Published online Dec 9, 2025. doi: 10.5409/wjcp.v14.i4.108854
Revised: May 28, 2025
Accepted: August 8, 2025
Published online: December 9, 2025
Processing time: 190 Days and 7.7 Hours
Excipients may improve the palatability of polyethylene glycol (PEG), the first-line treatment for childhood functional constipation (FC), leading to good com
To compare the developed PEG-based formula (PEG-Chula) to the commercial formula for treating childhood FC.
In this randomized controlled trial, we enrolled children aged < 18 years with FC diagnosed by the Rome IV criteria to receive PEG-Chula [four flavors: (1) Straw
Fifty-two children diagnosed with FC [median age: 4.21 (2.33, 7.88) years; 35 (67.31%) females] were enrolled. After the 8-week treatment, the mean weekly stool frequency increased in both groups, the mean change was 4.02 (95%CI: 3.09–4.95) in PEG-Chula and 3.78 (95%CI: 2.79–4.78) in commercial PEG compared to baseline (P < 0.001). The extent of stool consistency improvement did not differ significantly. The most preferred PEG-Chula flavor was rated more palatable than the commercial PEG. Treatment compliance correlated with medication palatability (r = 0.34, P = 0.013). No significant differences in adverse events were found.
Both PEG-based formulas are effective and safe for managing pediatric FC.
Core Tip: Functional constipation (FC), a common functional gastrointestinal disorder in children, affects the quality of life of these children and their families. A combination of osmotic laxatives and toilet training is essential for effective mana
- Citation: Tran DL, Sintusek P. Efficacy and palatability of the developed polyethylene glycol-based formula for the treatment of children with functional constipation. World J Clin Pediatr 2025; 14(4): 108854
- URL: https://www.wjgnet.com/2219-2808/full/v14/i4/108854.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i4.108854
Functional constipation (FC) has been identified as the predominant functional gastrointestinal disorder in children, with a global prevalence of 14.4%[1]. In Thailand, the prevalence of FC was approximately 2.4% in children below five years of age[2] and around 8.1% in adolescents[3]. In addition, up to 3% of children coming to general pediatricians’ appointments have constipation as a presenting complaint, whereas this rate significantly rises to 25% among all children visiting pediatric gastroenterologists[4]. Constipation also imposes a substantial financial burden in many countries[5,6] and also takes a toll on the quality of life of children and their families[7].
In recent years, the Rome criteria have been widely used as a helpful tool to detect FC in children. Although the duration of the symptoms was reduced from 2 months in Rome III to 1 month in Rome IV, to facilitate early detection and prompt treatment[8-10], the prevalence of FC in children is unchanged[11]. Fecal retention behavior is regarded as the primary pathophysiology of FC in infants and young children[12,13]. Therefore, the combination of toilet training and osmotic laxatives is the key to achieving treatment goals, which include establishing normal defecation and preventing recurrence[12,14,15].
Osmotic laxatives—including polyethylene glycol (PEG), lactulose, and milk of magnesium hydroxide (MOM) are recommended as the first-line pharmacological intervention according to the European Society of Pediatric Gastroenterology, Hepatology and Nutrition and the North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition guidelines[16]. PEG is the preferred option because it has shown superiority in enhancing the frequency of bowel movements and stool consistency and is receiving greater acceptance among children than lactulose or MOM[17,18].
However, the treatment adherence rate with PEG therapy was reportedly 37%[19] and more than 50% of children had issues with its palatability[20]. The association between adherence and the taste and convenience of medicine has been identified. The unpleasant taste leads patients avoiding the medication, especially in the pediatric population[21,22]. Therefore, taste concealment is essential for increasing medication adherence and treatment efficacy in children[22].
Several PEG-based formulas are currently available on the market. However, there is a paucity of high-quality research assessing the comparative efficacy, safety, and acceptance across these PEG-based products because the inactive in
This double-blind, randomized controlled trial (RCT) was conducted at The Clinical Research Center, Faculty of Medicine, Chulalongkorn University, from May 2024 to February 2025. The study protocol was approved by the Institutional Review Board of Chulalongkorn University (No. 0093/67) and registered at ClinicalTrials.gov (No. NCT06357897).
Children were included in this study when they met the following criteria: (1) Were aged from 6 months to 18 years old; (2) Were diagnosed with FC according to the Rome IV diagnostic criteria; and (3) Consent forms were signed. Written informed consent was obtained from guardians and patients aged over 12 years old, while informed assent was obtained from patients aged 7–12 years, following the Declaration of Helsinki. Children were excluded from the study if they had an organic cause of constipation (e.g., anorectal malformations, Hirschsprung disease, myelomeningocele, hypothyroidism, etc.), suspected gastrointestinal obstruction, received medication affecting bowel movement, had acute cardiac, liver, pulmonary disorder, and had a history of allergy to the medicine used in this study. Children could be withdrawn from the study when their parents/guardians requested treatment discontinuation for any reason.
A list of numbers randomly generated using a computer was prepared by another researcher without clinical involvement in the trial. Eligible patients were enrolled in the study. Then, patients were randomly assigned to the PEG-Chula and commercial PEG groups using a block size of 4 and a 1:1 allocation ratio within each block.
The participants and the doctor were blinded to the type of treatment received during the study. To ensure that the participants were blinded to the study medication, they received all sachets in an identical package.
We developed the PEG-Chula product based on the PEG 4000 Lavage product, which has been used for colonoscopy preparation and patients with constipation at King Chulalongkorn Memorial Hospital for over 30 years. Under pharmacologist consultations, we used a sugar and flavoring approach to improve the taste of the PEG 4000 Lavage product because it does not require extensive equipment or complex manufacturing processes[22].
We performed experiments with various sweeteners that are accepted for use in medicine and food currently available on the market. We chose stevia because of its advantages such as its natural origin, low-calorie nature, the absence of associated spikes in glycemia levels, and safety[24].
In terms of flavor, we concentrated on the flavors that children preferred. After conducting a pilot study with 24 voluntary participants to select the preferred flavors, we decided on four flavors, including apple, lychee, lychee plus rose, and strawberry, for this RCT study.
