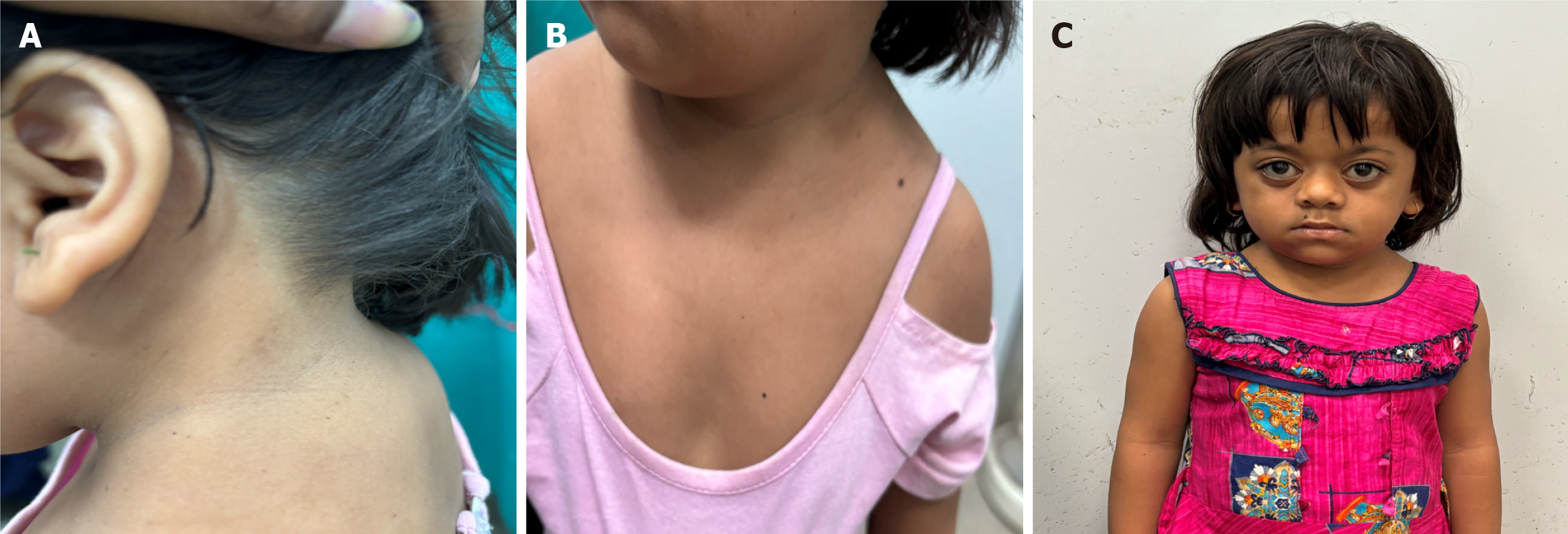
Published online Dec 9, 2025. doi: 10.5409/wjcp.v14.i4.108897
Revised: May 22, 2025
Accepted: August 4, 2025
Published online: December 9, 2025
Processing time: 189 Days and 22.1 Hours
Noonan syndrome (NS) is an autosomal dominant, multisystem disorder with a prevalence of 1 in 1000-2500. Multiple etiologies have been proposed for short stature in NS, including resistance to growth hormone (GH) and GH deficiency (GHD). Irrespective of the presence of GHD, NS is a Food and Drug Administration-approved indication for recombinant-GH therapy. Few case reports of combined anterior pituitary hormone deficiency (CPHD) in NS have been reported.
To describe the clinico-biochemical characteristics of NS with CPHD and to assess the response to recombinant GH therapy.
An ambispective case-control study was conducted to compare the clinico-hormonal profile and response to recombinant-GH in pediatric patients with NS and CPHD and pediatric patients with NS but without CPHD.
Five children with NS and CPHD were compared to 6 patients with NS but without CPHD. The most common anterior pituitary hormone involvement in combination with GHD was adrenocorticotrophic hormone deficiency causing hypocortisolemia (n = 3, 60%), followed by hypogonadotropic hypogonadism and secondary hypothyroidism (n = 1 each). Pituitary hypoplasia was seen in the magnetic resonance imaging of all patients with CPHD. Patients with NS and CPHD had lower standard deviation scores of height (-4.18 vs -2.52, P = 0.009), bodyweight, and body mass index but a slightly better first year response to recombinant GH (9.2 vs 5.5, P = 0.06). There were no differences in dysmorphisms and other anomalies between the two groups. Patients with NS and CPHD had a similar response to GH as patients with CPHD but without NS. One patient with NS and CPHD developed hypocortisolism after GH initiation.
Hypoplasia of the pituitary and GHD with involvement of other pituitary hormones may be seen in NS and may determine response to recombinant GH therapy.
Core Tip: Noonan syndrome (NS) can be associated with combined pituitary hormone deficiency (CPHD) and/or isolated growth hormone (GH) deficiency. Hypoplasia of the pituitary gland may contribute to CPHD in NS. Patients with NS and CPHD have decreased height and bodyweight but can have a better response to recombinant GH therapy than patients with NS but without CPHD. Children having a PTPN11 mutation with low insulin-like-growth-factor 1 should be tested for cortisol and thyroid function at baseline to rule out CPHD. If there is a suspicion for CPHD, the patient should undergo periodic testing for cortisol and thyroid function while receiving GH.
- Citation: Basu R, Bera S, Mondal S, Shah S, Swapnil K, Nanda R, Datta J, Mandal S, Goswami S, Baidya A, Sengupta N. Deficiency of anterior pituitary hormones in Noonan syndrome and its impact on response to growth hormone therapy. World J Clin Pediatr 2025; 14(4): 108897
- URL: https://www.wjgnet.com/2219-2808/full/v14/i4/108897.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i4.108897
Noonan syndrome (NS) is an autosomal dominant disorder with an incidence of 1:1000 to 1:2500 and affects multiple organ systems[1]. Clinical features include short stature, typical facial dysmorphism, congenital heart defects (most commonly pulmonary valve stenosis), typical chest deformities, cryptorchidism, coagulation defects and variable degrees of intellectual disability. Functional alterations of the Ras/mitogen-activated protein kinase (MAPK) signaling pathway (e.g., gain-of function germline mutations involving the different components of the Ras/MAPK pathway) are implicated in the pathogenesis of NS[2]. Mutations of the PTPN11 gene are present in up to 50% of NS cases, followed by mutations in SOS1 and RAF1 in up to 20%[3]. Few patients with NS patients have been shown to carry mutations in KRAS, BRAF, MEK1, and NRAS[3,4]. Causative gene defects are unknown in 30%-40% of NS cases[3].
