Published online Sep 14, 2018. doi: 10.5306/wjco.v9.i5.110
Peer-review started: March 30, 2018
First decision: May 2, 2018
Revised: June 11, 2018
Accepted: June 28, 2018
Article in press: June 28, 2018
Published online: September 14, 2018
Processing time: 168 Days and 2.8 Hours
To evaluate the efficacy and safety of the modified FOLFIRI3-aflibercept as second-line therapy in patients with metastatic colorectal cancer.
This is a retrospective multicenter cohort, evaluating the efficacy and safety of the association of aflibercept with FOLFIRI3 (day 1: aflibercept 4 mg/kg, folinic acid 400 mg/m2, irinotecan 90 mg/m2, 5-fluorouracil infusion 2400 mg/m2 per 46 h; day 3: irinotecan 90 mg/m2) in patients with previously treated metastatic colorectal cancer. The primary endpoint was overall response rate (ORR). Secondary endpoints were disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and safety.
Among 74 patients treated in four French centers, nine were excluded due to prior use of aflibercept (n = 3), more than one prior treatment line in irinotecan-naïve patients (n = 3), and inadequate liver function (n = 3). In the “irinotecan-naïve” patients (n = 30), ORR was 43.3% and DCR was 76.7%. Median PFS and OS were 11.3 mo (95%CI: 6.1-29.0) and 17.0 mo (95%CI: 13.0-17.3), respectively. The most common (> 5%) grade 3-4 adverse events were diarrhea (37.9%), neutropenia (14.3%), stomatitis and anemia (10.4%), and hypertension (6.7%). In the “pre-exposed irinotecan” patients (n = 35), 20 (57.1%) received ≥ 2 prior lines of treatment. ORR was 34.3% and DCR was 60.0%. Median PFS and OS were 5.7 mo (95%CI: 3.9-10.4) and 14.3 mo (95%CI: 12.8-19.5), respectively.
Minimally modified FOLFIRI has improvement dramatically the FOLFIRI3-aflibercept efficacy, whatever prior use of irinotecan. A prospective randomized trial is warranted to compare FOLFIRI-aflibercept to FOLFIRI3-aflibercept.
Core tip: Results obtained in this retrospective study show that minimally modified FOLFIRI has improvement dramatically the efficacy of the FOLFIRI3-aflibercept combination with high response rates and survivals in patients with previously treated metastatic colorectal cancer, whatever prior use of irinotecan. A prospective randomized trial is planned to compare FOLFIRI-aflibercept to FOLFIRI3-aflibercept.
- Citation: Carola C, Ghiringhelli F, Kim S, André T, Barlet J, Bengrine-Lefevre L, Marijon H, Garcia-Larnicol ML, Borg C, Dainese L, Steuer N, Richa H, Benetkiewicz M, Larsen AK, Gramont A, Chibaudel B. FOLFIRI3-aflibercept in previously treated patients with metastatic colorectal cancer. World J Clin Oncol 2018; 9(5): 110-118
- URL: https://www.wjgnet.com/2218-4333/full/v9/i5/110.htm
- DOI: https://dx.doi.org/10.5306/wjco.v9.i5.110
Standard second-line therapy in patients with previously treated metastatic colorectal cancer (mCRC) is doublet fluoropyrimidine-based chemotherapy with either irinotecan (FOLFIRI) or oxaliplatin (FOLFOX), depending on the regimen used in first-line, in association with antiangiogenic agents (e.g., bevacizumab, aflibercept, ramucirumab) or anti-EGFR agents in absence of RAS tumor gene mutation (e.g., cetuximab, panitumumab)[1-8].
The standard FOLFIRI regimen was optimized by splitting the dose of irinotecan on day 1 [half dose before 5-fluorouracil (5-FU)] and day 3 (half dose after 5-FU) in the so-called FOLFIRI3 regimen[9]. Drugs and doses are similar to FOLFIRI, except for suppression of the 5-FU bolus. The response rate was higher than that reported for FOLFIRI[9,10]. Based on these results, FOLFIRI3 became the second-line regimen of choice in some centers. Adding bevacizumab to FOLFIRI3 has shown promising efficacy results in two prior retrospective trials [response rate 22% and 35%, median progression-free survival (PFS) 7.0 and 6.2 mo, median overall survival (OS) 13.0 and 10.8 mo, respectively][11,12]. The addition of aflibercept to FOLFIRI in patients with pretreated mCRC increased response rate from 11% to 20% and improved median PFS from 4.7 to 6.9 mo [hazard ratio (HR) = 0.76] and median OS from 12.1 to 13.5 mo (HR = 0.82)[4]. Aflibercept was approved by the Food and Drug Administration on August 3, 2012 and by the European Medicines Agency on February 1, 2013 in combination with FOLFIRI for the treatment of patients with mCRC resistant to an oxaliplatin-containing regimen[13]. Based on the VELOUR study results and non-randomized FOLFIRI3 studies, we retrospectively analyzed the safety and efficacy of the FOLFIRI3-aflibercept combination as second or later-line therapy in patients with mCRC.
