Published online Jun 10, 2017. doi: 10.5306/wjco.v8.i3.190
Peer-review started: January 20, 2017
First decision: March 27, 2017
Revised: April 27, 2017
Accepted: May 3, 2017
Article in press: May 4, 2017
Published online: June 10, 2017
Processing time: 133 Days and 4.8 Hours
Large bowel cancer is a worldwide public health challenge. More than one third of patients present an advanced stage of disease at diagnosis and the liver is the most common site of metastases. Selection criteria for early diagnosis, chemotherapy and surgery have been recently expanded. The definition of resectability remains unclear. The presence of metastases is the most significant prognostic factor. For this reason the surgical resection of hepatic metastases is the leading treatment. The most appropriate resection approach remains to be defined. The two step and simultaneous resection processes of both primary and metastases have comparable survival long-term outcomes. The advent of targeted biological chemotherapeutic agents and the development of loco-regional therapies (chemoembolization, thermal ablation, arterial infusion chemotherapy) contribute to extend favorable results. Standardized evidence-based protocols are missing, hence optimal management of hepatic metastases should be single patient tailored and decided by a multidisciplinary team. This article reviews the outcomes of resection, systemic and loco-regional therapies of liver metastases originating from large bowel cancer.
Core tip: Improvements of colorectal cancer liver metastases (CRC-LM) treatment allows the down-staging of several patients. There is currently no agreement in the correct sequence of surgical resection of the primary cancer and metastatic disease. Surgical resection can be performed if the complete removal of cancer is achievable, leaving an adequate normal liver tissue. Neoadjuvant chemotherapy is widely accepted as primary therapy. Chemotherapy may lead to disease regression for unresectable CRC-LM, allowing resection and cure. The application of loco-regional therapies is increasing. They are recommended as third-line treatment for unresectable CRC-LM and have a palliative intent.
- Citation: Fiorentini G, Sarti D, Aliberti C, Carandina R, Mambrini A, Guadagni S. Multidisciplinary approach of colorectal cancer liver metastases. World J Clin Oncol 2017; 8(3): 190-202
- URL: https://www.wjgnet.com/2218-4333/full/v8/i3/190.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i3.190
Colorectal cancer (CRC) is an increasing global health issue[1,2] It is the most common gastro-intestinal tumor and the third most frequently diagnosed malignancy worldwide. It has a mortality rate of up to 10%[1,2]. Most recent epidemiological data show more than 1.4 million newly diagnosed CRC each year[1,2].
The liver is the most common site of CRC metastases with an incidence of 15%-20% at diagnosis. CRC patients have a > 50% probability of liver metastases development[3]. The majority of CRC liver metastases (CRC-LM) were defined not resectable in the past century. Surgery methods are considerably improved nowadays, resulting in cure or survival increase. CRC-LM resection rates are also increased[4]. Recent updating of resectability criteria of CRC-LM considerably improves outcomes, resulting in 5 and 10-year survival rates of 40% and 25% respectively[5,6].
Notwithstanding these good outcomes, the recurrence rate one year after metastasis resection is 30% and a recent study on CRC-LM survival after resection shows a 5-year survival of 16%-71%[7].
Neoadjuvant chemotherapy allow initially unresectable CRC-LM patients to have long term survival similar to those of resectable patients[8-12]. Chemotherapy efficacy, in terms of tumor reduction, is strongly correlated to resectability[10-13]. For this reason, chemotherapy associated to biological agents is increasingly used as resectability conversion of CRC-LM from unresectable to resectable. This method can efficiently increase downsizing rates[14,15].
Candidate selection for resection is still difficult and several CRC-LM patients are never referred to hepatobiliary multidisciplinary group[10,13]. For this reason CRC-LM patients need a multidisciplinary team for treatment decision. This team should include specialists from different disciplines: Oncology, surgery, radiology and radiotherapy. The purpose of this review is to examine the current management of CRC-LM, in order to better define potential advantages and limitations of the several available treatments.
The perioperative evaluation of a patient’s global health and liver function is essential to reduce postoperative complications. A dedicated multidisciplinary team should assess co-morbidities and patient’s performance status in order to decide a future treatment plan. Complete blood examination should be performed before surgery, to assess liver function [alanine aminotransferase (ALT), glutamic-oxalacetic transaminase (AST)], coagulation profile, bilirubin, creatinine and tumor markers, such as carcinoembryonic antigen (CEA).
Exclusion criteria for surgery include several factors to guarantee patient safety. They include advanced age, male gender, low serum albumin, presence of liver disease (hepatitis or alcoholic hepatitis), ascites, kidney or cardiologic impairment, bleeding syndromes, and chronic obstructive pulmonary disease[16-18].
