Published online Dec 9, 2025. doi: 10.5409/wjcp.v14.i4.107290
Revised: April 27, 2025
Accepted: July 7, 2025
Published online: December 9, 2025
Processing time: 221 Days and 4.4 Hours
Uveitis associated with juvenile idiopathic arthritis (U-JIA) is a vision-threatening condition. Estimates of prevalence of uveitis in patients with known juvenile idiopathic arthritis range from 11.6% to 30.0%. First-line treatment includes topical glucocorticoids; methotrexate (MTX) is used if topical corticosteroids are ineffective. In severe cases biological therapy like adalimumab may be prescribed. Complications, including vision loss, may be related to the disease and the ongo
To highlight the characteristics of operated U-JIA and to identify predictors of treatment failure.
A retrospective cohort study analyzed data from 68 pediatric patients (under 18 years old) with U-JIA between 2007 and 2023. The study focused on demogra
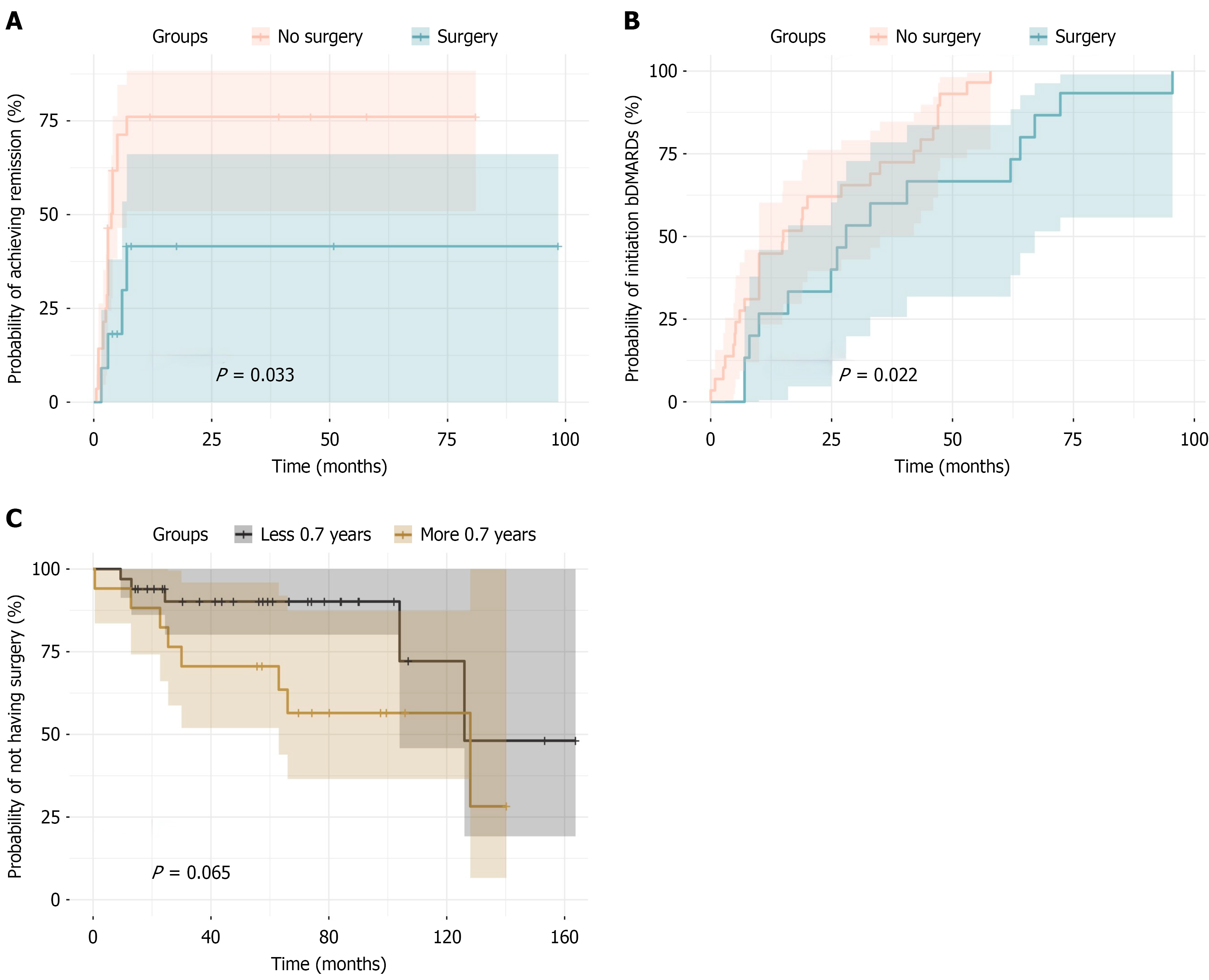
Eye surgery was performed on 17 (25%) patients with U-JIA. It was associated with an earlier onset of uveitis (P = 0.017), lower uveitis remission rate [odds ratio = 5.29, 95% confidence interval (CI): 1.23-24.90, P = 0.015], longer time to remission (P = 0.036), reduced probability of achieving remission on MTX (P = 0.033), and the necessity of the following treatment with biological disease-modifying antirheumatic drugs (odds ratio = 5.60; 95%CI: 1.11-55.19, P = 0.021) with similar efficacy with biological treatments in operated and non-operated cases. Kaplan-Meier curves showed a borderline difference in time to surgical intervention based on the MTX initiation cutoff (P = 0.065) although earlier MTX initiation might be associated with a higher likelihood of deferred surgery.
Operated patients exhibited an aggressive early-onset uveitis profile that needed early and more intensive treatment. Delayed and failed MTX treatment as well as delayed switching to biologics often required subsequent eye surgery.
