Published online Nov 18, 2025. doi: 10.5312/wjo.v16.i11.110426
Revised: July 9, 2025
Accepted: October 10, 2025
Published online: November 18, 2025
Processing time: 161 Days and 15.9 Hours
Spinal cord injury (SCI) imposes enduring physical impairments and substantial socio-economic burdens. These injuries are either traumatic incidents or ischemic but exhibit comparable clinical recoveries. This suggests shared underlying neuro
To investigate the relationship between the magnetic resonance imaging (MRI) biomarkers (tissue bridges) and clinical outcome in acute traumatic SCI.
In this prospective study adult patients with acute SCI who were examined clini
There were 47 patients with a mean age of 40.43 ± 10.73 years and male/female ratio of 34:13. There was a sig
The MRI imaging biomarkers in SCI patients demonstrated substantial improvement over time. There was a ne
Core Tip: This prospective study highlights the prognostic value of magnetic resonance imaging (MRI) biomarkers, particularly tissue bridges, in acute spinal cord injury (SCI). Quantitative MRI parameters, especially midsagittal tissue bridge dimensions assessed at 1 and 6 months, positively correlated with motor and sensory recovery. Conversely, greater spinal canal compromise and lesion size predicted poorer outcomes. These findings underscore the utility of early MRI-based assessment in predicting neurological recovery and guiding management in traumatic SCI.
- Citation: Singh R, Gautam S, Aggarwal S, Kaur S, Jain M. Correlation of magnetic resonance imaging biomarkers (tissue bridges) with neurological recovery following traumatic spinal cord injury. World J Orthop 2025; 16(11): 110426
- URL: https://www.wjgnet.com/2218-5836/full/v16/i11/110426.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i11.110426
Spinal cord injury (SCI) imposes enduring physical impairments and substantial socio-economic burdens. These injuries can stem from either traumatic incident like falls or non-traumatic causes such as ischemia. Interestingly, regardless of the injury's origin, patients with traumatic and ischemic thoracic and cervical SCI exhibit comparable clinical recoveries. This suggests shared underlying neurodegenerative mechanisms, such as neuronal cell death, demyelination, and axonal degeneration, regardless of etiology. However, while the neurodegenerative processes in cervical SCI have been ex
