliitis, and Enthesitis. Arthritis Care Res (Hoboken). 2019;71:717-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 229] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 8. | Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, DeWitt EM, Ilowite NT, Kimura Y, Laxer RM, Lovell DJ, Martini A, Rabinovich CE, Ruperto N. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken). 2011;63:465-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 639] [Cited by in RCA: 563] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 9. | Selvaag AM, Aulie HA, Lilleby V, Flatø B. Disease progression into adulthood and predictors of long-term active disease in juvenile idiopathic arthritis. Ann Rheum Dis. 2016;75:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 10. | Shih YJ, Yang YH, Lin CY, Chang CL, Chiang BL. Enthesitis-related arthritis is the most common category of juvenile idiopathic arthritis in Taiwan and presents persistent active disease. Pediatr Rheumatol Online J. 2019;17:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Nigrovic PA, Mannion M, Prince FH, Zeft A, Rabinovich CE, van Rossum MA, Cortis E, Pardeo M, Miettunen PM, Janow G, Birmingham J, Eggebeen A, Janssen E, Shulman AI, Son MB, Hong S, Jones K, Ilowite NT, Cron RQ, Higgins GC. Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis: report of forty-six patients from an international multicenter series. Arthritis Rheum. 2011;63:545-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 355] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 12. | Ravelli A, Consolaro A, Horneff G, Laxer RM, Lovell DJ, Wulffraat NM, Akikusa JD, Al-Mayouf SM, Antón J, Avcin T, Berard RA, Beresford MW, Burgos-Vargas R, Cimaz R, De Benedetti F, Demirkaya E, Foell D, Itoh Y, Lahdenne P, Morgan EM, Quartier P, Ruperto N, Russo R, Saad-Magalhães C, Sawhney S, Scott C, Shenoi S, Swart JF, Uziel Y, Vastert SJ, Smolen JS. Treating juvenile idiopathic arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2018;77:819-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 13. | Consolaro A, Ravelli A. Defining criteria for disease activity states in juvenile idiopathic arthritis. Rheumatology (Oxford). 2016;55:595-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997;40:1202-1209. [PubMed] [DOI] [Full Text] |
| 15. | Wallace CA, Ruperto N, Giannini E; Childhood Arthritis and Rheumatology Research Alliance; Pediatric Rheumatology International Trials Organization; Pediatric Rheumatology Collaborative Study Group. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31:2290-2294. [PubMed] |
| 16. | Press J, Neumann L, Uziel Y, Bolotin A, Buskila D. Assessment of quality of life of parents of children with juvenile chronic arthritis. Clin Rheumatol. 2002;21:280-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Mulligan K, Hirani SP, Harris S, Taylor J, Wedderburn LR, Newman S; WebParC Investigator group. The Effects of a Web-Based Tool for Parents of Children With Juvenile Idiopathic Arthritis: Randomized Controlled Trial. J Med Internet Res. 2022;24:e29787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 18. | Len C, Ferraz MB, Goldenberg J, Oliveira LM, Araujo PP, Quaresma MR, Terreri MT, Hilário MO. Pediatric Escola Paulista de Medicina Range of Motion Scale: a reduced joint count scale for general use in juvenile rheumatoid arthritis. J Rheumatol. 1999;26:909-913. [PubMed] |
| 19. | Viola S, Felici E, Magni-Manzoni S, Pistorio A, Buoncompagni A, Ruperto N, Rossi F, Bartoli M, Martini A, Ravelli A. Development and validation of a clinical index for assessment of long-term damage in juvenile idiopathic arthritis. Arthritis Rheum. 2005;52:2092-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Russo RA, Katsicas MM. Global damage in systemic juvenile idiopathic arthritis: preliminary early predictors. J Rheumatol. 2008;35:1151-1156. [PubMed] |
| 21. | van Dijkhuizen EH, Wulffraat NM. Early predictors of prognosis in juvenile idiopathic arthritis: a systematic literature review. Ann Rheum Dis. 2015;74:1996-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Nalbanti P, Kanakoudi-Tsakalidou F, Trachana M, Pratsidou-Gertsi P, Farmaki E, Bamidis P, Papachristou F. Juvenile idiopathic arthritis in the biologic era: predictors of the disease progression and need for early introduction of biologic treatment. Rheumatol Int. 2018;38:1241-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Nikishina I, Ruperto N, Kuzmina N, Shelepina T, Illarionova O, Salougina S, Kaleda M, Borodacheva O; Paediatric Rheumatology International Trials Organisation. The Russian version of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ). Clin Exp Rheumatol. 2001;19:S131-S135. [PubMed] |
| 24. | Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, He X, Maldonado-Cocco J, Orozco-Alcala J, Prieur AM, Suarez-Almazor ME, Woo P; International League of Associations for Rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390-392. [PubMed] |