For the commercial PEG group, we selected the PEG 4000 available in the Thailand market (Forlax containing PEG 4000, orange-grapefruit flavor, and saccharin sodium). The primary investigator prepared both PEG-Chula and Forlax into sachets labeled A, B, C, and D with 5 g or 10 g of PEG 4000. In the PEG-Chula group, label A was designated as strawberry, B as lychee, C as green apple, and D as a combination of lychee and rose flavors. In contrast, in the com
Eligible children were enrolled in the study and randomly assigned to receive PEG-Chula or the commercial PEG formula (Forlax) for eight weeks. The PEG 4000 dose was 0.5–1 g/kg/day for children without fecal impaction and 1–1.5 g/kg/day for children with fecal impaction. The children in each group received the PEG formula in identical packages. Children in both groups received counseling on toilet training, water intake, and fiber intake similarly. During the study, parents were instructed not to administer any laxatives to their children, except for the prescribed medication, and not to add medication to other juice to improve the taste.
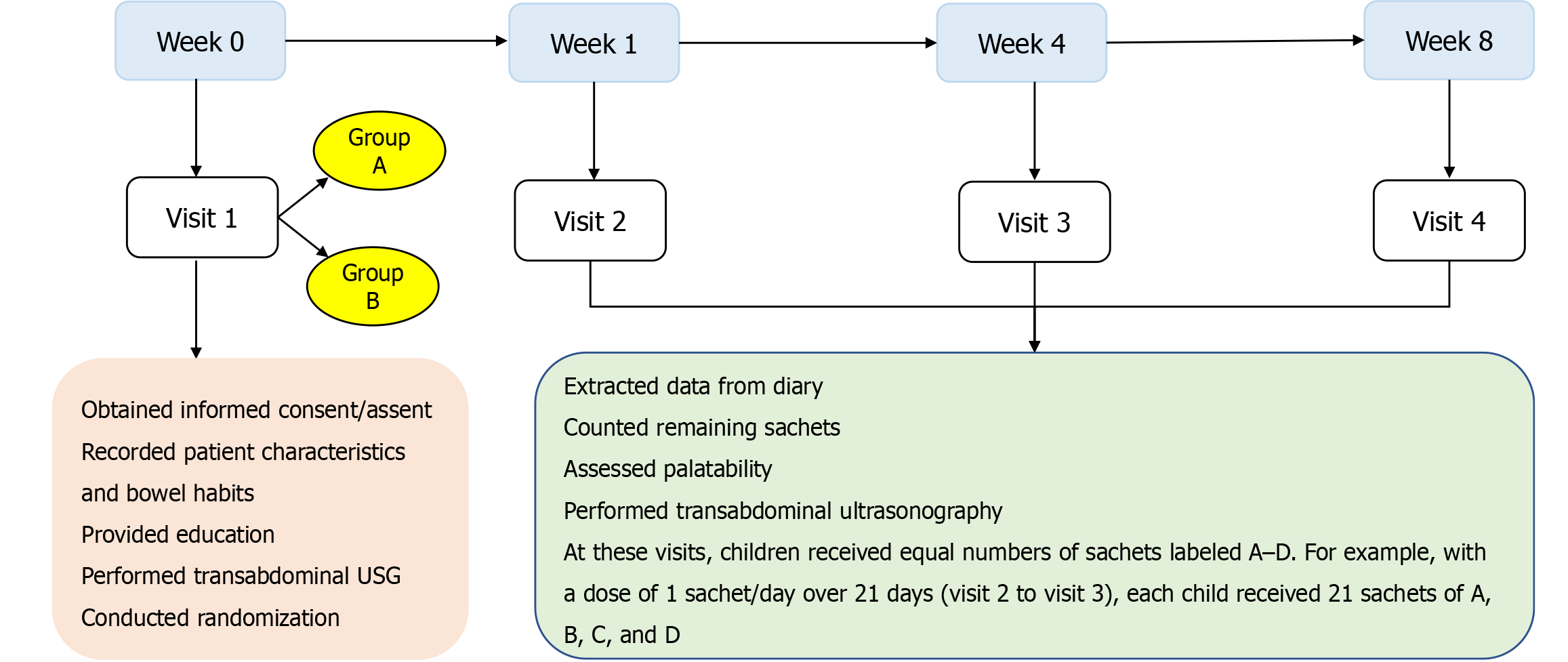
This study had a total of four visits. At visit 1 (day 0), the physician recorded the demographics, stool habits, and clinical features, and then the patients underwent a physical examination, anal position index measurement, and transabdominal ultrasonography (USG). Caregivers and patients received diary books to record the outcomes, including stool habits, compliance, adverse events, toilet training, and dietary intake. The children received medication labeled A, B, C, and D, with a total number of sachets sufficient for one week of treatment.
At follow-up visits, including visit 2 (week 1), visit 3 (week 4), and visit 4 (week 8), the stool output was identified via the patient diary, and caregivers were asked about the adverse events and medication compliance. From visit 2 (week 1) onward, the children received sachets labeled A, B, C, and D in quantities equal to the total number of days until the next visit. This allowed the children to choose their preferred flavor. The flowchart of the study procedure is shown in Figure 1.
The primary efficacy endpoint was improvement in stool frequency and stool consistency during the intervention period. The weekly stool frequency was recorded in patient diaries, while stool consistency was assessed for each bowel movement using the Bristol Stool scale by patients and caregivers[25]. The secondary outcomes were constipation-related symptom improvement, adverse events, and palatability[26]. Besides, compliance was assessed by recording in the diary and counting the remaining sachets. The palatability of the two formulas was evaluated using a 5-point facial hedonic scale in children aged > 4 years. For children aged ≤ 4 years, caregiver assessments were added to rate the medication’s palatability[27]. Parents reported any adverse events that occurred during the eight-week treatment period. Adverse events such as diarrhea, abdominal pain, nausea and vomiting, or bloating and flatulence were documented only if they did not precede the initiation of treatment.
The number of participants in this study was calculated based on the repeated measures design formula.