Although conventional magnetic resonance imaging (MRI) establishes prognostic indicators, it falls short as a neu
MRI serves as a valuable tool in clinical diagnostics and prognosis, particularly in SCI cases. T2-weighted scans obtained from the lesion epicenter offer critical insights into the spatiotemporal evolution of the injury, facilitating the quantification of various intramedullary processes such as edema, hemorrhage, and spinal cord compression, particularly in tetraplegic patients[5,6].
Conventional MRI captures macrostructural changes, but quantitative neuroimaging (qMRI) techniques offer precise microstructural insights. qMRI measures myelin, axonal density, iron deposition, and metabolic profiling, demonstrating evolving markers of neurodegeneration in the spinal cord and brain[7]. MRI is commonly used post-traumatic SCI to assess intramedullary damage. Lesion quantification reveals correlations between severity and clinical impairment. Weeks post-injury, preserved neuronal tissue forms midsagittal tissue bridges, crucial for electrophysiologic commu
Preserved tissue bridges adjacent to the lesion cavity in incomplete SCI patients facilitate electrophysiological in
This is a prospective study conducted at tertiary care centre in Northern India with approval from the institute ethics committee (IEC- BREC/22/TH/Ortho-08). The study period was extended from November 2022 to March 2024. Con
A sample size of 45 patients was considered necessary to detect statistical significances with an effect size of 0.67 at alpha 0.05 and power of 90% based on previous study by Huber et al[6]. Considering the total loss of patients as 10%, 50 consecutive patients were finally enrolled in the study. Three patients were lost to follow-up; and finally, 47 patients who completed minimum of six-month follow-up were evaluated.
Each patient was thoroughly examined clinically and was subjected to investigations like X-ray spine, computed tomography (CT), and MRI within 48 hours of injury. MRI was done in a 3.0 T (Discovery 750w of GE make, Milwaukee, WI, United States) superconductive MRI machine was used with gradient field 40 mT/m and gradient switching rate 150 mT/millisecond. Axial and sagittal images were acquired in order to accurately identify the midsagittal slice. The midsagittal slice was identified using multiplanar reconstruction on T2-weighted images, selecting the slice that best visualized the cerebral aqueduct, corpus callosum, and brainstem midline structures in true sagittal alignment. Mea