Up to 70% of patients with NS may present with short stature[3]. Possible mechanisms of short stature in NS include growth hormone (GH) deficiency (GHD), neurosecretory dysfunction, and GH resistance. The United States Food and Drug Administration approved the treatment of recombinant human (rh) GH for patients with NS and short stature in 2007. While GHD has been reported in several studies on NS, there have been few case reports on other pituitary cell lines in NS. Though the pathogenetic mechanisms linking NS with panhypopituitarism is not clear yet, it is known that the MAPK/ERK/Ras pathway plays a role during normal pituitary development, hinting at the fact that defects in this pathway might lead to combined pituitary hormone deficiency (CPHD) in patients with NS[5].
We incidentally encountered few cases of CPHD in NS in our institution, following which we decided to systemically look for CPHD in NS. The current study was the first to describe the clinico-biochemical characteristics of a unique cohort of patients with NS and CPHD and to assess their clinico-hormonal differences, including response to recombinant GH therapy (GHT), while comparing them to patients with NS but without CPHD and a cohort of individuals without NS but with CPHD due to structural defects or transcription factor defects of the pituitary gland.
An ambispective study was conducted on subjects diagnosed with definite NS based on the van-der-Burgt criteria (VDB), aged between 5 to 18 years, presenting to the Endocrinology Department of a tertiary care hospital in India[6]. We excluded those with suspected NS but not fulfilling the criteria for definite NS as per the VDB criteria and those already on recombinant GH or gonadal hormone replacement or on levothyroxine. All the subjects underwent clinical exa
The current study was an ambispective case-control study. For the cross-sectional part a total of 5 patients with NS who were found to have CPHD and 6 age-matched patients with NS who did not have CPHD were included for comparative analysis. Another group of 5 patients with CPHD due to structural defects of the pituitary or transcription factor deficits but not having NS were also included as an additional control group for comparison to the subjects with NS and CPHD. The longitudinal component of the ambispective study involved following up all the cases and controls for a period of 1 year while receiving recombinant GH at the same dose and monitoring their height, bodyweight, body mass index (BMI) and periodic reassessment of hormones including TSH, fT4, cortisol, and IGF-1. To ensure harmonization in data collection, all anthropometric measurements for the cases and controls were conducted by the same physician using the same equipment (Harpenden stadiometer, HBF-362 OMRON weighing scale) in the institute.
GHT and follow-up: The longitudinal part of the study involved assessment of response to rhGH in the different groups. After excluding the known absolute and relative contraindications of GHT, subjects with NS were started on sub
Diagnosis of definite NS was made using the VDB criteria of typical facies of NS plus one major criteria (any one out of the following five: Typical cardiac manifestations seen in NS including pulmonary stenosis or hypertrophic cardiomyopathy; Height < third percentile; chest wall deformity including pectus carinatum/excavatum; family history of definite NS in a first degree relative; and others including all three of mental retardation, cryptorchidism, and lymphatic dysplasia), or two minor criteria (cardiac defects other than the ones described before, height < tenth percentile, broad thorax, first degree relative with suggestive NS, or any one of the manifestations indicated as others), or suggestive plus two major or three minor criteria[6].
CPHD was diagnosed when two or more anterior pituitary hormone deficiencies were documented by hormonal tests with/without structural defects of the pituitary seen on magnetic resonance imaging (MRI)[9]. Hypocortisolemia was diagnosed when the basal cortisol was less than 3 µg/dL or the tetracosatide-stimulated and/or glucagon- stimulated cortisol was < 18.1 µg/dL without elevated levels of ACTH[10]. Those with cortisol between 3-15 µg/dL underwent stimulation testing with 250 µg of tetracosatide (Syntropac), and cortisol was tested after 1 h of the injection. GHD was diagnosed in subjects with IGF-1 < -2 standard deviation scores (SDS) for age and gender and structural defects of the pituitary seen or failure to stimulate GH after one GH stimulation test (GHST), or IGF-1 < -1 to -2 SDS with structural defects of the pituitary on imaging or failure to stimulate GH after two stimulation tests[11].
GHSTs: GHST was performed using oral clonidine and intramuscular glucagon using standard protocols following priming with estradiol valerate[11]. Both stimulation tests were done for those without pituitary structural defects seen on MRI while only clonidine stimulation was done for those with pituitary structural defects seen on MRI and in those with IGF-1 < - 2 SDS for age and gender. GHD was diagnosed when all the stimulated GH values were < 10 µg/dL[12].
The clonidine stimulation test was conducted following overnight fasting while lying down during the test and for 2 h afterward. At 8 am an intravenous cannula was inserted to collect a baseline GH sample, followed by administration of oral clonidine at a dose of 0.15 mg/m² (maximum 0.3 mg). Blood pressure was monitored every 30 min, and blood samples for GH were collected at 0, 30, 60, 90, and 120 min.
The glucagon stimulation test was done using intramuscular glucagon at a dose of 1 mg for adults and 15 µg/kg for children (up to 1 mg). Samples for GH and cortisol were collected at baseline followed by subsequent samples at 30, 60, 90, 120, 150, and 180 min. The patients were monitored for adverse effects, including nausea, abdominal pain, and hypoglycemia with observation maintained for at least 2 h after the test.
Priming with gonadal steroids was conducted in prepubertal boys and girls over 10 years of age using oral estradiol valerate (2 mg or 1 mg for those with bodyweights under 20 kg) for 3 consecutive nights immediately prior to the day of test.
Auxologic variables and Tanner staging: Height was measured with a Harpenden stadiometer to the nearest 0.1 cm with the head in the Frankfurt plane, and bodyweight was measured with the weighing platform of the HBF-362 OMRON machine. Sexual maturity rating was done using the Tanner scale based on genital development in males and breast development in females[13]. The SDS of height (Ht-SDS) and body weight (BW-SDS) at baseline and at different points while on GHT were obtained from the Indian Academy of Pediatrics (IAP) 2015 growth charts (Ht-SDS) and also from NS-specific growth charts[14,15]. Height velocity was the increase in height in cm in the initial 1 year after GHT initiation. Ht-SDS percent improvement was calculated using the formula (Ht-SDS after 1 year - Ht-SDS at baseline)/(Ht-SDS at baseline) × 100.
