Published online Jun 10, 2017. doi: 10.5306/wjco.v8.i3.285
Peer-review started: March 13, 2017
First decision: March 27, 2017
Revised: May 2, 2017
Accepted: May 12, 2017
Article in press: May 15, 2017
Published online: June 10, 2017
Processing time: 84 Days and 19.7 Hours
We are reporting a case of fatal radiation pneumonitis that developed six months following chemoradiation for limited stage small cell lung cancer. The patient was a 67-year-old man with a past medical history of Hashimoto’s thyroiditis and remote suspicion for CREST, neither of which were active in the years leading up to treatment. He received 6600 cGy delivered in 200 cGy daily fractions via intensity modulated radiation therapy with concurrent cisplatin/etoposide followed by additional chemotherapy with dose-reduced cisplatin/etoposide and carboplatin/etoposide and then received prophylactic cranial irradiation. The subsequent months were notable for progressively worsening episodes of respiratory compromise despite administration of prolonged steroids and he ultimately expired. Imaging demonstrated bilateral interstitial and airspace opacities. Autopsy findings were consistent with pneumonitis secondary to chemoradiation as well as lymphangitic spread of small cell carcinoma. The process was diffuse bilaterally although his radiation was delivered focally to the right lung and mediastinum.
Core tip: Radiation pneumonitis is an uncommon but serious complication from radiation therapy which can on rare occasions be fatal. This report not only documents the details of such a case but also includes pathologic confirmation and computed tomography images. Although the radiation field was limited to the right lung and mediastinum, the process was also noted to be bilateral and diffuse.
- Citation: Osborn VW, Leaf A, Lee A, Garay E, Safdieh J, Schwartz D, Schreiber D. Bilateral diffuse grade 5 radiation pneumonitis after intensity modulated radiation therapy for localized lung cancer. World J Clin Oncol 2017; 8(3): 285-288
- URL: https://www.wjgnet.com/2218-4333/full/v8/i3/285.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i3.285
Pneumonitis is an inflammatory lung reaction marked by dyspnea, cough, and occasional fever. It can occur following radiation therapy as a result of cytokine production[1,2], and patients are at increased risk of developing pneumonitis if they have a history of chronic lung disease or smoking[3], or if they received concurrent chemotherapy[3,4]. Rarely, it can be fatal. In the following case report we examine a patient who developed fatal pneumonitis six months after receiving concurrent chemoradiation for small cell lung cancer (SCLC).
A 67-year-old man with a 40 pack-year smoking history initially presented with chills and a productive cough and was given antibiotics for presumed pneumonia. When his condition did not improve, a computed tomography (CT) of the chest was performed and revealed a large right hilar mass with extensive mediastinal adenopathy as well as surrounding infiltrate and atelectasis. Bronchial brushings and a right hilar node FNA were consistent with SCLC. The remainder of the workup, including brain magnetic resonance imaging (MRI), bone scan and positron emission tomography (PET)-CT, was negative for distant metastatic disease, establishing a diagnosis of limited stage (LS) disease. His medical history was significant for numerous coexisting medical conditions including a remote history of suspected but unconfirmed connective tissue disorder (CREST), colitis, esophagitis, duodenitis, livedo reticularis, Hashimoto’s thyroiditis, multinodular goiter, arthritis, glaucoma, hypertension, multifocal motor neuropathy and atrioventricular (AV) nodal reentry tract for which he had undergone AV nodal ablation. Of note, neither the Hashimoto’s nor CREST were active for multiple years leading up to his diagnosis of SCLC. The latter diagnosis had been suspected by the Rheumatology Service but after a negative workup, he was discharged from their clinic.
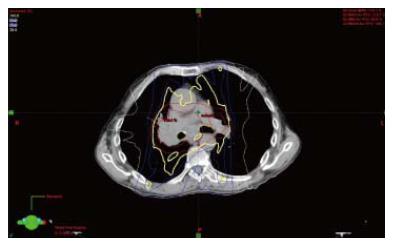
After completion of staging, he was advised to undergo definitive chemoradiation. He was also advised to re-establish follow up with the Rheumatology Service, but declined. After a detailed discussion of the potential for increased risk of complications from radiation with an underlying connective tissue disorder, he elected to proceed. He was treated with intensity modulated radiation therapy to the right lung and mediastinum in 33 daily fractions of 200 cGy to a total dose of 6600 cGy with two cycles of concurrent cisplatin (cis) and etoposide. After 3000 cGy, another CT was performed to allow for decrease in treatment field after initial response. RT was completed in 8 wk and 1 d. A representative image from his intensity modulated radiation therapy (IMRT) radiation plan is presented in (Figure 1). His treatment course was complicated by pancytopenia (for which he received filgrastim and one unit of packed red blood cells), as well as dysphagia and odynophagia. He received two cycles of chemotherapy during the radiation and two cycles in the adjuvant setting after concurrent chemotherapy and radiation therapy, though the last three cycles were dose-reduced because of hematologic toxicities. During chemotherapy he was treated for clostridium difficile colitis and was briefly admitted for generalized weakness. Approximately three months after completion of thoracic RT, he received prophylactic cranial irradiation (PCI) which was given as 10 fractions of 250 cGy.