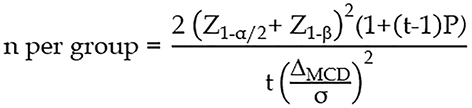
The significance level was set at 0.05, corresponding to Z1-α/2 = 1.96, while the power was set at 80% (Z1-β = 0.84). The pooled standard deviation was calculated as 2.85. The number of repeated measurements in the current study was set at 8. The correlation between time points and stool frequency was assumed to be weak, and a value of 0.25 was chosen. We anticipated that the difference in the number of bowel movements between the PEG-Chula and the commercial PEG would not exceed 1.4 times/week[28]. Accounting for a 10% dropout rate, the final sample size required for each group was determined to be 26 children with FC.
The following three study populations were evaluated: (1) The safety population, comprising patients who received at least one dose of the study medication; (2) The intention-to-treat (ITT) population, which includes all patients in the safety population with at least one post-treatment measure of stool frequency and consistency; and (3) The per-protocol (PP) population, consisting of ITT patients without major protocol deviations. The primary efficacy analysis was performed using the ITT population, while the sensitivity analysis was performed using the PP population. Our safety analysis included the safety population.
Data were managed using REDCap electronic data capture tools[29] hosted at the Chula Data Management Center, Faculty of Medicine, Chulalongkorn University.
For the primary outcomes, stool frequency and consistency were compared between the PEG-Chula and Forlax groups using a mixed-effects model for repeated measures. The model included the intervention group as a fixed effect and used an unstructured covariance matrix to account for correlations within repeated measures of the same subject, assuming independence between patients. The results were illustrated graphically to demonstrate weekly trends in outcomes in the different treatment groups.
For secondary outcomes, continuous variables were reported as the mean ± SD for normally distributed continuous data and the median (interquartile range) for non-normally distributed continuous data. To control for type I error due to multiple comparisons, the Bonferroni correction was applied where appropriate. Group comparisons were conducted using independent t-tests for normally distributed continuous data and the Mann-Whitney U test for non-normally distributed continuous data. Categorical variables were presented as frequencies and proportions, with comparisons made using the χ2 test or Fisher’s exact test. Statistical analyses were performed using STATA version 18.
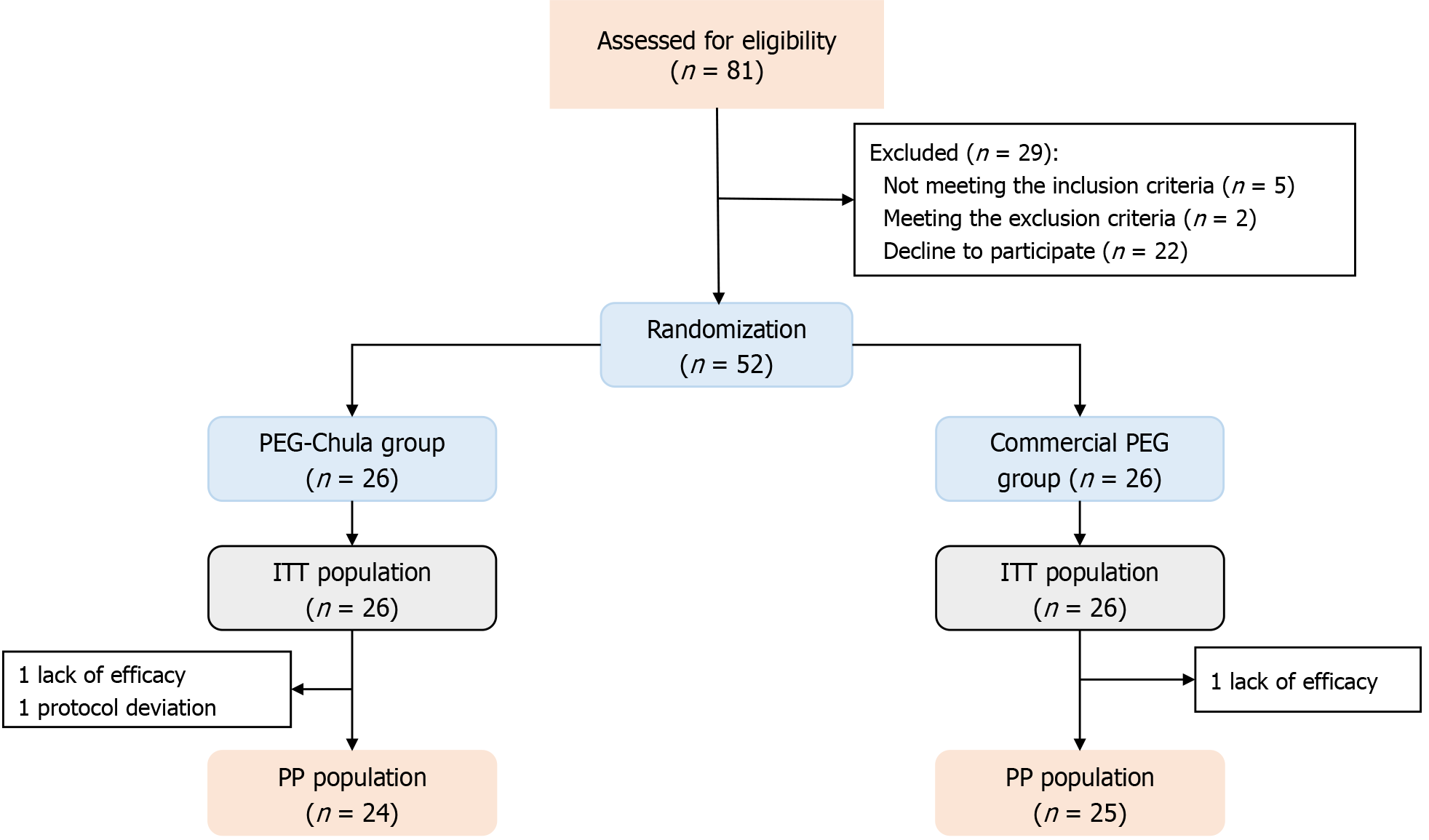
Eighty-one children were screened for eligibility, and 52 were randomized to receive either the PEG-Chula or the commercial PEG formula. One child in the commercial PEG group was excluded due to the unavailability of outcome measurements. Additionally, two patients in the PEG-Chula group were also excluded, one due to missing outcome measurements and another due to protocol deviation. Ultimately, 49 patients completed the study (Figure 2).
