Published online Dec 9, 2025. doi: 10.5409/wjcp.v14.i4.108823
Revised: May 21, 2025
Accepted: August 25, 2025
Published online: December 9, 2025
Processing time: 191 Days and 0.6 Hours
Dual-energy CT (DECT) is an advancement in CT technology that allows for the acquisition of images at two different energy levels. Two main post-processing tools, which form the backbone of DECT, include material decomposition and virtual monoenergetic images. Material decomposition helps in the generation of virtual nonenhanced, iodine, pulmonary lung blood volume, lung vessel, au
Core Tip: Dual-energy CT has a wide range of applications in vascular, brain, chest, abdomen, skeletal, and oncological imaging in children. Concerns have always been raised about the potential risks of ionizing radiation in the pediatric population from CT scans. Amongst the recent advancements in CT technology, dual-energy CT stands out because of its ability to provide enhanced diagnostic information, reduce radiation doses, and facilitate faster scans, making it a highly promising imaging investigation in children.
- Citation: Saini S, Bhatia A, Bansal A, Saxena AK, Sodhi KS. Dual-energy computed tomography in children: Technique and clinical applications. World J Clin Pediatr 2025; 14(4): 108823
- URL: https://www.wjgnet.com/2219-2808/full/v14/i4/108823.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i4.108823
Dual-energy CT (DECT) allows for the acquisition of images at two different energy levels. It is based on the fact that materials behave differently with respect to their attenuation at high-energy and low-energy spectra due to the photoelectric effect. Apart from generating two image datasets at two different energy levels, a blended contrast image dataset is also generated for interpretation. Studies have shown that the radiation dose of DECT is not significantly higher than that of single-energy CT (SECT)[1-5]. This is especially important in children, who are more sensitive to the hazardous effects of radiation. By using the least amount of radiation to achieve the principles of DECT, the radiation dose can be minimized to a great extent[6,7]. The mean CT dose index in children in the dual-energy technique approximates to 3.7 mGy while for SECT to about 4.4 mGy[2]. Further, an inherent limitation of conventionally used SECT is that structures having different tissue compositions, such as calcification, blood products, and iodine, can sometimes be indistinguishable because of the overlap in their respective attenuation.
Magnetic resonance imaging (MRI) is a radiation-free alternative to CT that can be considered especially in the evaluation of the head and neck, spine, pelvis, hepatobiliary system, and chest. Recently, unenhanced MRI has also been found in a study to be useful for the initial evaluation of abdominal emergencies in children. However, in head and neck emergencies, CT takes precedence over MRI. MRI is also compromised due to increased sensitivity to motion-related artifacts, poorer spatial resolution compared with that of CT, and limited compatibility with equipment used in the intensive care unit[8]. In addition the anatomy as well as pathologies of paranasal sinuses and temporal bones are better assessed on CT than MRI. Therefore, considering the potential benefits of DECT in children, we discussed its applications in the pediatric population in this article.
Different vendors use different technologies for the generation of dual-energy image datasets[9,10]. These include the following.
There is an acquisition of two different helical datasets, one followed by another in the same scanner at different energy spectra or subsequent rotations at alternating tube voltages. Its disadvantage is the delay between the acquisitions, making misregistration due to motion unavoidable.
There is a single X-ray tube, which rapidly alternates between high-energy and low-energy kilo-voltage to generate two different sets of images. Its main disadvantages are the long acquisition time and the high dose[9].
There are two layers of detectors, one is sensitive to low-energy photons and the other to high-energy photons. The top layer is yttrium-based for absorption of low-energy data, and the bottom layer is gadolinium-oxysulfide-based for high-energy data. The advantages are a lack of misregistration and cross-scatter, whereas decreased spectral separation is a drawback[10,11].
This technique uses two filters, tin and gold, to split the 120 tube kilovoltage peak (kVp) X-ray beam into two different energy levels. Decreased spectral separation is a limitation here also[10,11].
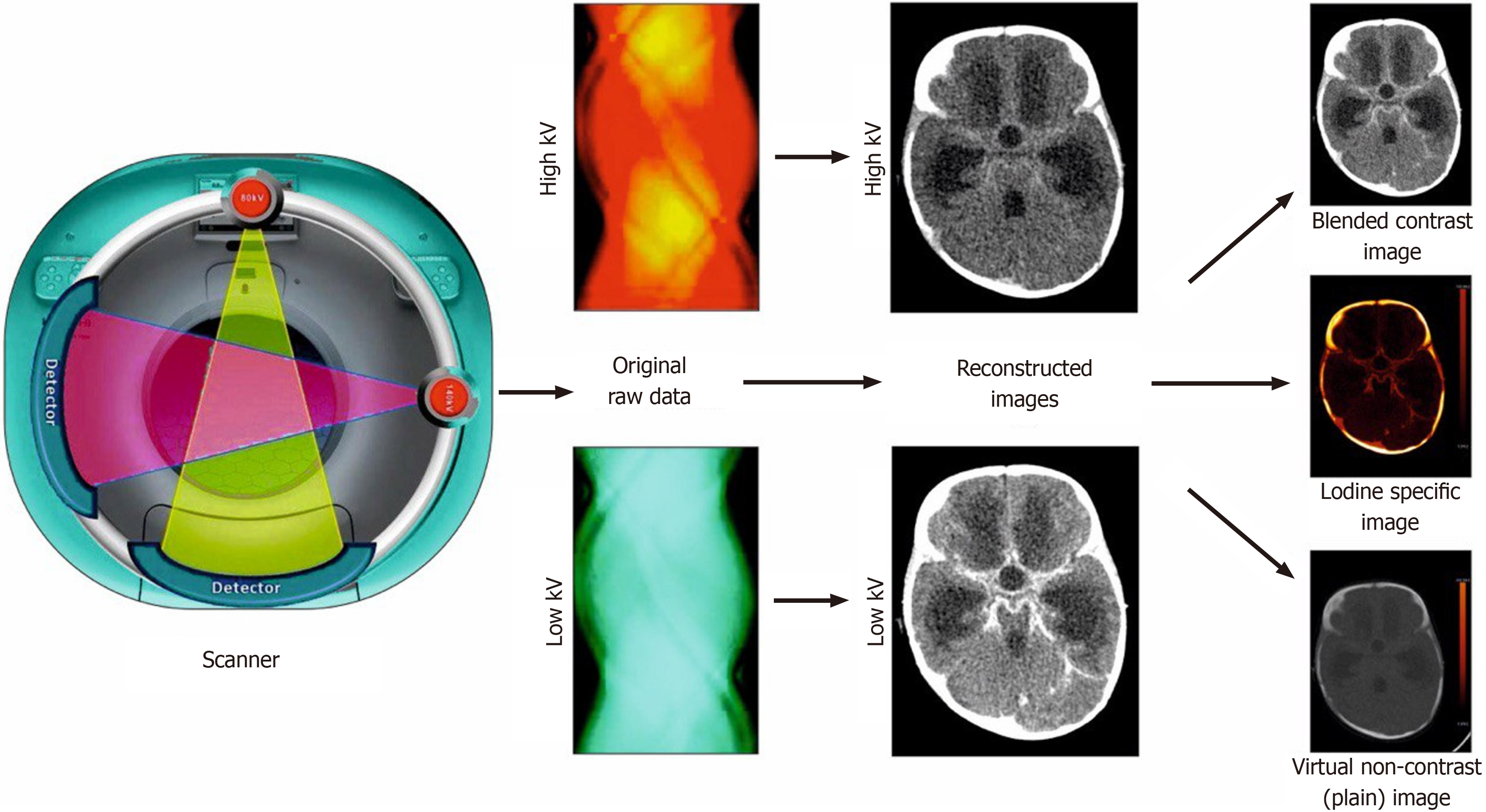
There are two different X-ray tubes in one gantry to generate X-ray photons at two different energy levels (mostly 80 kV and 140 kV) simultaneously (Figure 1). The advantage of this technique is the ability to choose voltage, current, and filters for each of the tubes independent of one another. The disadvantage is the need for correction of cross-scatter radiation[9].
The material decomposition process is able to identify and differentiate materials based on their elemental composition and attenuation properties, allowing for the assessment of their concentration and distribution in body tissues. With the help of photoelectric and Compton scattering effects, it is able to generate material-specific maps. The two main types of material decomposition approaches that are available include two-material and three-material decomposition. The material chosen for decomposition in dual source systems depends on clinical application. For example in abdominal scans, three material decomposition is conducted using iodine, fat, and soft tissue. For chest scans decomposition is achieved using soft tissue, air, and iodine. For single-source systems like rapid kV switching and dual-layer scanner, a two-material decomposition algorithm is used. It typically uses iodine and water and generates iodine density and water density images[11-13].