| Axial TR | Slice gap | Slice thickness (mm) | FOV (cm) | NEX | TE | ||
| T1 | Plan | 650 | 1 | 4 | 1 | 2 | 10 |
| T2 | Sagittal | 3841 | 1 | 4 | 0.80 | 2 | 102 |
| T1 | Sagittal | 2671 | 1 | 4 | 1 | 2 | 24 |
| T2 | Axial | 6847 | 1 | 4 | 0.80 | 3 | 112 |
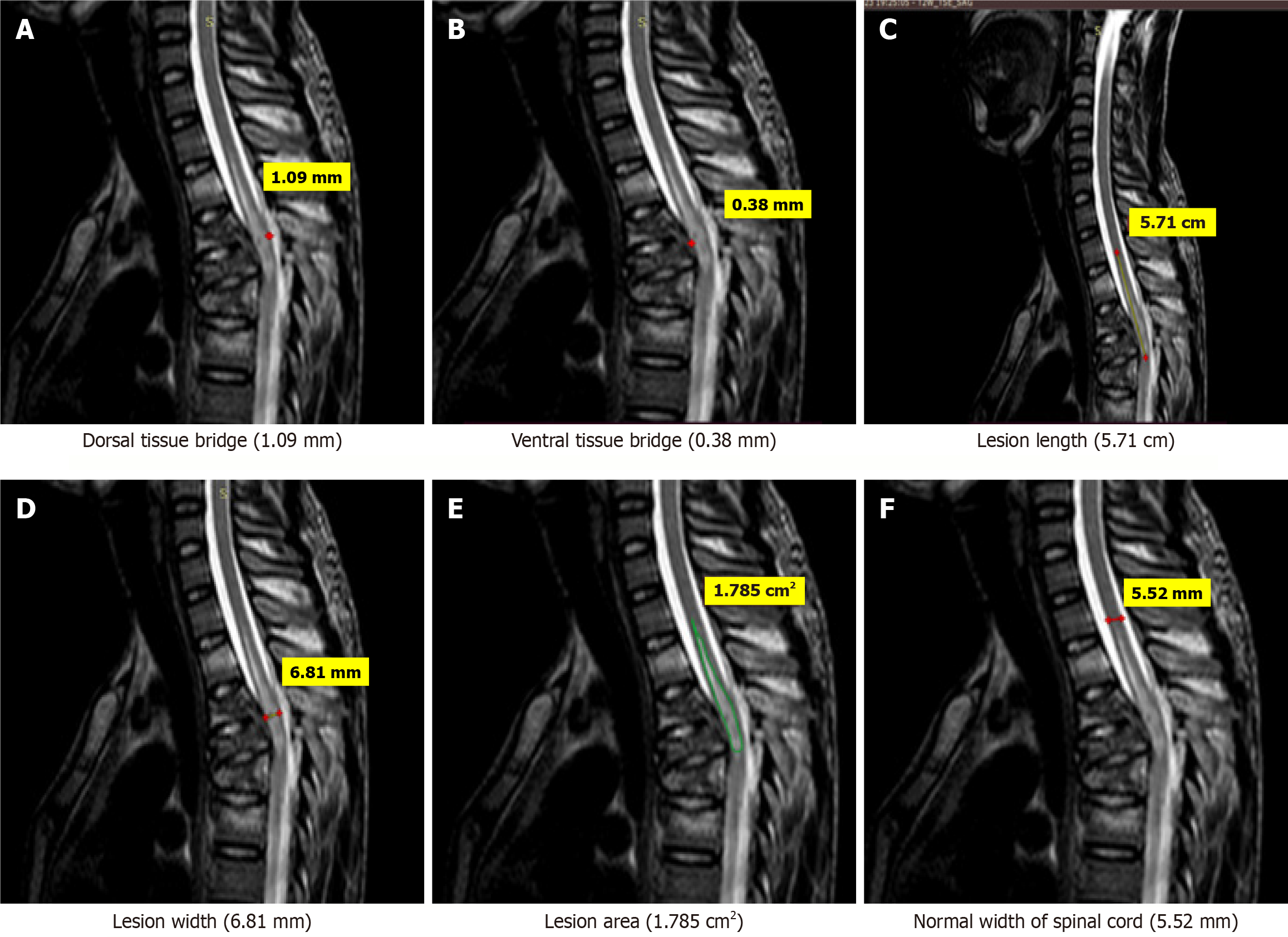
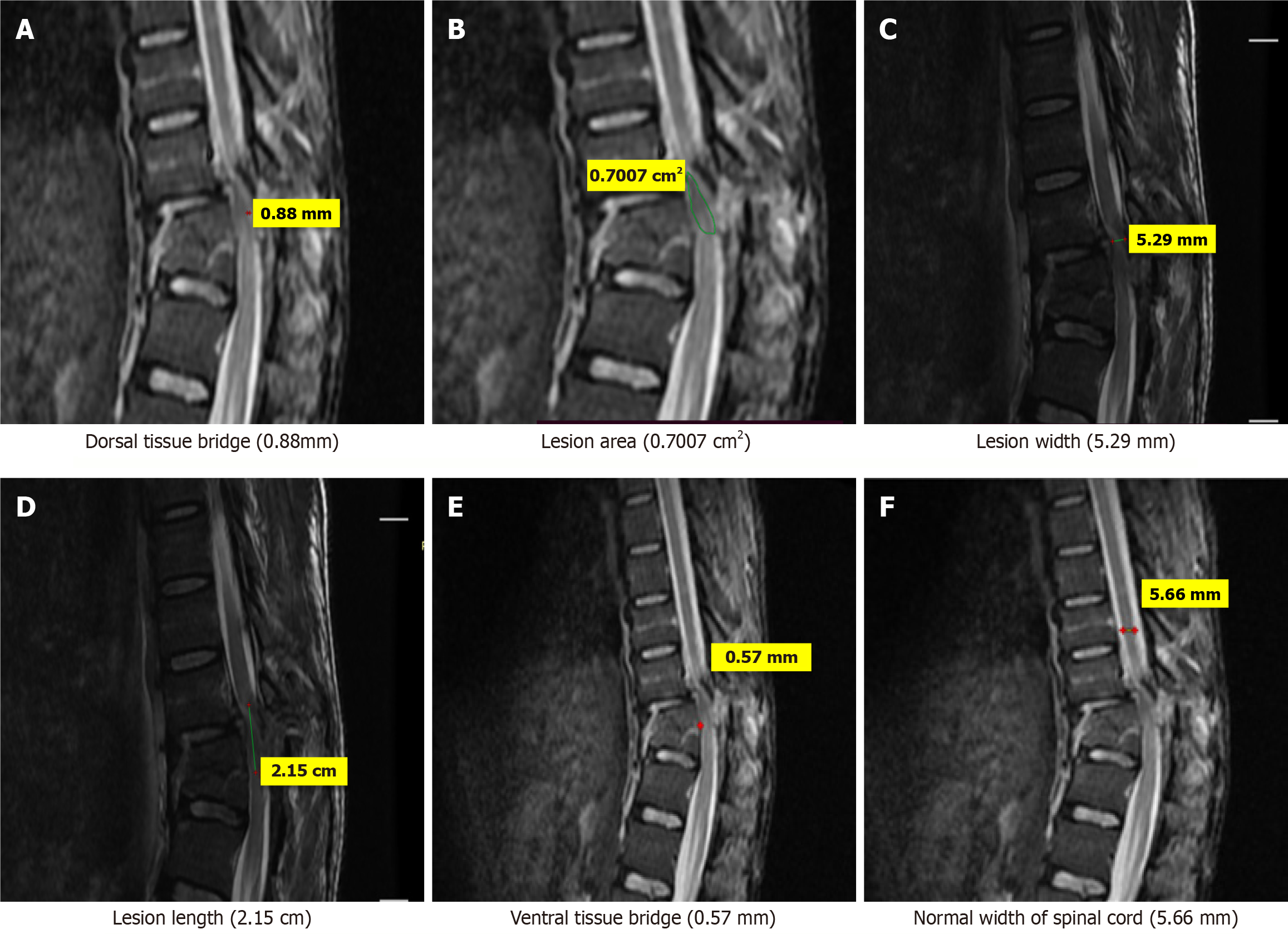
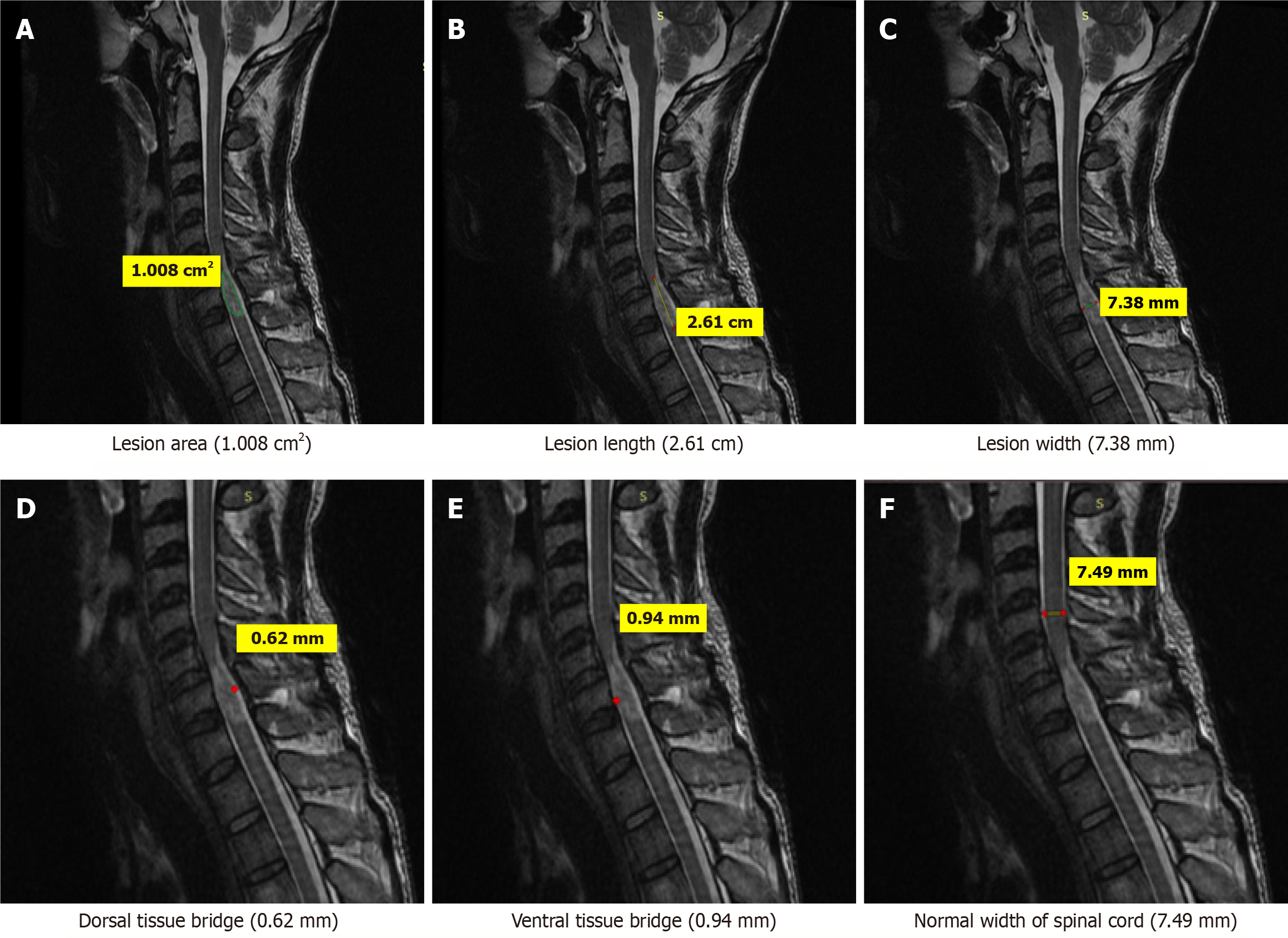
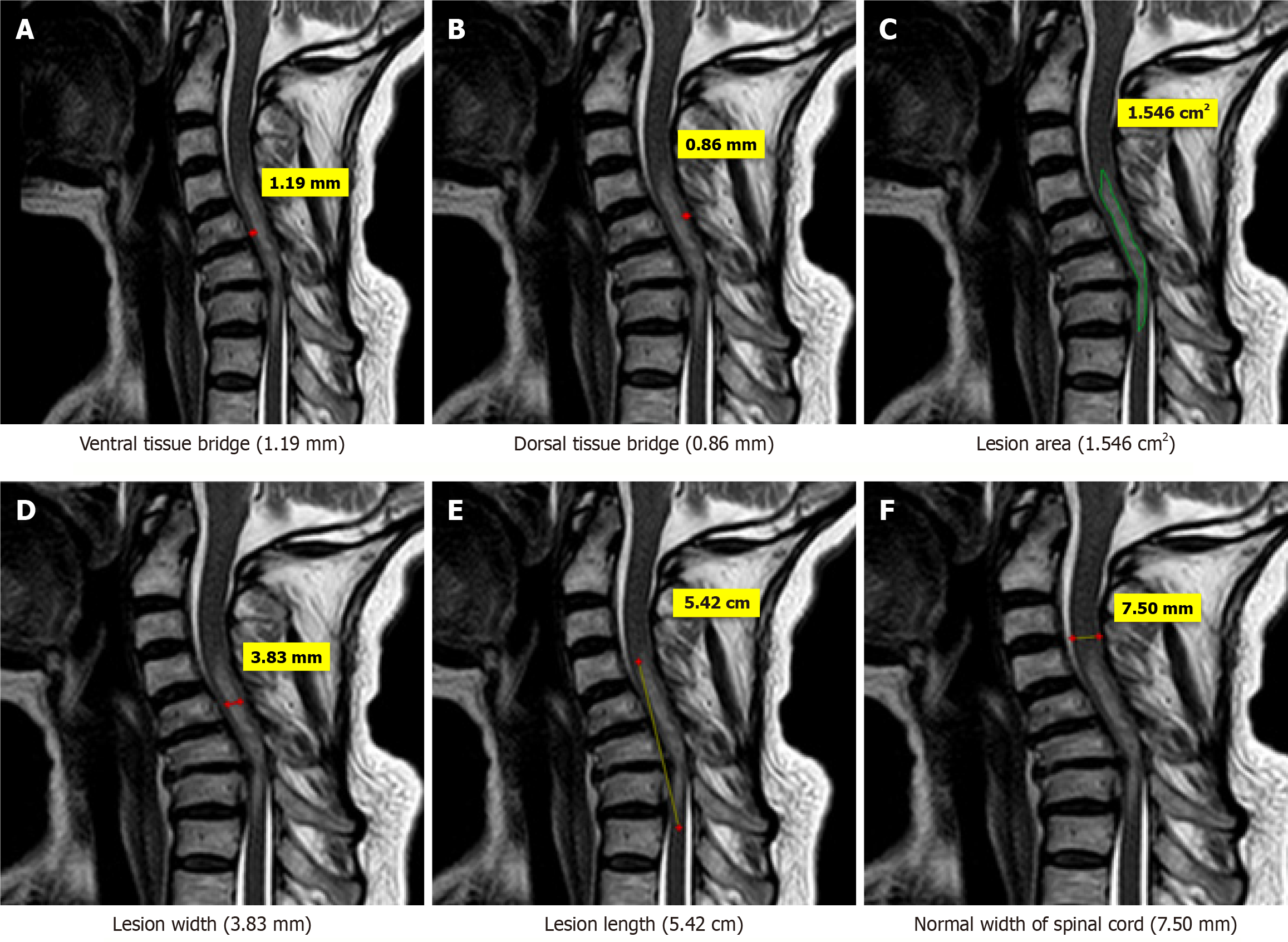
One rater (radiologist), blinded to patient identity and scan time point, assessed the anterior-posterior width, rostro-caudal length, and total area of the lesion, and the width of tissue bridges on the midsagittal slice.
Clinical assessment (sensory score, motor score and zone of partial preservation) were done at the time of admission, 3rd day, 7th day, one month, 3 months, and 6 months as per international guidelines (ASIA impairment scale)[10].
Spinal stability for thoracolumbar injuries was evaluated according to thoracolumbar injury classification and severity score classification. Unstable spine was taken up for spinal surgery (stabilisation alone or stabilisation with decom
Patients were followed up at one, 3 and 6 months. Clinical evaluation and plain radiography were done at each follow up. MRI was done at one month, 3-months and 6-months follow up. Neurological recovery was documented as per ASIA impairment scale (AIS)[10] and following outcome were measured and assessed.
The following radiological measurements was done in subsequent follow ups.
Kyphotic deformities (on sagittal view X-ray): (1) Sagittal index (SI): SI is segmental kyphosis at the vertebrae level adjusted for the baseline sagittal contour in the normal spine. Kyphotic deformity (KD) at the fracture motion segment level minus normal contour [NC; SI = KD - NC] was calculated. The baseline sagittal curve/Level was estimated using a 5° angle in thoracic segments, 0 degree at thoracolumbar junction, and 10° lumbar segments; (2) Regional kyphosis: The measurement of an angle created between the lines drawn on the superior end plate of the upper normal vertebra and the inferior end plate of the lower normal vertebra[13]; and (3) Gardener segmental KD: It was measured as the angle formed from lines drawn on the lower end plate of the adjacent normal vertebrae.