Morbidity and mortality after liver resection is often due to inadequate function of remnant liver, leading to liver failure. Morbidity and mortality rates are around 61% and 11%, respectively[16,17].
The remnant liver cannot sustain metabolic, synthetic, and detoxifying functions if reduced below a critical liver volume[18]. Liver volume is not the best index for liver functionality assessment[16-20]. Patients with concomitant liver disease may have impaired liver regeneration capacity due to cirrhosis, steatosis, or jaundice obstruction[20].
Most chemotherapeutic agents (5-fluorouracil, irinotecan, oxaliplatin) can result in hepatic damage and modification of liver regeneration[11,19-22].
Morbidity and mortality after liver resection may be improved by measuring the intake of 99mTc mebrofenin of tumor-free liver in a pre-operative setting, in order to assess the risk of liver failure and liver failure-related mortality after partial liver resection[17].
During liver regeneration induced by partial hepatectomy, normally quiescent hepatocytes start to replicate in order to restore the original liver. Several genes are involved in liver regeneration, including cytokine, growth factor and metabolic genes[21]. Several studies show that recurrence and progression are directly proportional to the amount of liver resected[22,23].
Neoadjuvant chemotherapy may induce hepatic changes, such as steatohepatitis, hepatic sinusoidal obstruction and periportal inflammation, negatively affecting patient outcome[20,21] and increasing the risk of liver failure and death after major liver resection. A normal liver can bear an extensive resection. Severely compromised livers, on the contrary, cannot tolerate even a minor hepatectomy[8,9,19]. For this reason, monitoring the functionality of surrounding tumor-free liver needs to be highly considered for selection of surgical method.
CRC-LM radiological study is necessary for assessment of surgical resectability. This can be performed using any of these main radiological methods: Magnetic resonance imaging (MRI), computed tomography (CT) and positron emission tomography (PET) scan[24]. Liver metastases can be detected as hypoattenuating lesions, when using contrast-enhanced normal or multidetector CT scans with a sensitivity rate of 85% and 90%, respectively[25]. MRI performed with liver-specific contrast agents has > 90% sensitivity in cases of underlying liver disease (steatosis, cirrhosis) or very small lesions (< 1 cm). For this reason MRI is better than CT for metastasis detection[26].
Specificity of CT, MRI and PET is very high: 95%, 93%, and 97% respectively. PET scan is useful to obtain whole body map, to identify extrahepatic disease (EHD) and to assess resectability[24]. A recent study showed that the FDG PET scan is the best radiological modality for detecting CRC-LM. It can have high false negative rates in patients recently treated with chemotherapy[17,18]. The association of CT to FDG PET scans is highly recommended because it improves the sensitivity up to 97%[24].
Nowadays, also intraoperative ultrasound (IOUS) is a mandatory surgical tool to confirm preoperative investigations by CT or MRI and for detection of missed lesions[27].
The preferred therapy for CRC-LM is surgery, providing up to 50% survival at five-years[28]. Patient selection criteria for resectability are not standardized and still controversial in clinical practice. The American Hepato-Pancreato-Biliary Association (AHPBA) consensus on definition of resectability is currently accepted by most liver surgeons[28,29]. Main CRC-LM resection criteria of AHPBA are: Presence of disease confined to the liver as identified after surgery of primitive cancer; disease in a single hepatic lobe; < 3 nodules; the largest size of nodules < 5 cm in diameter; margin FLR > 1 cm. According to these criteria, however, less than 10% of patients would be indicated for resection.
The classification of resectable disease is broader nowadays, increasing the number of resections[30]. Current guidelines generally agree that resection should be performed for liver metastases only[12,30,31], but hepatectomy and resections of concomitant extrahepatic disease are considered[32]. The remaining liver must be undamaged and at least 20% or 25% of the whole hepatic volume, and have a full functional vascular and biliary in- and out- flow. In this case also multiple resection can be performed[8,14,30]. The survival advantage of repeated resection is close to that after the surgery of primary hepatic disease[33]. Hepatic resection safety depends on: Age of patients, performance score, and concomitant hepatic impairments. Resection is contraindicated when the following are observed: Non resectable extra hepatic tumor; wide involvement of parenchyma; or patient’s poor general conditions.
Possible prognostic factors of resection outcome of CRC-LM are: Age, sex, synchronous or metachronous hepatic metastases, tumor size, number and distribution of LM, primary tumor stage, extrahepatic distant metastases, surgical margin, type of primary hepatic tumor surgery and previous tumor pharmacological therapy, levels of tumor markers.