Hormone assays: Hormonal assays were done using Roche Cobas e411 autoanalyzer (Roche Diagnostics, Switzerland). Hormonal assays for prolactin, luteinizing hormone, follicle stimulating hormone, TSH, IGF-1, and ACTH were done using the solid phase, competitive electrochemiluminescent immunometric assay, except fT4, cortisol, and total testosterone, which were assessed by competitive immunoassays. All hormonal assays for the subjects were conducted in the same machine Roche Cobas e411 autoanalyzer in the institute and using the same assay methods at baseline and at follow-up visits to ensure harmonization. The IGF-1 SDS for each subject was calculated according to normal IGF-1 Levels for age and sex based on national normative data[16]. Percentage improvement in IGF-1 SDS was calculated as (IGF-1 SDS after 1 year - IGF-1 SDS at baseline)/(IGF-1 SDS at baseline) × 100.
Genetic testing: Next generation sequencing for complete coding regions and splice site junctions of genes on the Illumina sequencing platform to a mean depth of > 80-100 × and a read length of 2 × 100/2 × 150 was utilized to confirm NS. The sequences obtained were aligned to reference sequences based on NCBI RefSeq transcripts and human genome build GRCh37/UCSC hg19. Data was filtered and analyzed to identify variants of interest and interpreted in the context of a single most damaging clinically relevant transcript as indicated for variant details. The Human Genome Variation Society guidance was followed in the reporting of pathogenic variants.
Radiological assessment: MRI was done using thin (2-3 mm) sections to view the hypothalamo-pituitary region in sagittal T1, coronal T1, and coronal T2 or axial T1-weighted slices before and after gadolinium contrast injection (0.1 mmol/kg). Transabdominal ultrasonography was done using a Philips HD 7 model machine, using a curvilinear ul
All statistical analyses were done using GraphPad v.8 for Mac. Quantitative variables were expressed as mean (standard deviation) or median [interquartile range (IQR) in which IQR was represented as (25th percentile, 75th percentile)] for non-parametric parameters. Categorical variables were expressed in terms of prevalence (%). Comparison between two groups was done using with Mann-Whitney U test for the non-normally distributed continuous variables and the χ2 test with Fisher’s correction where appropriate for categorical variables. Correlations were tested using Spearman’s r. A P value less than 0.05 (P < 0.05) was considered significant. The study was cleared by the institutional ethics committee, vide NRSMC/IEC/45/2021 dated September 22, 2021.
In the current study we reported the presence of CPHD in 5 patients with NS and compared their clinico-biochemical profile to 6 other patients with NS but without CPHD and 5 patients with CPHD (all having GHD) but without NS. The clinico-biochemical profile of the cases is summarized in Table 1.
| Parameter | Median (IQR) or n (%) (No. of cases with NS = 11) |
| Age | 10.5 (7.8, 13.0) |
| Height (cm) | 117.5 (104.0, 124.5) |
| Ht-SDS | -3.43 (-4.18, -2.52) |
| Noonan specific Ht-SDS | -1.08 (-1.97, -0.61) |
| Bodyweight (kg) | 18 (14, 25) |
| Bodyweight SDS | -2.93 (-4.38, -2.22) |
| BMI (kg/m2) | 13.77 (12.69, 15.18) |
| BMI-SDS | -1.68 (-3.52, -1.00) |
| IGF-1 (ng/mL) | 162 (135, 200) |
| IGF-1 SDS | -1.21 (-1.82, 0.22) |
| Height velocity in first year after GH | 9.2 (7.5, 9.8) |
| Low IGF-1 (< -2 SDS) | 4 |
| GH deficiency seen on stimulation tests | 5 |
| IGF-1 SDS (%) increment in 1 year | 75.21 (51.33, 83.85) |
| Genetic test ( n = 6) | PTPN11 mutation: 5 |
| Cardiac defects (n = 8) | Pulmonary stenosis, n = 4 |
| ASD; n = 3 | |
| HCM, n = 1 | |
| Chest deformity | 9 |
| Family history | 1 |
| Cryptorchidism | 3/5 males |
| Mental retardation | 2 |
| Coagulation defects | 1 |
| Lymphatic dysplasia | 3 |
| Neuropsychological impairments, language delay | 3 |
A total of 11 patients with NS (5 with CPHD and 6 without CPHD) were included for the study, the median age of the entire cohort was 10.5 years (7.8, 13.0). The median height was 117.5 cm (104, 124.5), weight was 18.0 kg (14.0, 25.0), and BMI was 13.77 kg/m2 (12.69, 15.18). Diagnosis of NS was made in all cases using the VDB criteria for definite NS, and the genetic test was performed in 6 patients. Five patients showed pathogenic mutations in different exons of the PTPN11 gene (Supplementary Table 1) One patient with definite NS and meeting four major criteria for NS, including the characteristic facies, pulmonary stenosis, pectus carinatum, and horse-shoe kidneys, was unexpectedly found to have a POU1F1 mutation (Figure 1). No other NS-causing mutation was identified on whole exome sequencing for this patient.
The median Ht-SDS calculated from IAP 2015 reference data was -3.43 (-4.18, -2.52), and the median Ht-SDS calculated from NS-specific growth charts was -1.08 (-1.97, -0.61). The median IGF-1 was 162 (135, 200) with median IGF-1 SDS being -1.21 (-1.82, 0.22). Four patients (36.4%) had IGF-1 below –2 SDS for age, gender, and pubertal status. Hypogonadotropic hypogonadism was seen in 4 out of the 8 patients (50%) who had attained pubertal age. Following GHST, GHD was documented in 5 patients. Secondary hypocortisolism was documented in 4 patients while 2 patients had documented secondary hypothyroidism.
Out of the 5 patients with CPHD, the most common pattern of involvement was a combination of GHD and secondary hypocortisolism (n = 3, 60%). One case had a combination of GHD and hypogonadotropic hypogonadism, and another had a combination of GHD with secondary hypothyroidism. Hypoplasia of the pituitary gland was seen on MRI in all cases of CPHD except one case with GHD and secondary hypocortisolism. All cases of NS without CPHD had normal pituitary dimensions on MRI.