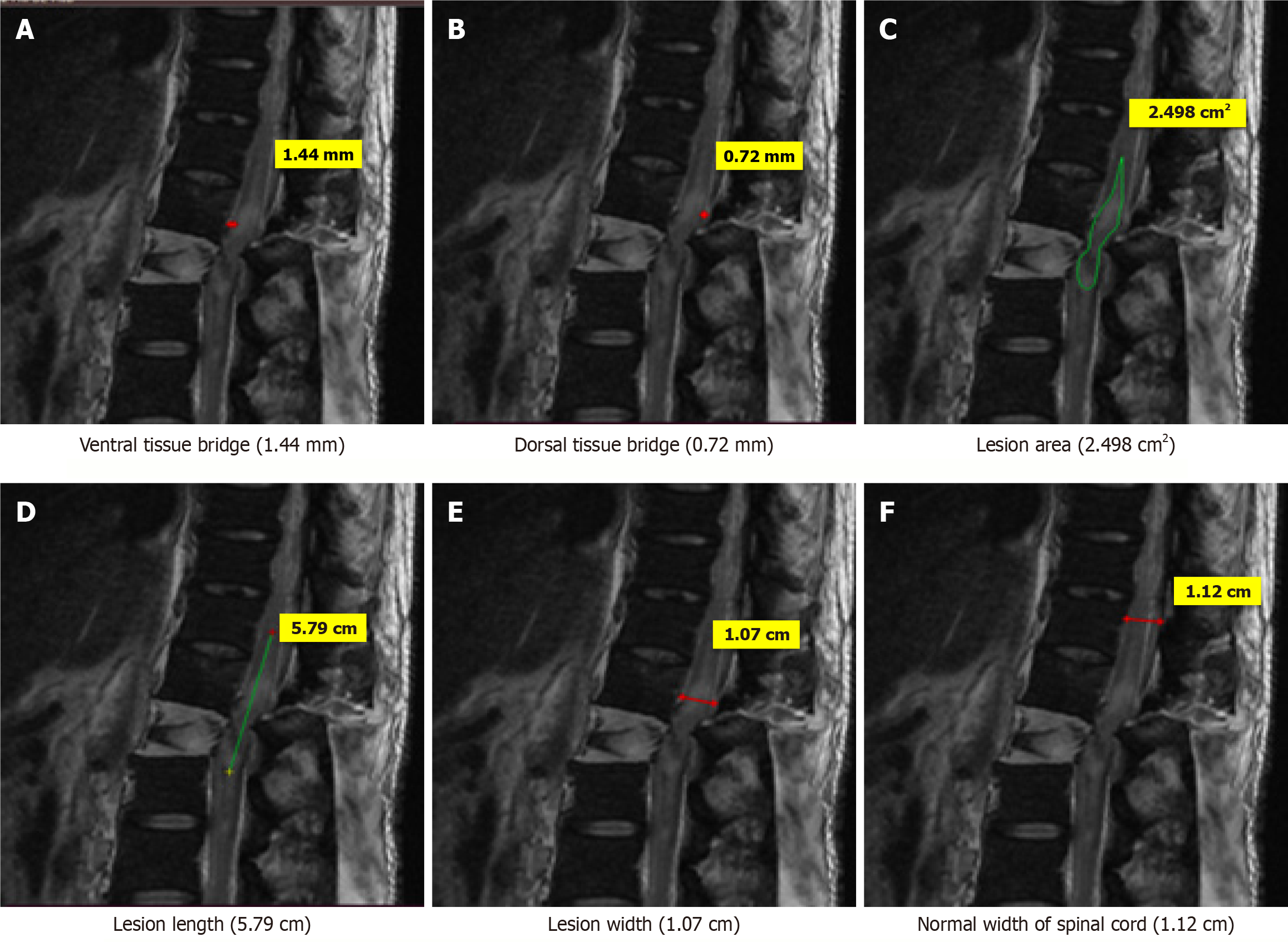
MRI parameters: These were measured on MRI as per following criteria: Three quantitative measures were used: Maximum canal compromise (MCC), maximum spinal cord compression (MSCC) and length of lesion. Mid-sagittal T1- and T2-weighted imaging were used to determine the MCC and MSCC respectively as described by Fehlings et al[14]. The values were ascertained by measuring the distance of the canal or spinal cord one segment above and below the lesion to compute the average distance. The distance was subsequently measured at the lesion site and expressed as a percentage of the average. The length, width and area of the lesion were determined on T2-weighted images. The length was defined as the distance between the most superior and most inferior extent of the lesion. The midsagittal T2-weighted image was utilised to measure the width of the ventral and dorsal tissue bridges, defined as the shortest distance from the cystic cavity to the ventral and dorsal edges of the spinal canal, respectively, at a right angle to the rostro-caudal orientation of the spinal cord, with the total width of the tissue bridges being the sum of both mea
A neurological examination was conducted at each follow-up assessment. An enhancement in motor power, restoration of sensation, and recovery of bladder and bowel function were observed, and graded to the ASIA score[10].
There were 47 patients with mean age of 40.43 ± 10.73 years and male is female ration of 34:13. The detail of demography are given in Table 2. The details of symptomatology among the subjects in tabulated in Table 3. The comparison motor index score (MIS) and sensory index score (SIS) at the 1-month, 3-month and 6-month intervals is outlined in Table 4. Table 5 shows distribution of subjects according to neurological parameters on clinical examination. The radiological indices at various time intervals are outlined in Table 6. Table 7 gives the correlation coefficients the radiological indices in relation to tissue bridges at 1 and 6 months. Table 8 compares the length, width, and area of the lesion at different time intervals. Finally, the regression analysis between various clinical measures at 6 months and MR parameters at 1 month is given in Table 9.
| Variable | Frequency | Percentage (%) | |
| Age (year) | < 30 | 10 | 21.3 |
| 31-40 | 9 | 19.1 | |
| 41-50 | 22 | 46.8 | |
| > 60 | 6 | 12.8 | |
| Gender | Female | 13 | 27.7 |
| Male | 34 | 72.3 | |
| Socio economic status | Lower class | 17 | 36.2 |
| Lower middle class | 21 | 44.7 | |
| Upper middle class | 9 | 19.1 | |
| Marital status | Married | 43 | 91.5 |
| Unmarried | 4 | 8.5 | |
| Educational status | Higher sec certificate | 7 | 14.9 |
| Secondary | 3 | 6.4 | |
| Middle school | 4 | 8.5 | |
| Primary school | 12 | 25.5 | |
| Illiterate | 21 | 44.7 | |
| Level of injury | Cervical | 8 | 17.02 |
| Upper dorsal (up to D5) | 8 | 17.02 | |
| Lower dorsal (D6-D11) | 16 | 34.04 | |
| Thoracolumbar area (D12-L2) | 15 | 31.91 | |
| Mode of injury | Diving injury | 4 | 8.5 |
| Fall | 7 | 14.9 | |
| Fall from height | 14 | 29.8 | |
| Motorcycle injury | 9 | 19.1 | |
| RSA | 13 | 27.7 | |
| Pain | Initial | 1 month | 3 months | 6 months | P value1 | |||||
| No. of cases | Percentage (%) | No. of cases | Percentage (%) | No. of cases | Percentage (%) | No. of cases | Percentage (%) | |||
| Pain score (VAS) | No | 0 | 0.0 | 20 | 42.6% | 47 | 100.0 | 47 | 100.0 | < 0.001 |
| Mild | 0 | 0.0 | 22 | 46.8% | 0 | 0.0 | 0 | 0.0 | ||
| Moderate | 0 | 0.0 | 5 | 10.6% | 0 | 0.0 | 0 | 0.0 | ||
| Severe | 47 | 100.0 | 0 | 0.0% | 0 | 0.0 | 0 | 0.0 | ||
| Swelling | 47 | 100.00 | 5 | 10 | 4 | 8.50 | 3 | 6.40 | < 0.001 | |
| Deformity | 5 | 10.60 | 5 | 10.60 | 5 | 10.60 | 5 | 10.60 | < 0.001 | |
| Weakness-lower limb | 47 | 100.00 | 47 | 100.00 | 10 | 21.30 | 10 | 21.30 | 0.008 | |
| Weakness-upper limb | 5 | 10.60 | 0 | 0.00 | 3 | 6.40 | 3 | 6.40 | < 0.001 | |
| Incontinence | 47 | 100.00 | 3 | 6.40 | 0 | 0.00 | 0 | 0.00 | < 0.001 | |
| Retention | 47 | 100.00 | 3 | 6.40 | 0 | 0.00 | 0 | 0.00 | ||
| MIS | Mean | SD | t value | P value1 | Difference | 95% confidence interval of the difference | ||||
| Mean | SD | SE mean | Lower | Upper | ||||||
| MIS upper limb (month) | 1 | 44.32 | 6.29 | |||||||
| 3 | 44.85 | 6.31 | -5.080 | 0.001 | -0.53 | 0.72 | 0.10 | -0.74 | -0.32 | |
| 6 | 45.68 | 5.75 | -6.643 | 0.001 | -1.36 | 1.41 | 0.20 | -1.77 | -0.95 | |
| MIS lower limb | 1 | 31.21 | 14.57 | |||||||
| 3 | 34.57 | 13.74 | -5.601 | 0.001 | -3.36 | 4.11 | 0.60 | -4.57 | -2.15 | |
| 6 | 38.23 | 13.63 | -6.466 | 0.001 | -7.02 | 7.44 | 1.09 | -9.21 | -4.84 | |
| Total (MIS) | 1 | 75.53 | 14.99 | |||||||
| 3 | 79.43 | 15.13 | -6.699 | 0.001 | -3.89 | 3.98 | 0.58 | -5.06 | -2.72 | |
| 6 | 83.91 | 15.54 | -8.358 | 0.001 | -8.38 | 6.88 | 1.00 | -10.40 | -6.36 | |
| SIS light touch (R + L) | 1 | 92.60 | 17.41 | |||||||
| 3 | 94.74 | 17.24 | -12.121 | 0.001 | -2.15 | 1.22 | 0.18 | -2.51 | -1.79 | |
| 6 | 99.85 | 17.88 | -11.647 | 0.001 | -7.26 | 4.27 | 0.62 | -8.51 | -6.00 | |
| SIS pin prick (R + L) | 1 | 93.55 | 17.32 | |||||||
| 3 | 96.21 | 17.35 | -8.109 | 0.001 | -2.66 | 2.25 | 0.33 | -3.32 | -2.00 | |
| 6 | 101.13 | 17.58 | -11.678 | 0.001 | -7.57 | 4.45 | 0.65 | -8.88 | -6.27 | |
| Total SIS | 1 | 186.15 | 34.50 | |||||||
| 3 | 190.96 | 34.30 | -12.632 | 0.001 | -4.81 | 2.61 | 0.38 | -5.57 | -4.04 | |
| 6 | 200.98 | 35.16 | -14.144 | 0.001 | -14.83 | 7.19 | 1.05 | -16.94 | -12.72 | |
| Variables | 1 month | 3 months | 6 months | P value1 | ||||