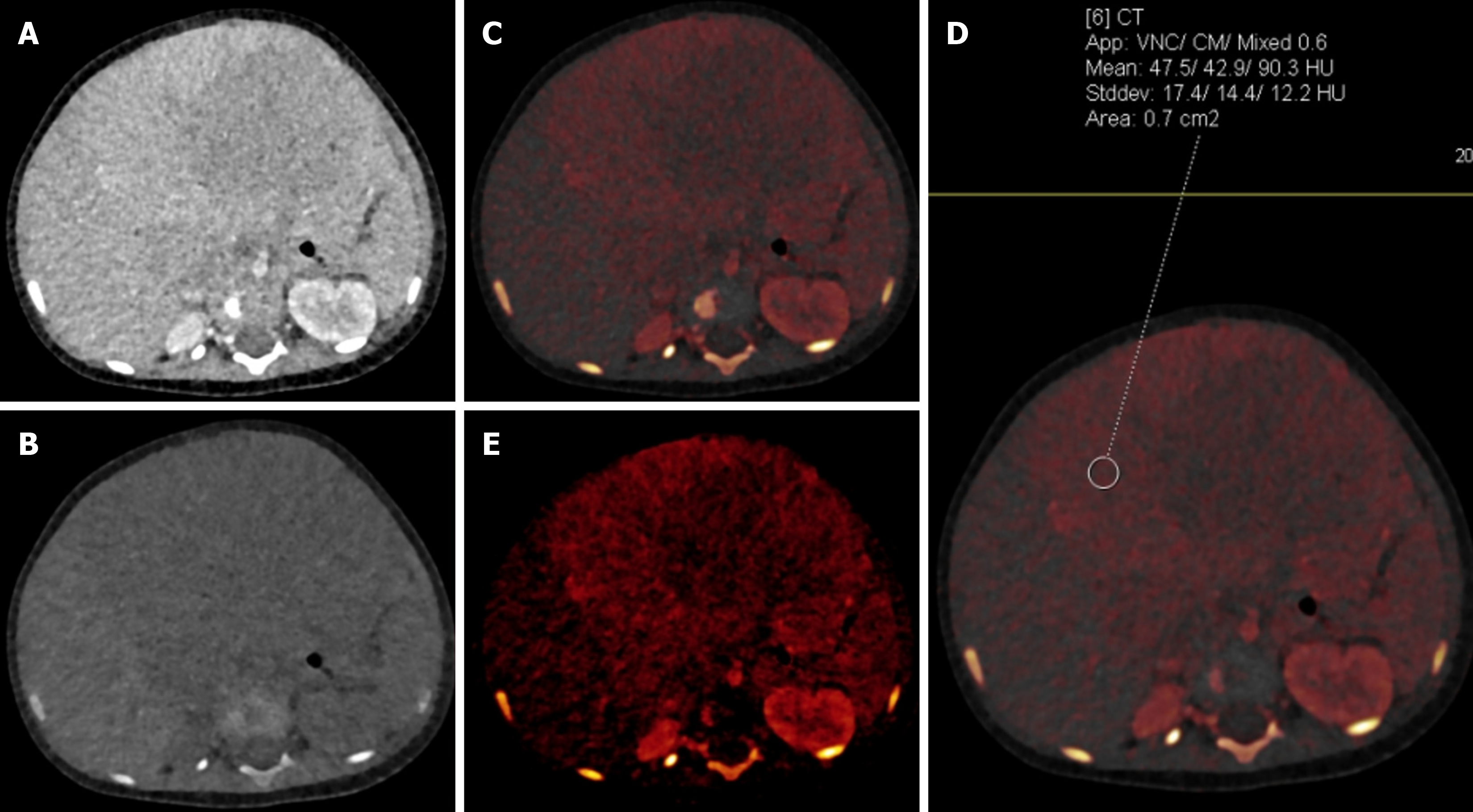
Based on this decomposition process, mathematical algorithms are able to remove the iodine from scanned voxels, thereby generating iodine-less or virtual unenhanced images. This would help in reducing the overall radiation dose and study duration[11]. Similarly, iodine-only images will help in quantitative as well as qualitative assessment of iodine contrast distribution in body tissue. Iodine levels can be shown using either Hounsfield units (HU) or concentration (Figures 1 and 2)[13].
These images are virtually generated by extrapolating datasets acquired at low-energy and high-energy values to simulate images at a particular kiloelectron volt, which generally ranges between 40 keV to 200 keV. Images at low kiloelectron volt have improved contrast and high noise, whereas images at high kiloelectron volt have reduced noise and low contrast resolution (Figure 3)[11,12].
Virtual monoenergetic images (VMIs) at low energy values demonstrate higher contrast in the vessels even at lower doses of contrast (Figure 4) due to their energy level being closer to the K-edge value of iodine[9]. In children with suboptimal contrast enhancement due to technical factors, these images may help in visualizing the vessels without compromising the diagnostic quality. High-energy VMIs lack the low-energy photos that are responsible for the generation of artifacts in the form of streaks around metal implants. These images will be particularly helpful in pediatric patients who have spinal fusion implants or osteosynthetic material[13].
The unique body composition, size, and physiological aspects of children warrant the need for optimization of DECT protocols. These differences can be utilized to tailor the protocols as follows.
Children have relatively higher organ size, lower body fat, and higher tissue water content compared with adults. As a result of this, a lower kVp (70 kVp-80 kVp) can be used to reduce the radiation dose and boost the iodine contrast. Phantom studies have shown that lowering the tube voltage does not lead to a significant increase in noise to hamper the overall quality of images[14]. This trade-off between noise and iodine contrast can be tackled with the use of iterative techniques or artificial intelligence (AI) to increase overall image quality[15].
Automatic exposure control and filtering: Modern scanners automatically adjust tube current to patient size (CARE dose or equivalent), ensuring only as many photons as needed. Tin filtration on the high-energy source (as on Siemens dual-source scanners) “cuts out unnecessary low energy photons” and increases dose efficiency by sharpening the energy spectra. For example Tabari et al[4] reported 30%-40% lower size-specific dose estimation for chest and abdomen-pelvic using dual energy against matched single energy scans.
The ability of DECT to derive virtual non-contrast (VNC) images from post-contrast scans allows the generation of true unenhanced phases. This can halve the number of scans needed. In oncologic studies using VNC images helped reduce total dose length product by a factor of 5-6 compared with earlier multiscan protocols[3].
Better detection of iodine uptake at lower energy voltage has the potential to lower the requirement of iodinated contrast agent for scanning. It is important particularly in imaging newborns and infants as they can tolerate only a lower volume of contrast due to their faster metabolic rate and developing renal excretory system. It is helpful for patients in whom it is difficult to establish the peripheral access and who are at risk of contrast-induced nephropathy. One of the recent studies conducted on pediatric and adult phantom models comparing SECT and DECT demonstrated a reduction in dose of iodinated contrast material ranging from 22%-52% at various VMIs while maintaining the contrast-to-noise ratio. A higher reduction in contrast dose, as high as 74%, was achieved using dual layer CT[16].
Weight-based contrast administration protocols are to be followed. For newborns, neonates, and patients weighing < 10 kg, a smaller-gauge catheter, such as 24 G, is preferred with an injection rate of 0.5-1.0 mL/second. As with the increase in age and weight, a larger gauge canula with a higher injection rate is to be used (e.g., in patients > 30-40 kg, a 20-gauge canula can be used with 3.0-5.0 mL/s injection rate)[17].
In summary protocol adjustments for pediatric DECT include using low kVp, tin filters, automatic dose modulation, fewer phases (with VNC/iodine maps), iterative and AI reconstruction, and judicious contrast dosing. These measures collectively maintain or improve image quality while substantially lowering radiation exposure.
At our institution the Siemens SOMATOM Force scanner with dual-source dual-energy technique is used to acquire image datasets. The protocols used vary depending on the area of interest to be scanned as well as the specific indication. Patients are adequately centered as the field of view (FOV) of the DECT is limited (35 cm approximately). The contrast dose is generally kept at 1 mL per kilogram of the child’s weight. In most cases only a single post-contrast phase acquired in dual-energy mode suffices. In cases where there is a need for a second phase (in suspected contrast leak or oncology), the same is added. A low-energy (70 kVp or 80 kVp) and high-energy (140 kVp or 150 kVp) image and a blended image that combines the high-energy and low-energy data (50% from each data set usually) are generated at the CT console. Post-processing, like the creation of VNC, iodine-specific, iodine overlay, or VMIs, is done afterwards on the reporting workstation, depending on the clinical indication[11,12].
Dual energy is preferred for assessment of congenital cardiac (tetralogy of Fallot, transposition of great arteries) and vascular anomalies (coarctation, arteritis, etc.), producing high-quality three-dimensional images aiding in surgical planning[18]. VMIs derived using dual energy have the potential of reducing the overall radiation dose and contrast volume[19].
A comparative study between single-energy and dual-energy cardiac CT in pediatric patients showed promising results for DECT. It achieved a 40% reduction in contrast volume while maintaining comparable image quality across both groups. The 45 keV setting emerged as the most representative VMI for dual-energy imaging. Notably, DECT also led to a 64% reduction in radiation dose (mean: 0.4 ± 0.2 mSv). Diagnostic accuracy improved from 74% with SECT to 81% with dual energy, highlighting its superior diagnostic performance[19].
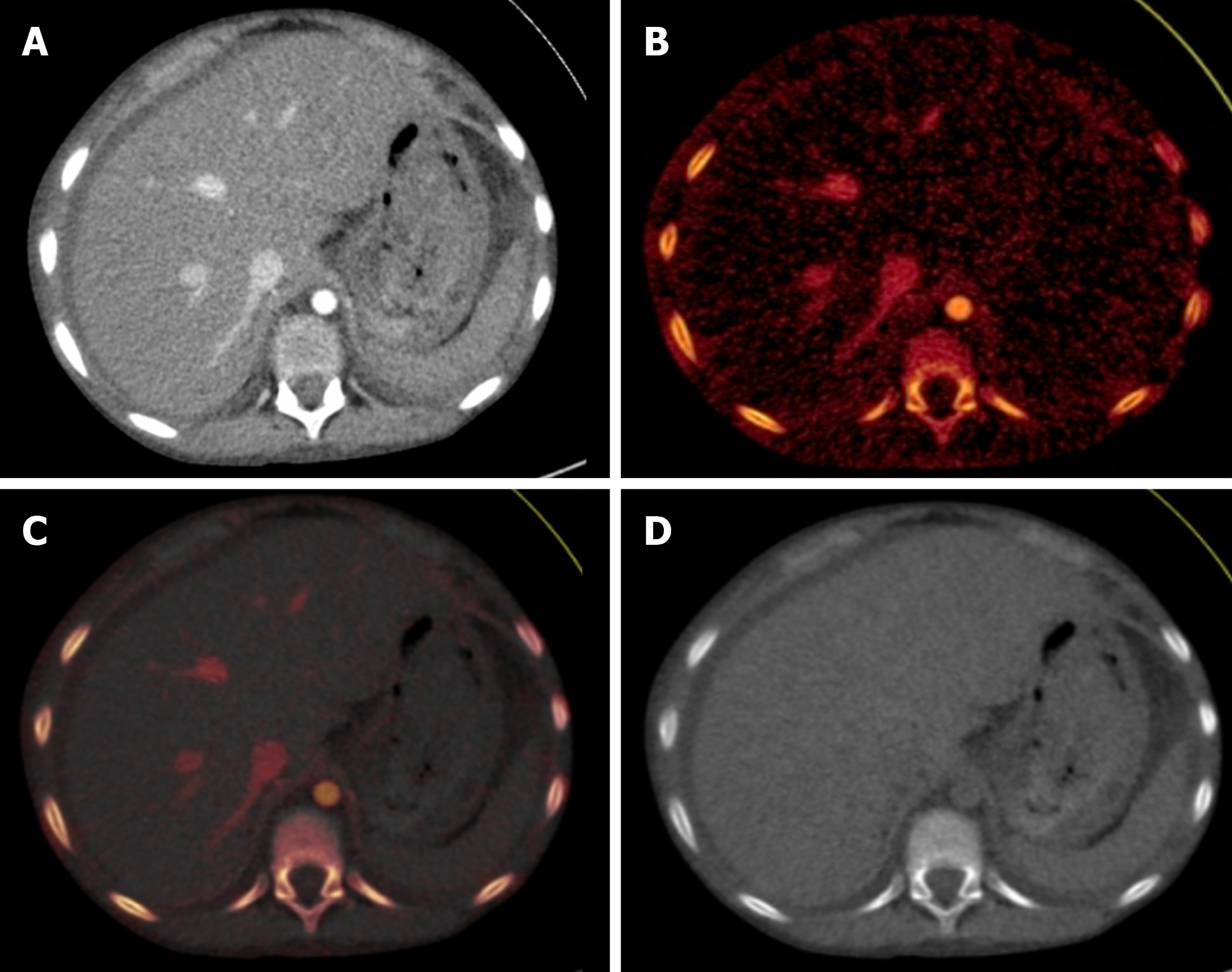
The application of bone removal aids in better detection of abnormalities in the vasculature (Figure 5). Although SECT also offers a threshold-based bone removal tool, this is sometimes not accurate. This is because these tools rely on particular attenuation values to differentiate bone from soft tissue, and errors can occur that result in incomplete bone removal and false removal of vessel parts[18,20].