This study was a retrospective, multicenter cohort, conducted in four French institutions (Centre Georges François Leclerc, Franco-British Hospital, University Hospital Besançon, and Saint-Antoine University Hospital) from September 2014 to December 2016.The main objective of this study was to evaluate the efficacy and safety profile of the aflibercept-FOLFIRI3 combination.
All patients with previously treated mCRC and with FOLFIRI3-aflibercept administered from September 2014 to December 2016 were included. During the inclusion period, the decision to give FOLFIRI3-aflibercept to each patient or another treatment regimen was at physician’s discretion. Prior use of bevacizumab was allowed, but prior exposure to aflibercept was not permitted. Patients were divided into two subgroups depending on the prior use of irinotecan and the number of previous treatment lines for metastatic disease: (1) “irinotecan-naive" population including patients with no more than one prior line of treatment for metastatic disease; and (2) the “irinotecan pre-exposed” population including patients for whom the number of prior treatment lines for metastatic disease was not restricted.
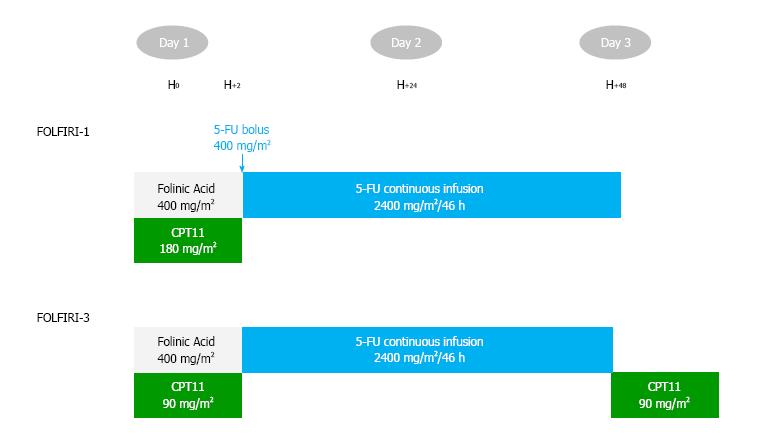
Treatment cycles were given intravenously every 14 d, as follows: Aflibercept 4 mg/kg over 1-h infusion (day 1), folinic acid 400 mg/m² over 2-h infusion (day 1), irinotecan 90 mg/m² over 60-90 min infusion (day 1), followed by continuous 5-FU 2400 mg/m² as a 46-h infusion (days 1 to 3), then irinotecan 90 mg/m² over 60-90 min infusion (day 3; Figure 1). Treatment information (date of treatment, doses) was collected using CHIMIO® 5.4 (Computer Engineering, Paris, France) or BPC (GCS Emosist, Région Franche-Comté, France) softwares.
Treatment efficacy was evaluated with tumor response, PFS, and OS. The objective response rate (ORR) was defined as the proportion of patients having either complete response (CR) or partial response (PR) according to RECIST version 1.1. The best ORR was defined as the best response recorded from the start of treatment until progressive disease (PD). Disease control rate (DCR) was the sum of ORR and stable disease (SD). PFS was defined as the time from the date of starting treatment to the date of progression or death (from any cause). OS was defined as the time from the date of starting treatment to the date of patient death (from any cause) or to the last date the patient was known to be alive. Toxicity was evaluated according to the United States National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03.
Follow-up and survival were estimated using the reverse Kaplan-Meier method and the Kaplan-Meier method, respectively, and were described using median with 95% confidence interval (CI). Qualitative variables were described using percent and means and continuous variables using medians (minimum-maximum). The cut-off date for statistical analysis was June 15, 2017. The final analysis was performed on the irinotecan-naïve and irinotecan pre-exposed populations.