Core Tip: Uveitis, a vision-threatening condition associated with juvenile idiopathic arthritis, requires rapid control of intraocular inflammation. There is limited information in the literature regarding patients with juvenile idiopathic arthritis-associated uveitis who have undergone surgical treatment, making our study a unique contribution. According to the obtained data, patients who have undergone surgical treatment for uveitis or its complications require more aggressive therapy, including the use of biological disease-modifying antirheumatic drugs. Additionally, it is important to consider that early methotrexate administration in this patient group may be associated with a decreased probability of surgical inter
- Citation: Yakovlev AA, Gaidar EV, Sorokina LS, Nikitina TN, Chikova IA, Kalashnikova OV, Kostik MM. Features of patients with uveitis associated with juvenile idiopathic arthritis required eye surgery. World J Clin Pediatr 2025; 14(4): 107290
- URL: https://www.wjgnet.com/2219-2808/full/v14/i4/107290.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i4.107290
Uveitis is a diverse group of over 30 different clinical entities characterized by inflammation of the uveal tract, parts of the eye, or adjacent structures[1]. Uveitis in children is predominantly non-infectious and is often associated with immune-mediated diseases, such as juvenile idiopathic arthritis (JIA), juvenile seronegative spondyloarthropathies, tubulointerstitial nephritis and uveitis syndrome, sarcoidosis, Blau syndrome, and Behcet’s disease[2]. Estimates of the prevalence of uveitis in patients with known JIA range from 11.6% to 30.0%[3,4]. Because JIA is the most common childhood rheumatic disease[5], a significant number are at risk for uveitis because JIA is the most common identifiable cause of childhood uveitis[2]. Rigorous epidemiological data are lacking; the estimated incidence in the Russian Federation is 1.5-2.0 cases per 100000 children, and the prevalence is 8-11 cases per 1 million children[6].
Currently, first-line therapy for uveitis involves the use of topical glucocorticoids. Conventional disease-modifying antirheumatic drugs (DMARDs), such as methotrexate (MTX), can be used if treatment with topical corticosteroids fails or the patient has a risk factor for a complicated course[7,8]. MTX is the most commonly prescribed conventional DMARD for pediatric uveitis; however, 27%-48% of patients treated with MTX fail to achieve inflammation control[9]. In cases of severe or refractory disease, biological DMARDs (bDMARDs), including adalimumab and others, may be initiated[7]. However, when therapy is insufficient, complications might occur. The sequelae of uveitis associated with JIA (U-JIA) can occur not only due to illness but also due to treatment and often lead to suboptimal visual outcomes[10].
Studies indicate that structural complications, such as posterior synechiae, active anterior chamber inflammation, and prior ophthalmic surgeries, are significantly associated with an increased risk of vision loss to ≤ 20/50 and ≤ 20/200[11]. The incidence of new ophthalmic complications during follow-up has been reported as 0.15 per eye-year. Surgical intervention is often required to improve visual outcomes and quality of life although it may be associated with certain risks[12]. However, modern surgical techniques, including improved microsurgery instruments, advanced intraocular lens design, and materials, have resulted in an increased success rate in uveitis cataract surgery. The surgical intervention requirement may reflect more severe forms of uveitis that need a different approach described in standard treatment algorithms. We conducted the present study to investigate the demographic, clinical, and laboratory characteristics of children with U-JIA who required eye surgery due to uveitis.
This retrospective cohort study included the medical records of patients under the age of 18 years with U-JIA who received treatment in the pediatric rheumatology and pediatric ophthalmology clinics of Saint-Petersburg State Pediatric Medical University from September 2007 to May 2023. The study flowchart is presented in Figure 1. Inclusion criteria were: (1) Diagnosis of U-JIA; (2) Age under 18 years; and (3) Monitoring for over 1 year at our clinics. Exclusion criteria were: (1) Diagnosed with systemic diseases other than JIA, such as systemic lupus erythematosus, vasculitis, systemic connective tissue disorders, and autoimmune inflammatory conditions; (2) Uveitis caused by infection or other etiologies; (3) Masquerade syndromes; (4) Patients with blind eye with nil vision, phthisical or pre-phthisical eye; and (5) Any bDMARD treatment effective for uveitis (e.g., adalimumab or infliximab) before the onset of uveitis. Treatment with etanercept was allowed prior to uveitis onset.
The diagnosis of JIA was established based on the International League of Associations for Rheumatology criteria[13]. Ocular involvement was assessed through an ophthalmologic examination using slit-lamp biomicroscopy and classified according to the standardization of uveitis nomenclature (SUN) criteria[14]. All patients underwent a fundus examination under pharmacological mydriasis, and in doubtful cases the posterior pole of the eye was examined using a Goldman lens. Additionally, an ultrasound examination of the eyes and optical coherence tomography of the posterior segment of the eye was performed. In addition to these procedures, all patients with uveitis also underwent eye tonometry. All patients were examined by an experienced ophthalmologist (Nikitina TN) with more than 30 years of experience in pediatric uveitis. Each patient was also evaluated by a rheumatologist to confirm or exclude the diagnosis of JIA and to assign the appropriate disease subtype. Two pediatric rheumatologists (Yakovlev AA and Sorokina LS) independently reviewed the medical records. In cases where discrepancies were observed in the determination of the JIA subtype, the medical records were further scrutinized by a third expert (Kostik MM), who provided a final decision regarding the subtype of JIA.