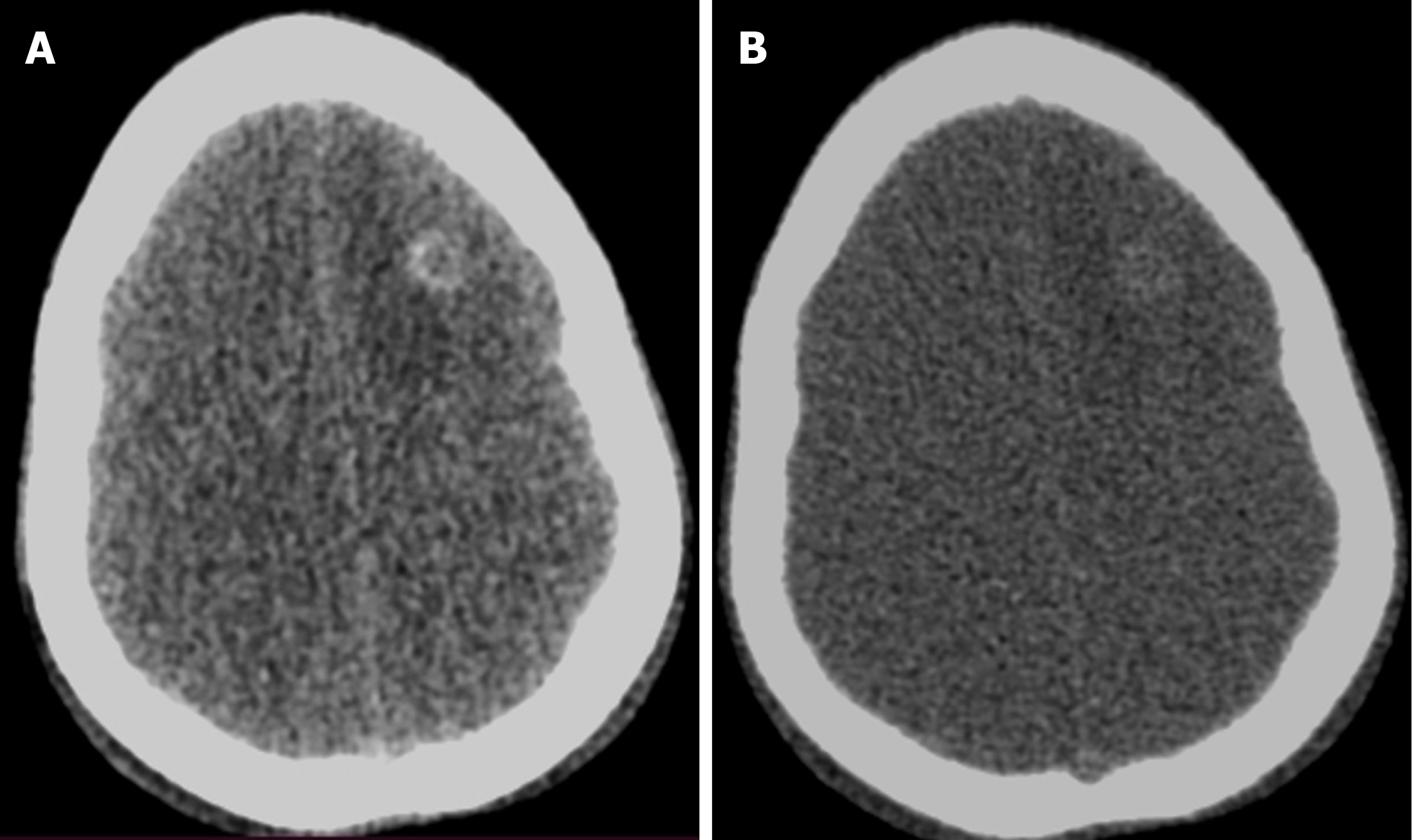
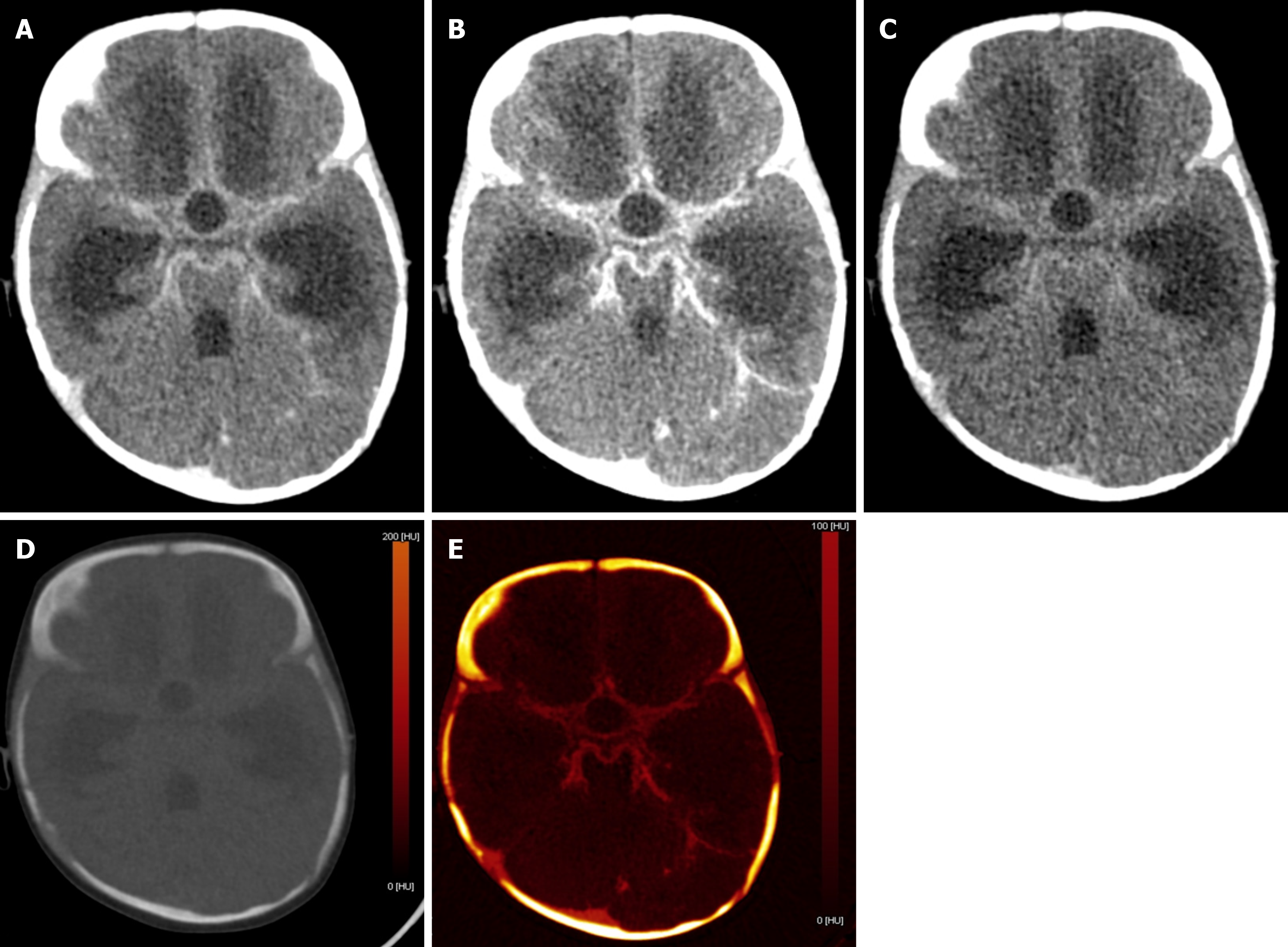
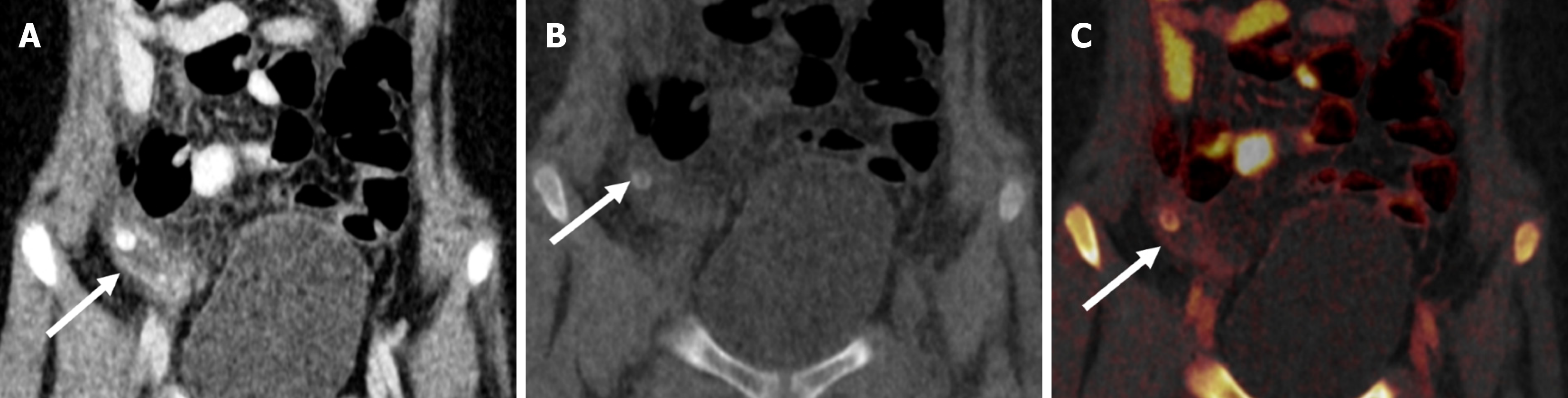
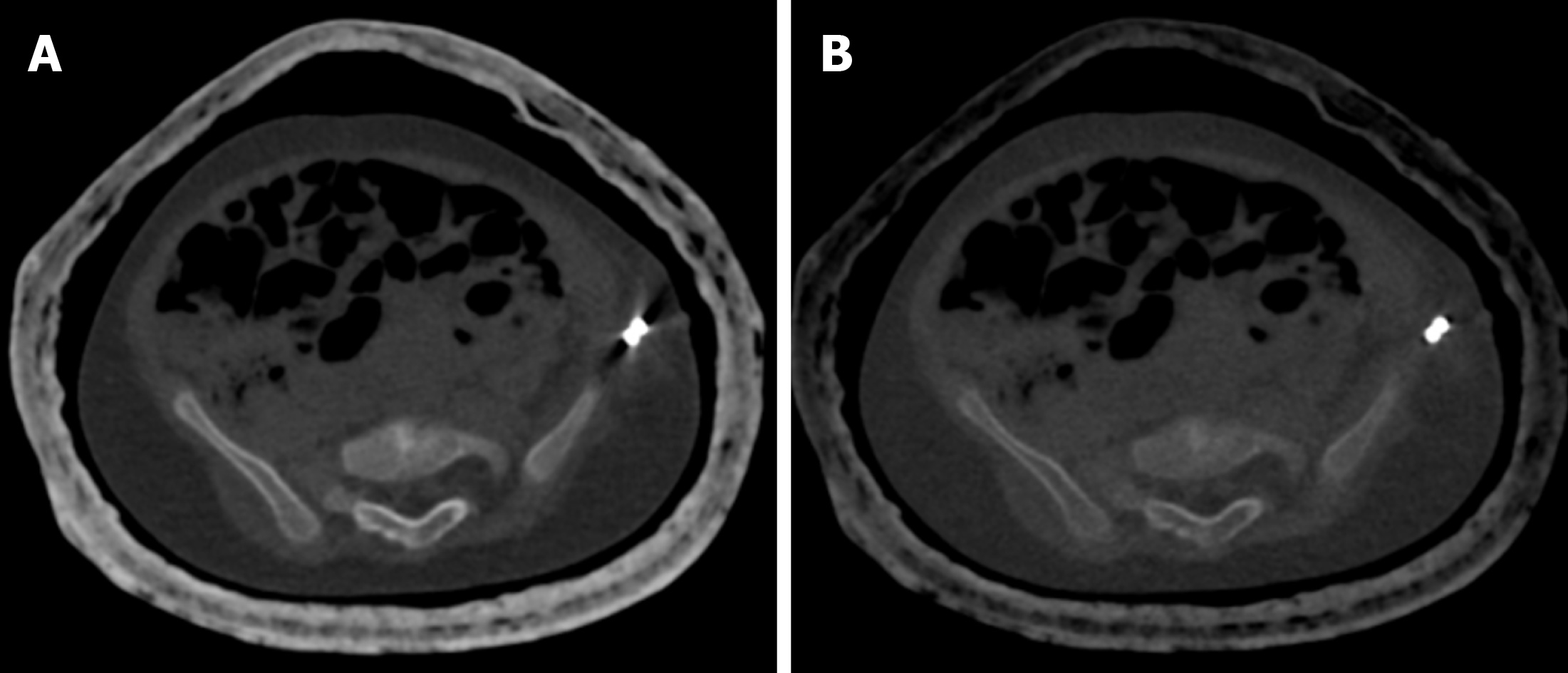
In children presenting with altered sensorium with a history of both fever and prior trauma, intracranial hemorrhage and infectious etiology are among the main possibilities. However, the former indication warrants a non-contrast CT and the latter, contrast-enhanced CT. This leads to higher radiation exposure, which can be avoided by the VNC images generated by DECT (Figure 6). Iodine overlay maps can aid in the detection of subtly enhancing tumors, which may be the cause of hemorrhage[21,22]. Low-energy reconstructions can help in the detection of subtle lesions, edema, and ischemia due to increased soft tissue contrast as well as increasing contrast-to-noise ratios (CNR) on angiographic imaging.
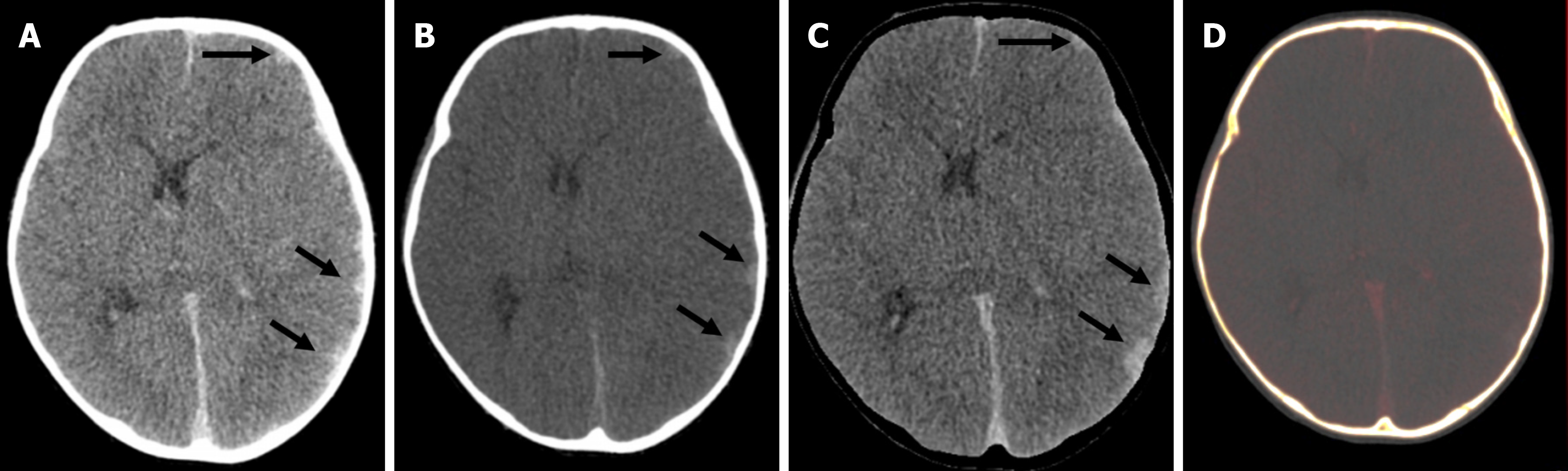
Compared with conventional images, low-energy VMIs offer superior image quality. In unenhanced scans performed with dual-layer CT, VMIs in the 45–75 keV range demonstrated significantly higher CNR and signal-to-noise ratio than standard images. Among children under 6 years of age, the grey-white matter CNR was markedly improved at 50 keV VMIs (2.20 ± 0.84) compared with conventional images (1.11 ± 0.70). Based on these findings, 50 keV VMIs are optimal for evaluating brain parenchyma[23]. Higher energy reconstructions can reduce image artifacts from aneurysm clips and coils, spinal hardware, and dental amalgam[24]. A bone removal tool makes the lesions more conspicuous, more so in the case of lesions located in close proximity to the bony surfaces. Detection of small epidural/subarachnoid hemorrhages is also improved by the bone removal application (Figure 7)[21,22].