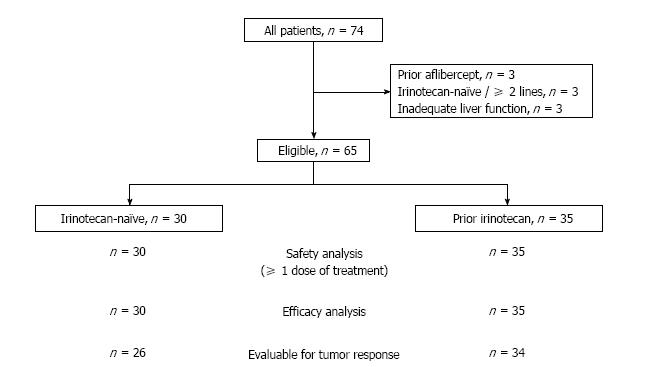
A total of 74 patients were treated (Figure 2). Nine patients were excluded from the analysis due to: Prior use of aflibercept (n = 3), more than one prior line of treatment in irinotecan-naïve patients (n = 3), or inadequate liver function (pretreatment alkaline phosphatase level > 7 × upper normal limit, n = 3). Thirty patients did not receive prior irinotecan (the irinotecan-naïve population) and 35 were previously exposed to irinotecan (the pre-exposed population).
Overall, 25 (38.5%) patients had an Eastern Cooperative Oncology Group performance status 0. The mean age was 63.1 years (range: 31.9-82.1); 23 (35.4%) had a single metastatic site, 49 (75.4%) had RAS mutated tumors, and two (3.1%) had BRAF mutated tumors. Prior use of bevacizumab and anti-EGFR were reported in 47 (72.3%) and 6 (9.2%) patients, respectively.
In the irinotecan-naïve population, five patients did not receive first-line therapy for metastatic disease (n = 4, early relapse after FOLFOX adjuvant therapy or n = 1, radiochemotherapy). In irinotecan pre-exposed population, 20 (57.1%) patients received more than two prior lines of treatment. Various irinotecan regimens (FOLFIRINOX, n = 21; FOLFIRI, n = 10; FOLFIRI3, n = 4) were previously given. The portion of patients with increased level of lactate dehydrogenase in the irinotecan pre-exposed population was higher than in that with the irinotecan-naïve patients (56.0% vs 17.6%; P = 0.027; Table 1).
| Characteristics | Irinotecan-naive cohort | Irinotecan-pre-exposed cohort | P-value |
| Age (yr) | 0.793 | ||
| < 70 | 21 (70.0) | 23 (65.7) | |
| ≥ 70 | 9 (30.0) | 12 (34.3) | |
| Sex | 0.623 | ||
| Male | 16 (53.3) | 21 (60.0) | |
| Female | 14 (46.7) | 14 (40.0) | |
| ECOG PS | 0.227 | ||
| 0 | 14 (46.7) | 11 (31.4) | |
| 1 | 13 (43.3) | 15 (42.9) | |
| 2 | 3 (10.0) | 9 (25.7) | |
| Time to metastasis | 0.314 | ||
| Metachronous | 9 (30.0) | 15 (42.9) | |
| Synchronous | 21 (70.0) | 20 (57.1) | |
| No. of metastatic sites | 0.118 | ||
| 1 | 14 (46.7) | 9 (25.7) | |
| > 1 | 16 (53.3) | 26 (74.3) | |
| No. of prior lines for metastatic disease | |||
| - | |||
| 0 | 5 (16.7) | 0 (0.0) | |
| 1 | 25 (83.3) | 15 (42.9) | |
| > 1 | - | 20 (57.1) | |
| Prior drug exposure | 0.699 | ||
| Oxaliplatin | 29 (96.7) | 35 (100.0) | |
| Bevacizumab | 18 (60.0) | 29 (82.9) | |
| Anti-EGFR | 2 (6.7) | 4 (11.4) |
In the irinotecan-naïve population, chemotherapy drugs (irinotecan and 5-FU) were given at standard dose in 12 (40.0%) patients. A lower dose of irinotecan and 5-FU were given in 15 (50.0%) and 4 (13.3%) patients, respectively. The median number of cycles was 8 (range: 1-19), and the median treatment duration was 3.7 mo (95%CI: 2.4-5.7). Dose reductions during treatment were performed in 7 (23.3%), 13 (43.3%), and 6 (20.0%) patients for aflibercept, irinotecan, and 5-FU, respectively. Granulocyte colony-stimulating factor (G-CSF) was given as primary prophylaxis in 14 (46.7%) patients and as secondary prevention in 3 (10.0%) patients. Erythropoietin was used in 5 (17.9%) patients. At the time of analysis, the treatment was still ongoing in 2 patients. The main reasons for stopping therapy were the occurrence of a limiting adverse event in 14 (46.7%) patients (diarrhea, n = 8; bleeding, n = 1; bowel perforation, n = 1; asthenia, n = 1; other, n = 3) or progression in 11 (36.7%) patients.