| No. of cases | Percentage (%) | No. of cases | Percentage (%) | No. of cases | Percentage (%) | |||
| ASIA score | A | 10 | 21.3 | 2 | 4.3 | 2 | 4.3 | 0.548 |
| B | 0 | 0 | 0 | 0 | 0 | 0 | ||
| C | 10 | 21.3 | 12 | 25.5 | 9 | 19.1 | ||
| D | 22 | 46.8 | 28 | 59.6 | 21 | 44.7 | ||
| E | 5 | 10.6 | 5 | 10.6 | 15 | 31.9 | ||
| Voluntary anal contraction | 0 | 14 | 29.8 | 17 | 36.2 | 14 | 29.8 | 0.857 |
| 1 | 22 | 46.8 | 22 | 46.8 | 22 | 46.8 | ||
| 2 | 11 | 23.4 | 8 | 17.0 | 11 | 23.4 | ||
| Temperature | 8 | 17.0 | 4 | 8.5 | 0 | 0.0 | 0.345 | |
| Deep anal pressure | 12 | 25.5 | 4 | 8.5 | 0 | 0.0 | 0.548 | |
| Superficial reflexes | Abdominal reflex (absent) | 18 | 40 | 4 | 8.0 | 4 | 8.0 | < 0.001 |
| Babinski reflex (absent) | 47 | 100 | 47 | 100 | 47 | 100 | - | |
| Patellar (absent) | 18 | 40.0 | 8 | 16.0 | 6 | 12.0 | < 0.05 | |
| Ankle (absent) | 18 | 40.0 | 8 | 16.0 | 4 | 8.0 | < 0.01 | |
| Clonus (absent) | 47 | 100 | 47 | 100 | 47 | 100 | - | |
| Radiological variables | Time interval in months | Mean | SD | t value | P value1 | Difference | 95% confidence interval of the difference | |||
| Mean | SD | SE mean | Lower | Upper | ||||||
| Sagittal index | 1 | 10.30 | 3.20 | |||||||
| 3 | 5.41 | 2.19 | 12.468 | 0.000 | 4.88 | 2.68 | 0.39 | 4.09 | 5.67 | |
| 6 | 4.70 | 1.56 | 12.140 | 0.000 | 5.60 | 3.16 | 0.46 | 4.67 | 6.52 | |
| Regional kyphosis | 1 | 17.40 | 2.60 | |||||||
| 3 | 16.68 | 2.12 | 2.174 | 0.035 | 0.72 | 2.28 | 0.33 | 0.05 | 1.39 | |
| 6 | 16.76 | 2.08 | 2.028 | 0.048 | 0.65 | 2.19 | 0.32 | 0.00 | 1.29 | |
| Gardener segmental kyphotic deformity | 1 | 16.00 | 0.42 | |||||||
| 3 | 15.47 | 1.63 | 2.477 | 0.017 | 0.53 | 1.47 | 0.21 | 0.10 | 0.96 | |
| 6 | 15.47 | 1.64 | 2.452 | 0.018 | 0.53 | 1.49 | 0.22 | 0.10 | 0.97 | |
| MRI parameters at 1 month | 1 month | 6 months | |||||
| Spearman’s rho | Sagittal index | Regional kyphosis | Gardener segmental kyphotic deformity | Sagittal index | Regional kyphosis | Gardener segmental kyphotic deformity | |
| Ventral tissue bridge | Correlation coefficient | 0.006 | 0.108 | 0.226 | -0.161 | 0.134 | 0.073 |
| P value | 0.969 | 0.470 | 0.126 | 0.280 | 0.369 | 0.628 | |
| Dorsal tissue bridge | Correlation coefficient | 0.024 | 0.047 | -0.181 | -0.055 | -0.182 | -0.094 |
| P value | 0.871 | 0.753 | 0.224 | 0.714 | 0.221 | 0.529 | |
| Total width of tissue bridges | Correlation coefficient | 0.037 | 0.082 | 0.152 | -0.112 | -0.019 | 0.045 |
| P value | 0.806 | 0.583 | 0.307 | 0.452 | 0.901 | 0.766 | |
| Midsagittal tissue bridge ratios | Correlation coefficient | 0.005 | -0.011 | -0.095 | 0.046 | -0.116 | -0.076 |
| P value | 0.973 | 0.940 | 0.525 | 0.760 | 0.436 | 0.612 | |
| MRI parameters | Mean | SD | t value | P value1 | Difference | 95% confidence interval of the difference | |||
| Mean | SD | SE mean | Lower | Upper | |||||
| Lesion length (mm) | |||||||||
| 1 month | 33.96 | 27.09 | |||||||
| 3 months | 18.43 | 14.68 | 4.808 | 0.001 | 15.53 | 22.14 | 3.23 | 9.03 | 22.03 |
| 6 months | 14.14 | 15.59 | 4.837 | 0.001 | 19.82 | 28.09 | 4.10 | 11.57 | 28.07 |
| Lesion width (mm) | |||||||||
| 1 month | 4.65 | 1.76 | |||||||
| 3 months | 3.94 | 1.65 | 4.900 | 0.001 | 0.71 | 0.99 | 0.14 | 0.42 | 1.00 |
| 6 months | 3.57 | 1.73 | 6.957 | 0.001 | 1.07 | 1.06 | 0.15 | 0.76 | 1.39 |
| Lesion area (mm2) | |||||||||
| 1 month | 110.66 | 83.41 | |||||||
| 3 months | 80.05 | 32.29 | 2.532 | 0.015 | 30.61 | 82.88 | 12.09 | 6.27 | 54.94 |
| 6 months | 73.35 | 37.22 | 2.700 | 0.010 | 37.31 | 94.76 | 13.82 | 9.49 | 65.14 |
| Clinical measure at 6 months; dependent variable | MR parameter at 1 month; independent variable | R | R square | P value1 | Standardized regression coefficients | 95% confidence interval | |
| Lower bound | Upper bound | ||||||
| MIS upper limb | MSCC | 0.454 | 0.206 | 0.001 | -1.442 | -2.291 | -0.592 |
| MCC | 0.135 | 0.018 | 0.365 | -0.232 | -0.744 | 0.279 | |
| Lesion length (mm) | 0.098 | 0.010 | 0.513 | 0.021 | -0.043 | 0.084 | |
| Lesion width (mm) | 0.030 | 0.001 | 0.840 | 0.099 | -0.879 | 1.076 | |
| Lesion area (mm2) | 0.217 | 0.047 | 0.142 | 0.015 | -0.005 | 0.035 | |
| Total width of tissue bridges | 0.301 | 0.090 | 0.040 | 3.755 | 0.179 | 7.332 | |
| Midsagittal tissue bridge ratios | 0.342 | 0.117 | 0.019 | 25.095 | 4.408 | 45.781 | |
| MIS lower limb | MSCC | 0.194 | 0.038 | 0.192 | -1.459 | -3.676 | 0.759 |
| MCC | 0.144 | 0.021 | 0.333 | -0.588 | -1.798 | 0.622 | |
| Lesion length (mm) | 0.242 | 0.059 | 0.101 | -0.122 | -0.268 | 0.025 | |
| Lesion width (mm) | 0.368 | 0.135 | 0.011 | -2.841 | -4.997 | -0.685 | |
| Lesion area (mm2) | 0.286 | 0.082 | 0.051 | -0.047 | -0.094 | 0.000 | |
| Total width of tissue bridges | 0.209 | 0.044 | 0.159 | 6.182 | -2.512 | 14.876 | |
| Midsagittal tissue bridge ratios | 0.146 | 0.021 | 0.328 | 25.355 | -26.281 | 76.991 | |
| Total (MIS) | MSCC | 0.338 | 0.114 | 0.020 | -2.900 | -5.326 | -0.475 |
| MCC | 0.177 | 0.031 | 0.235 | -0.820 | -2.193 | 0.552 | |
| Lesion length (mm) | 0.176 | 0.031 | 0.237 | -0.101 | -0.271 | 0.069 | |
| Lesion width (mm) | 0.311 | 0.097 | 0.033 | -2.743 | -5.255 | -0.231 | |
| Lesion area (mm2) | 0.171 | 0.029 | 0.251 | -0.032 | -0.087 | 0.023 | |
| Total width of tissue bridges | 0.294 | 0.087 | 0.045 | 9.937 | 0.250 | 19.625 | |
| Midsagittal tissue bridge ratios | 0.255 | 0.065 | 0.084 | 50.450 | -7.101 | 108.000 | |
| SIS light touch | MSCC | 0.359 | 0.129 | 0.013 | -3.543 | -6.312 | -0.775 |
| MCC | 0.402 | 0.162 | 0.005 | -2.149 | -3.618 | -0.679 | |
| Lesion length (mm) | 0.145 | 0.021 | 0.330 | -0.096 | -0.292 | 0.100 | |