Of the 52 participants, the median age was 4.21 (2.33, 7.88) years, with 25 children (48.08%) aged under 4 years old. Thirty-five children (67.31%) were female. The median weight and height were 16.10 (11.80, 16.85) kg and 99.50 (88.50, 125.30) cm, respectively. The median duration of FC was 19.50 (12.00, 44.00) months. Additionally, 25 children (48.08%) had received prior treatment, including lactulose (n = 20), PEG (n = 5), enemas (n = 8), and bisacodyl (n = 1). Fecal impaction was detected in one child in the PEG-Chula group and two children in the commercial PEG group. The participants’ demographics and baseline features did not differ significantly between the two groups (Table 1).
| Variables | PEG-Chula | Commercial PEG |
| Age (years) | 4.38 (2.90, 7.00) | 3.92 (2.25, 9.91) |
| Age group (6 months-4 years) | 11 (42.30) | 14 (53.80) |
| Sex, males | 11 (42.30) | 6 (23.10) |
| Weight (kg) | 16.1 (12.20, 25.20) | 15.3 (11.50, 30.10) |
| Height (cm) | 101 (90.00, 124.50) | 97.75 (84.50, 135.00) |
| FC in family | 15 (57.70) | 16 (61.54) |
| Previous treatment for FC | 14 (53.90) | 11 (42.30) |
| Duration of constipation (months) | 21 (12, 48) | 16.5 (12, 30) |
| Frequency of defecation (time/week) | 1.96 ± 0.58 | 2.21 ± 0.74 |
| Stool consistency (Bristol Stool scale) | 2.31 ± 0.78 | 2.08 ± 0.82 |
| Painful defecation (Facial Pain scale) | 3.65 ± 1.16 | 3.48 ± 1.01 |
| Clogged toilet for toilet-trained children (n = 42) | 13 (59.09) | 10 (50.00) |
| Presence of fecal mass by examination | 2 (7.69) | 1 (3.85) |
| Blood coating the stool | 11 (42.31) | 8 (30.77) |
| Anal position index | ||
| Male | 0.51 ± 0.06 | 0.5 ± 0.05 |
| Female | 0.38 ± 0.09 | 0.37 ± 0.06 |
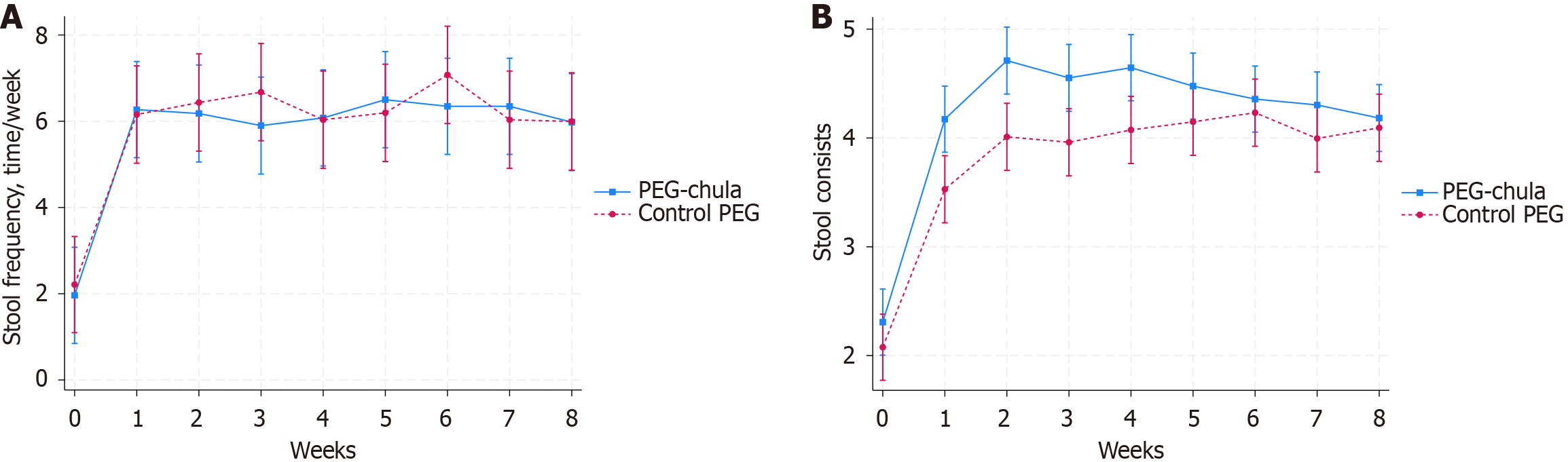
The mean stool frequency per week at baseline was 1.96 ± 0.58 in the PEG-Chula group and 2.21 ± 0.74 in the commercial PEG group (P = 0.18). After treatment, the mean stool frequency significantly increased in both groups compared to baseline. The improvement of stool frequency was not significantly different in the PEG-Chula group compared to the commercial PEG group in each week (Table 2, Figure 3A).