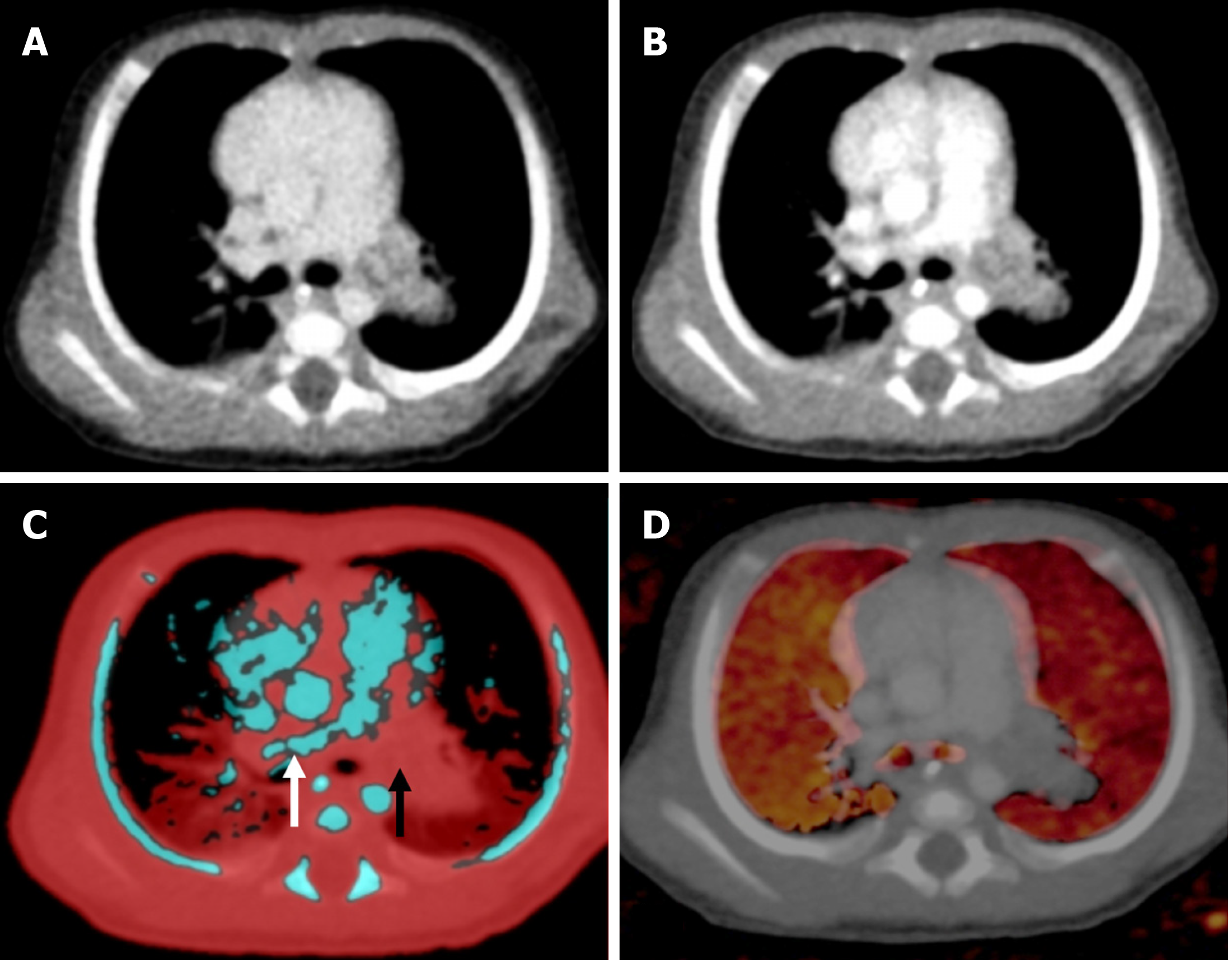
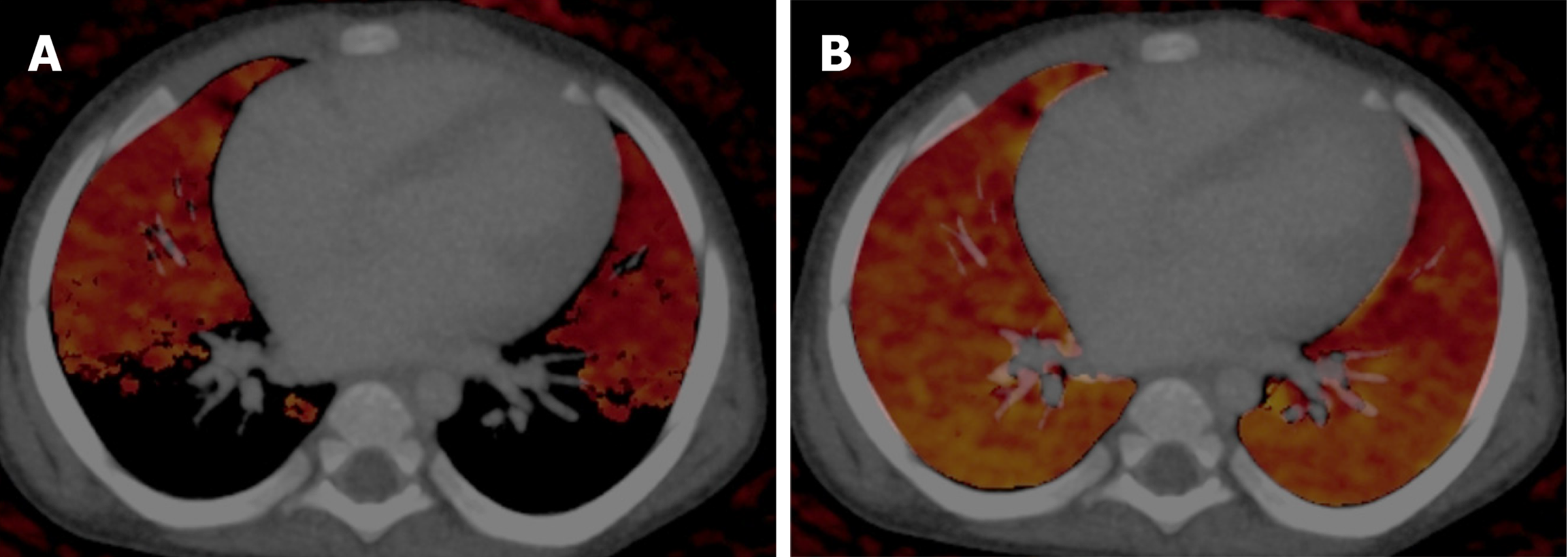
DECT has been found to have high sensitivity, specificity, and diagnostic accuracy in the detection of acute pulmonary thromboembolism[25,26]. In pulmonary thromboembolism the pulmonary blood volume (PBV) images allow for the detection of perfusion defects in the lung parenchyma, and the lung vessel images are used for picking up thrombi/emboli. Areas of infarcts show no or low iodine content compared with the surrounding normal lung on PBV images (images that are generated for visual assessment and quantification of iodine uptake on lung images), thereby enhancing the level of confidence in making a diagnosis. On lung vessel images, pulmonary arteries are color-coded blue, whereas thrombi appear as areas of red within the blue pulmonary arteries (Figure 8). It also enhances the visualization of arteriovenous malformation. Combined images can also be generated to show both the thrombi as well as perfusion defects[27].
By virtue of the generation of PBV images, it also has a potential role in congenital heart diseases with hypoplastic or absent pulmonary arteries[11]. The PBV images can help differentiate various parenchymal abnormalities, such as atelectasis, infarcts, pneumonias, and airway abnormalities. Pulmonary infarctions show homogeneous low iodine distribution on iodine or PBV images, whereas pneumonia demonstrates heterogeneously decreased or increased iodine distribution. Homogeneously increased iodine distribution occurs in atelectasis[28]. PBV can be used as a surrogate for pulmonary perfusion, especially in neonates and infants, in diagnosing conditions such as congenital lobar overinflation, hypoplastic lung, or bronchopulmonary dysplasia. In a recent study perfusion imaging conducted with DECT has been advocated as an alternative to single-photon emission computed V/Q scan in infants[29].
An important pitfall is the false perfusion defect due to the selection of the wrong maximum HU value for PBV images in younger children. It should be kept between -300 HU to -200 HU (Figure 9)[11].
The uses of virtual unenhanced images in the chest include the differentiation of calcifications, talc, and enhanced thoracic structures. DECT can also help in distinguishing benign lung lesions from malignant lesions by using iodine-specific images. In the pediatric population this may be useful in the confirmation of diagnosis of congenital malformations, like pulmonary airway malformation (Figure 10) or duplication cysts with proteinaceous debris, which may resemble a complex solid cystic mass at times and pose a diagnostic challenge. Demonstration of a non-iodine-containing lesion favors a complex cyst or benign solid mass while demonstration of iodine within a mediastinal mass raises the suspicion for malignancy[30]. In a study by Lee et al[31], significant differences in iodine concentrations between benign and malignant tumors were found, while there was no significant difference in attenuation values. The best cutoff iodine value for differentiating benign from malignant tumors was found to be 1.40 mg/mL for the early phase DECT and 1.58 mg/mL for the delayed phase[31]. In addition to this DECT is also useful for treatment planning and assessment of response to treatment in chest oncology[30].
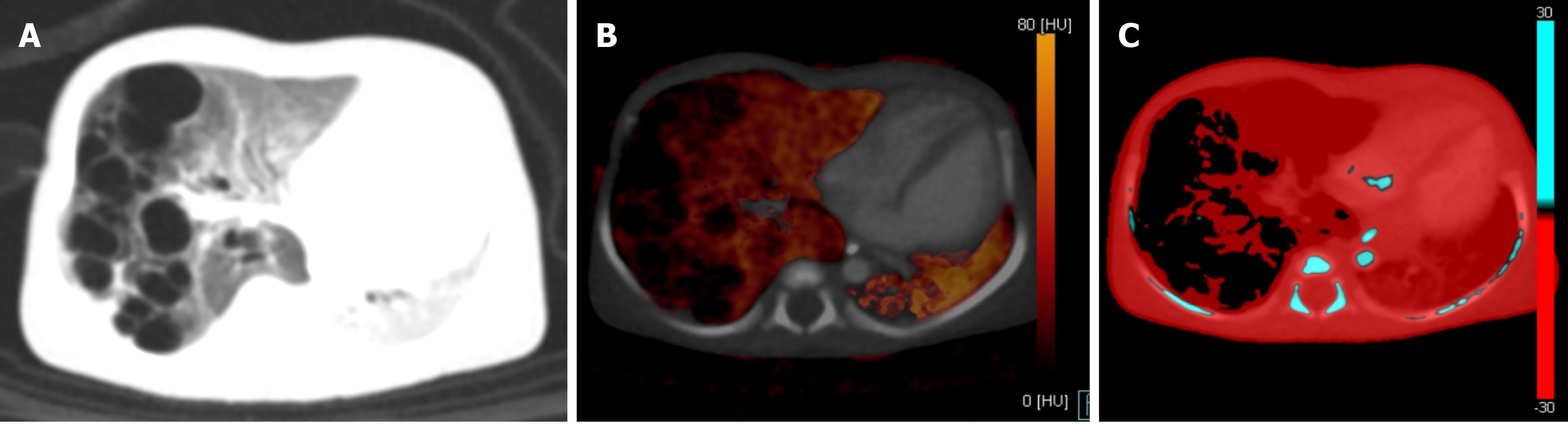
Liver: Small hepatic lesions in children with primary malignancy elsewhere can be benign granulomas or cysts and may be confused with hypovascular metastases on a SECT. An iodine map can show non-enhancement in the benign lesions, thus resolving the dilemma[17,32].