Fong et al[34] report data from 1001 CRC-LM patients who were candidates for resection. These data led to the identification of seven criteria for worse prognosis prediction after resection. Five of these criteria are actually used for the Clinical Risk Score (CRS) that is a preoperative scoring system. These criteria are: Disease-free interval from primary to metastases < 12 mo; largest hepatic tumor > 5 cm in diameter; node-positivity; number of lesions > 1; and CA 19-9 > 200 ng/mL. Positive prognosis after surgery corresponds to a score < 2. Scores of 3-4 indicate that patients are candidates for resection followed by adjuvant therapy. Prognosis is poor when the score is five. The appropriateness of CRS is proved. CRS can predict patients’ response and OS[35].
A new method has been recently introduced in the CRC-LM resectability criteria assessment[5]. Resection criteria are different. They depend less on the size, number, and location of the metastases. They give more importance to the volume and function of the future liver remnant (FLR), which should be > 25% estimated normal liver parenchyma or 30% in the presence of impaired liver function[36]. Metastases are considered resectable if the excision of all metastatic lesions can be obtained with an adequate FLR[37] and the presence of EHD is currently no longer considered as a contraindication[5]. The new requirements for LM resection are: R0 resection achievement of intrahepatic and extra hepatic disease; adequate FRL; and > 2 adjacent liver segments to be spared with blood and bile inflow and outflow preservation[31,37].
The best sequence and timing of CRC-LM resection is still under debate and many options are available. The use of up front chemotherapy is increasing. Strong evidence is missing and there are currently no randomized controlled trials comparing the different approaches[38].
The classic surgical method is “primary first”, whose suggested sequence is to firstly resect the primary CRC, then to administer the chemotherapy and after 3-6 mo to eventually resect the LM. This approach is indicated for patients with advanced or symptomatic CRC, important comorbidities, or inadequate FLR. In cases of advanced CRC, indeed, the chemotherapy may be associated with high complication rates and the insurgence of disease progression may lead to unresectability[39]. Any delay correlated to complications during surgery of CRC may also increase the risk of progression occurrence for some patients[40,41]. A possible benefit of this method can be the possibility to identify previously occult LM that may become visible during adjuvant chemotherapy. This allows avoidance of the morbidity of a liver resection.
Another surgical method is the “synchronous resection of LM and primary CRC”. This approach can avoid delays in chemotherapy treatment that can be started earlier if no complications occur after surgery. The possible disadvantage of this method is the increased postoperative morbidity and mortality because of infection resulting from bacterial contamination of the surgical field[36]. For this reason this approach is indicated for patients who can tolerate long operative times[6].
The third available surgical method is the “alternative staged liver-first” approach that firstly resect the LM, then administer 3-6 cycles of chemotherapy, and at last resect the primary CRC. Adjuvant chemotherapy can be administered in between both procedures. Recent data report that this method is indicated for selected patients with advanced CRC-LM, and when neo-adjuvant and adjuvant chemotherapy may have better results[9,12].
The utility of neoadjuvant chemotherapy for CRC-LM is unclear even if there is the tendency to use it frequently[15]. There are many advantages of neo-adjuvant treatment such as increasing tumor sensitivity, downstaging large or multiple liver lesions, increasing resectability, and treating micrometastases[8,9,11]. This therapy also allows better planning for the date of surgical resection.
On the other hand, neo-adjuvant chemotherapy can delay surgical treatment, which may be detrimental for patients, increasing the risk of disease progression[12,15]. This chemotherapy can also induce liver toxicity, such as steatohepatitis, increasing postoperative mortality. It can also mask metastases on preoperative imaging, as is observed in 5%-25% of cases[42].