| Variables | PEG-Chula group | Commercial group | Between group | |||
| mean ± SD | Mean change from baseline (95%CI) | mean ± SD | Mean change from baseline (95%CI) | Mean difference (95%CI) | P value | |
| Stool frequency (time/week) | ||||||
| Baseline (n = 52) | 1.96 ± 0.58 | - | 2.21 ± 0.74 | - | - | 0.181 |
| Week 1 (n = 51) | 6.27 ± 2.97 | 4.31 (3.39-5.23) | 6.16 ± 4.35 | 3.94 (2.95-4.94) | 0.36 (-0.99 to 1.72) | 0.597 |
| Week 2 (n = 50) | 6.20 ± 2.24 | 4.22 (3.29-5.15) | 6.44 ± 3.98 | 4.22 (3.23-5.22) | -0.01 (-1.36 to 1.35) | 0.993 |
| Week 3 (n = 50) | 5.92 ± 2.33 | 3.94 (3.01-4.87) | 6.68 ± 3.8 | 4.46 (3.47-5.46) | -0.53 (-1.88 to 0.83) | 0.448 |
| Week 4 (n = 51) | 6.08 ± 2.42 | 4.12 (3.20-5.03) | 6.04 ± 3.88 | 3.82 (2.83-4.82) | 0.29 (-1.06 to 1.64) | 0.671 |
| Week 5 (n = 51) | 6.50 ± 2.67 | 4.54 (3.62-5.46) | 6.20 ± 3.58 | 3.98 (2.99-4.98) | 0.56 (-0.8 to 1.91) | 0.420 |
| Week 6 (n = 51) | 6.35 ± 2.81 | 4.38 (3.47-5.3) | 7.08 ± 3.5 | 4.86 (3.87-5.86) | -0.48 (-1.83 to 0.87) | 0.488 |
| Week 7 (n = 51) | 6.35 ± 2.42 | 4.38 (3.47-5.3) | 6.04 ± 2.91 | 3.82 (2.83-4.82) | 0.56 (-0.79 to 1.91) | 0.415 |
| Week 8 (n = 50) | 6.00 ± 2.14 | 4.02 (3.09-4.95) | 6.00 ± 3.07 | 3.78 (2.79-4.78) | 0.23 (-1.12 to 1.59) | 0.736 |
| Stool consistency | ||||||
| Baseline (n = 52) | 2.31 ± 0.78 | - | 2.08 ± 0.82 | - | - | 0.30 |
| Week 1 (n = 51) | 4.17 ± 1.02 | 1.87 (1.55-2.18) | 3.53 ± 1.15 | 1.45 (1.12-1.78) | 0.41 (-0.04 to 0.87) | 0.075 |
| Week 2 (n = 50) | 4.70 ± 0.74 | 2.40 (2.08-2.72) | 4.01 ± 0.96 | 1.93 (1.61-2.26) | 0.47 (0.01 to 0.93) | 0.045 |
| Week 3 (n = 50) | 4.55 ± 0.92 | 2.24 (1.92-2.57) | 3.96 ± 0.87 | 1.88 (1.56-2.21) | 0.36 (-0.10 to 0.82) | 0.123 |
| Week 4 (n = 51) | 4.65 ± 0.83 | 2.34 (2.02-2.66) | 4.08 ± 0.78 | 2.00 (1.67-2.32) | 0.34 (-0.11 to 0.80) | 0.142 |
| Week 5 (n = 51) | 4.48 ± 0.54 | 2.17 (1.85-2.49) | 4.15 ± 0.88 | 2.07 (1.74-2.40) | 0.10 (-0.36 to 0.55) | 0.675 |
| Week 6 (n = 51) | 4.36 ± 0.57 | 2.05 (1.73-2.37) | 4.23 ± 0.79 | 2.16 (1.83-2.48) | -0.11 (-0.56 to 0.35) | 0.650 |
| Week 7 (n = 51) | 4.30 ± 0.49 | 2.00 (1.68-2.31) | 4.00 ± 0.88 | 1.92 (1.59-2.25) | 0.08 (-0.38 to 0.53) | 0.740 |
| Week 8 (n = 50) | 4.18 ± 0.38 | 1.87 (1.55-2.2) | 4.10 ± 0.73 | 2.02 (1.69-2.34) | -0.14 (-0.60 to 0.32) | 0.546 |
Stool consistency at baseline was 2.31 ± 0.78 in the PEG-Chula group and 2.08 ± 0.82 in the commercial PEG group (P = 0.30). After 8-week treatment, the stool consistency was also significantly increased in both groups compared to baseline (P < 0.001). The stool consistency was significantly higher in the PEG-Chula group compared to the commercial PEG group only at week 2 (P = 0.045). No significant differences were observed during the other weeks (Table 2, Figure 3B).
Table 3 presents the improvement of related symptoms after the 8-week treatment period. The dose of PEG 4000 administered during the treatment period did not differ significantly between the groups [0.58 (0.39, 0.63) vs 0.6 (0.38, 0.82), P = 0.62]. Patient compliance in the PEG-Chula group [94.17 (88.60, 100)] was higher than that in the commercial PEG group [90.1 (77.67, 98.10)], although the difference was not statistically significant (P = 0.33). The number of days with defecation per week was similar between the two groups (5.19 ± 1.23 vs 4.95 ± 1.54, P = 0.52). Based on the Facial Pain scale, children in the PEG-Chula group showed a greater reduction in pain during defecation compared to those in the control group, although this difference was not statistically significant (-2.41 ± 1.41 vs -1.87 ± 1.29, P = 0.17). Improvement in the related symptoms of constipation, including withholding behaviors, large-diamater stool, fecal incontinence, abdominal pain, appetite loss, and anal fissures, did not differ significantly between the two groups. By the end of the 8-week treatment period, 98% of the children no longer met the Rome IV criteria for constipation, and one child in the commercial PEG group still met the Rome IV criteria (less than 2 defecations per week, stool retention, and large-diameter stool).