From the medical records the following information was extracted: (1) Demographic information. Sex, JIA subtype, age at JIA onset, and number of active joints at JIA onset; (2) Uveitis characteristics. Age at onset of uveitis, anatomical type of uveitis at onset, laterality of eye involvement at onset, type of uveitis presentation (acute or insidious), and presence of U-JIA before, concurrent with, or following joint involvement. Individuals for whom it was challenging to determine the onset of uveitis or those without an apparent red eye-syndrome or whom the ophthalmologist diagnosed uveitis during routine examination of the patient with JIA without any evident clinical signs were classified as having an asymptomatic onset. Red eye refers to the condition known as red eye syndrome, which includes symptoms such as eye redness, photophobia, pain, tearing, headache, and other clinically manifesting symptoms. The term insidious was used to describe clinically silent uveitis; (3) The presence of complications at the initial examination was recorded, including the specific type when available. In cases where no information on complications was documented, patients were classified as having no complications; (4) History of ophthalmic surgery, date and type of surgery, and time before eye surgery; (5) Laboratory characteristics. Presence of human leukocyte antigen-B27 antigen, antinuclear antibody positivity, erythrocyte sedimentation rate at onset, and C-reactive protein at onset; and (6) Treatment characteristics. Time to MTX initiation, remission on MTX, flare on MTX, time to first flare on MTX, time to bDMARD initiation, remission on bDMARD, flare on bDMARD, time to first flare on bDMARD, time between MTX and bDMARD initiation, and time to intraocular corticosteroids injection. We compared patients with operated and non-operated U-JIA for the subgroup analysis.
Statistical analysis was performed using R (version 4.4.0, released April 24, 2024) within the RStudio environment (version 2024.04.2, build 764). Categorical variables were presented as absolute counts. Comparisons between independent categorical variables were conducted using 2 × 2 contingency tables and Fisher’s exact test. The distribution of continuous variables was assessed with the Shapiro-Wilk test. Quantitative data were reported as medians with interquartile ranges (25th and 75th percentiles). For normally distributed data comparisons between two independent groups were made using the independent samples t-test; otherwise, the Mann-Whitney U test was used. Survival analysis was conducted using Kaplan-Meier estimates and the Cox proportional hazards model to assess the probability of achieving key outcomes, including remission on MTX and initiation of biologic DMARDs. Survival analysis with Kaplan-Meier curves and the Cox proportional hazards model was performed to estimate the probability of achieving the event (remission on MTX and initiation of bDMARDs). Likelihood ratio (LR), Wald, and Score tests were employed to assess the validity of the Cox proportional hazards model. We used the Schoenfeld residuals test to verify the assumption of proportional hazards for the model. Due to the small sample size, a modification of the Cox proportional hazards model, penalized using the Firth method, was applied. A P value less than 0.05 was considered significant. Receiver operating characteristic (ROC) analysis was also used to identify cutoff values for quantitative variables. The results of the ROC analysis were evaluated in comparison with the random guessing model using DeLong’s test.