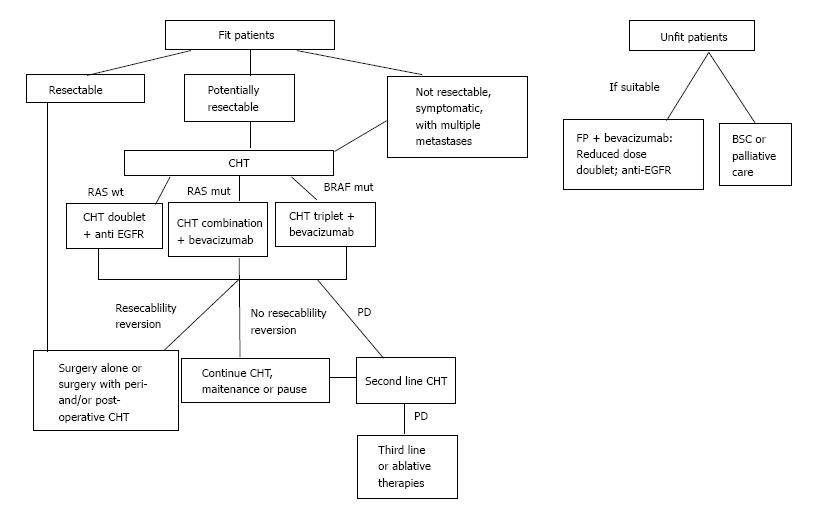
Perioperative chemotherapy is widely used for patients with unresectable disease (Table 1 and Figure 1) with the purpose of reducing disease progression, which occurs in 50%-70% of patients after surgery[3]. A multicentre randomized trial compared surgery alone with perioperative chemotherapy (6 cycles of preoperative and post operative FOLFOX4) in 364 unresectable CRC-LM patients. The results of this study showed no significant differences in five-year OS for the two groups; nevertheless, progression-free survival (PFS) increased by 7.3% at 3 years in the perioperative chemotherapy group[43]. The rate of post-operative complications is also increased and is directly proportional to the length of therapy. For this reason, it is suggested that only 6 cycles of chemotherapy for no longer than 3 mo should be performed, in order to reduce toxicity[28], especially for patients who need a major hepatectomy[44].
| Perioperative treatment |
| It is defined by technical criteria for resection and prognostic considerations |
| It may not be necessary in patients with clearly resectable disease and favourable prognosis, in this case upfront resection is justified |
| It should administer FOLFOX or CAPOX to patients with resectable disease and unclear (probably unfavourable) |
| Targeted agents should not be used in resectable patients with prognostic indication for perioperative treatment |
| It should be considered when prognostic and resectability criteria are unclearly defined, and in patients with synchronous onset of metastases |
| Adjuvant chemotherapy is not strongly indicated for patients with favourable oncological and surgical criteria, who did not receive any neoadjuvant chemotherapy |
| Adjuvant chemotherapy is indicated for patients with unfavourable criteria |
| Adjuvant treatment with FOLFOX or CAPOX is recommended for patients who have not received any previous chemotherapy, unless patients already received oxaliplatin-based adjuvant chemotherapy |
| The choice of chemotherapy type should consider patients’ clinical conditions and therapy preferences |
| Conversion therapy |
| A chemotherapy regimen leading to high response rates and/or a large tumour shrinkage is recommended for potentially resectable patients |
| The best drug combination to use is still not clear because only few trials have addressed this issue: |
| RAS wild-type patients may benefit from a cytotoxic doublet plus an epidermal growth factor receptors agents antibody (best benefit/risk), and from the combination of FOLFOXIRI plus bevacizumab and, to a lesser extent, from a cytotoxic doublet plus bevacizumab |
| RAS mutant patients may benefit from a cytotoxic doublet plus bevacizumab or FOLFOXIRI plus bevacizumab |
| Patients must be re-evaluated regularly (every 2-3 mo) to prevent the overtreatment of resectable patients |
Patients with more than 3 lesions, and tumor diameter greater than 3 cm are clearly indicated for this treatment. The surgery of lesions should be done 4-8 wk after the neo-adjuvant chemotherapy. In summary, the advantages of neo-adjuvant chemotherapy outnumber the disadvantages, and we are in favor of its utilization.
The ultimate dilemma after complete CRC-LM resection is the rate of recurrence that is reported as high as 60% after complete surgical excision. Several studies show the benefits of adjuvant therapy such as FOLFOX4 (folinic acid, fluorouracil, and oxaliplatin), resulting in longer disease-free-survival (DFS)[45] than liver resection alone.
Adjuvant chemotherapy also increases OS when compared to surgery alone, even if the difference is not statistically significant[46,47].
The classic adjuvant chemotherapeutic drugs are: 5-fluorouracil/leucovorin (5-FU/LV), capecitabine, oxaliplatin and irinotecan[47]. New molecular-targeted agents are now available. They include anti-angiogenic drugs (bevacizumab, regorafenib and aflibercept) and anti-epidermal growth factor receptors agents (anti-EGFR), such as cetuximab and panitumumab. These agents are widely used as adjuvant treatment without any evidence of clinical benefit[48].
Adjuvant chemotherapy after metastasectomy is generally recommended by clinicians, even if the best regimen protocol is still unclear, and should be considered in a patient dependent manner[24]. There, efficacy of adjuvant chemotherapy on OS for resectable CRC-LM is still under discussion[45]. The National Comprehensive Cancer Network (NCCN) guidelines suggests the use of more than one chemotherapy line[48]. Most study agree that 5-FU/LV with or without oxaliplatin should always be used as first-line[47]. More recently, however, the use of combination therapy is increasing, and several combinations have emerged.