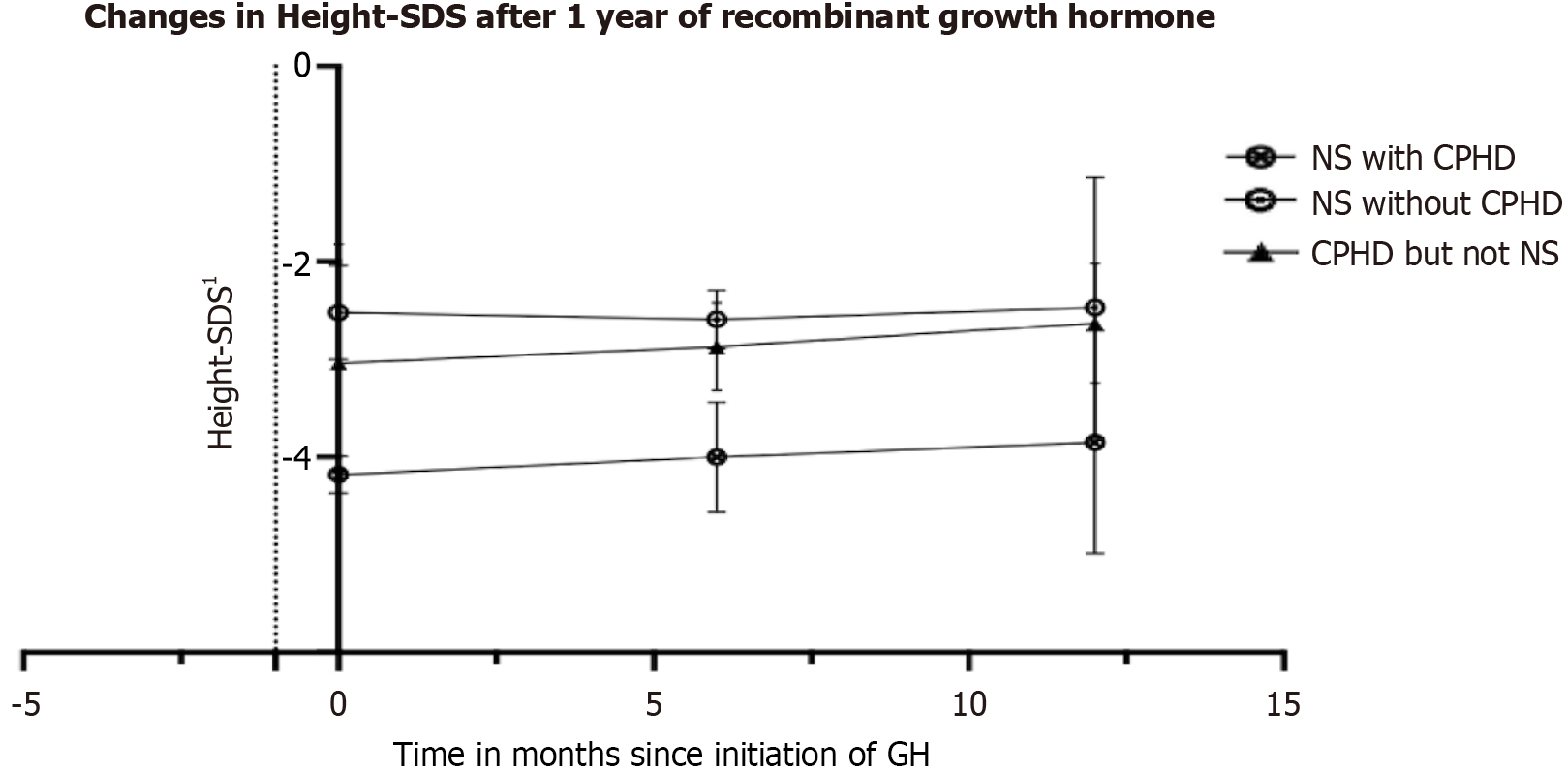
Children with NS and CPHD (n = 5) were shorter, had lower Ht-SDS as per national standard reference IAP charts (-4.18 vs -2.52, P = 0.009) and NS-specific charts (-1.99 vs -1.04, P = 0.02), had lower BW-SDS (-5.05 vs -2.22, P = 0.05), had lower BMI-SDS (-3.54 vs -1, P = 0.009), and had slightly lower IGF-1 SDS for age (-1.71 vs 0.21, P = 0.07). Notably, the first-year height velocity was numerically higher for patients with NS and CPHD (9.2 cm vs 5.5 cm, P = 0.06) (Figure 2). There were no differences in the prevalence of genetic mutations in PTPN11, skeletal deformities including chest defects, congenital defects of the heart, kidneys, or coagulation system, and neuropsychologic impairment between the two groups (Table 2).
| Parameter | NS with CPHD (n = 5) | NS without CPHD (n = 6) | P value |
| Age | 12.0 (11.0, 14.0) | 8.8 (7.4, 10.1) | 0.360 |
| Height (cm) | 117.5 (89.0, 126.0) | 114.0 (106.5, 122.3) | 0.790 |
| Ht-SDS | -4.18 (-4.36, -4.17) | -2.52 (-2.76, -2.27) | 0.009 |
| Noonan specific Ht-SDS | -1.99 (-2.30, -1.59) | -0.61 (-0.74, 0.40) | 0.020 |
| Bodyweight (kg) | 18 (10, 19) | 19 (15, 27) | 0.430 |
| Bodyweight SDS | -5.05 (-6.32, -3.63) | -2.22 (-2.81, -1.99) | 0.050 |
| BMI (kg/m2) | 13.37 (11.34, 13.77) | 14.66 (13.21, 15.23) | 0.330 |
| BMI-SDS | -3.54 (-3.96, -3.49) | -1.00 (-1.59, -0.50) | 0.009 |
| IGF-1 (ng/mL) | 149 (86, 179) | 175 (154, 206) | 0.330 |
| IGF-1 SDS | -1.59 (-1.94, -1.10) | 0.22 (-1.00, 1.17) | 0.070 |
| Height velocity in first year with GH | 9.2 (8.4, 9.6) | 5.5 (4.3, 6.0) | 0.067 |
| Ht-SDS (%) increment in 1 year | 11.70 (5.09, 17.49) | 3.32 (-3.82, 13.90) | 0.430 |
| Low IGF-1 (< -2 SDS) | 2 (40) | 2 (33) | 0.990 |
| GH deficiency seen on stimulation tests | 4 (80) | 1 (17) | 0.080 |
| IGF-1 SDS (%) increment in 1 year | 78.45 (48.35, 89.26) | 64.76 (59.53, 69.98) | 0.860 |
| Genetic test ( n = 5) | PTPN11 mutation: 3; POU1F1: 1 | PTPN11 mutation: 2 | > 0.990 |
| Cardiac defects | 4 (80); Pulmonary stenosis: 3; ASD: 2 | 3 (50); Pulmonary regurgitation: 1; ASD: 1; ASH: 1 | 0.550 |
| Chest deformity | 4 (80) | 5 (83) | > 0.990 |
| Family history | 0 | 1 | |
| Cryptorchidism | 50% of males | 60% of males | > 0.990 |
| Mental retardation | 1 (20) | 1 (17) | > 0.990 |
| Coagulation defects | 0 | 1 (17) | |
| Lymphatic dysplasia | 1 (20) | 2 (33) | > 0.990 |
| Neuropsychological impairments, language delay | 1 (20) | 2 (33) | > 0.990 |
Comparison of CPHD with NS and CPHD without NS: After comparing the patients with NS and CPHD to patients with CPHD but without NS, there were no differences in the prevalence of GHD, central hypocortisolism, or secondary hypothyroidism. Children in the former group had lower BW-SDS (-5.05 vs -2.10, P = 0.03) and BMI-SDS (-3.54 vs – 0.37, P = 0.008). Baseline Ht-SDS, IGF-1, and improvement in Ht-SDS% and IGF-1% after 1 year of GHT were similar in both groups (Figure 2, Supplementary Table 2).