Bowel: Diseases like Crohn’s disease have an active phase and a fibrotic phase that need to be differentiated to decide the treatment[11]. By measuring the iodine density and keeping a cutoff value of 2.5 mg/mL, DECT is advantageous over conventional CT by pinpointing regions of active inflammation within the affected bowel. DECT with an iodine overlay imaging can confirm or exclude the presence of gangrenous change that is visualized as an area of markedly decreased enhancement or reduced iodine uptake (Figure 11)[33,34]. In a small cohort of 21 infants, DECT demonstrated 100% sensitivity and specificity in detecting bowel ischemia associated with necrotizing enterocolitis with surgical findings serving as the reference standard[35].
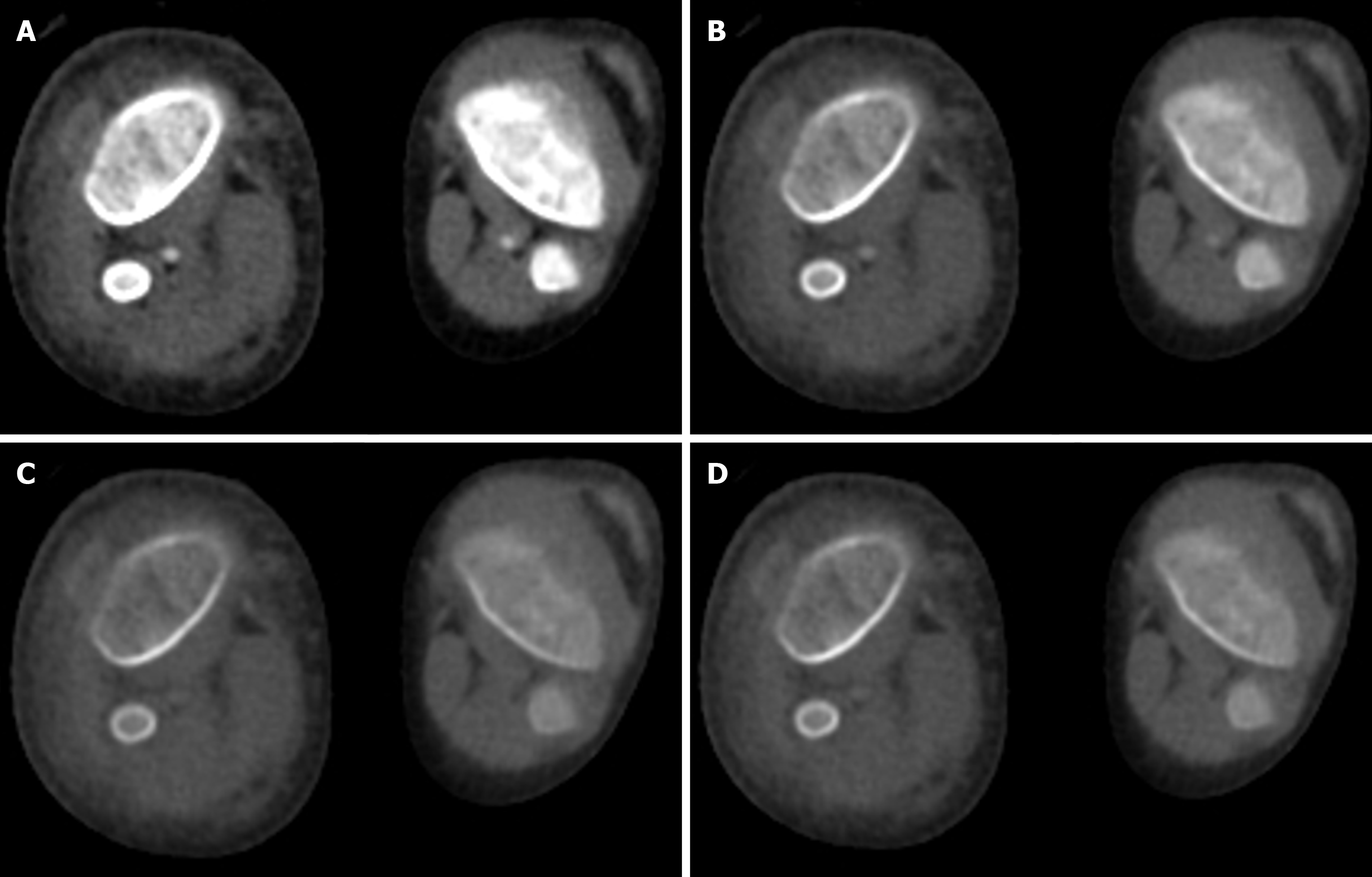
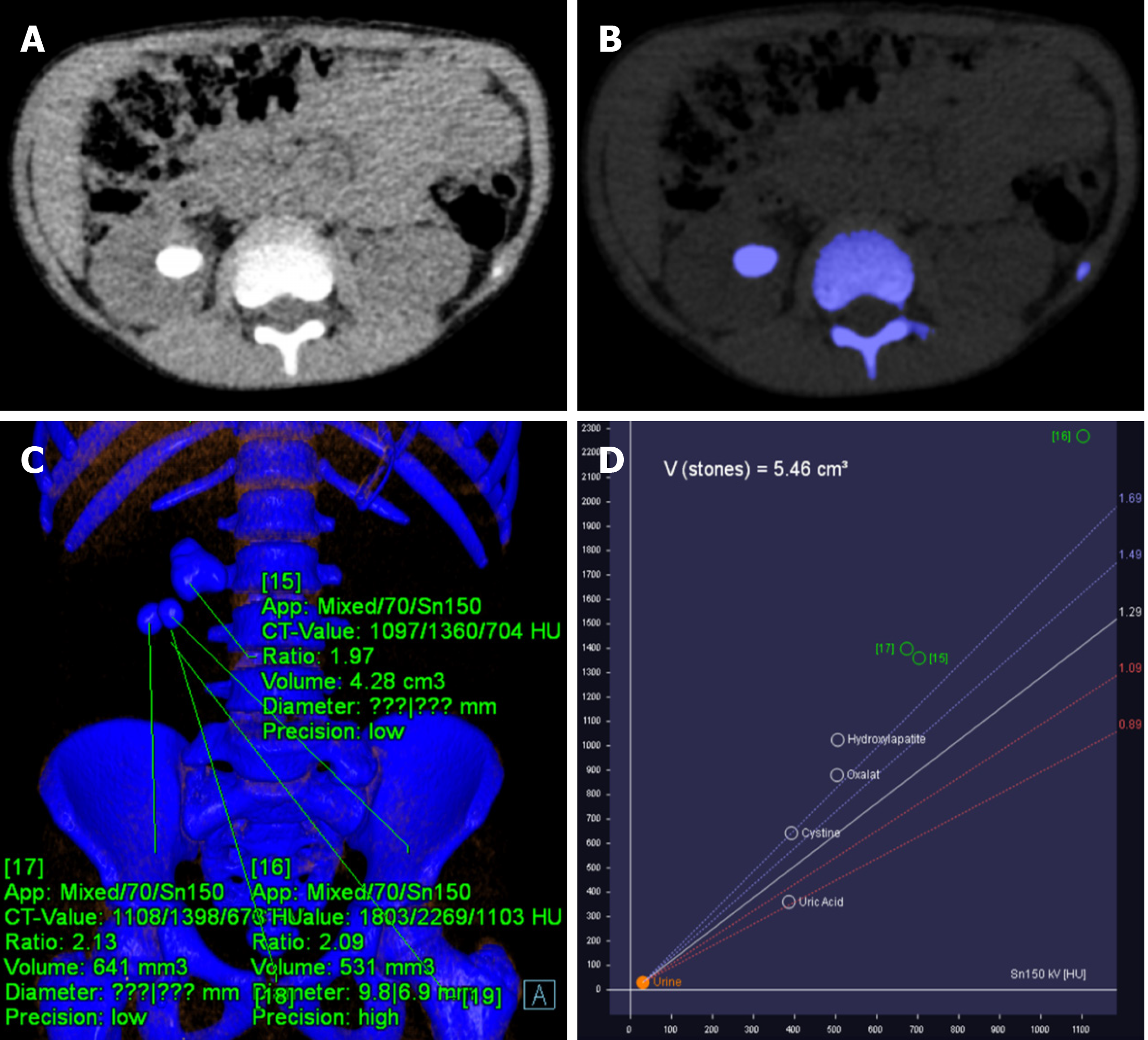
Due to its material decomposition ability, DECT is able to differentiate uric acid from non-uric acid stones (calcium oxalate, calcium phosphate, struvite, cysteine, and hydroxyapatite) (Figure 12). Low-energy and high-energy attenuation ratios are < 1.1 for uric acid, 1.1–1.24 for cystine, and > 1.24 for calcified stones[36]. This has implications in the management as the uric acid calculi are managed medically using alkalizing agents, whereas non-uric acid stones are treated either by lithotripsy or surgery. DECT parameters such as attenuation value at different spectral energies and effective atomic number are correlated with outcomes of lithotripsy[37].
Assessment of congenital anomalies of the urinary tract, tumors, and blunt trauma to the abdomen with suspected renal injury often requires multiphasic examination, including acquisition of non-contrast and delayed excretory phase. Addition of these phases for complete evaluation leads to increased radiation dose and overall increase in contrast volume administration. DECT, by virtue of generating virtual unenhanced images, can omit the need for a non-contrast phase. In a randomized controlled trial, Chen et al[38] demonstrated that DECT urography with a split-bolus technique significantly reduced radiation dose by 48%-56% in pediatric patients. This study also showed superior corticomedullary phase image quality based on improved CNR and signal-to-noise ratio values[38].