| 25. | Klein A, Minden K, Hospach A, Foeldvari I, Weller-Heinemann F, Trauzeddel R, Huppertz HI, Horneff G. Treat-to-target study for improved outcome in polyarticular juvenile idiopathic arthritis. Ann Rheum Dis. 2020;79:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Guzman J, Oen K, Loughin T. Predicting disease severity and remission in juvenile idiopathic arthritis: are we getting closer? Curr Opin Rheumatol. 2019;31:436-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Guzman J, Henrey A, Loughin T, Berard RA, Shiff NJ, Jurencak R, Benseler SM, Tucker LB; ReACCh-Out Investigators. Predicting Which Children with Juvenile Idiopathic Arthritis Will Have a Severe Disease Course: Results from the ReACCh-Out Cohort. J Rheumatol. 2017;44:230-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Shoop-Worrall SJW, Verstappen SMM, McDonagh JE, Baildam E, Chieng A, Davidson J, Foster H, Ioannou Y, McErlane F, Wedderburn LR, Thomson W, Hyrich KL. Long-Term Outcomes Following Achievement of Clinically Inactive Disease in Juvenile Idiopathic Arthritis: The Importance of Definition. Arthritis Rheumatol. 2018;70:1519-1529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Triaille C, Quartier P, De Somer L, Durez P, Lauwerys BR, Verschueren P, Taylor PC, Wouters C. Patterns and determinants of response to novel therapies in juvenile and adult-onset polyarthritis. Rheumatology (Oxford). 2024;63:594-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 30. | Shoop-Worrall SJW, Wu Q, Davies R, Hyrich KL, Wedderburn LR. Predicting disease outcomes in juvenile idiopathic arthritis: challenges, evidence, and new directions. Lancet Child Adolesc Health. 2019;3:725-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Ganeva M, Fuehner S, Kessel C, Klotsche J, Niewerth M, Minden K, Foell D, Hinze CH, Wittkowski H. Trajectories of disease courses in the inception cohort of newly diagnosed patients with JIA (ICON-JIA): the potential of serum biomarkers at baseline. Pediatr Rheumatol Online J. 2021;19:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Rosina S, Natoli V, Santaniello S, Trincianti C, Consolaro A, Ravelli A. Novel biomarkers for prediction of outcome and therapeutic response in juvenile idiopathic arthritis. Expert Rev Clin Immunol. 2021;17:853-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Eng SW, Duong TT, Rosenberg AM, Morris Q, Yeung RS; REACCH OUT and BBOP Research Consortia. The biologic basis of clinical heterogeneity in juvenile idiopathic arthritis. Arthritis Rheumatol. 2014;66:3463-3475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Correll CK, Shrader P, Dennos A, Phillips T, Shiff NJ, Verstegen RHJ, Beukelman T; Childhood Arthritis and Rheumatology Research Alliance Registry Investigators. Effectiveness and Safety of High-Dose Biologics in Juvenile Idiopathic Arthritis in the Childhood Arthritis and Rheumatology Research Alliance. Arthritis Care Res (Hoboken). 2022;74:1770-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Giancane G, Consolaro A, Lanni S, Davì S, Schiappapietra B, Ravelli A. Juvenile Idiopathic Arthritis: Diagnosis and Treatment. Rheumatol Ther. 2016;3:187-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 36. | Russo RA, Katsicas MM. Patients with very early-onset systemic juvenile idiopathic arthritis exhibit more inflammatory features and a worse outcome. J Rheumatol. 2013;40:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Al-Mayouf SM, Hashad S, Khawaja K, Alrasheedi A, Abdwani R, Abushhaiwia A, AlSuwaiti M, Alzyoud R, Al Abrawi S, Asiri A, Alshaikh M, Sharif E, Muzaffer M, Alsewairi W, Zlenti M, Kawaja E, Almutairi M, Majeed M, Lotfy H, AlMarri M, Almutairi N; Pediatric Arab Rheumatology Group. Cumulative Damage in Juvenile Idiopathic Arthritis: A Multicenter Study From the Pediatric Rheumatology Arab Group. Arthritis Care Res (Hoboken). 2021;73:586-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Rypdal V, Arnstad ED, Aalto K, Berntson L, Ekelund M, Fasth A, Glerup M, Herlin T, Nielsen S, Peltoniemi S, Zak M, Rygg M, Rypdal M, Nordal E; Nordic Study Group of Pediatric Rheumatology (NoSPeR). Predicting unfavorable long-term outcome in juvenile idiopathic arthritis: results from the Nordic cohort study. Arthritis Res Ther. 2018;20:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Sarma PK, Misra R, Aggarwal A. Outcome in patients with enthesitis related arthritis (ERA): juvenile arthritis damage index (JADI) and functional status. Pediatr Rheumatol Online J. 2008;6:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Sarma PK, Misra R, Aggarwal A. Physical disability, articular, and extra-articular damage in patients with juvenile idiopathic arthritis. Clin Rheumatol. 2008;27:1261-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Solari N, Viola S, Pistorio A, Magni-Manzoni S, Vitale R, Ruperto N, Ullmann N, Filocamo G, Martini A, Ravelli A. Assessing current outcomes of juvenile idiopathic arthritis: a cross-sectional study in a tertiary center sample. Arthritis Rheum. 2008;59:1571-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Tangcheewinsirikul S, Sukharomana M, Charuvanij S. Disability and disease-related damage in Thai children and adolescents with juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2023;21:68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 43. | Menon NVB, Peethambaran G, Puthiyapurayil AT, Nambudakath C, Arakkal R. Clinical profile and juvenile arthritis damage index in children with juvenile idiopathic arthritis: A study from a tertiary care center in south India. Int J Rheum Dis. 2018;21:871-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Susic GZ, Stojanovic RM, Pejnovic NN, Damjanov NS, Soldatovic II, Jablanovic DB, Sefik Bukilica MN. Analysis of disease activity, functional disability and articular damage in patients with juvenile idiopathic arthritis: a prospective outcome study. Clin Exp Rheumatol. 2011;29:337-344. [PubMed] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/