Correlation of GH response: The Ht-SDS% improvement showed no correlation with age, IGF-1 SDS, IGF-1 SDS% improvement or with the maximum GH levels during GHST. Figure 2 shows the change in Ht-SDS% at 6 months and at 1 year after GHT in the three groups.
In the current study we reported the presence of CPHD in a group of children with NS and compared their clinico-biochemical parameters and response to GH to patients with NS without CPHD and to patients with CPHD without NS. There have been multiple studies on GHD in NS, but CPHD has been rarely reported in NS.
Several mechanisms have been proposed to explain short stature in NS, including impaired GH release, impaired functioning of the GH/IGF-1 axis, and GH resistance as well as the presence of comorbidities like congenital heart diseases[17-20]. Emerging evidence suggests an impact of the underlying molecular cause on stature as evidenced by more severely impaired growth in patients carrying PTPN11, RAF1, and KRAS pathogenic variants than in those with SOS1 variants[17,20]. Patients with NS and PTPN11 mutations have been reported to have lower IGF-1 and higher GH levels, suggestive of mild GH resistance[21]. In the current study we found GHD on GHST in up to 46% of the patients with NS though low IGF-1 was found in only one-third of the cohort. Previous studies have also shown that up to three-fourths of children with NS fail to mount peak GH response after provocation[22]. One of the older studies on rhGHT in NS reported that in spite of having low IGF-1, most children with NS had normal GH response to standard provocation tests and normal overnight GH secretory profile[23]. However, existing guidelines do not recommend testing or monitoring spontaneous GH secretion before starting GHT in NS, and NS is an indication for GHT irrespective of the presence of GHD. However, whether the response to GHT varies between NS with and without GHD and whether the dose of GH administered should be guided by the presence or absence of GHD in NS remains a controversial area. Because 1 patient with NS developed central hypocortisolism after GHT, attention should focus on the need to screen for CPHD in some subjects with NS at baseline and during GHT. GHT in patients with suspected CPHD needs close monitoring of thyroid function and cortisol levels at periodic intervals.
We found no differences in the growth rate and Ht-SDS or IGF-1% increase in children with NS and CPHD and children with CPHD without NS after 1 year of rhGHT. One study compared growth rate during the initial year of GHT between patients with NS and GHD and patients with isolated GHD. The authors reported a significantly lower growth rate in patients with NS and GHD than the latter[24]. Another study showed Ht-SDS in NS to be similar to Turner Syndrome but significantly lower than idiopathic GHD. The change in prepubertal Ht-SDS was related to the duration of pre-pubertal rhGHT[25]. However, another study showed patients with NS and GHD had a similar growth response to rhGHT as patients with idiopathic GHD[26].
In the study by Ahmed et al[23], the mean height velocity in the first year of GHT ranged between 4.8-7.4 cm/year, and the improvement in height velocity-SDS ranged from + 0.20 to + 3.75. These values are similar to the height velocity and Ht-SDS improvement in the cohort of NS without CPHD in our study. We found that children having NS and CPHD had somewhat higher first year height velocity and improvement in Ht-SDS. We failed to find any correlation of Ht-SDS improvement with stimulated GH or IGF-1 increase. In another study, although the mean overnight GH was low in a large proportion of patients with NS, neither the overnight GH parameters nor the provocative test results were correlated to the height velocity or response to GHT though height velocity correlated with rise in IGF-1[27]. Overall, our results suggested that unlike in NS without CPHD, in cases of NS and CPHD or isolated GHD, the predominant mechanism for short stature was low GH pulsatile secretion rather than resistance to GH action at the level of hepatocytes.
PTPN11 gene mutations are the most commonly detected and seen in more than 50% of the patients with NS, whereas 25% can have mutations in other genes of the RAS kinase pathway[28]. Overall, more than 90% of the mutations known to cause NS involve the PTPN11, SOS1, RAF1, and RIT1 genes[4,29]. Mutation in the PTPN11 gene results in a gain of function of the Src homology region 2-domain phosphatase-2 (SHP-2) protein, a negative regulator of the GH receptor signaling pathway[30,31]. The pathogenetic mechanism of NS involves gain-of-function pathogenic mutations of mediators in the RAS/MAPK signaling cascade. Gain-of-function mutations in these proteins destabilize the autoinhibitory mechanisms responsible for maintaining them in their catalytically inactive conformation. Similarly, inactivating pathogenic mutations in genes like loss-of-function mutations in the SPRED1, SPRED2, neurofibromin, and LZTR1 genes, which code for the proteins involved in negative regulation of the RAS/MAPK cascade, may also cause NS[32,33].
In our study in all but one of the patients for whom exome sequencing was done, pathogenic mutation in the PTPN11 gene was found. We could not check for any association with the genotype due to lack of a non-PTPN11 gene in our cohort. Notably, one female with a diagnosis of definite NS as per the VDB criteria with typical facies, skeletal stigmata including pectus carinatum as well as pulmonary stenosis had a detectable pathogenic mutation in the POU1F1 gene. NS due to POU1F1 has not been reported before. While POU1F1 is an important transcription factor for pituitary development and mutation in POU1F1 is known to cause pituitary hypoplasia and CPHD, its involvement in the RAS pathway is not clear. Very few systematic studies about NS have been published from India, and most of them are descriptive about the phenotype. PTPN11 mutation has been reported in up to 65% among children with NS from India[34,35]. Results of prior studies reporting the association of the genotype with response to rhGH in NS are mostly controversial with both better and poor response reported in NS with PTPN11 mutation[26,36,37]. Since none of our patients had a non-PTPN11 mutation detected, we could not study the role of genotype in determining the response to GHT.