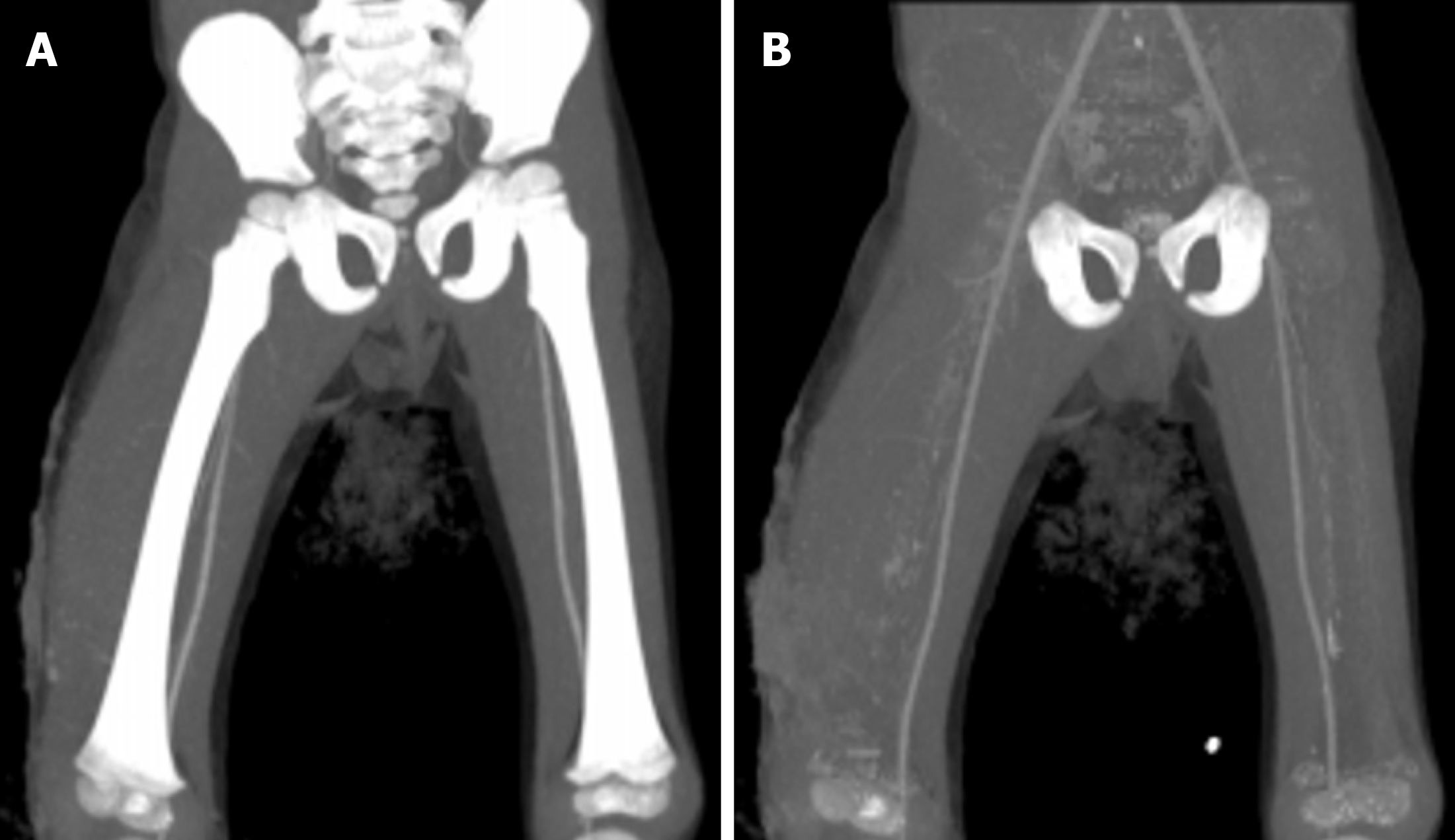
Corrective surgeries for scoliosis or developmental dysplasia of the hip joint in children involve the insertion of metallic implants, which can lead to a lot of streak artifacts. These streak artifacts hinder the visualization of surrounding soft tissues. VMIs at high kiloelectron volt (110 keV-130 keV), in addition to the iterative metal artifact reduction algorithms, can reduce these streak artifacts (Figure 13)[39].
Hyperdense masses in organs like the liver, kidney, or brain can often pose a challenge given their resemblance to a hematoma. VNC images can easily demonstrate the baseline attenuation of masses (Figure 14), including the presence of any underlying hemorrhage or calcification, which can then be compared with attenuation on contrast-enhanced scans. Another application is the use of iodine-specific images for the assessment of treatment response. The viable tumors show higher iodine uptake compared with post-treatment non-viable or fibrotic lesions[40,41]. DECT iodine overlay images can be a key imaging parameter to assess the response to chemotherapy as newer drugs reduce the blood supply to the tumor. These drugs do not significantly change the size; however, a change in attenuation or iodine uptake is noticeable. These changes are readily picked up by iodine overlay images. Literature in this direction is still developing, especially in pediatric patients[30].
Clinically relevant artifacts that interfere with the interpretation of dual-energy images are beam hardening and photon starvation. Beam hardening, caused by selective attenuation of low-energy photons, produces streaking and cupping near metal implants while photon starvation results in complete attenuation of all photons by the foreign body, causing zero transmission projections[13,10]. Beam hardening and photon starvation occur more at low kilo-electron volt (e.g., 80 keV) energies, which degrades the image quality[42]. On color-coded maps these streak artifacts may render the images non-diagnostic.
Appropriate window/Level settings are a must for the demonstration of correct color-coded iodine maps[42,43]. In iodine quantification, there are no well-validated thresholds of iodine concentration to define true enhancement[42,43]. Sometimes, VNC images may show some residual iodine in vessels or renal collecting systems that could not be subtracted. This may mimic pathology and confound interpretation[42,43]. Calcium remains bright on VNC images as well as color-coded iodine maps. Therefore, color-coded maps must be viewed in conjunction with VNC images so as not to confuse with iodine[42,43].
Different dual-energy systems available on the market have their specific limitations that are clinically relevant. A dual-source system has an inherent difference in the FOV of the two X-ray tubes with a smaller FOV available with higher tube current and a larger FOV available with lower tube current. This incongruity in FOV limits the use of dual-source CT in the evaluation of larger body size and cases that require increased anatomical coverage. This difference can result in non-processing of tissues falling between smaller and larger FOV. Due to this, appropriate centering in the gantry during the acquisition is of paramount importance[13]. Another limitation that comes with dual-source systems is with echocardiogram gating. For faster, relatively motionless acquisition in heart and dynamic lung images, the application of dual energy mode is traded off with super quick acquisition due to higher pitch[44]. Similarly, in rapid kV switching systems, there is low photon output at low voltage, compromising image quality due to higher noise. To overcome this higher tube current is required, therefore increasing the total radiation dose. This can also be overcome with the use of deep learning models and iterative reconstruction algorithms[13].
Owing to the unique body composition and continuous growth of children, there is a requirement for age and weight-based protocol optimization. Children have relatively higher organ size, lower body fat, and higher tissue water content compared with adults. As a result of this, a lower tube voltage (70-80 kVp) can be used to reduce the radiation dose and boost the iodine contrast[14]. Lower tube voltage results in an increase in noise of diagnostic images. AI driven algorithms can be used for denoising; however, there is currently limited resource availability in this regard, especially in pediatric patients.
Children are more vulnerable to the effects of ionizing radiation during CT examinations. This increased sensitivity is due to their higher rate of mitosis and longer life expectancy[45]. Given the continuous changes in body composition during growth, there is a critical need to develop age-specific and body composition-specific imaging protocols to minimize unnecessary radiation exposure in line with the application of as low as reasonably possible. Moreover, there is limited validation of DECT protocols in neonates and pediatric populations who are critically ill in which unique challenges such as altered image quality and increased motion artifacts may impact diagnostic performance.
There is a long learning curve associated with the interpretation of iodine maps and the generation of VMIs from spectral images that are generated for the radiologists.
Deep learning models can be employed for the optimization of acquisition protocols, and further reduction of radiation dose can be achieved by deep learning image reconstruction. In a recent systematic review on the role of AI in the optimization of the radiation dose, a 36%-70% reduction in radiation dose was demonstrated without sacrificing image quality. The major leap in this direction can be achieved by training these models to develop the algorithms producing diagnostic quality images with ultra-low dose protocol using spectral imaging[15]. Another study showed that VMIs when combined with deep learning models for reducing the noise could offer a 19.6% reduction in radiation dose with maintenance of diagnostic quality images[46].