| Secondary end points | PEG-Chula | Commercial PEG | P value |
| Dose of PEG 4000 (g/kg) (n = 51) | 0.58 (0.39, 0.63) | 0.6 (0.38, 0.82) | 0.62 |
| Compliance (n = 51) | 94.17 (88.60, 100) | 90.1 (77.67, 98.10) | 0.33 |
| Days with defecations per week (n = 51) | 5.19 ± 1.23 | 4.95 ± 1.54 | 0.52 |
| Change from baseline of painful defecation (n = 51) | -2.41 ± 1.41 | -1.87 ± 1.29 | 0.17 |
| Withholding behaviours after treatment (n = 40) | 3 (15.79) | 8 (38.09) | 0.161 |
| Large stool diameter after treatment (n = 36) | 2 (10.53) | 1 (5.88) | 11 |
| Fecal incontinence after treatment (n = 6) | 1 (20) | 0 (0) | 11 |
| Abdominal pain after treatment (n = 13) | 0 (0) | 1 (16.67) | 0.461 |
| Bloating/flatulence after treatment (n = 11) | 1 (11.11) | 0 (0) | 11 |
| Appetite loss after treatment (n = 9) | 0 (0) | 1 (33.33) | 0.331 |
| Anal fissure after treatment (n = 22) | 3 (25) | 1 (10) | 0.591 |
| Fulfill Rome IV criteria for functional constipation after treatment (n = 51) | 0 | 1 (4) | 0.301 |
| Fecal contents detected by transabdominal ultrasonography (n = 51) | 8 (30.77) | 7 (28) | 0.82 |
The median ratings for PEG-Chula with strawberry, lychee, green apple, and lychee-rose flavors were 3.00 (2.50, 4.00), 4.00 (3.20, 5.00), 3.60 (2.60, 4.00), and 4.00 (3.00, 4.00), respectively. In comparison, the commercial PEG formula received a median rating of 3.90 (3.50, 4.10) (Table 4). The palatability rating of the preferred flavor in the PEG-Chula group was higher than that in the commercial PEG formula group (P = 0.004). The lychee flavor of PEG-Chula was the best favorite flavor chosen by 13 (50%) children. Additionally, a statistically significant positive correlation was observed between treatment compliance and medication palatability (r = 0.35, P = 0.013) (Supplementary Figure 1). There was no statistically significant difference in palatability between children aged less than four years and those aged above four years (Supplementary Table 1).
| Intervention group | Palatability | P value | |
| PEG-Chula group | Strawberry | 3.00 (2.50, 4.00) | 0.12 |
| Lychee | 4.00 (3.20, 5.00) | 0.44 | |
| Green apple | 3.60 (2.60, 4.00) | 0.27 | |
| Lychee-rose | 4.00 (3.00, 4.00) | 0.80 | |
| Most preferred flavor | 4.30 (4.00, 5.00) | 0.004 | |
| Commercial PEG group | Orange-grapefruit | 3.90 (3.50, 4.10) | 1 |
Adverse events were reported in 5 (19.23%) patients in the PEG-Chula group and 3 (11.54%) patients in the commercial PEG group (P = 0.7). Since all reported adverse events were mild, no patient discontinued treatment due to the adverse events (Table 5).
| Adverse events | PEG-Chula (n = 26) | Commercial PEG (n = 26) |
| Number of patients who reported adverse events | 5 (19.23) | 3 (11.54) |
| Mild | 5 (19.23) | 3 (11.54) |
| Moderate | 0 | 0 |
| Severe | 0 | 0 |
| Any adverse events | ||
| Diarrhoea | 3 (11.54) | 2 (7.69) |
| Abdominal pain | 2 (7.69) | 1 (3.85) |
This study investigated a head-to-head comparison between two PEG 4000-based formulas—the commercially available and the locally produced formulas. PEG was recommended as the first-line treatment because it demonstrated superior effectiveness to other osmotic laxatives[16,17]. Selecting a preferred flavor PEG-based formula for children may provide additional benefits in managing constipation[23]. Therefore, our study was based on the hypothesis that the better-tasting flavor of PEG-based formulas could increase treatment adherence and the effectiveness of constipation management in children.
Per the primary outcomes of this study, stool frequency and consistency improved significantly after an 8-week treatment period. The PEG-Chula was non-inferior and non-superior compared to the commercial PEG formula. At each week, although the stool frequency and consistency in the PEG-Chula group were higher than those in the control group, the differences may not be clinically meaningful. Besides, there were no significant differences in the improvement of symptoms associated with constipation. The dose of PEG 4000 did not differ significantly between the two formulas.
An RCT comparing PEG 4000 without electrolytes and PEG 3350 plus electrolytes showed that children in the PEG 4000 group had superior stool frequency improvement than those in the PEG plus electrolyte group (9.20 ± 3.20 vs 7.80 ± 2.40, P = 0.025). Besides that, the PEG 4000 formula had a better taste than PEG 3350 plus electrolyte (P < 0.001), and the compliance with PEG 4000 was also higher than that with PEG 3350 plus electrolyte[28]. However, in this study, parents of children in the PEG 4000 group were allowed to dissolve the medication in other fluids to improve its taste, and the study outcomes were not adjusted for potential confounders such as sex, age, or medication palatability.
Conversely, the study conducted by Llerena et al[30] also showed that the change in stool frequency and stool consistency did not differ significantly between PEG 4000 and PEG 3350 plus electrolytes. However, to increase compliance in children, parents could dissolve the medication in a different beverage to improve acceptance in both groups. Although the active ingredient used in both studies was the same, the acceptance of children played an important role in the outcomes. These findings suggested that the better palatability formula may have superior efficacy due to increased acceptance and compliance in children.
In our study, there were no significant differences in the palatability of strawberry, lychee, green apple, and lychee-rose in the PEG-Chula group compared to the orange-grapefruit flavor in the control group. However, the rating palatability of the most preferred formula in PEG-Chula was higher than that in the commercial PEG group (P = 0.004). In our study, we observed a weak correlation between treatment compliance and medication palatability (r = 0.35, P = 0.013). This finding suggests a potentially important trend, as poor palatability can negatively affect adherence, while good palatability has been demonstrated to improve compliance[31,32]. A palatable formula may enhance adherence in long-term treatment settings[33]. Although a trend toward better compliance was found in the PEG-Chula [94.17 (88.60, 100) vs 90.1 (77.67, 98.10), P = 0.33], the two groups had the same treatment efficacy. This may be explained by the fact that, in this study, we provided education and compliance reinforcement for guardians. However, in real clinical practice, guardians may be less motivated to adhere to treatment compared to what happens in a controlled clinical trial setting. Notably, cross-sectional studies have demonstrated that the adherence rate to PEG therapy among children with constipation is approximately 30%–40%. Additionally, treatment satisfaction and convenience were found to be associated with compliance in children[19,34].