There have been case reports published about CPHD in NS[38-40]. A group of authors reported panhypopituitarism in NS, and based on their findings of dissociated response of prolactin to TRH and chlorpromazine suggested that the pituitary hormone deficiencies may be secondary to hypothalamic dysfunction rather than an intrinsic pituitary problem[39]. The pathogenetic mechanisms behind the crosstalk between the two axes are unclear. The RAS-RAF-MEK-ERK pathway is an important downstream signaling pathway of the EGF receptor and plays an important role in cell proliferation, growth, and survival.
The PTPN11 product SHP-2 is a cytoplasmic protein with tyrosine phosphatase actions that plays an important role in the signaling pathway of multiple growth factors including IGF-1, insulin, leptin, and cytokines. SHP-2 activity is necessary during embryonal development and embryos nullizygous for SHP-2 have demonstrated severe defects in gastrulation and mesodermal patterning. SHP-2 also plays a role in relieving inhibitory tyrosine phosphorylation events in Jak2 required for Jak2 activity, Stat5 phosphorylation, and induction of transcriptional activity. SHP-2 has been found to be activated by the prolactin receptor[41]. A role of SHP-2 has also been established in postmitotic forebrain neurons to control energy balance and metabolism, and its phosphatase action is a critical signaling component of leptin receptors in the hypothalamus[42].
The RAS/MAPK signaling transduction pathway has been shown to play an important role in regulation of lactotroph-specific gene expression in the pituitary[43,44]. The Ras pathway via Raf kinase, MEK, and ERK can influence tran
Although CPHD has been reported in NS, it has been rarely reported in other forms of RASopathies. There have been reports of BRAF mutations in a group of children with congenital hypopituitarism and clinical features of RASopathies[47]. The authors have also used phosphoproteomic analyses and mass spectrometry techniques and identified that activating mutations in BRAF lead to an increase in B-Raf kinase activity leading to hyperphosphorylation of the RAS/MAPK and JAK/STAT pathways. Increased levels of phosphorylated ERK indicated that the BRAF mutations were activating. Although there was appropriate expression of pituitary specification markers like Lhx3, Pitx1, and Hesx1, the authors observed an impairment of cell lineage determination with increased expression of POMC1 but decreased expression of PIT1. They also found in pituitary models evidence of impaired terminal differentiation of hormone-producing cells.
In another case report septo-optic dysplasia with hypopituitarism was associated in patients with a known RASopathy, Cardio-Facio-Cutaneous syndrome, in patients who had mutations in BRAF[48]. Overall, there seems to be an underreported and unexplored pathway linking the RAS pathway to the pituitary development pathways, and further studies in this regard are warranted.
We encountered one case with definite NS as per the VDB criteria who was found to have a defect in the POU1F1 gene. While this might be a coincidental finding, no other NS-causing mutation was identified on whole exome sequencing for this patient. The POU1F1 gene encoding pituitary transcription factor 1 (PIT-1) is involved in the development of pituitary cells. The mechanism of its crosstalk with the Ras-Raf-MAPK pathway is yet unknown although there is suggestion that Ras signaling can impact the activation of the prolactin promoter, which is regulated by PIT-1[49]. RAS signaling proceeds via RAF/MEK/ERK1/2 activation to translocate ERK1/2 into nucleus. This step is important for increased PIT-1 (POU1F1) expression and thereby pituitary development and transcriptional activation of GH, PRL, and TSH genes leading to synthesis of these anterior pituitary hormones. Decreased expression of PIT-1 has been seen in the pituitary lineage of those with BRAF mutations. Thus, POU1F1 might be the link between CPHD and RASopathy like NS. Our patient had a pathogenic mutation in POU1F1 but had clinical features of RASopathy typical of NS. While there is no established role in POU1FI upstream in RAS pathway, it might be that some of the clinical manifestations of the RASopathy are manifest because of POU1F1 expression defects occurring downstream.
To the best of our knowledge, this is the first study to report the presence of CPHD in a group of children with NS and compare them to a group of patients with NS without CPHD and CPHD without NS. Anterior pituitary function testing, including ACTH-stimulated cortisol levels and GHST, and pituitary imaging was done for all and genetic mutation analysis were available for the majority. The response to GH in the first year was also studied and compared in the participants.
The limitations of the study included a small sample size, a single-center study, and the lack of long-term follow-up data on the effects of GH. The very small sample size limits the power of the statistical analyses and its generalizability. Bayesian statistical analysis was also not applied due to lack of reliable prior information since there has been no previous systematic studies on the prevalence of pituitary deficiencies in NS. The POU1F1 mutation found in our patient was intriguing, but a functional analysis of the mutation could not be done and the exact mechanism of its relationship to RASopathies was not clear from the existing literature. Sequencing could not be done for all the patients, and the absence of non-PTPN11 mutations in the patients with NS limited analysis of any genotype-phenotype correlation. IGFBP3 Levels could not be tested even for children below 6 years of age although a vast majority of the children had a low BMI suggestive of malnutrition, affecting IGF-1 generation. Posterior pituitary function was not tested.