In the irinotecan pre-exposed population, chemotherapy drugs (irinotecan and 5-FU) were given at standard dose in 22 (62.9%) patients. A lower dose of irinotecan and 5-FU were given in 10 (28.6%), and 6 (17.1%) patients, respectively. The median number of cycles was 6 (range: 1-20), and the median treatment duration was 3.5 mo (95%CI: 2.1-5.6). Dose reductions during treatment were performed in 5 (14.3%), 9 (25.7%), and 7 (20.0%) patients for aflibercept, irinotecan, and 5-FU, respectively. G-CSF was given as primary prophylaxis in 13 (37.1%) patients and as secondary prevention in 2 (5.7%) patients. Erythropoietin was used in 3 (8.6%) patients. At the time of analysis, the treatment was still ongoing in 5 patients. The main reasons for stopping therapy were disease progression in 22 (62.9%) patients and the occurrence of limiting adverse events in 3 (8.6%) patients (diarrhea, n = 2; skin reactions, n = 1).
In the irinotecan-naïve population, 4 (13.3%) patients were not evaluated for tumor response due to an early stop for limiting toxicity. ORR was reported in 13 patients [43.3%, intention-to-treat (ITT); 50.0%, evaluable patients] without CR, and DCR was reported in 23 patients (76.7%, ITT; 88.5%, evaluable patients). Three (10.0%) patients had PD at the first tumor evaluation (Table 2).
| Irinotecan-naïve, n = 30 | Prior irinotecan, n = 35 | |||||
| Response rate | ||||||
| n | % | % | n | % | % | |
| (ITT) | (evaluable) | (ITT) | (evaluable) | |||
| CR | 0 | 0 | 0 | 1 | 2.8 | 2.9 |
| PR | 13 | 43.3 | 50 | 11 | 31.4 | 32.4 |
| SD | 10 | 33.3 | 38.5 | 9 | 25.7 | 26.5 |
| PD | 3 | 10 | 11.5 | 13 | 37.1 | 38.2 |
| NE | 4 | 13.3 | - | 1 | 2.8 | - |
| ORR | 13 | 43.3 | 50 | 12 | 34.3 | 35.3 |
| DCR | 23 | 76.7 | 88.5 | 21 | 60 | 61.8 |
| Survivals | ||||||
| median, mo | 95%CI | median, mo | 95%CI | |||
| PFS | 11.3 | 6.1-29.0 | 5.7 | 3.9-10.4 | ||
| OS | 17.0 | 13.0-17.3 | 14.3 | 12.8-19.5 | ||
In the irinotecan pre-exposed population, one patient was not evaluable for tumor response (switch to intra-arterial chemotherapy after 2 treatment cycles). ORR was reported in 12 patients (34.3%, ITT; 35.3%, evaluable patients) including one CR, and DCR was reported in 21 patients (60.0%, ITT; 61.8%, evaluable population). Thirteen (37.1%) patients had PD at the first tumor evaluation (Table 2). Among seven patients refractory to irinotecan, 1 (2.9%) had PR with FOLFIRI3-aflibercept, 2 (5.7%) had stable disease, 3 (8.6%) had PD, and 1 (2.6%) was not evaluable (Table 3).