A recent study on FOLFIRI (5-FU/LV and irinotecan) vs 5FU/LV after R0 (complete resection) of CRC-LM does not report any difference in OS and median DFS. FOLFIRI improves DFS, but causes more frequent grade 3/4 toxic adverse events (47% vs 30%)[49].
We suggest the use of adjuvant chemotherapy in patients with multiple lesions that are found in more than 3 liver segments, where the surgery, even if radical, may not be able to remove undetected tumor deposits.
Patients with unreserctable CRC-LM from diagnosis should receive chemotherapy in order to downstage the disease and allow the surgery (Figure 1 and Table 2).
| Trial name | Chemotherapy type | Control | n | KRAS status | Overall response | Conversion to resection | R0 resection |
| BEAT[61] | FOLFOX/XELOX/FOLFIRI or fluoropyrimidines + bevacizumab | No | 1914 | Not selected | NA | 11.80% | NA |
| First BEAT[62] | FOLFOX/XELOX + bevacizumab | Placebo | 1914 | Not selected | 38% | 11.80% | 6.3% vs 4.9% |
| OPUS[70] | FOLFOX + cetuximab | FOLFOX | 233 | Wilde type | 61% vs 37% | 9% | 4.7% vs 2.4% |
| POCHER[72] | Chr IFLO + cetuximab | No | 43 | Wild type | 79% | 60% | 25.70% |
| PRIME[77] | FOLFOX + panitumumab | FOLFOX | 591 | Wild type | 57% vs 48% | 31% vs 22% | 29% vs 17% |
| CELIM[11] | FOLFOX6 + cetuximab | FOLFIRI + cetuximab | 106 | Wild type | 68% vs 57% | 43% | 38% vs 30% |
| BOXER[63] | CAPOX + bevacizumab | No | 47 | Not selected | 78% | 40% | NA |
| Loupakis et al[55] | FOLFOXIRI + bevacizumab | FOLFIRI + bevacizumab | 508 | Not selected | 65% vs 53% | 15% vs 12% | NA |
| Ye et al[73] | FOLFIRI + cetuximab | FOLFOX + cetuximab | 177 | Wild type | 57% vs 29% | 26% vs 7% | NA |
| CRYSTAL[71] | FOLFIRI + cetuximab | FOLFIRI | 599 | Wilde type | 47% vs 39% | 16% | 4.8% vs 1.7% |
| OLIVIA[79] | FOLFOXIRI + bevacizumab | FOLFOX + bevacizumab | 80 | Not selected | 81% vs 62% | 61% vs 49% | 49% vs 23% |
About 70% of patients with CRLM are unresectable at diagnosis[4]. They have a complicated disease, often requiring a combination of loco-regional therapy (chemoembolization, hepatic arterial infusion, ablation or radiation).
Perioperative chemotherapy is widely used also for unresectable CRC-LM, even if there is no proof of OS improvement[50]. Systemic chemotherapy remains the first-line therapy. FOLFOXIRI followed by surgical resection has a 70.4% response rate, and 19% of patients obtain R0. OS at 5 and 8 years are 42% and 33% respectively, and 29% of patients are disease free at 5 years[51].
Downstaging of unresectable CRC-LM ranges from 5% to 38%. This is due to multiple factors including disease extension, type and duration of chemotherapy[51]. The purpose of the “conversion chemotherapy” in unresectable CRC-LM patients is to convert their disease to resectable, and is often the first line treatment. Standard regimens include FOLFIRI or FOLFOX that induce downstaging in 7%-40% of patients[12]. Giacchetti’s group reports that FOLFOX reduces the LM dimension by more than 50% in 59% of non-resectable CRC-LM, resulting in 38% of CR[52]. FOLFOXIRI allows 36% of R0 in LM patients[53]. The METHEP trial reports that FOLFIRINOX seems to be better therapy for CRC-LM than the others, bringing to resection 67% of cases with a survival > 48 mo. These results confirm that OS is greater for patients after R0 or R1 surgery, 65.2 mo vs 18.3 mo of not-operated or R2 patients[54].