Our findings suggested that CPHD and hypoplastic anterior pituitary can exist in patients with NS having PTPN11 mutations, most commonly with a combination of secondary hypocortisolism and GHD. Patients with NS and CPHD were considerably shorter but had a slightly better response to GH than patients with NS but without CPHD. Patients with NS and CPHD responded to GHT similarly to children with CPHD without NS. It is necessary to check for se
| 1. | Mendez HM, Opitz JM. Noonan syndrome: a review. Am J Med Genet. 1985;21:493-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 284] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Aoki Y, Niihori T, Narumi Y, Kure S, Matsubara Y. The RAS/MAPK syndromes: novel roles of the RAS pathway in human genetic disorders. Hum Mutat. 2008;29:992-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 258] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 3. | Lee BH, Kim JM, Jin HY, Kim GH, Choi JH, Yoo HW. Spectrum of mutations in Noonan syndrome and their correlation with phenotypes. J Pediatr. 2011;159:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Ko JM, Kim JM, Kim GH, Yoo HW. PTPN11, SOS1, KRAS, and RAF1 gene analysis, and genotype-phenotype correlation in Korean patients with Noonan syndrome. J Hum Genet. 2008;53:999-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Haston S, Pozzi S, Carreno G, Manshaei S, Panousopoulos L, Gonzalez-Meljem JM, Apps JR, Virasami A, Thavaraj S, Gutteridge A, Forshew T, Marais R, Brandner S, Jacques TS, Andoniadou CL, Martinez-Barbera JP. MAPK pathway control of stem cell proliferation and differentiation in the embryonic pituitary provides insights into the pathogenesis of papillary craniopharyngioma. Development. 2017;144:2141-2152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | van der Burgt I. Noonan syndrome. Orphanet J Rare Dis. 2007;2:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 237] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 7. | Osio D, Dahlgren J, Wikland KA, Westphal O. Improved final height with long-term growth hormone treatment in Noonan syndrome. Acta Paediatr. 2005;94:1232-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Rohrer TR, Abuzzahab J, Backeljauw P, Birkegård AC, Blair J, Dahlgren J, Júlíusson PB, Ostrow V, Pietropoli A, Polak M, Romano A, Ross J, Sävendahl L, Miller BS. Long-Term Effectiveness and Safety of Childhood Growth Hormone Treatment in Noonan Syndrome. Horm Res Paediatr. 2020;93:380-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Jakobsen LK, Jensen RB, Birkebæk NH, Hansen D, Christensen AR, Bjerrum MC, Christesen HT. Diagnosis and Incidence of Congenital Combined Pituitary Hormone Deficiency in Denmark-A National Observational Study. J Clin Endocrinol Metab. 2023;108:2475-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 10. | Fleseriu M, Hashim IA, Karavitaki N, Melmed S, Murad MH, Salvatori R, Samuels MH. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101:3888-3921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 621] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 11. | Grimberg A, DiVall SA, Polychronakos C, Allen DB, Cohen LE, Quintos JB, Rossi WC, Feudtner C, Murad MH; Drug and Therapeutics Committee and Ethics Committee of the Pediatric Endocrine Society. Guidelines for Growth Hormone and Insulin-Like Growth Factor-I Treatment in Children and Adolescents: Growth Hormone Deficiency, Idiopathic Short Stature, and Primary Insulin-Like Growth Factor-I Deficiency. Horm Res Paediatr. 2016;86:361-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 470] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 12. | Vyas V, Kumar A, Jain V. Growth Hormone Deficiency in Children: From Suspecting to Diagnosing. Indian Pediatr. 2017;54:955-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | De Sanctis V, Elhakim IZ, Soliman AT, Elsedfy H, Elalaily R, Millimaggi G. Methods for Rating Sexual Development in Girls. Pediatr Endocrinol Rev. 2016;14:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 14. | Indian Academy of Pediatrics Growth Charts Committee; Khadilkar V, Yadav S, Agrawal KK, Tamboli S, Banerjee M, Cherian A, Goyal JP, Khadilkar A, Kumaravel V, Mohan V, Narayanappa D, Ray I, Yewale V. Revised IAP growth charts for height, weight and body mass index for 5- to 18-year-old Indian children. Indian Pediatr. 2015;52:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 291] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 15. | A Clinical Guideline: Noonan Syndrome Guideline Development Group. Noonan Syndrome. 2019;159-188. [DOI] [Full Text] |
| 16. | Dehiya RK, Bhartiya D, Kapadia C, Desai MP. Insulin like growth factor-I, insulin like growth factor binding protein-3 and acid labile subunit levels in healthy children and adolescents residing in Mumbai suburbs. Indian Pediatr. 2000;37:990-997. [PubMed] |
| 17. | Cessans C, Ehlinger V, Arnaud C, Yart A, Capri Y, Barat P, Cammas B, Lacombe D, Coutant R, David A, Baron S, Weill J, Leheup B, Nicolino M, Salles JP, Verloes A, Tauber M, Cavé H, Edouard T. Growth patterns of patients with Noonan syndrome: correlation with age and genotype. Eur J Endocrinol. 2016;174:641-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Nakaguma M, Jorge AAL, Arnhold IJP. Noonan syndrome associated with growth hormone deficiency with biallelic LZTR1 variants. Genet Med. 2019;21:260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Otten BJ, Noordam C. Growth in Noonan syndrome. Horm Res. 2009;72 Suppl 2:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Stagi S, Ferrari V, Ferrari M, Priolo M, Tartaglia M. Inside the Noonan "universe": Literature review on growth, GH/IGF axis and rhGH treatment: Facts and concerns. Front Endocrinol (Lausanne). 2022;13:951331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Binder G, Neuer K, Ranke MB, Wittekindt NE. PTPN11 mutations are associated with mild growth hormone resistance in individuals with Noonan syndrome. J Clin Endocrinol Metab. 2005;90:5377-5381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Soliman AT, Rajab A, el Zalabany M, alSalmi I, Fattah MA. Defective growth hormone (GH) secretion and short-term treatment in Noonan syndrome. Indian J Pediatr. 1998;65:741-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Ahmed ML, Foot AB, Edge JA, Lamkin VA, Savage MO, Dunger DB. Noonan's syndrome: abnormalities of the growth hormone/IGF-I axis and the response to treatment with human biosynthetic growth hormone. Acta Paediatr Scand. 1991;80:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Zavras N, Meazza C, Pilotta A, Gertosio C, Pagani S, Tinelli C, Bozzola M. Five-year response to growth hormone in children with Noonan syndrome and growth hormone deficiency. Ital J Pediatr. 2015;41:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Romano AA, Dana K, Bakker B, Davis DA, Hunold JJ, Jacobs J, Lippe B. Growth response, near-adult height, and patterns of growth and puberty in patients with noonan syndrome treated with growth hormone. J Clin Endocrinol Metab. 2009;94:2338-2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Seok EM, Park HK, Rho JG, Kum CD, Lee HS, Hwang JS. Effectiveness of growth hormone therapy in children with Noonan syndrome. Ann Pediatr Endocrinol Metab. 2020;25:182-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Noordam C, van der Burgt I, Sweep CG, Delemarre-van de Waal HA, Sengers RC, Otten BJ. Growth hormone (GH) secretion in children with Noonan syndrome: frequently abnormal without consequences for growth or response to GH treatment. Clin Endocrinol (Oxf). 2001;54:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Noordam K. Expanding the genetic spectrum of Noonan syndrome. Horm Res. 2007;68 Suppl 5:24-27. [PubMed] [DOI] [Full Text] |