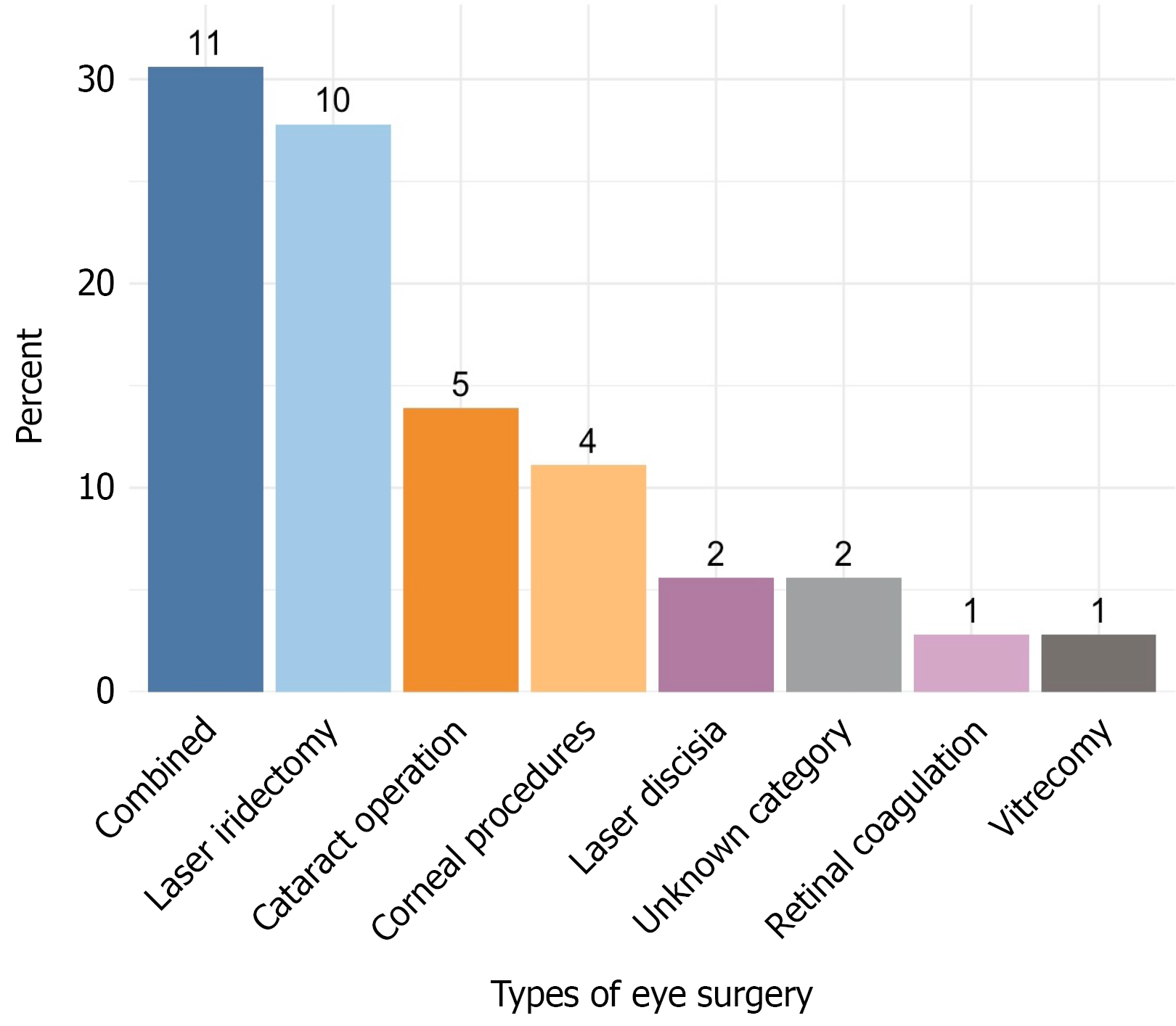
The study included 68 unique participants (107 affected eyes), comprising 44 females (64.7%) and 24 males (35.3%). The mean age at the time of uveitis diagnosis was 7.2 years with a median age of 7.0 years (range 1.8-16.8 years). The mean time to surgery was 4.4 years, and the median time was 2.5 years (range, 1.1 to 6.6 years). A total of 17 patients underwent 36 surgical interventions: Cataract removal, 5 (13.9%); corneal procedures, 4 (11.1%); laser discission, 2 (5.5%); laser iridectomy, 10 (27.8%); retinal laser coagulation, 1 (2.8%); vitrectomy, 1 (2.8%); and the exact information about 2 (5.5%) surgical interventions was incomplete. Laser discission refers to yttrium-aluminum-garnet laser discission or yttrium-aluminum-garnet laser capsulotomy, a microsurgical procedure to treat secondary cataracts. Retinal laser coagulation refers to retinal laser photocoagulation.
Elective surgery to improve vision, mainly cataract extraction or combined operations, cataract extraction with synechiotomy, or cataract extraction with vitrectomy, was undertaken in 11 out of 17 patients (64.7%). Patients who underwent elective surgery were in drug remission at the time of surgery (6 months without exacerbation, cell reaction zero according to the SUN criteria). Emergency surgery was performed in 4 out of 17 patients (23.5%) due to a sharp increase in intraocular pressure, pain syndrome, and the presence of iris bombe. These patients underwent surgery regardless of whether they had intraocular inflammation or not. Information about 2 remaining patients (11.8%) was lost. Combined surgical interventions were defined as procedures in which multiple operations were simultaneously carried out involving several anatomical structures of the eye in 11 (30.6%) patients.
In the preoperative period, two main strategies were used to suppress ocular inflammation: (1) A short course of oral glucocorticoids at a dose of 1 mg/kg (60 mg maximum) for 3-7 days before surgery; or (2) Intravenous pulse therapy with methylprednisolone at a dose of 10-30 mg/day (1000 mg maximum) for 3 consecutive days. In the postoperative period depending on the degree of intraocular inflammation and individual clinical considerations, one of the following regimens was employed: (1) Pulse therapy with methylprednisolone at the same dose; (2) A tapering course of oral glucocorticoids starting at 1 mg/kg (maximum 60 mg); or (3) A combination of both approaches.
If therapy with biologic drugs and MTX was prescribed before the operation, it did not stop either before, during, or after the surgical stage of treatment. The descriptive characteristics of the cohort are presented in Table 1. The eye surgery type distribution are presented in Figure 2. Patients who underwent eye surgery had an earlier age of uveitis onset (P = 0.017) and a trend (P = 0.057) toward later MTX administration. The remission rate (P = 0.015) as well as the time to remission (P = 0.036) and the probability of the remission achievement on MTX was lower (P = 0.033) in operated children (Figure 3A). Patients who did not undergo surgery had approximately five times higher odds of achieving remission with MTX compared with those who underwent surgery [odds ratio = 5.29, 95% confidence interval (CI): 1.23-24.90, P = 0.015]. Patients who underwent eye surgery were significantly more likely to require treatment with bDMARDs (odds ratio = 5.6; 95%CI: 1.11-55.19, P = 0.021). The data are presented in Table 2 and Figure 3A.