The use of bevacizumab is increasing for unresectable CRC-LM[55,56], even if the benefits are extremely limited. A slight gain in response rate is observed when bevacizumab is associated with FOLFOXIRI as first line chemotherapy. The association of bevacizumab to first and second line chemotherapy for CRC-LM improves PFS[57-60] and OS in some studies[59,60]. Available data on the efficacy of bevacizumab associated to perioperative chemotherapy are limited. This may be due to concerns about possible complications in wound healing after resection[61,62]. The Bevacizumab Expanded Access Trial reports good feasibility of LM surgery after first-line chemotherapy associated to bevacizumab, resulting in resection rates of 11.8% and 6% of R0[63]. Bevacizumab association with FOLFOX, however, obtains higher resection rates (16.1%) than with FOLFIRI (9.7%), and higher R0 (6.3%) than FOLFOX plus placebo (4.9%) (P = 0.24)[62]. Neoadjuvant capecitabine and oxaliplatin (CAPOX) plus bevacizumab resulted in 40% of CRC-LM resectability conversion[63]. Loupakis et al[55] report 64% of tumor response and 15% of rate of resection of CRC-LM after FOLFOXIRI plus bevacizumab, vs 53% and 12% respectively after FOLFIRI/bevacizumab.
Transarterial chemoembolization with irinotecan combined with FOLFOX plus bevacizumab chemotherapy results in a response rate of 78%, and allows resection of 35% of non resectable CRC-LM, offering a new cure option to these patients[64].
A recent report by Stremitzer et al[65] shows that mutated BRAF/RAS are correlated to a poor outcome after CRC-LM surgery. This is in agreement with the results of other 3 studies[66-69]. These important evidences support the application of newer methods for the therapy of liver metastases, associating biological molecular aspects (biological resectability) to the other clinical and pathological indexes for the selection of good surgical candidates and the prediction of their outcomes.
Anti-EFGR agents such as cetuximab and panitumumab are effective alone as well as in association with chemotherapy in CRC-LM that are RAS (both KRAS and NRAS) wild type[69]. Some randomized trials report the effects of cetuximab for the therapy of unresectable CRC-LM. The OPUS trial[70] showed that the association of FOLFOX-4 plus cetuximab as up front therapy doubled R0 (4.7%). The CRYSTAL study[71] showed that the association of FOLFIRI plus cetuximab as up front therapy increased the R0 resection rate from 3.7% to 7.0%. The CELIM trial[11] reported that neoadjuvant treatment with FOLFIRI plus cetuximab or FOLFOX6 resulted in 34% of R0 resections. Other studies also report that chemotherapy containing cetuximab significantly improves R0 in unresectable CRC-LM with KRAS wild-type[72,73]. There are differences in resection rates among the above studies. Overall response rate is in the range 60%-79%, however, resection rates after chemotherapy/cetuximab are very variable (Table 2). These discrepancies may be due to the fact that the resection rate is defined and determined by clinical conditions of the patients and not by specialist oncologists in CRYSTAL and OPUS studies. Resection evaluation is done by a multidisciplinary team in the other trials.
The COIN[74] and NORDIC VII[75] trials report no advantage for the association of oxaliplatin based chemotherapy/cetuximab in first-line treatment of CRC-LM, independently from K-RAS status.
Resection rates of first-line FOLFIRI/panitumumab treatment of CRC-LM are 15% and 7% in the KRAS wild type (WT) and mutant groups respectively[76]. FOLFOX4 plus panitumumab results in 32% of R0 resections vs 28% of those receiving only FOLFOX4[77]. A post hoc analysis of the PRIME study on RAS WT (KRAS, NRAS) shows that panitumumab/FOLFOX can convert to resection 31% of initially unresectable CRC-LM patients and lead to 29% of R0 (Table 2)[78]. A further analysis of PRIME trial also shows that NRAS mutations are indications of non-response to panitumumab[77]. For this reason, it is extremely important to analyze other types of mutations in the RAS gene to improve patient selection for anti EGFR therapy.
The OLIVIA trial studies FOLFOXIRI + bevacizumab vs mFOLFOX-6 + bevacizumab and reports an overall resection rate of 61% vs 49%, with R0 resection rates of 49% vs 23%[79].
In conclusion “biologically directed” chemotherapy reduces the number and size of unresectable lesions. It also allows rescue of 15%-35% of patients, bringing them to surgery. These therapies are increasingly used worldwide.
Extra hepatic disease (EHD) has a poor prognosis[28]. Most common sites of EHD from CRC are lymph nodes, lungs, peritoneum, brain and bone. EHD is currently no longer a contraindication to metastasis resection, and patients after surgery have[5] longer DFS and five-year-survival rates compared to those receiving only chemotherapy[5,6].
OS after lymph node resection is different according to their site and number[80]. Celiac or aorto-caval lymph node resections are associated with a worse outcome when compared to hepatic pedicle nodes, and mediastinal lymph nodes have a worse median survival than intra-thoracic ones[80]. A high number of lymph nodes positive for metastases have also a poor outcome[80].
In conclusion, the treatment of EHD is substantially palliative, aiming to improve the quality of life[81].