| 29. | El Bouchikhi I, Belhassan K, Moufid FZ, Iraqui Houssaini M, Bouguenouch L, Samri I, Atmani S, Ouldim K. Noonan syndrome-causing genes: Molecular update and an assessment of the mutation rate. Int J Pediatr Adolesc Med. 2016;3:133-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Tajan M, Paccoud R, Branka S, Edouard T, Yart A. The RASopathy Family: Consequences of Germline Activation of the RAS/MAPK Pathway. Endocr Rev. 2018;39:676-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 31. | Padidela R, Camacho-Hübner C, Attie KM, Savage MO. Abnormal growth in noonan syndrome: genetic and endocrine features and optimal treatment. Horm Res. 2008;70:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Motta M, Fasano G, Gredy S, Brinkmann J, Bonnard AA, Simsek-Kiper PO, Gulec EY, Essaddam L, Utine GE, Guarnetti Prandi I, Venditti M, Pantaleoni F, Radio FC, Ciolfi A, Petrini S, Consoli F, Vignal C, Hepbasli D, Ullrich M, de Boer E, Vissers LELM, Gritli S, Rossi C, De Luca A, Ben Becher S, Gelb BD, Dallapiccola B, Lauri A, Chillemi G, Schuh K, Cavé H, Zenker M, Tartaglia M. SPRED2 loss-of-function causes a recessive Noonan syndrome-like phenotype. Am J Hum Genet. 2021;108:2112-2129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 33. | Johnston JJ, van der Smagt JJ, Rosenfeld JA, Pagnamenta AT, Alswaid A, Baker EH, Blair E, Borck G, Brinkmann J, Craigen W, Dung VC, Emrick L, Everman DB, van Gassen KL, Gulsuner S, Harr MH, Jain M, Kuechler A, Leppig KA, McDonald-McGinn DM, Can NTB, Peleg A, Roeder ER, Rogers RC, Sagi-Dain L, Sapp JC, Schäffer AA, Schanze D, Stewart H, Taylor JC, Verbeek NE, Walkiewicz MA, Zackai EH, Zweier C; Members of the Undiagnosed Diseases Network, Zenker M, Lee B, Biesecker LG. Autosomal recessive Noonan syndrome associated with biallelic LZTR1 variants. Genet Med. 2018;20:1175-1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 158] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 34. | Narayanan DL, Pandey H, Moirangthem A, Mandal K, Gupta R, Puri RD, Patil SJ, Phadke SR. Hotspots in PTPN11 Gene Among Indian Children With Noonan Syndrome. Indian Pediatr. 2017;54:638-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Gupta R, Goyal M, Gupta A. Noonan syndrome: Clinical and molecular profile with review of literature. Saudi J Health Sci. 2024;13:28-34. [DOI] [Full Text] |
| 36. | Jo KJ, Kim YM, Yoon JY, Lee YJ, Han YM, Yoo HW, Kim HS, Cheon CK. Comparison of effectiveness of growth hormone therapy according to disease-causing genes in children with Noonan syndrome. Korean J Pediatr. 2019;62:274-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Jorge AAL, Edouard T, Maghnie M, Pietropoli A, Kelepouris N, Romano A, Zenker M, Horikawa R. Outcomes in growth hormone-treated Noonan syndrome children: impact of PTPN11 mutation status. Endocr Connect. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Törnhage CJ, Lithner F, Eriksson UJ. Panhypopituitarism, neurosensory deafness and Noonan's syndrome in a child of a diabetic mother: role of maternal hypoglycaemia during pregnancy in induction of congenital lesions. Diabet Med. 1998;15:620-621. [PubMed] [DOI] [Full Text] |
| 39. | Ross JL, Shenkman L. Noonan's syndrome and hypopituitarism. Am J Med Sci. 1980;279:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 40. | Abstracts of the ESPE 61 Annual Society Meeting. Horm Res Paediatr. 2023;1-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Berchtold S, Volarevic S, Moriggl R, Mercep M, Groner B. Dominant negative variants of the SHP-2 tyrosine phosphatase inhibit prolactin activation of Jak2 (janus kinase 2) and induction of Stat5 (signal transducer and activator of transcription 5)-dependent transcription. Mol Endocrinol. 1998;12:556-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Zhang EE, Chapeau E, Hagihara K, Feng GS. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc Natl Acad Sci U S A. 2004;101:16064-16069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 215] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 43. | Bradford AP, Conrad KE, Tran PH, Ostrowski MC, Gutierrez-Hartmann A. GHF-1/Pit-1 functions as a cell-specific integrator of Ras signaling by targeting the Ras pathway to a composite Ets-1/GHF-1 response element. J Biol Chem. 1996;271:24639-24648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Bradford AP, Conrad KE, Wasylyk C, Wasylyk B, Gutierrez-Hartmann A. Functional interaction of c-Ets-1 and GHF-1/Pit-1 mediates Ras activation of pituitary-specific gene expression: mapping of the essential c-Ets-1 domain. Mol Cell Biol. 1995;15:2849-2857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Cakir M, Grossman AB. Targeting MAPK (Ras/ERK) and PI3K/Akt pathways in pituitary tumorigenesis. Expert Opin Ther Targets. 2009;13:1121-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Derwich A, Sykutera M, Bromińska B, Rubiś B, Ruchała M, Sawicka-Gutaj N. The Role of Activation of PI3K/AKT/mTOR and RAF/MEK/ERK Pathways in Aggressive Pituitary Adenomas-New Potential Therapeutic Approach-A Systematic Review. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Gualtieri A, Kyprianou N, Gregory LC, Vignola ML, Nicholson JG, Tan R, Inoue SI, Scagliotti V, Casado P, Blackburn J, Abollo-Jimenez F, Marinelli E, Besser REJ, Högler W, Karen Temple I, Davies JH, Gagunashvili A, Robinson ICAF, Camper SA, Davis SW, Cutillas PR, Gevers EF, Aoki Y, Dattani MT, Gaston-Massuet C. Activating mutations in BRAF disrupt the hypothalamo-pituitary axis leading to hypopituitarism in mice and humans. Nat Commun. 2021;12:2028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Gregory L, Besser R, Temple K, Davies J, Dattani M. Mutations in BRAF are associated with septo-optic dysplasia and cardiofaciocutaneous syndrome. EJEA. 2015. [DOI] [Full Text] |
| 49. | Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351 Pt 2:289-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 781] [Cited by in RCA: 799] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/