| Parameter | Result |
| Sex, females | 44 (64.7) |
| HLA-B27-positivity | 7 (22.6) |
| ANA positivity | 41 (61.2) |
| Type of arthritis | |
| Oligoarthritis | 43 (63.2) |
| ERA | 11 (16.2) |
| Psoriatic arthritis | 2 (2.9) |
| Undifferentiated arthritis | 12 (17.7) |
| Age at onset of uveitis, years | 7.0 (4.4-9.6) |
| Patients who underwent eye surgery | 17 (25.0) |
| Time to eye surgery, years | 2.5 (1.1-6.6) |
| MTX therapy | 55.0 (80.9) |
| Time to MTX initiation, years | 0.33 (0.08-1.48) |
| Remission achieved on MTX | 39 (70.9) |
| Time to remission on MTX, years | 0.33 (0.25-0.58) |
| Patients who flared on MTX | 25 (46.3) |
| Time to first flare on MTX, years | 0.6 (0.3-1.1) |
| bDMARDs received | 44 (64.7) |
| Time to initiation of bDMARD therapy, years | 1.62 (0.65-3.67) |
| Remission achieved on bDMARD therapy | 35 (77.8) |
| Time to remission, years | 0.2 (0.1-0.4) |
| Flare on bDMARD therapy | 18 (52.9) |
| Time to first flare on bDMARD therapy, years | 1.6 (0.8-2.5) |
| Received intraocular corticosteroids | 32 (47.1) |
| Time to intraocular corticosteroid injection, years | 0.08 (0.00-1.80) |
| Time between MTX and bDMARD therapy, years | 1.0 (0.4-1.5) |
| Parameter | Eye surgery, n = 17 | No eye surgery, n = 51 | P value |
| Sex, female | 11 (64.7) | 33 (64.7) | 1.000 |
| Age at arthritis onset, years | 3.1 (2.2-5.9) | 6.5 (3.0-10.7) | 0.056 |
| Age at uveitis onset, years | 4.9 (3.7-8.2) | 7.8 (4.7-10.8) | 0.017 |
| Anatomical type of uveitis | |||
| Posterior | 1 (5.9) | 3 (5.9) | 0.553 |
| Panuveitis | 5 (29.4) | 8 (15.7) | |
| Pars planitis | 0 (0.0) | 2 (3.9) | |
| Anterior | 11 (64.7) | 38 (74.5) | |
| Acute manifested uveitis | 6 (35.3) | 21 (41.2) | 0.779 |
| Unilateral uveitis | 6 (35.3) | 23 (45.1) | 0.577 |
| Complications at first examination | 7 (41.2) | 14 (27.5) | 0.366 |
| ESR at onset, mm/hour | 24.0 (19.5-41.5) | 18.5 (8.3-34.3) | 0.551 |
| CRP at onset, mg/L | 18.0 (9.5-75.5) | 2.1 (1.0-14.7) | 0.358 |
| Active joints | 1.0 (1.0-1.5) | 2.0 (1.0-2.0) | 0.109 |
| Age at uveitis onset under 7 years | 12 (70.6) | 22 (43.1) | 0.091 |
| ANA-positive | 9 (56.3) | 32 (62.7) | 0.770 |
| HLA-B27-positive | 2 (25.0) | 5 (21.7) | 1.000 |
| Time to MTX initiation, years | 0.75 (0.30-1.70) | 0.25 (0.10-0.70) | 0.057 |
| Time to remission on MTX, years | 0.58 (0.40-1.10) | 0.28 (0.20-0.40) | 0.036 |
| Time to first flare, years | 0.59 (0.50-1.10) | 0.53 (0.30-1.00) | 0.679 |
| Remission on MTX | 6 (42.9) | 33 (80.5) | 0.015 |
| Loss of disease control | 5 (38.5) | 20 (48.8) | 0.545 |
| Intraocular corticosteroid injections | 9 (52.9) | 24 (47.1) | 0.782 |
| bDMARD therapy | 15 (88.2) | 29 (56.9) | 0.021 |
| Time to bDMARD initiation, years | 2.3 (1.1-5.3) | 1.3 (0.5-3.5) | 0.065 |
| Remission on bDMARDs | 11 (73.3) | 24 (80.0) | 0.710 |
| Time to remission on bDMARDs, years | 0.17 (0.08-0.30) | 0.20 (0.08-0.40) | 0.810 |
| Flare on bDMARDs | 8 (66.7) | 10 (45.5) | 0.297 |
| Time to first flare on bDMARDs, years | 1.0 (0.3-2.0) | 1.9 (1.4-3.0) | 0.323 |
ROC analysis identified a cutoff value of 0.69 years for MTX initiation with a sensitivity of 75%, specificity of 61%, and an area under the curve of 0.68 (95%CI: 0.51-0.84). Analyzing this threshold using Fisher’s exact test revealed significant differences between the groups (P = 0.021, 95%CI: 0.04-0.93). Patients who underwent surgery were five times more likely to initiate MTX later than 0.69 years compared with those without surgery. A ROC analysis identified 0.375 years as the optimal cutoff for time to remission on MTX (sensitivity: 71%, specificity: 72%, area under the curve = 0.72, 95%CI: 0.53-0.90). Fisher’s exact test confirmed significant differences between groups (P = 0.027, 95%CI: 0.02-0.87) with patients undergoing surgery being 6.3 times more likely to have a remission time exceeding this threshold. These findings aligned with the Cox model, indicating that surgical intervention reduced the likelihood of remission on MTX. Due to the small sample size and to obtain a more precise estimation, a Firth-penalized Cox model was constructed, demonstrating similar results (hazard ratio = 2.7, 95%CI: 0.12-0.95, LR test P = 0.038), indicating that surgical intervention reduced the chances of achieving remission on MTX by approximately three-fold.
The Kaplan-Meier curves showed that the time to initiation in the non-surgical group was significantly shorter (P = 0.022; Figure 3B). However, no statistically significant differences in the time to remission on bDMARD therapy were observed. Due to the small sample size, a Firth-penalized Cox model was constructed to obtain a more precise estimation, yielding similar results (hazard ratio = 2.2, 95%CI: 0.21-0.93, LR test P = 0.029). The probability of the achievement of remission on bDMARDs was similar to operated and non-operated eyes (P = 0.72). Additionally, Kaplan-Meier curves were constructed to determine the probability of having surgical intervention based on the cutoff value of MTX initiation (0.70 years). The Kaplan-Meier curves (Figure 3C) illustrate that the time to surgical intervention in these groups reached borderline significance (P = 0.065).
The majority of studies on U-JIA have focused on identifying predictors of uveitis occurrence in patients with JIA. Gender is currently considered one of the significant risk factors[4]. Other significant risk factors include antinuclear antibody positivity, an age of onset before 7 years, an oligoarticular JIA category, and a disease duration of less than 4 years[2,7,15,16]. However, significantly fewer studies have focused on the stratification of patients with U-JIA.