| FOLFIRI3-aflibercept | ||||||
| CR/PR | SD | PD | NE | All | ||
| Prior irinotecan-based regimen | CR/PR | 5 | 3 | 7 | 0 | 15 (42.8) |
| SD | 4 | 4 | 2 | 0 | 10 (28.6) | |
| PD | 1 | 2 | 3 | 1 | 7 (20.0) | |
| NE | 2 | 0 | 1 | 0 | 3 (8.6) | |
| All | 12 (34.3) | 9 (25.7) | 13 (37.1) | 1 (2.8) | 35 | |
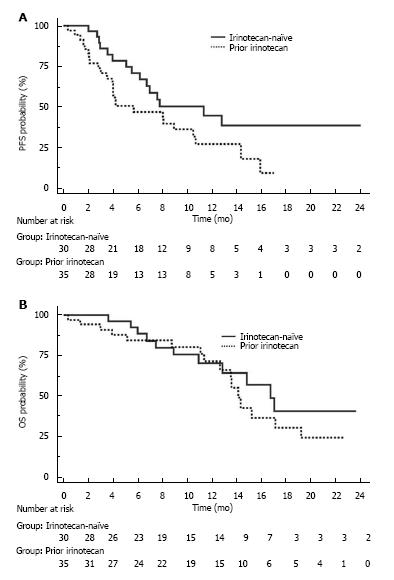
The median follow-up was 13.6 mo (95%CI: 9.6-17.7) in the irinotecan-naïve population and 14.2 mo (95%CI: 11.0-21.5) in the pre-exposed population (P = 0.692). In the irinotecan-naïve population, median PFS was 11.3 mo (95%CI: 6.1-29.0) and median OS was 17.0 mo (95%CI: 13.0-17.3; Figure 3A). A lower starting dose of irinotecan (< 90 mg/m²) did not impact PFS (P = 0.518) and OS (P = 0.311), but decreased the incidence of severe neutropenia (0.0% vs 30.8%, respectively, P = 0.041). In the irinotecan pre-exposed population, median PFS was 5.7 mo (95%CI: 3.9-10.4) and median OS was 14.3 mo (95%CI: 12.8 -19.5; Figure 3B).
In the irinotecan-naïve cohort, 17 (56.7%) patients experienced grade ≥ 3 toxicity (Table 4). The most common (≥ 5%) grade 3-4 adverse events were diarrhea (n = 11, 36.7%), neutropenia (n = 4, 13.3%), anemia and mucositis (n = 3, 10.0%), and nausea and hypertension (n = 2, 6.7%). Any grade hemorrhage was reported in 4 (13.8%) patients, gastrointestinal perforation in 1 (3.3%) patient, and arterial thromboembolic event in 1 (3.3%) patient.
| SOC | PT | Irinotecan-naïve | Prior irinotecan | All |
| Any | 17 (56.7) | 15 (42.9) | 32 (49.2) | |
| Blood | Neutropenia | 4 (13.3) | 1 (2.9) | 5 (7.7) |
| Anemia | 3 (10.0) | 0 (0.0) | 3 (4.6) | |
| Thrombocytopenia | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Gastrointestinal | Nausea | 2 (6.7) | 0 (0.0) | 2 (3.1) |
| Vomiting | 1 (3.3) | 0 (0.0) | 1 (1.5) | |
| Mucositis | 3 (10.0) | 3 (8.6) | 6 (9.2) | |
| Diarrhea | 11 (36.7) | 9 (25.7) | 20 (30.8) | |
| Vascular | Hypertension | 2 (6.7) | 4 (11.4) | 6 (9.2) |
In the irinotecan pre-exposed cohort, 15 (42.9%) patients experienced grade ≥ 3 toxicity (Table 4). The most common (≥ 5%) grade 3-4 adverse events were diarrhea (n = 9, 25.7%), hypertension (n = 4, 11.4%), and mucositis (n = 3, 8.6%).
Salvage surgery for metastatic disease was performed in 7 (10.0%) patients (n = 4, liver; n = 1, lung; n = 1, liver and lung; n = 1, peritoneum). A complete (R0) resection and liver pathological complete response were observed in all and one patient, respectively.
To our knowledge, this is the first report evaluating the FOLFIRI3-aflibercept combination in patients with previously treated mCRC. The response rate, which is a strong indicator of treatment efficacy, was unusually high not only in irinotecan-naïve patients (43%, ITT; 50%, evaluable), but also in irinotecan pre-exposed patients (34%, ITT, 35%, evaluable).
The median 11.3 mo PFS and median 17.0 mo OS in the irinotecan-naïve population receiving FOLFIRI3-aflibercept as second-line therapy compared favorably to the FOLFIRI-aflibercept combination in the pivotal phase III VELOUR study (response rate 19.8%, median 7.2 mo PFS, median 13.2 mo OS) and the FOLFIRI3 regimen without targeted agent (response rate 17%-23%, median 4-7 mo PFS, median 9-12 mo OS)[4,9,10,14].