| Lesion width (mm) | 0.341 | 0.116 | 0.019 | -3.458 | -6.318 | -0.598 | |
| Lesion area (mm2) | 0.337 | 0.114 | 0.021 | -0.072 | -0.133 | -0.012 | |
| Total width of tissue bridges | 0.077 | 0.006 | 0.608 | 2.985 | -8.646 | 14.616 | |
| Midsagittal tissue bridge ratios | 0.069 | 0.005 | 0.645 | 15.748 | -52.577 | 84.073 | |
| SIS pin prick | MSCC | 0.353 | 0.125 | 0.015 | -3.427 | -6.155 | -0.700 |
| MCC | 0.333 | 0.111 | 0.022 | -1.748 | -3.236 | -0.261 | |
| Lesion length (mm) | 0.086 | 0.007 | 0.565 | -0.056 | -0.250 | 0.138 | |
| Lesion width (mm) | 0.322 | 0.104 | 0.027 | -3.206 | -6.037 | -0.375 | |
| Lesion area (mm2) | 0.264 | 0.070 | 0.073 | -0.056 | -0.117 | 0.005 | |
| Total width of tissue bridges | 0.064 | 0.004 | 0.670 | 2.434 | -9.008 | 13.876 | |
| Midsagittal tissue bridge ratios | 0.084 | 0.007 | 0.576 | 18.770 | -48.307 | 85.846 | |
| Total SIS | MSCC | 0.359 | 0.129 | 0.013 | -6.971 | -12.413 | -1.528 |
| MCC | 0.371 | 0.137 | 0.010 | -3.897 | -6.828 | -0.966 | |
| Lesion length (mm) | 0.117 | 0.014 | 0.434 | -0.152 | -0.539 | 0.235 | |
| Lesion width (mm) | 0.334 | 0.112 | 0.022 | -6.664 | -12.301 | -1.027 | |
| Lesion area (mm2) | 0.303 | 0.092 | 0.038 | -0.128 | -0.248 | -0.007 | |
| Total width of tissue bridges | 0.071 | 0.005 | 0.636 | 5.419 | -17.459 | 28.297 | |
| Midsagittal tissue bridge ratios | 0.077 | 0.006 | 0.607 | 34.518 | -99.736 | 168.773 | |
Few case illustrations are given in Figures 1, 2, 3, 4 and 5.
We found the mean age of patients in present study was 40.23 ± 10.73 year. Pfyffer et al[2] had a mean of 51.20 ± 20.07 in 2019, but Singh et al[15] had a mean age of 37.32 ± 13.31 in 2020. Male were more affected in our study similar to previous study by Singh et al[15], but contrary to study by Pfyffer et al[2]. The study population predominantly consisted of individuals with low socioeconomic status and high illiteracy rates, reflective of the regional demographics in Northern India. These factors can influence health-seeking behavior, time to presentation, and access to care, which in turn may affect injury outcomes. The majority (> 90%) were married. Fall from height, accounting for nearly a third of the cases and road traffic accidents (RTAs) represented about 27.7% of cases. These findings were consistent with other studies that have also identified falls and RTA as leading causes of SCIs[16,17]. In developed countries, RTAs tend to be the primary cause, whereas fall from height remain prevalent in developing countries like those represented in this study[14].
We found SCI at various levels, with a notable concentration at levels D4-D5, L1, and D12-L1. These findings align with previous studies that also observed clustering of SCI patients around the D12 and L1 levels. Research by Chadha and Bahadur[18] noted D12-L1 as the most common level in 80% of cases, while studies by Liu et al[19] and Knop et al[20] reported L1 as predominant in 40% and 55% of cases, respectively[19,20]. Additionally, Singh et al[21] found L1 to be the most involved level in 36% of cases, followed by D12 in 24%. The increased incidence can be attributed to transition from fixed to mobile segments and from thoracic kyphosis to lumbar lordosis, making it a zone of higher biomechanical stresses during trauma. Importantly, injuries at levels D12 and L1, and above, often result in poorer neurological outcomes due to the anatomical endpoint of the spinal cord around the lower border of L1 or upper L2 level. The mean time elapsed between injury and imaging was 21.38 ± 6.28 hours. It ranged between 12-45 hours. In a study by Shimada et al[22], all patients were imaged within 48 hours of injury. Most patients were imaged rapidly after injury; as cord lesions evolve during the early phase, it should assist in efforts to correlate neurological findings with those on MRI.
The study assessed pain outcomes over a period from initial assessment up to six months among 47 the patients. Initially, all the patients reported severe pain. There was gradual but statistically significant (P < 0.001) improvement during first six months. Weakness in the lower limbs was universally reported initially and persisted in a significant (P = 0.008) proportion of cases up to the 3-month mark, after which a gradual decrease was observed. Weakness in the upper limbs, while less common, was present in a subset of cases with cervical spine injury at the beginning and showed a slight increase in prevalence at the 3-month follow-up (P < 0.001). Incontinence and retention exhibited similar patterns, being prevalent initially and gradually decreasing over time, with a notable decrease observed at the 6-month mark (P < 0.001). Tenderness and deformity were consistently observed in all cases across all time points, indicating their persistent presence throughout the observation period.
In present study we had incomplete SCI with preserved motor function below the injury site in majority of the cases. There were improvements noted across most ASIA categories (P = 0.548).
Time interval: Similar findings were noted by Singh et al[21], Narasinga Rao et al[23], and Butt et al[24], varying degrees of neurological recovery were observed among patients with traumatic SCIs. These findings underscore that the extent of initial spinal cord trauma correlates closely with subsequent neurological deficits and recovery outcomes. Patients with incomplete lesions generally showed more substantial neurological improvement compared to those with complete injuries.