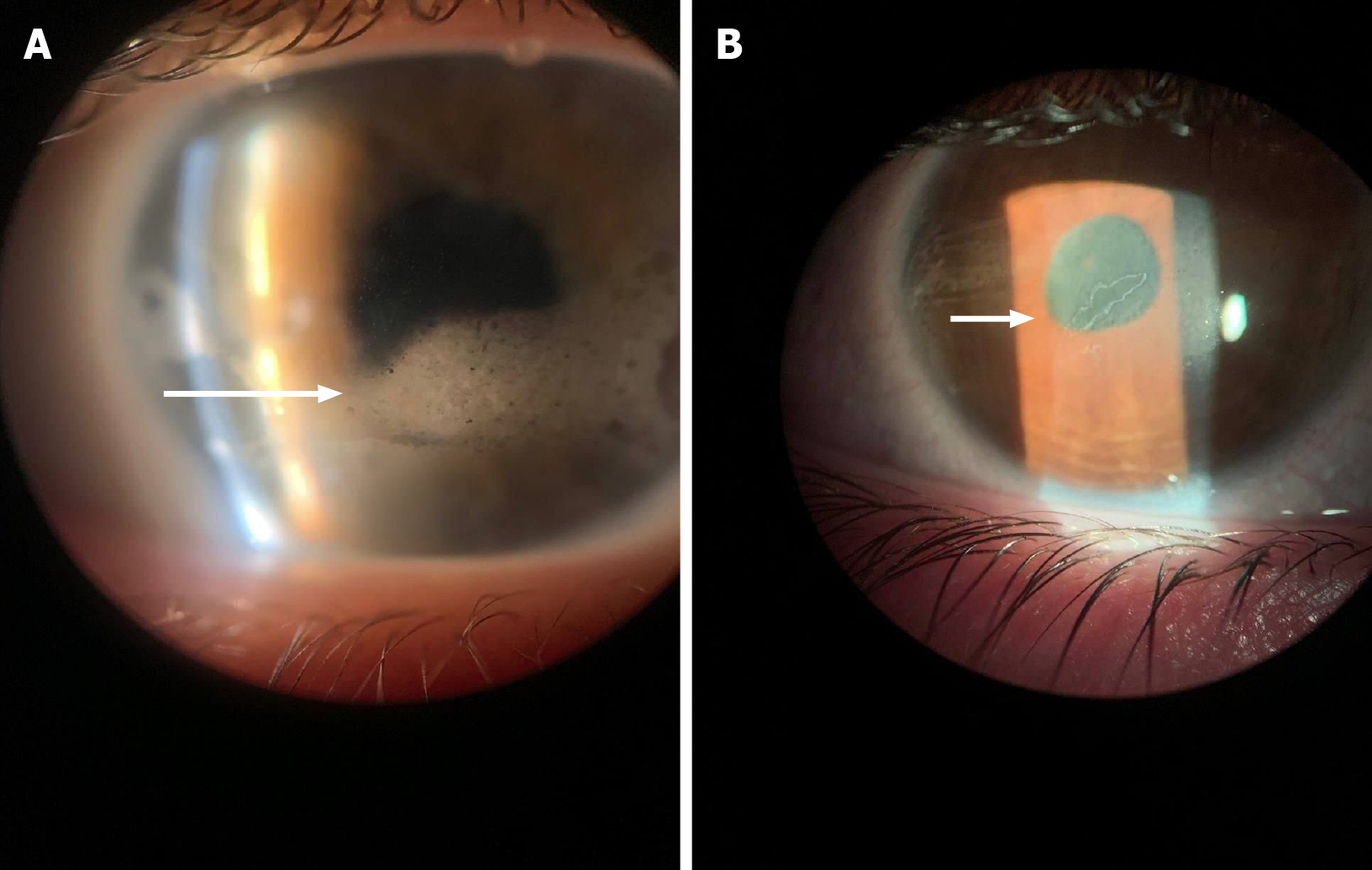
Our study aimed to investigate the clinical and demographic characteristics of patients who underwent surgical treatment. Patients who required surgical intervention were characterized by a more severe and aggressive course of uveitis, as evidenced by an earlier age of onset of uveitis (P = 0.002) as well as a significantly lower likelihood of achieving remission with conventional cytostatic therapy using MTX (P = 0.015). The surgical treatment requirement was likely associated with the early onset of uveitis, delays in initiating timely therapy with MTX, and potentially late diagnosis, contributing to the rapid progression of the disease and the development of complications [e.g., band keratopathy (Figure 4A) or posterior synechia (Figure 4B)].
However, in our study, no significant differences were observed in the presence of complications at the initial examination, contrasting the findings of a cohort study[11]. The observed differences were most likely attributable to variations in methodology. The referenced study was a multicenter investigation with a relatively large sample size, assessing risk factors for vision loss in U-JIA. Our study did not pursue this objective and was characterized by a significantly smaller sample size. The mentioned study demonstrated that the presence of posterior synechiae, active inflammation in the anterior chamber (≥ 1 cell), and ophthalmic surgeries were statistically significantly associated with an increased risk of new vision loss to levels of ≤ 20/50 and ≤ 20/200. The incidence of at least one new ophthalmic complication during follow-up was reported as 0.15 per eye-year. However, this rate was notably lower at 0.04 per eye-year among eyes without complications at the initial examination.
It has been reported that patients undergoing surgical treatment exhibited a significantly earlier disease onset (P = 0.002). This observation, interpreted cautiously, suggests that a longer disease duration may be linked to a higher likelihood of requiring surgical intervention. However, it is important to note that surgical intervention may be just another marker of severe uveitis. It has been well established that persistent inflammation in the anterior segment as observed in U-JIA can elevate aqueous outflow resistance by promoting the accumulation of inflammatory debris and causing damage to the trabecular meshwork[17]. However, some studies do not report a statistically significant difference in the onset of uveitis between operated and non-operated children[18]. It is also important to note that we could not prove that earlier MTX initiation had a protective effect[19].