After eight weeks of treatment, although none of the children exhibited clinical signs of fecal impaction, transabdominal USG detected fecal contents in 8 (30.70%) children in the PEG-Chula group and 7 (28%) children in the commercial PEG group. This finding suggests that the assessment of fecal contents using transabdominal USG could serve as a more sensitive and objective marker to diagnose stool retention in children than abdominal palpation alone. This raises the question of whether fecal contents and rectal diameter detected by transabdominal USG can be utilized as a biomarker for monitoring treatment response or predicting long-term outcomes in pediatric constipation[35,36]. Further studies are warranted to evaluate the diagnostic accuracy and prognostic value of USG assessment in this context.
We observed some adverse events during the 8-week treatment period. The rate of adverse events did not differ significantly between the two groups [5 (19.23%) vs 3 (11.54%)], and all of them were mild. Consistent with findings from other studies, the most commonly reported adverse symptoms associated with PEG were diarrhea or abdominal pain[28,33].
To the best of our knowledge, this is the first study conducted to evaluate the role of inactive ingredients, such as flavor and sweeteners, in PEG 4000 formulas for treating childhood FC. The findings of this study encourage pediatric centers and pharmaceutical companies to develop locally produced PEG formulas with flavors preferred by children in specific regions, potentially optimizing treatment adherence and effectiveness in the management of pediatric FC.
However, this study has some limitations that should be acknowledged. First, although we tried to standardize the external appearance and labeling of both interventions to ensure a double-blind design, some caregivers could still detect differences in smell and taste if they deliberately sampled the medication before administering it to the child. This may have introduced bias in the perception of treatment effectiveness or adherence. Second, we enrolled children across a wide age range (6 months to 18 years), and the relatively small sample size resulted in variability in flavor preferences among different age groups, and the study may be underpowered to detect significant differences in secondary outcomes. Third, while this study assessed short-term efficacy and tolerability, the long-term adherence and effectiveness were not fully explored. Further studies with larger sample sizes, extended follow-up periods, and stricter control of confounders are needed to validate our findings and provide a more comprehensive understanding of the impact of flavoring agents and sweeteners in PEG-based pediatric FC treatments.
Both PEG-based formulas are effective and safe in the management of pediatric FC. Palatability aligned with child preference is the key to improving compliance in clinical practice. Therefore, exploring multiple flavor options with the same active ingredient is essential to identifying the most appealing taste for children, ensuring better compliance and reducing their boredom with taking medication daily during long-term treatment.
We would like to express our sincere gratitude to the Maha Chakri Sirindhorn Clinical Research Center under the Royal Patronage and Clinical Sciences program, Faculty of Medicine, Chulalongkorn University, for their invaluable support. We are especially thankful to Samsung Thailand for generously providing the ultrasound machine used in this study, and to Insent Company for their assistance in taste testing the formulas. We are also deeply thankful to Assoc. Prof. Rossarin Tansawat and Assoc. Prof. Warangkana Warisnoicharoen for their expert consultation and guidance in developing the polyethylene glycol-Chula formula, and Dr. Yuda Chongpison for her statistical analysis consultation. In addition, we gratefully acknowledge the contributions of our research team—Mr. Santirat Sopee, Ms. Arisa Ama, Ms. Nittikarn Pinyoying, and Ms. Pakpine Phunnoi for their dedication and assistance in data collection.
| 1. | Tran DL, Sintusek P. Functional constipation in children: What physicians should know. World J Gastroenterol. 2023;29:1261-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (36)] |
| 2. | Osatakul S, Puetpaiboon A. Use of Rome II versus Rome III criteria for diagnosis of functional constipation in young children. Pediatr Int. 2014;56:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Siajunboriboon S, Tanpowpong P, Empremsilapa S, Lertudomphonwanit C, Nuntnarumit P, Treepongkaruna S. Prevalence of functional abdominal pain disorders and functional constipation in adolescents. J Paediatr Child Health. 2022;58:1209-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Benninga MA, Voskuijl WP, Taminiau JA. Childhood constipation: is there new light in the tunnel? J Pediatr Gastroenterol Nutr. 2004;39:448-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (4)] |
| 5. | Ansari H, Ansari Z, Lim T, Hutson JM, Southwell BR. Factors relating to hospitalisation and economic burden of paediatric constipation in the state of Victoria, Australia, 2002-2009. J Paediatr Child Health. 2014;50:993-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Liem O, Harman J, Benninga M, Kelleher K, Mousa H, Di Lorenzo C. Health utilization and cost impact of childhood constipation in the United States. J Pediatr. 2009;154:258-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 210] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 7. | Vriesman MH, Rajindrajith S, Koppen IJN, van Etten-Jamaludin FS, van Dijk M, Devanarayana NM, Tabbers MM, Benninga MA. Quality of Life in Children with Functional Constipation: A Systematic Review and Meta-Analysis. J Pediatr. 2019;214:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 8. | Koppen IJ, Nurko S, Saps M, Di Lorenzo C, Benninga MA. The pediatric Rome IV criteria: what's new? Expert Rev Gastroenterol Hepatol. 2017;11:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (22)] |
| 9. | Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Functional Disorders: Children and Adolescents. Gastroenterology. 2016;S0016-5085(16)00181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 858] [Article Influence: 85.8] [Reference Citation Analysis (5)] |