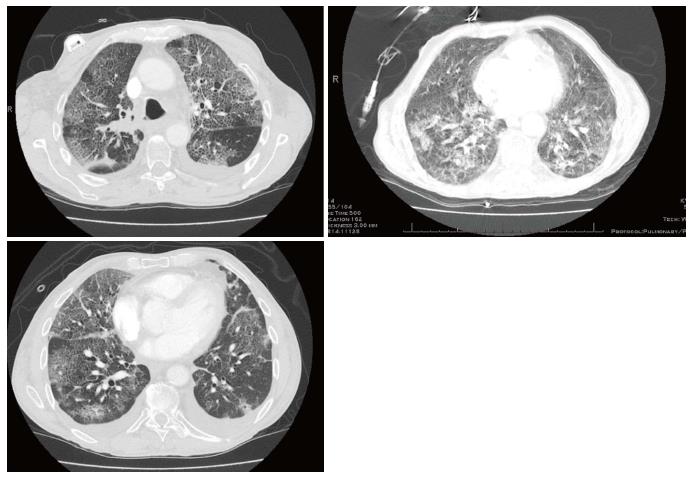
During PCI, he required admission due to inability to tolerate daily travel. Shortly after completion of PCI he developed recurrent clostridium difficile colitis and within weeks of completion of PCI he was readmitted and remained hospitalized for two months. While admitted, he experienced episodes of hypoxemic respiratory failure requiring repeated use of a nonrebreather and for which he underwent intubation twice. Chest imaging demonstrated development of worsening bilateral interstitial and airspace opacities (Figure 2). He was aggressively treated with broad spectrum antibiotics and high dose steroids. Eventually he developed tachycardia, respiratory distress, hypotension and suspected disseminated intravascular coagulation. In accordance with his family’s wishes he underwent palliate extubation and expired shortly thereafter.
An autopsy was performed and the report described extensive, diffuse, bilateral alveolar damage consistent with post-radiation changes, as well as small cell carcinoma in multiple foci within septal capillaries and contiguous alveolar spaces.
Radiation pneumonitis is an uncommon complication of chemoradiation for lung cancer but one which can be fatal in almost 2% of patients[4]. It has been previously been described as having two types of presentations: “Classical” vs “sporadic”. The former is attributed to local cytokine production within the radiated field, while the latter is likened to a hypersensitivity reaction and can be out of proportion to volume irradiated or manifest its effects outside of the treated field. It has even been proposed that the majority of patients develop subclinical lymphocytic alveolitis following lung radiation, but that acute pneumonitis only develops in the fraction that have some genetic or environmental predisposition[5]. Our literature search did not reveal any specific associations between connective tissue disorders and pneumonitis, however in the event that our patient did have a true diagnosis of a connective tissue disorder, one could postulate that it could have served as such a predisposing factor for him.
Although certain radiation dose parameters have also been found to be associated with increased risk for radiation pneumonitis, including mean lung dose (MLD), volume of lung receiving 20 Gy (V20) and possibly 5 Gy (V5), this patient’s parameters were within recommendations. His MLD was 1822 cGy, V20 28%, and V5 69.5%. Qualitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) guidelines, as well as others, indicate that mean lung dose of 13 Gy results in a 10% rate of symptomatic pneumonitis, MLD of 20 Gy results in 20% risk, and V20 of ≤ 30%-31% keeps the risk below 20%[6,7]. The current Radiation Therapy Oncology Group protocols recommend V20 not to exceed 40% and MLD of no more than 20 Gy[8]. Not only did our patient’s plan meet all of the recommended criteria, it was essentially unilateral, targeted at the right hilar mass and mediastinum. His presentation is therefore more consistent with the development of “sporadic” radiation pneumonitis, given that his ultimate condition was spatially diffuse and out of proportion to what would have been expected from the doses received by his normal tissues.
Further complicating this patient’s condition was the presence of lymphangitic spread of tumor which may have contributed to compromise of the patient’s lung function. Additionally, he had a history of both a possible CREST and autoimmune disease (Hashimoto’s Thyroiditis). Connective tissue disorders have been described as potential predisposing factors for increased toxicity from radiation therapy, and the mechanism of sporadic radiation pneumonitis itself is in some ways analogous to an autoimmune reaction with cytokine-mediated destruction[9]. However in this case the autoimmune diseases had not been active for years and the collagen vascular disease, though suspected, had not been officially diagnosed, so it is difficult to evaluate whether the patient’s toxicity could be attributed to these medical issues.