In the irinotecan pre-exposed population, patients received FOLFIRI3-aflibercept as salvage therapy. Yet, median PFS and OS were 5.7 mo and 14.3 mo, respectively, and were comparable to figures observed in second-line trials[8,15].
A high portion (27%) of patients had to stop the FOLFIRI3-aflibercept combination because of limiting toxicity, mainly diarrhea. Its frequency (38%) was twice as common as in the VELOUR study (19%), but in the same range as in previous studies using FOLFIRI3. It has been demonstrated that severe diarrhea induced by aflibercept is due to microscopic colitis, which can be managed successfully using oral budesonide and/or mesalamine treatment[16,17]. Placental growth factor (PlGF) could play a role in the occurrence of diarrhea. The absence of PlGF blocks dextransodium sulfate-induced colonic mucosal angiogenesis and increases mucosal hypoxia[18,19]. Knockout of PlGF aggravates disease course in acute colitis[20]. Neutropenia and stomatitis were at a lower incidence than in the FOLFIRI-aflibercept arm of the pivotal VELOUR study (13.3% vs 36.7%, neutropenia; 10.0% vs 13.8%, stomatitis), which can be explained by deletion of the 5-FU bolus in the FOLFIRI3 regimen and use of G-CSF in 49% of patients. In the irinotecan-naïve population, a lower starting dose of irinotecan (< 90 mg/m²) did not impact treatment efficacy, but decreased the incidence of severe neutropenia (0.0% vs 30.8%, P = 0.041).
The conversion to surgery of metastasis in second-line is another key finding that could modify the strategy in patients suitable for salvage surgery in case of response (sequential doublets versus triplets).The main limitation of this study is the retrospective design with a low number of patients. In conclusion, the combination of aflibercept and FOLFIRI3 in our study shows the encouraging efficacy results with high response rates and longer survivals in patients with previously treated mCRC, whatever the prior exposition to irinotecan. A randomized trial is warranted to compare FOLFIRI-aflibercept to FOLFIRI3-aflibercept.
FOLFIRI3 is the second-line regimen of choice in patients with previously treated mCRC in some centers. Adding bevacizumab to FOLFIRI3 has shown promising efficacy results in two prior retrospective trials. The addition of aflibercept to FOLFIRI in patients with pretreated mCRC increased response rate from 11% to 20% and improved median PFS from 4.7 to 6.9 mo.
The phase III VELOUR and non-randomized FOLFIRI3 studies results provide a backbone for our study.
The main objective of the study is to evaluate the safety and efficacy of the FOLFIRI3-aflibercept combination as second or later-line therapy in patients with mCRC.
Patients with previously treated mCRC were given the aflibercept-FOLFIRI3 combination and were divided into “irinotecan-naive” population including patients with no more than one prior line of treatment for metastatic disease, and the “irinotecan pre-exposed” population including patients for whom the number of prior treatment lines for metastatic disease was not restricted. The primary endpoint was overall response rate (ORR). Secondary endpoints were disease control rate, progression-free survival (PFS), overall survival (OS), and safety. Toxicity was evaluated according to the United States National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03.
Minimally modified FOLFIRI has improvement dramatically the efficacy of the FOLFIRI3-aflibercept combination with high response rates (43% in irinotecan-naïve patients and 34% in irinotecan pre-exposed patients) and survivals (median PFS: 11.3 mo, OS: 17.0 mo and PFS: 5.7 mo and OS: 14.3 mo, respectively) in patients with previously treated mCRC, whatever prior use of irinotecan.
The combination of aflibercept and FOLFIRI3 shows encouraging efficacy results in patients with previously treated mCRC.
A prospective randomized trial is planned to compare FOLFIRI-aflibercept to FOLFIRI3-aflibercept.