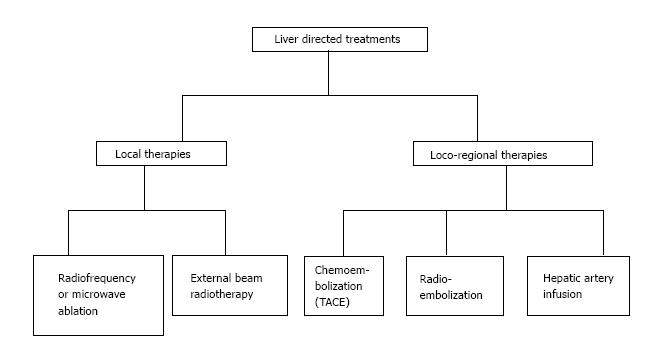
Loco-regional therapies (Figure 2) are indicated for patients that are elderly, have a poor performance status, refusing surgery or chemotherapy, or refractory to chemotherapy. They also allow chemo-holidays with suspension of chemotherapy, and prolong the non-treatment period in between different chemotherapy lines. This reduces the treatment costs in respect to systemic chemotherapy.
In the last years new strategies have been developed in order to overcome several problems: High percentage of unresectable CRC-LM at diagnosis, high recurrence rates and presence of extensive disease. These methods increase the number of patients indicated for non surgical procedures.
Ablation techniques include radiofrequency ablation (RFA), Microwave ablation and external beam radiotherapy (EBRT). RFA is widely used and allows the application of extreme temperature to ablate the lesion with minimal toxicity (< 1%) in the surrounding liver tissue. RFA results in mortality and morbidity < 10% independently from the administration route[82]. The “heat sink effect” is however a major disadvantage of RFA and may cause important hepatic or vascular injury. For this reason, RFA is not indicated for unresectable tumors, lesions near blood vessels or the diaphragm because of the high risk of perforation. Another disadvantage of RFA is the recurrence rate that is higher when the tumor is > 3 cm or when treatment is delivered percutaneously[82,83].
Microwave ablation uses high frequency microwave radiation to induce coagulation with necrosis of lesions. This method, however, is not well known and there are several concerns about its feasibility[84]. Available data on this method show a 6% local recurrence rate[85].
Improvements in imaging methods have increased the use of EBRT[86]; that, however, has a low therapeutic window, and toxicity is still a major issue. EBRT is safe (at 60 Gy) and effective for liver tumors in general and in selected patients[87,88].
Hepatic artery infusion (HAI) is indicated for patients with unresectable lesions when physicians want to associate an intra-arterial with an endovenous treatment.
The advantage of HAI is to minimize the toxicity to normal liver tissue, because the chemotherapeutic agents are injected directly to the tumor[89]. Potential risks of this method are treatable complications related to catheter and pump placement, or life-threatening complications such as biliary sclerosis, hepatotoxicity and systemic toxicity. For this reason it should be performed by experienced hospitals[89-91].
Intravenously 5-FU and intra hepatic artery oxaliplatin are successfully used[92] for unresectable CRC-LM. Best results concerning survival and response rates are obtained with floxuridine based HAI[93].
The comparison of OS between HAI therapy and systemic therapy alone (15.9 mo vs 12.4 mo) does not show any difference, however, there was a great response rate in favor of HIA (43% vs 18%)[94].
In conclusion, HAI has interesting results; however it is a cumbersome method because it requires the implantation of an infusion pump.
Trans-arterial chemoembolization (TACE) is increasingly used for unresectable CRC-LM, improving survival and tumor response[95]. TACE is indicated for unresectable CRC-LM as third line therapy, and allows the attainment of important palliative results.
The use of drug-eluting beads for TACE increases efficacy, while reducing adverse events due to systemic drug leakage or liver toxicity[95-98]. The advantage of these beads is the direct delivery of toxic drugs inside the arterial capillary bed of the tumor, releasing the drug in a controlled manner. In this way the systemic exposure to toxic drugs is reduced, their local concentration is increased and a greater tissue necrosis than classic TACE with lipiodol is obtained[99,100].
The indication for TACE is presence of multinodular LM, absence of extra hepatic disease, refractory to systemic chemotherapy[101].
Recent reports show that TACE with irinotecan (DEBIRI) for the treatment of CRC-LM is effective, feasible and has limited side effects[95-101]. Systemic chemotherapy (FOLFIRI) is compared to DEBIRI for the therapy of refractory CRC-LM in some studies. This comparison shows that DEBIRI is statistically better than FOLFIRI in terms of OS, PFS, time to extra-hepatic progression, and quality of life[95].
The association of cetuximab and TACE with irinotecan is an improvement in the treatment of CRC-LM, because these drugs are efficacious and have acceptable, and not cumulative, toxicities[102].