The main findings of our study included the identification of an association between surgical intervention and the efficacy of MTX. Most ophthalmic surgical procedures for uveitis are performed due to complications associated with previous or current inflammatory activity. The need for surgical intervention often reflects insufficient control of the disease through conservative therapy and can serve as a clinical marker of a more aggressive or refractory disease. We want to emphasize that patients who require surgery despite ongoing systemic therapy may have a more severe form of the disease that is less amenable to standard immunosuppressive therapy regimens. However, surgical treatment was more often required for patients who had delayed MTX treatment, failed to achieve remission on MTX (possibly due to a later administration), and later switched to biological therapy. The results of our study may help identify patients who may benefit from an earlier escalation of biologic therapy, particularly if remission does not occur within the expected timeframe (e.g., 4.5 months after starting MTX).
Our personal experience (about 300 patients with uveitis) showed that if a patient needs surgery, he has a poor visual prognosis even though the operation has been performed, and often surgical treatment leads to a series of subsequent eye operations, which is ultimately associated with a marked loss of visual function. Early administration of MTX and early switching to biologic therapy, particularly in cases of insufficient effectiveness and persistent intraocular inflammation, will in our opinion effectively control uveitis, postpone the need for surgical treatment if necessary, and preserve visual function for a more extended period. Surgical treatment of the eyes in patients with uveitis is often a last resort measure that one wants to avoid.
The German guideline recommends that therapy should be intensified if remission is not achieved within 16 weeks[8]. Although the limited sample size and wide 95%CI preclude definitive conclusions, this study may serve as a starting point for further investigation and refinement of treatment approaches for severe uveitis. The Kaplan-Meier analysis in our study showed that patients who required eye surgery received bDMARD therapy significantly later. In contrast a higher proportion of surgical patients eventually received bDMARDs (88.2% vs 56.9%, P = 0.021). It may seem contradictory, but surgical patients are more likely to require it despite the delay, potentially indicating differences in disease severity or treatment management between the groups. The delay in bDMARD therapy might be connected with a higher probability of surgical intervention.
The delay in bDMARD administration is most likely due to ophthalmologists underestimating the consequences of uncontrolled inflammation as they usually do not prescribe biologic therapies. A rheumatologist prescribes it, but an ophthalmologist recommends it since a rheumatologist cannot independently examine the eye. Insufficient control of the disease in the early stages can lead to a serious course of uveitis and the development of complications such as cataracts and glaucoma, which increase the likelihood of surgical intervention.
Our study was limited by several factors, primarily its retrospective nature, which poses a significant constraint due to the potential loss of critical information. Additionally, the relatively small sample size reduced the statistical power and limited the generalizability of its findings. A limited sample size increases the risk of type II errors, whereby actual effects may go undetected. Additionally, the quality of medical records posed a limitation, resulting in incomplete data and potentially compromising the accuracy of the findings. Another important limitation was that data on complications were only recorded at the initial examination and restricted our understanding of their progression over time. The lack of stratification further hindered our ability to draw more detailed conclusions. However, it is important to note that the 95%CIs were relatively wide. For more precise conclusions future studies with larger sample sizes are needed. The identified discrepancies may be attributed to several factors. Specifically, due to the retrospective nature of our study, data on visual acuity were unavailable and prevented us from considering this factor in evaluating therapeutic outcomes.
Additionally, complications at the initial examination were not stratified into groups; only the presence or absence of complications was recorded. Furthermore, it was impossible to assess the frequency of complications during follow-up as this parameter was recorded only once at the time of the first examination. The lack of access to comprehensive ophthalmological data monitoring (surgical protocols, visual activity, eyesight assessments, intraocular pressure, and visual outcomes) decreased the importance of the study results. The lack of clear definitions or standardization could lead to inconsistencies in data collection and analysis, ultimately impacting our conclusions.
In this study potential confounding variables that may influence outcomes were not systematically assessed. These included the course of arthritis, which may necessitate more intensive therapy and impact the control of ocular inflammation, temporal variations in treatment protocols, and disparities in healthcare access, particularly access to biologic therapies. The presence of such factors could bias the results and affect the interpretation of findings. Future research incorporating a more comprehensive analysis that adjusts for these confounders is warranted to enhance the robustness and reliability of the conclusions.
U-JIA is a challenging condition that requires a thorough investigation of its risk factors and clinical characteristics. Surgical intervention may be a surrogate marker of severe uveitis course. Patients with early onset of uveitis and arthritis, delayed and failed MTX treatment, and delayed switching to biologics often required subsequent eye surgery. Early and timely diagnosis of uveitis, careful monitoring of disease activity according to the SUN criteria, timely administration of MTX, and earlier switching to biologic therapy in cases of insufficient control of uveitis with MTX may be modifiable risk factors for the need and timing of surgery, resulting in better visual outcomes. Studies are needed for the long-term assessment of uveitis outcomes to search for early markers of an unfavorable outcome. Improved eye healthcare service, a collaboration between pediatric rheumatologists and ophthalmologists, correct estimation of current eye inflammation, and encouraging earlier administration of MTX and/or biologics to patients with risk factors of unfavorable visual outcomes or having predictors of complicated/Loss-control uveitis course may decrease the requirements in eye surgery and provide better visual outcomes.