| 1. | Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2282] [Cited by in RCA: 2215] [Article Influence: 100.7] [Reference Citation Analysis (1)] |
| 2. | Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB 3rd; Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1738] [Article Influence: 91.5] [Reference Citation Analysis (2)] |
| 3. | Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 919] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 4. | Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 1055] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 5. | Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy DC, Van Cutsem E, Grothey A. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 689] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 6. | Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F, Punt CJ. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706-4713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 763] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 7. | Mishima H, Oba K, Sakamoto J, Muro K, Yoshino T, Hyodo I, Maehara Y. FOLFIRI plus bevacizumab 5 mg/kg versus 10 mg/kg as second-line therapy in patients with metastatic colorectal cancer who have failed first-line bevacizumab plus oxaliplatin-based therapy: a randomized phase III study (EAGLE Study). Jpn J Clin Oncol. 2012;42:134-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Mabro M, Louvet C, André T, Carola E, Gilles-Amar V, Artru P, Krulik M, de Gramont A; GERCOR. Bimonthly leucovorin, infusion 5-fluorouracil, hydroxyurea, and irinotecan (FOLFIRI-2) for pretreated metastatic colorectal cancer. Am J Clin Oncol. 2003;26:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Mabro M, Artru P, André T, Flesch M, Maindrault-Goebel F, Landi B, Lledo G, Plantade A, Louvet C, de Gramont A. A phase II study of FOLFIRI-3 (double infusion of irinotecan combined with LV5FU) after FOLFOX in advanced colorectal cancer patients. Br J Cancer. 2006;94:1287-1292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Chibaudel B, Maindrault-Gœbel F, Bachet JB, Louvet C, Khalil A, Dupuis O, Hammel P, Garcia ML, Bennamoun M, Brusquant D. PEPCOL: a GERCOR randomized phase II study of nanoliposomal irinotecan PEP02 (MM-398) or irinotecan with leucovorin/5-fluorouracil as second-line therapy in metastatic colorectal cancer. Cancer Med. 2016;5:676-683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Ghiringhelli F, Vincent J, Guiu B, Chauffert B, Ladoire S. Bevacizumab plus FOLFIRI-3 in chemotherapy-refractory patients with metastatic colorectal cancer in the era of biotherapies. Invest New Drugs. 2012;30:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Bourges O, Chibaudel B, Bengrine-Lefevre L, Afchain P, Tournigand C, Perez-Staub N, Maindrault-Goebel F, Larsen AK, Louvet C, de Gramont A. FOLFIRI-3 + bevacizumab after the first-line in metastatic colorectal cancer. Colon and Rectum. American Society of Clinical Oncology Gastrointestinal Cancers (ASCO-GI) Symposium. 2009;Abstract 417. |
| 13. | European Medicines Agency (EMA). Zaltrap® (Aflibercept). Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/002532/WC500139486.pdf. |
| 14. | Bidard FC, Tournigand C, André T, Mabro M, Figer A, Cervantes A, Lledo G, Bengrine-Lefevre L, Maindrault-Goebel F, Louvet C. Efficacy of FOLFIRI-3 (irinotecan D1,D3 combined with LV5-FU) or other irinotecan-based regimens in oxaliplatin-pretreated metastatic colorectal cancer in the GERCOR OPTIMOX1 study. Ann Oncol. 2009;20:1042-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Chong DQ, Manalo M, Imperial M, Teo P, Yong G, Ng M, Tan IB, Choo SP, Chua C. Safety and efficacy of aflibercept in combination with fluorouracil, leucovorin and irinotecan in the treatment of Asian patients with metastatic colorectal cancer. Asia Pac J Clin Oncol. 2016;12:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Ghiringhelli F, Vincent J, Beltjens F, Bengrine L, Ladoire S. Fluorouracil, leucovorin and irinotecan associated with aflibercept can induce microscopic colitis in metastatic colorectal cancer patients. Invest New Drugs. 2015;33:1263-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Miehlke S, Madisch A, Kupcinskas L, Petrauskas D, Böhm G, Marks HJ, Neumeyer M, Nathan T, Fernández-Bañares F, Greinwald R. Budesonide is more effective than mesalamine or placebo in short-term treatment of collagenous colitis. Gastroenterology. 2014;146:1222-1230.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Zhou Y, Tu C, Zhao Y, Liu H, Zhang S. Placental growth factor enhances angiogenesis in human intestinal microvascular endothelial cells via PI3K/Akt pathway: Potential implications of inflammation bowel disease. Biochem Biophys Res Commun. 2016;470:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Kim KJ, Cho CS, Kim WU. Role of placenta growth factor in cancer and inflammation. Exp Mol Med. 2012;44:10-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Hindryckx P, Waeytens A, Laukens D, Peeters H, Van Huysse J, Ferdinande L, Carmeliet P, De Vos M. Absence of placental growth factor blocks dextran sodium sulfate-induced colonic mucosal angiogenesis, increases mucosal hypoxia and aggravates acute colonic injury. Lab Invest. 2010;90:566-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Tsushima T, Velenik V, Zhang J S- Editor: Ji FF L- Editor: A E- Editor: Tan WW