DECT has expanded the horizons of the currently available applications of SECT in the pediatric population. There is no significant increase in radiation exposure, making it safe for use in children. There are certain pitfalls that need to be kept in mind to derive the maximum benefit from it. Further, DECT has more common uses in adults that may not translate to actual clinical practice in pediatric imaging.
| 1. | Weinman JP, Mirsky DM, Jensen AM, Stence NV. Dual energy head CT to maintain image quality while reducing dose in pediatric patients. Clin Imaging. 2019;55:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Siegel MJ, Curtis WA, Ramirez-Giraldo JC. Effects of Dual-Energy Technique on Radiation Exposure and Image Quality in Pediatric Body CT. AJR Am J Roentgenol. 2016;207:826-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Agostini A, Mari A, Lanza C, Schicchi N, Borgheresi A, Maggi S, Giovagnoni A. Trends in radiation dose and image quality for pediatric patients with a multidetector CT and a third-generation dual-source dual-energy CT. Radiol Med. 2019;124:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Tabari A, Gee MS, Singh R, Lim R, Nimkin K, Primak A, Schmidt B, Kalra MK. Reducing Radiation Dose and Contrast Medium Volume With Application of Dual-Energy CT in Children and Young Adults. AJR Am J Roentgenol. 2020;214:1199-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Siegel MJ, Mhlanga JC, Salter A, Ramirez-Giraldo JC. Comparison of radiation dose and image quality between contrast-enhanced single- and dual-energy abdominopelvic computed tomography in children as a function of patient size. Pediatr Radiol. 2021;51:2000-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Sodhi KS, Khandelwal N, Saxena AK, Singh M, Agarwal R, Bhatia A, Lee EY. Rapid lung MRI in children with pulmonary infections: Time to change our diagnostic algorithms. J Magn Reson Imaging. 2016;43:1196-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Solomon DZ, Ayalew B, Dellie ST, Admasie D. Justification and Optimization Principles of ALARA in Pediatric CT at a Teaching Hospital in Ethiopia. Ethiop J Health Sci. 2020;30:761-766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Arora V, Kaur T, Singh K. The role of magnetic resonance imaging in acute abdominal pain in paediatric age group. Egypt J Radiol Nucl Med. 2022;53:36. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Johnson TR. Dual-energy CT: general principles. AJR Am J Roentgenol. 2012;199:S3-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 385] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 10. | Odedra D, Narayanasamy S, Sabongui S, Priya S, Krishna S, Sheikh A. Dual Energy CT Physics-A Primer for the Emergency Radiologist. Front Radiol. 2022;2:820430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Siegel MJ, Ramirez-Giraldo JC. Dual-Energy CT in Children: Imaging Algorithms and Clinical Applications. Radiology. 2019;291:286-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 12. | Greffier J, Villani N, Defez D, Dabli D, Si-Mohamed S. Spectral CT imaging: Technical principles of dual-energy CT and multi-energy photon-counting CT. Diagn Interv Imaging. 2023;104:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 165] [Reference Citation Analysis (0)] |
| 13. | Gallo-Bernal S, Peña-Trujillo V, Gee MS. Dual-energy computed tomography: pediatric considerations. Pediatr Radiol. 2024;54:2112-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 14. | Ogbole GI. Radiation dose in paediatric computed tomography: risks and benefits. Ann Ib Postgrad Med. 2010;8:118-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Ng CKC. Artificial Intelligence for Radiation Dose Optimization in Pediatric Radiology: A Systematic Review. Children (Basel). 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Wang J, Duan X, Mahmood U, McKenney SE, Brady SL. An adult and pediatric size-based contrast administration reduction phantom study for single and dual-energy CT through preservation of contrast-to-noise ratio. J Appl Clin Med Phys. 2024;25:e14340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Kamps SE, Otjen JP, Stanescu AL, Mileto A, Lee EY, Phillips GS. Dual-Energy CT of Pediatric Abdominal Oncology Imaging: Private Tour of New Applications of CT Technology. AJR Am J Roentgenol. 2020;214:967-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Peña-Trujillo V, Gallo-Bernal S, Tung EL, Gee MS. Pediatric Applications of Dual-Energy Computed Tomography. Radiol Clin North Am. 2023;61:1069-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Xie M, Wang H, Tang S, Chen M, Li T, He L. Application of dual-energy CT with prospective ECG-gating in cardiac CT angiography for children: Radiation and contrast agent dose. Eur J Radiol. 2024;170:111229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Schulz B, Kuehling K, Kromen W, Siebenhandl P, Kerl MJ, Vogl TJ, Bauer R. Automatic bone removal technique in whole-body dual-energy CT angiography: performance and image quality. AJR Am J Roentgenol. 2012;199:W646-W650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Kim SJ, Lim HK, Lee HY, Choi CG, Lee DH, Suh DC, Kim SM, Kim JK, Krauss B. Dual-energy CT in the evaluation of intracerebral hemorrhage of unknown origin: differentiation between tumor bleeding and pure hemorrhage. AJNR Am J Neuroradiol. 2012;33:865-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Choi Y, Shin NY, Jang J, Ahn KJ, Kim BS. Dual-energy CT for differentiating acute intracranial hemorrhage from contrast staining or calcification: a meta-analysis. Neuroradiology. 2020;62:1617-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Tan Z, Zhang L, Sun X, Yang M, Makamure J, Wu H, Wang J. Dual-Layer Detector Head CT to Maintain Image Quality While Reducing the Radiation Dose in Pediatric Patients. AJNR Am J Neuroradiol. 2023;44:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Gibney B, Redmond CE, Byrne D, Mathur S, Murray N. A Review of the Applications of Dual-Energy CT in Acute Neuroimaging. Can Assoc Radiol J. 2020;71:253-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Abdellatif W, Ebada MA, Alkanj S, Negida A, Murray N, Khosa F, Nicolaou S. Diagnostic Accuracy of Dual-Energy CT in Detection of Acute Pulmonary Embolism: A Systematic Review and Meta-Analysis. Can Assoc Radiol J. 2021;72:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Rapp JB, Biko DM, Siegel MJ. Dual-Energy CT for Pediatric Thoracic Imaging: A Review. AJR Am J Roentgenol. 2023;221:526-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Hoey ET, Mirsadraee S, Pepke-Zaba J, Jenkins DP, Gopalan D, Screaton NJ. Dual-energy CT angiography for assessment of regional pulmonary perfusion in patients with chronic thromboembolic pulmonary hypertension: initial experience. AJR Am J Roentgenol. 2011;196:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Goo HW. Initial experience of dual-energy lung perfusion CT using a dual-source CT system in children. Pediatr Radiol. 2010;40:1536-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Ramirez-Suarez KI, Barrera CA, Otero HJ, Biko DM, States LJ, Servaes S, Zhu X, Davis JC, Piccione J, Rapp JB. Pilot study for comparative assessment of dual-energy computed tomography and single-photon emission computed tomography V/Q scanning for lung perfusion evaluation in infants. Pediatr Pulmonol. 2022;57:702-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Siegel MJ, Bhalla S, Cullinane M. Dual-Energy CT Material Decomposition in Pediatric Thoracic Oncology. Radiol Imaging Cancer. 2021;3:e200097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Lee SH, Hur J, Kim YJ, Lee HJ, Hong YJ, Choi BW. Additional value of dual-energy CT to differentiate between benign and malignant mediastinal tumors: an initial experience. Eur J Radiol. 2013;82:2043-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Coursey CA, Nelson RC, Boll DT, Paulson EK, Ho LM, Neville AM, Marin D, Gupta RT, Schindera ST. Dual-energy multidetector CT: how does it work, what can it tell us, and when can we use it in abdominopelvic imaging? Radiographics. 2010;30:1037-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 278] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 33. | Elbanna KY, Mohammed MF, Chahal T, Khosa F, Ali IT, Berger FH, Nicolaou S. Dual-Energy CT in Differentiating Nonperforated Gangrenous Appendicitis From Uncomplicated Appendicitis. AJR Am J Roentgenol. 2018;211:776-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Singh R, Rai R, Mroueh N, Kambadakone A. Role of Dual Energy Computed Tomography in Inflammatory Bowel Disease. Semin Ultrasound CT MR. 2022;43:320-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Çağlar Ö, Cesur E, Sade R, Fırıncı B, Kara M, Çelikkaya ME, Oral A, Yiğiter M, Özmen S. Dual energy CT in necrotizing enterocolitis; a novel diagnostic approach. Turk J Med Sci. 2021;51:2575-2583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 36. | Hidas G, Eliahou R, Duvdevani M, Coulon P, Lemaitre L, Gofrit ON, Pode D, Sosna J. Determination of renal stone composition with dual-energy CT: in vivo analysis and comparison with x-ray diffraction. Radiology. 2010;257:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 37. | Tu B, Jia J, Yu L, Li H, Wang D. Correlative investigation between routine clinical parameters of dual-energy computed tomography and the outcomes of extracorporeal shock wave lithotripsy in children with urolithiasis: a retrospective study. Abdom Radiol (NY). 2021;46:4881-4887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 38. | Chen H, Deng Z, Tan T, Li S, Qian F, He L, Tang S. Application value of split-bolus contrast injection combined with dual-energy CT scanning technology in pediatric CTU imaging. Eur J Radiol. 2025;184:111949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 39. | Kim YJ, Cha JG, Kim H, Yi JS, Kim HJ. Dual-Energy and Iterative Metal Artifact Reduction for Reducing Artifacts Due to Metallic Hardware: A Loosening Hip Phantom Study. AJR Am J Roentgenol. 2019;212:1106-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Meyer M, Hohenberger P, Apfaltrer P, Henzler T, Dinter DJ, Schoenberg SO, Fink C. CT-based response assessment of advanced gastrointestinal stromal tumor: dual energy CT provides a more predictive imaging biomarker of clinical benefit than RECIST or Choi criteria. Eur J Radiol. 2013;82:923-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Guerrini S, Bagnacci G, Perrella A, Meglio ND, Sica C, Mazzei MA. Dual Energy CT in Oncology: Benefits for Both Patients and Radiologists From an Emerging Quantitative and Functional Diagnostic Technique. Semin Ultrasound CT MR. 2023;44:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 42. | Wortman JR, Sodickson AD. Pearls, Pitfalls, and Problems in Dual-Energy Computed Tomography Imaging of the Body. Radiol Clin North Am. 2018;56:625-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Parakh A, Lennartz S, An C, Rajiah P, Yeh BM, Simeone FJ, Sahani DV, Kambadakone AR. Dual-Energy CT Images: Pearls and Pitfalls. Radiographics. 2021;41:98-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 44. | Rapp JB, Biko DM, White AM, Ramirez-Suarez KI, Otero HJ. Spectral imaging in the pediatric chest: past, present and future. Pediatr Radiol. 2022;52:1910-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Granata C, Sofia C, Francavilla M, Kardos M, Kasznia-Brown J, Nievelstein RA, Olteanu BS, Owens C, Salerno S, Sorantin E, Apine I. Let's talk about radiation dose and radiation protection in children. Pediatr Radiol. 2025;55:386-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 46. | Lee S, Choi YH, Cho YJ, Lee SB, Cheon JE, Kim WS, Ahn CK, Kim JH. Noise reduction approach in pediatric abdominal CT combining deep learning and dual-energy technique. Eur Radiol. 2021;31:2218-2226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/