| 1. | Van Gelder RN, Sen HN, Tufail A, Lee AY. Here Comes the SUN (Part 2): Standardization of Uveitis Nomenclature for Disease Classification Criteria. Am J Ophthalmol. 2021;228:A2-A6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Maleki A, Anesi SD, Look-Why S, Manhapra A, Foster CS. Pediatric uveitis: A comprehensive review. Surv Ophthalmol. 2022;67:510-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 3. | Angeles-Han ST, Pelajo CF, Vogler LB, Rouster-Stevens K, Kennedy C, Ponder L, McCracken C, Lopez-Benitez J, Drews-Botsch C, Prahalad S; CARRA Registry Investigators. Risk markers of juvenile idiopathic arthritis-associated uveitis in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. J Rheumatol. 2013;40:2088-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Moradi A, Amin RM, Thorne JE. The role of gender in juvenile idiopathic arthritis-associated uveitis. J Ophthalmol. 2014;2014:461078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 983] [Cited by in RCA: 1102] [Article Influence: 58.0] [Reference Citation Analysis (1)] |
| 6. | Katargina LA, Brzheskiy VV, Guseva MR, Denisova EV, Drozdova EA, Zhukova OV, Nikishina IP, Starikova AV. The federal clinical guidelines on “The diagnostics and treatment of uveitis associated with juvenile idiopathic arthritis”. Russ Ped Ophthal. 2016;11:102-111. [DOI] [Full Text] |
| 7. | Angeles-Han ST, Ringold S, Beukelman T, Lovell D, Cuello CA, Becker ML, Colbert RA, Feldman BM, Holland GN, Ferguson PJ, Gewanter H, Guzman J, Horonjeff J, Nigrovic PA, Ombrello MJ, Passo MH, Stoll ML, Rabinovich CE, Sen HN, Schneider R, Halyabar O, Hays K, Shah AA, Sullivan N, Szymanski AM, Turgunbaev M, Turner A, Reston J. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Screening, Monitoring, and Treatment of Juvenile Idiopathic Arthritis-Associated Uveitis. Arthritis Care Res (Hoboken). 2019;71:703-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 8. | Heiligenhaus A, Minden K, Tappeiner C, Baus H, Bertram B, Deuter C, Foeldvari I, Föll D, Frosch M, Ganser G, Gaubitz M, Günther A, Heinz C, Horneff G, Huemer C, Kopp I, Lommatzsch C, Lutz T, Michels H, Neß T, Neudorf U, Pleyer U, Schneider M, Schulze-Koops H, Thurau S, Zierhut M, Lehmann HW. Update of the evidence based, interdisciplinary guideline for anti-inflammatory treatment of uveitis associated with juvenile idiopathic arthritis. Semin Arthritis Rheum. 2019;49:43-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Cann M, Ramanan AV, Crawford A, Dick AD, Clarke SLN, Rashed F, Guly CM. Outcomes of non-infectious Paediatric uveitis in the era of biologic therapy. Pediatr Rheumatol Online J. 2018;16:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Thorne JE, Woreta F, Kedhar SR, Dunn JP, Jabs DA. Juvenile idiopathic arthritis-associated uveitis: incidence of ocular complications and visual acuity loss. Am J Ophthalmol. 2007;143:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Gregory AC 2nd, Kempen JH, Daniel E, Kaçmaz RO, Foster CS, Jabs DA, Levy-Clarke GA, Nussenblatt RB, Rosenbaum JT, Suhler EB, Thorne JE; Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study Research Group. Risk factors for loss of visual acuity among patients with uveitis associated with juvenile idiopathic arthritis: the Systemic Immunosuppressive Therapy for Eye Diseases Study. Ophthalmology. 2013;120:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Bajraktari G, Jukić T, Kalauz M, Oroz M, Bertetić AR, Vukojević N. Evaluation of postoperative outcomes after cataract surgery in patients with Juvenile Idiopathic Arthritis-Associated Uveitis. Eur J Ophthalmol. 2025;35:1109-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, He X, Maldonado-Cocco J, Orozco-Alcala J, Prieur AM, Suarez-Almazor ME, Woo P; International League of Associations for Rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390-392. [PubMed] |
| 14. | Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2474] [Cited by in RCA: 3372] [Article Influence: 160.6] [Reference Citation Analysis (0)] |
| 15. | Martini A, Ravelli A, Avcin T, Beresford MW, Burgos-Vargas R, Cuttica R, Ilowite NT, Khubchandani R, Laxer RM, Lovell DJ, Petty RE, Wallace CA, Wulffraat NM, Pistorio A, Ruperto N; Pediatric Rheumatology International Trials Organization (PRINTO). Toward New Classification Criteria for Juvenile Idiopathic Arthritis: First Steps, Pediatric Rheumatology International Trials Organization International Consensus. J Rheumatol. 2019;46:190-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 328] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 16. | Sen ES, Ramanan AV. Juvenile idiopathic arthritis-associated uveitis. Clin Immunol. 2020;211:108322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Sijssens KM, Rothova A, Berendschot TT, de Boer JH. Ocular hypertension and secondary glaucoma in children with uveitis. Ophthalmology. 2006;113:853-9.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | van Meerwijk CLLI, Wieringa WG, de Boer JH, Jansonius NM, Los LI. Factors Associated With Glaucoma Surgery in Pediatric Non-Infectious Uveitis. Ocul Immunol Inflamm. 2023;31:2018-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Iannone C, Costi S, Germinario S, Amati A, Gattinara M, Chighizola C, Miserocchi E, Marino A, Caporali R. Ab1719 The Protective Role of Methotrexate in Patients with Juvenile Idiopathic Arthritis and Uveitis: A Retrospective Study on over 100 Patients. Ann Rheum Dis. 2024;83:2235-2236. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/