This case is notable for striking imaging findings of diffuse interstitial and alveolar processes (Figure 2) as well as pathologic confirmation of diagnosis of a rare complication from radiation for lung cancer. Limitations are akin to those of any case report, in that it is anecdotal. The patient had multiple processes occurring in the lungs as determined by autopsy, including lymphangitic spread of tumor as well as pneumonia so the fatal respiratory failure may not be entirely attributable to radiation pneumonitis. Furthermore, the patient received concurrent chemotherapy and additional cycles of chemotherapy after radiation which may have resulted in its own toxicity.
This is a case report of grade 5 radiation pneumonitis in a patient with a potential history of connective tissue disease and/or autoimmune disease who also developed lymphangitic spread of tumor. Standard of care chemoradiation was provided to this patient and all of the radiation dose parameters were well within commonly accepted ranges. Furthermore, connective tissue disorder diagnosis was in question and autoimmune disorder was not active. Despite appropriate precautions, he still developed fatal pneumonitis. Further research is needed to develop a better understanding of the interplay of all of these factors.
This is a case report of grade 5 radiation pneumonitis in a patient with a potential history of connective tissue disease and/or autoimmune disease who also developed lymphangitic spread of tumor after receiving chemoradiation with intensity modulated radiation therapy (IMRT) technique for limited stage small cell lung cancer.
Grade 5 radiation pneumonitis and lymphangitic spread of tumor developed after chemoradiation for small cell lung cancer.
Differential included pneumonitis, lymphangitic spread of tumor, pneumonia, or other interstitial and/or airspace disease.
Chest X-ray and computed tomography showed worsening bilateral interstitial and airspace opacities.
Autopsy examination of lung tissue demonstrated extensive, diffuse, bilateral alveolar damage consistent with post-radiation changes, as well as small cell carcinoma in multiple foci within septal capillaries and contiguous alveolar spaces.
Initial therapy consisted of IMRT radiation therapy with concurrent and adjuvant chemotherapy. For his pneumonitis, he was treated with steroids, antibiotics, non-invasive and later mechanical ventilation.
Standard of care chemoradiation was provided to this patient and all of the radiation dose parameters were well within commonly accepted ranges. Furthermore connective tissue disorder diagnosis was in question and autoimmune disorder was not active. Despite appropriate precautions, he still developed fatal pneumonitis in addition to lymphangic tumor spread. Further research is needed to develop a better understanding of the interplay of all of these factors.
The authors present a case report showing a patient with a fatal radiation pneumonitis 6 mo after radiation for limited stage of small cell lung cancer. The article is well explained and implemented.
| 1. | Anscher MS, Marks LB, Shafman TD, Clough R, Huang H, Tisch A, Munley M, Herndon JE, Garst J, Crawford J. Risk of long-term complications after TFG-beta1-guided very-high-dose thoracic radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:988-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Anscher MS, Thrasher B, Zgonjanin L, Rabbani ZN, Corbley MJ, Fu K, Sun L, Lee WC, Ling LE, Vujaskovic Z. Small molecular inhibitor of transforming growth factor-beta protects against development of radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2008;71:829-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Zhang XJ, Sun JG, Sun J, Ming H, Wang XX, Wu L, Chen ZT. Prediction of radiation pneumonitis in lung cancer patients: a systematic review. J Cancer Res Clin Oncol. 2012;138:2103-2116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Palma DA, Senan S, Tsujino K, Barriger RB, Rengan R, Moreno M, Bradley JD, Kim TH, Ramella S, Marks LB. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85:444-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 510] [Cited by in RCA: 517] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 5. | Morgan GW, Breit SN. Radiation and the lung: a reevaluation of the mechanisms mediating pulmonary injury. Int J Radiat Oncol Biol Phys. 1995;31:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 189] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, Bentzen SM, Nam J, Deasy JO. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10-S19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1352] [Cited by in RCA: 1223] [Article Influence: 76.4] [Reference Citation Analysis (1)] |
| 7. | Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109-122. [PubMed] |
| 8. | CALGB 30610 Phase III Comparison of Thoracic Radiotherapy Regimens in Patients with Limited Small Cell Lung Cancer also Receiving Cisplatin or Carboplatin and Etoposide (Protocol Update #11 4/15/16, Accessed December 12, 2016). Available from: http://www.kccop.org/pdfs/FullProtocol-159116480.pdf. |
| 9. | Giaj-Levra N, Sciascia S, Fiorentino A, Fersino S, Mazzola R, Ricchetti F, Roccatello D, Alongi F. Radiotherapy in patients with connective tissue disorders. Lancet Oncology. 2016;17:e109-e177. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Arcangeli S, Freixinet J, Nacak M, Sugawara I S- Editor: Song XX L- Editor: A E- Editor: Lu YJ