Similarly, we had improvement in MIS upper limb scores (44.32 ± 6.29 to 45.68 ± 5.7 at 6 months) and lower limb (31.21 ± 14.57, 38.23 ± 13.63 at 6 months). The study by Steeves et al[25] investigated the pattern of MIS improvement over time in individuals with SCI. Improvements occurred in MIS within the first six-month post-injury. Most people recover similar motor points or levels regardless of initial cervical motor level. We also observed improvement in SIS for light touch (92.60 to 99.85 at 6 months) and pin prick (93.55 to 101.13 at 6 months). On the contrary, Vasquez et al[26] found a disparity (P < 0.001) between light touch (64.5 ± 3.2, mean ± SE) and pin prick (54.7 ± 2.9) AIS sensory scores.
We had KD correction as evidenced by improvement in radiological indices. The same has been observed in various studies[15,21,27-30]. However, there is a weak correlation between the measurements of SI, regional kyphosis, with the MRI biomarkers and midsagittal tissue bridges at 1 month and 6 months. The weak correlations between sagittal alignment (e.g., segmental kyphosis, spinal index) and tissue bridge dimensions suggest that spinal angulation alone may not strongly influence intramedullary tissue preservation. This may reflect the multifactorial nature of secondary SCI, in which vascular compromise, edema, and microstructural disruption play more prominent roles than gross alignment. Moreover, KD correction may not necessarily reverse internal cord damage already established at the time of injury. Previous studies have similarly shown limited direct association between alignment correction and neurological recovery (e.g., Fehlings et al[14], 1999). These findings underscore the need to evaluate both mechanical stability and neuroanatomical preservation as independent but complementary targets in spinal trauma management.
Unlike study by Hayashi et al[31] who assessed MSCC on MRI, the present study used an objective method to quantify MR images obtained from patients with SCI. We found improvement in MSCC (13.12 to 12.26) MSCC (P = 0.063) was predictive of a poor neurological outcome.
Chandra et al[18] found that cord compression severity affected prognosis and was an important outcome measure.
Compared to study conducted by Pfyffer et al[2] on 12 patients with SCI who underwent longitudinal follow-up scans showed a 5.68 mm2 decrease in lesion area per month in 2019. In 2017, Aarabi et al[32] reported ASI grade conversion had statistically significant relationship with admission ASI grade, lesion length and haemorrhage. Intramedullary lesion was the sole and strongest indicator of AIS grade conversion[32]. In present study lesion length, width, and area exhibited mixed correlations with MISs and SISs for light touch and pin prick. Notably, lesion width and area show strong negative correlations with all MISs and SIS scores at the 6-month interval (P < 0.05). Pfyffer et al[8] also reported significant negative correlation of one-month post-SCI lesion area and length with lower extremity motor score, light touch, and pinprick scores at one year of injury.
Over months, there were minor fluctuations in these widths, with some segments showing slight increases while others remained stable. Ventral tissue bridge at 1 month showed statistically significant positive correlations with MIS at 6-month time interval (r = 0.294, P = 0.004); while statistically non-significant positive correlations with SIS light touch (r = 0.77, P = 0.188), SIS pin prick (r = 0.264, P = 0.384), and Total SIS (r = 0.303, P = 0.184) were observed at 6-month time interval. Ventral tissue bridge at 6-month showed statistically significant positive correlations with MIS at 6-month time interval (r = 0.165, P = 0.006).
Like this study, Vallotton et al[9] found that ventral tissue bridge width predicted better lower extremity motor scores at 12 months (r = 0.41, P = 0.035) independent of baseline clinical score and dorsal tissue bridges. Midsagittal tissue bridge ratios were also associated with six-minute walk distance (r = 0.68, P = 0.03), according to O'Dell et al[33]. Contrary to the findings of the present study, Pfyffer et al[8] documented an increased width of ventral tissue bridges—a surrogate for spinothalamic tract functionality—at one month following SCI, which correlated with the onset and persistence of neuropathic pain and heightened pin-prick sensitivity.
Dorsal tissue bridges at I month did not show any statistically significant correlation with MIS at 6-month time interval (r = 0.294, P = 0.771); while SIS pin prick (r = 0.264, P = 0.039) and total SIS (r = 0.303, P = 0.05) showed statistically significant correlation at 6-month time interval. No such correlations of these parameters were observed with dorsal tissue bridges at 6-month. Vallotton et al[9] also reported greater width of dorsal tissue bridges predicted better light-touch score at 12 months (r = 0.40, P = 0.045) independently of baseline clinical score and ventral tissue bridges. These findings can be explained based on spaciotemporal orientation of sensory fibres (tactile, vibrational, and touch) in the dorsal column of the spinal cord.
Midsagittal tissue bridge ratios exhibited positive correlations with MISs and SIS scores at the 6-month interval, though not all correlations were statistically significant (P < 0.05). Pfyffer et al[2] also reported improved pinprick scores (P = 0.004, n = 21, r = 0.610) at 1-year post-injury was associated with wider midsagittal tissue bridges at 1-month post-SCI. Moreover, there was a trend toward improved light touch scores with wider midsagittal tissue bridges (P = 0.082, n = 21, r = 0.398).
The results of the present study revealed a significant positive correlation between midsagittal tissue bridge ratios and the MIS - upper limb (P = 0.019), suggesting that greater presence or extent of these tissue bridges aligns with improved motor function recovery over time. Similarly, Pfyffer et al[2] also reported improved LEMS (P = 0.022, n = 21, r = 0.508) and pinprick scores (P = 0.004, n = 21, r = 0.610) at 1year post-injury were linked with wider midsagittal tissue bridges at 1-month post-SCI.
Smith et al[34] conducted a study involving 136 participants with cervical SCI, establishing a significant correlation between wider ventral tissue bridges and pinprick scores (r = 0.31, P < 0.001). Moreover, broader dorsal tissue bridges exhibited substantial correlations with light touch scores at discharge from inpatient rehabilitation for the motor complete SCI group, while ventral tissue bridges were significantly associated with discharge pinprick scores (n = 56, r = 0.39, P = 0.003), and dorsal tissue bridges were significantly correlated with discharge light touch scores (n = 56, r = 0.39, P = 0.002). In the motor incomplete SCI group, ventral tissue bridges exhibited a significant correlation with discharge pinprick scores (n = 80, r = 0.26, P = 0.023), while dorsal tissue bridges demonstrated a significant correlation with discharge light touch scores (n = 80, r = 0.26, P = 0.022).
The findings of the present study suggest that MRI biomarkers, such as tissue bridges, can be used to predict the likely prognosis of recovery following SCI. This information can aid clinicians and physiotherapists in personalizing rehabilitation protocols for individual patients.
An important aspect of this study is the thorough evaluation of different MRI parameters in relation to clinical and neurological outcomes. This study incorporates objective measurements, statistical significance, thorough analysis, clinical relevance, predictive values, and recommendations for future research. By leveraging these strengths, such a study can provide valuable insights into the prognosis and management of SCIs. However, this study has several li
Additional factors such as the age of the patient, their overall health, and the presence of other medical conditions can also have an impact on the final clinical and neurological outcome.