| 10. | Benninga MA, Faure C, Hyman PE, St James Roberts I, Schechter NL, Nurko S. Childhood Functional Gastrointestinal Disorders: Neonate/Toddler. Gastroenterology. 2016;S0016-5085(16)00182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 388] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 11. | Játiva-Mariño E, Rivera-Valenzuela MG, Velasco-Benitez CA, Saps M. The prevalence of functional constipation in children was unchanged after the Rome IV criteria halved the diagnosis period in Rome III. Acta Paediatr. 2019;108:2274-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Rial R, Uc A. Functional constipation. In: Kleinman RE, Goulet O-J, Mieli-Vergani G, Sanderson IR, Sherman PM, Shneider BL, editors. Walker's pediatric gastrointestinal disease. United States: People's Medical Publishing House, 2018: 991-1005. |
| 13. | Koppen IJN, Benninga MA. Functional Constipation and Dyssynergic Defecation in Children. Front Pediatr. 2022;10:832877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (3)] |
| 14. | Vriesman MH, Benninga MA. Functional Constipation and Fecal Incontinence. In: Wyllie R, Hyams JS, Kay M. Pediatric Gastrointestinal and Liver Disease. Netherlands: Elsevier, 2021. [DOI] [Full Text] |
| 15. | Leung AK, Hon KL. Paediatrics: how to manage functional constipation. Drugs Context. 2021;10:2020-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Tabbers MM, DiLorenzo C, Berger MY, Faure C, Langendam MW, Nurko S, Staiano A, Vandenplas Y, Benninga MA; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition; North American Society for Pediatric Gastroenterology. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58:258-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 675] [Article Influence: 56.3] [Reference Citation Analysis (1)] |
| 17. | Gordon M, MacDonald JK, Parker CE, Akobeng AK, Thomas AG. Osmotic and stimulant laxatives for the management of childhood constipation. Cochrane Database Syst Rev. 2016;2016:CD009118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (2)] |
| 18. | Chen SL, Cai SR, Deng L, Zhang XH, Luo TD, Peng JJ, Xu JB, Li WF, Chen CQ, Ma JP, He YL. Efficacy and complications of polyethylene glycols for treatment of constipation in children: a meta-analysis. Medicine (Baltimore). 2014;93:e65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Koppen IJN, van Wassenaer EA, Barendsen RW, Brand PL, Benninga MA. Adherence to Polyethylene Glycol Treatment in Children with Functional Constipation Is Associated with Parental Illness Perceptions, Satisfaction with Treatment, and Perceived Treatment Convenience. J Pediatr. 2018;199:132-139.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 20. | Venables R, Batchelor H, Hodson J, Stirling H, Marriott J. Determination of formulation factors that affect oral medicines acceptability in a domiciliary paediatric population. Int J Pharm. 2015;480:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Thompson C, Lombardi D, Sjostedt P, Squires L. Best Practice Recommendations Regarding the Assessment of Palatability and Swallowability in the Development of Oral Dosage Forms for Pediatric Patients. Ther Innov Regul Sci. 2015;49:647-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Al-Japairai K, Hamed Almurisi S, Almonem Doolaanea A, Mahmood S, Alheibshy F, Alobaida A, Abdul-Halim N, Chatterjee B. A review on taste masked multiparticulate dosage forms for paediatric. Int J Pharm. 2023;632:122571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 23. | Szojda MM, Mulder CJ, Felt-Bersma RJ. Differences in taste between two polyethylene glycol preparations. J Gastrointestin Liver Dis. 2007;16:379-381. [PubMed] |
| 24. | Orellana-Paucar AM. Steviol Glycosides from Stevia rebaudiana: An Updated Overview of Their Sweetening Activity, Pharmacological Properties, and Safety Aspects. Molecules. 2023;28:1258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 25. | Koppen IJN, Velasco-Benitez CA, Benninga MA, Di Lorenzo C, Saps M. Using the Bristol Stool Scale and Parental Report of Stool Consistency as Part of the Rome III Criteria for Functional Constipation in Infants and Toddlers. J Pediatr. 2016;177:44-48.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14:9-17. [PubMed] |
| 27. | Mistry P, Stirling H, Callens C, Hodson J, Batchelor H; SPaeDD-UK project. Evaluation of patient-reported outcome measurements as a reliable tool to measure acceptability of the taste of paediatric medicines in an inpatient paediatric population. BMJ Open. 2018;8:e021961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Savino F, Viola S, Erasmo M, Di Nardo G, Oliva S, Cucchiara S. Efficacy and tolerability of peg-only laxative on faecal impaction and chronic constipation in children. A controlled double blind randomized study vs a standard peg-electrolyte laxative. BMC Pediatr. 2012;12:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38562] [Cited by in RCA: 40072] [Article Influence: 2357.2] [Reference Citation Analysis (0)] |
| 30. | Llerena E, Varea Calderón V, Pujol Muncunill G, Hernandez Hernandez K, Sosa Giraldo FJ, Suarez Fuentes T, Martín de Carpi J. [Comparison of the effectiveness and safety of polyethylene glycol with and without electrolytes in the treatment of chronic constipation]. An Pediatr (Barc). 2016;85:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Winnick S, Lucas DO, Hartman AL, Toll D. How do you improve compliance? Pediatrics. 2005;115:e718-e724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | El-Rachidi S, LaRochelle JM, Morgan JA. Pharmacists and Pediatric Medication Adherence: Bridging the Gap. Hosp Pharm. 2017;52:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 33. | Bekkali NLH, Hoekman DR, Liem O, Bongers MEJ, van Wijk MP, Zegers B, Pelleboer RA, Verwijs W, Koot BGP, Voropaiev M, Benninga MA. Polyethylene Glycol 3350 With Electrolytes Versus Polyethylene Glycol 4000 for Constipation: A Randomized, Controlled Trial. J Pediatr Gastroenterol Nutr. 2018;66:10-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Steiner SA, Torres MR, Penna FJ, Gazzinelli BF, Corradi CG, Costa AS, Ribeiro IG, de Andrade EG, do Carmo Barros de Melo M. Chronic functional constipation in children: adherence and factors associated with drug treatment. J Pediatr Gastroenterol Nutr. 2014;58:598-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 35. | Nunes NC, de Abreu GE, Dourado ER, Veiga ML, Nacif A, Calasans MTA, Braga AANM, Barroso U Jr. Association between rectal diameter and response to treatment with parasacral transcutaneous electrical nerve stimulation and behavioral changes in children and adolescents with bladder and bowel dysfunction. Int Braz J Urol. 2023;49:688-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Joensson IM, Siggaard C, Rittig S, Hagstroem S, Djurhuus JC. Transabdominal ultrasound of rectum as a diagnostic tool in childhood constipation. J Urol. 2008;179:1997-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/