The MRI imaging biomarkers in SCI patients demonstrated substantial improvement over time. There was a negative correlation between neurological recovery, MSCC, MCC, and lesion dimensions (lesion length, lesion width, and lesion area). Higher canal compromise and lesion dimensions were associated with a poorer outcome. The evaluation of the midsagittal tissue bridge (including the ventral tissue bridge, dorsal tissue bridge, total width of the tissue bridge, and tissue bridge ratios) at 1 and 6 months showed a positive correlation with the neurological recovery. The current study underscores the potential of MRI imaging biomarkers to predict neurological recovery in SCI, providing confidence for their use in clinical practice and research. Further research is necessary to confirm these findings and investigate their clinical implications for improving patient care and rehabilitation strategies in the management of SCIs.
| 1. | Scivoletto G, Laurenza L, Mammone A, Foti C, Molinari M. Recovery following ischemic myelopathies and traumatic spinal cord lesions. Spinal Cord. 2011;49:897-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Pfyffer D, Huber E, Sutter R, Curt A, Freund P. Tissue bridges predict recovery after traumatic and ischemic thoracic spinal cord injury. Neurology. 2019;93:e1550-e1560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Freund P, Weiskopf N, Ashburner J, Wolf K, Sutter R, Altmann DR, Friston K, Thompson A, Curt A. MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: a prospective longitudinal study. Lancet Neurol. 2013;12:873-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 228] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 4. | Filli L, Engmann AK, Zörner B, Weinmann O, Moraitis T, Gullo M, Kasper H, Schneider R, Schwab ME. Bridging the gap: a reticulo-propriospinal detour bypassing an incomplete spinal cord injury. J Neurosci. 2014;34:13399-13410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Kumar Y, Hayashi D. Role of magnetic resonance imaging in acute spinal trauma: a pictorial review. BMC Musculoskelet Disord. 2016;17:310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Huber E, Lachappelle P, Sutter R, Curt A, Freund P. Are midsagittal tissue bridges predictive of outcome after cervical spinal cord injury? Ann Neurol. 2017;81:740-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Cummins DP, Connor JR, Heller KA, Hubert JS, Kates MJ, Wisniewski KR, Berliner JC, O'Dell DR, Elliott JM, Weber KA 2nd, Smith AC. Establishing the inter-rater reliability of spinal cord damage manual measurement using magnetic resonance imaging. Spinal Cord Ser Cases. 2019;5:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Pfyffer D, Vallotton K, Curt A, Freund P. Tissue bridges predict neuropathic pain emergence after spinal cord injury. J Neurol Neurosurg Psychiatry. 2020;91:1111-1117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Vallotton K, Huber E, Sutter R, Curt A, Hupp M, Freund P. Width and neurophysiologic properties of tissue bridges predict recovery after cervical injury. Neurology. 2019;92:e2793-e2802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, Johansen M, Jones L, Krassioukov A, Mulcahey MJ, Schmidt-Read M, Waring W. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34:535-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1502] [Cited by in RCA: 1663] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 11. | McCormack T, Karaikovic E, Gaines RW. The load sharing classification of spine fractures. Spine (Phila Pa 1976). 1994;19:1741-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 534] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 12. | Vaccaro AR, Zeiller SC, Hulbert RJ, Anderson PA, Harris M, Hedlund R, Harrop J, Dvorak M, Wood K, Fehlings MG, Fisher C, Lehman RA Jr, Anderson DG, Bono CM, Kuklo T, Oner FC. The thoracolumbar injury severity score: a proposed treatment algorithm. J Spinal Disord Tech. 2005;18:209-215. [PubMed] |
| 13. | Farcy JP, Weidenbaum M, Glassman SD. Sagittal index in management of thoracolumbar burst fractures. Spine (Phila Pa 1976). 1990;15:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 168] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Fehlings MG, Rao SC, Tator CH, Skaf G, Arnold P, Benzel E, Dickman C, Cuddy B, Green B, Hitchon P, Northrup B, Sonntag V, Wagner F, Wilberger J. The optimal radiologic method for assessing spinal canal compromise and cord compression in patients with cervical spinal cord injury. Part II: Results of a multicenter study. Spine (Phila Pa 1976). 1999;24:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Singh R, Magu S, Baskar A, Rohilla RK, Kaur K, Kaur S. Correlation of Clinical Findings in Acute Spinal Injury Patients with Magnetic Resonance Including Diffusion Tensor Imaging and Fiber Tractography. Spine Surg Relat Res. 2020;4:305-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Birua GJS, Munda VS, Murmu NN. Epidemiology of Spinal Injury in North East India: A Retrospective Study. Asian J Neurosurg. 2018;13:1084-1086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Tator CH. Biology of neurological recovery and functional restoration after spinal cord injury. Neurosurgery. 1998;42:696-707; discussion 707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Chadha M, Bahadur R. Steffee variable screw placement system in the management of unstable thoracolumbar fractures: a Third World experience. Injury. 1998;29:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Liu S, Xie YY, Wang B. Role and prospects of regenerative biomaterials in the repair of spinal cord injury. Neural Regen Res. 2019;14:1352-1363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 20. | Knop C, Fabian HF, Bastian L, Rosenthal H, Lange U, Zdichavsky M, Blauth M. Fate of the transpedicular intervertebral bone graft after posterior stabilisation of thoracolumbar fractures. Eur Spine J. 2002;11:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Singh R, Rohilla RK, Kamboj K, Magu NK, Kaur K. Outcome of pedicle screw fixation and monosegmental fusion in patients with fresh thoracolumbar fractures. Asian Spine J. 2014;8:298-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B, Stram DO, Gerbing RB, Lukens JN, Matthay KK, Castleberry RP. The International Neuroblastoma Pathology Classification (the Shimada system). Cancer. 1999;86:364-372. [PubMed] |
| 23. | Narasinga Rao K, Vijaya Saradhi M, Purohit A. Factors affecting long-term outcome in acute cervical cord injury. Indian J Neurotrauma. 2010;7:149-155. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Butt MF, Farooq M, Mir B, Dhar AS, Hussain A, Mumtaz M. Management of unstable thoracolumbar spinal injuries by posterior short segment spinal fixation. Int Orthop. 2007;31:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Steeves JD, Kramer JK, Fawcett JW, Cragg J, Lammertse DP, Blight AR, Marino RJ, Ditunno JF Jr, Coleman WP, Geisler FH, Guest J, Jones L, Burns S, Schubert M, van Hedel HJ, Curt A; EMSCI Study Group. Extent of spontaneous motor recovery after traumatic cervical sensorimotor complete spinal cord injury. Spinal Cord. 2011;49:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 26. | Vasquez N, Gall A, Ellaway PH, Craggs MD. Light touch and pin prick disparity in the International Standard for Neurological Classification of Spinal Cord Injury (ISNCSCI). Spinal Cord. 2013;51:375-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Yue JJ, Sossan A, Selgrath C, Deutsch LS, Wilkens K, Testaiuti M, Gabriel JP. The treatment of unstable thoracic spine fractures with transpedicular screw instrumentation: a 3-year consecutive series. Spine (Phila Pa 1976). 2002;27:2782-2787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Shin TS, Kim HW, Park KS, Kim JM, Jung CK. Short-segment Pedicle Instrumentation of Thoracolumbar Burst-compression Fractures; Short Term Follow-up Results. J Korean Neurosurg Soc. 2007;42:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Singh R, Kumar RR, Setia N, Magu S. A prospective study of neurological outcome in relation to findings of imaging modalities in acute spinal cord injury. Asian J Neurosurg. 2015;10:181-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Defino HL, Canto FR. Low thoracic and lumbar burst fractures: radiographic and functional outcomes. Eur Spine J. 2007;16:1934-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Hayashi K, Yone K, Ito H, Yanase M, Sakou T. MRI findings in patients with a cervical spinal cord injury who do not show radiographic evidence of a fracture or dislocation. Paraplegia. 1995;33:212-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Aarabi B, Sansur CA, Ibrahimi DM, Simard JM, Hersh DS, Le E, Diaz C, Massetti J, Akhtar-Danesh N. Intramedullary Lesion Length on Postoperative Magnetic Resonance Imaging is a Strong Predictor of ASIA Impairment Scale Grade Conversion Following Decompressive Surgery in Cervical Spinal Cord Injury. Neurosurgery. 2017;80:610-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 33. | O'Dell DR, Weber KA, Berliner JC, Elliott JM, Connor JR, Cummins DP, Heller KA, Hubert JS, Kates MJ, Mendoza KR, Smith AC. Midsagittal tissue bridges are associated with walking ability in incomplete spinal cord injury: A magnetic resonance imaging case series. J Spinal Cord Med. 2020;43:268-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Smith AC, O'Dell DR, Thornton WA, Dungan D, Robinson E, Thaker A, Gisbert R, Weber KA 2nd, Berliner JC, Albin SR. Spinal Cord Tissue Bridges Validation Study: Predictive Relationships With Sensory Scores Following Cervical Spinal Cord Injury. Top Spinal Cord Inj Rehabil. 2022;28:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/