The TACE methodology is constantly improving, in particular, the last innovation is the introduction of new embolics for drug delivery. Among the new types of microspheres there are polyethylene glycol (PEG) microspheres (LifePearls, Terumo), that are more resistant to stress and attrition. The advantages of these embolics are increased suspension time, better catheter deliverability and drug retention and release[103].
In a recent study we show the data of TACE with PEG embolics for the treatment of 20 cases of non resectable liver tumors and metastases from colorectal carcinoma, breast cancer and uveal melanoma. Irinotecan and doxorubicin are used for PLC and LM respectively. More than 80% of cases respond to TACE patients. We observe 63% of CR, and 37% PR. The chemoembolization procedure is well tolerated by all the patients with only mild or moderate adverse events. These results indicate that PEG embolics-TACE is effective and tolerable for the therapy of hepatic primary and metastatic cancer[103].
In the last decade radioembolization (RE) with Yttrium 90 (Y90) has been widely used for the treatment of CRC-LM that are refractory to chemotherapy[104]. Objective tumor response rates of RE are 33%-48% in second line[105,106] and 10%-48% in third line[107-109]. Survival and progression free survival are also improved after RE application as third line[109]. RE with Y90 has, however, a low recommendation in the last ESMO guidelines[110].
The treatment decision is very challenging for CRC-LM patients that are refractory to chemotherapy. Several patients are unfit and have a biologically unfavorable progression often associated to comorbidities. Palliative care with chemo- or radio-embolization is indicated in these cases, in order to avoid too aggressive therapies.
The involvement of a multidisciplinary approach should be promoted in order to obtain the best CRC-LM management and outcomes, and to reduce peri-operative morbidity and mortality, prolonging OS and rising resection rates[110,111].
For this reason, the multidisciplinary team management of CRC-LM is growing in most Western countries[112]. The team includes different types of specialists including: Liver surgeons; interventional radiologists specialized in hepatobiliary disease; an oncologist; a pathologist; and a case manager nurse. They have to discuss each case to ensure resectability appropriateness and lead to down-staging wherever possible. The team should be consulted about the choice of chemotherapy combination and type of targeted agents and care to be used, timing of chemo-holidays, and follow up.
Medical oncologists select the most active treatment for the shortest time combining chemotherapy to targeted drugs, in order to reduce tumor size without damaging the normal liver. The definition of the acceptable FRL should be performed by a radiologist and a liver surgeon. Repeating the resection is safe and effective, obtaining survival rates close to those after first resection[112,113]. Finally the case manager nurse or the practitioner are important in patient’s management, because they provide indications on the follow up and assistance.
Recent improvements of CRC-LM treatment allows the down-staging of several patients, resulting in increased number of patients cured or living with longer disease control. There is currently no agreement about the correct sequence of surgical resection of the primary cancer and metastatic disease, however, the neoadjuvant chemotherapy is widely accepted as up front treatment.
Surgical resection can be performed if the complete removal of cancer is achievable leaving an adequate FRL. The use of adjuvant chemotherapy is highly suggested, even if standardized protocols are still unclear. The use of chemotherapy may lead to disease regression for unresectable CRC-LM, allowing resection and cure.
The application of loco-regional therapies is increasing, resulting in high tumor response, however, they are not recommended as first-line treatment in case of unresectable CRC-LM.
| 1. | World Health Organization. International Agency for Research in Cancer. Globocan 2012 estimated cancer incidence, mortality and prevalence worldwide 2012. [accessed 2016 Aug 20]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. |
| 2. | Zhang YL, Zhang ZS, Wu BP, Zhou DY. Early diagnosis for colorectal cancer in China. World J Gastroenterol. 2002;8:21-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Renehan AG, Egger M, Saunders MP, O’Dwyer ST. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ. 2002;324:813. [PubMed] |
| 4. | Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982-999. [PubMed] |
| 5. | Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist. 2008;13:51-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 329] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 6. | Hadden WJ, de Reuver PR, Brown K, Mittal A, Samra JS, Hugh TJ. Resection of colorectal liver metastases and extra-hepatic disease: a systematic review and proportional meta-analysis of survival outcomes. HPB (Oxford). 2016;18:209-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, Hess K, Curley SA. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818-825; discussion 825-827. [PubMed] |
| 8. | Bismuth H, Adam R, Lévi F, Farabos C, Waechter F, Castaing D, Majno P, Engerran L. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509-520; discussion 520-522. [PubMed] |
| 9. | Nordlinger B, Benoist S. Benefits and risks of neoadjuvant therapy for liver metastases. J Clin Oncol. 2006;24:4954-4955. [PubMed] |
| 10. | Van Cutsem E, Nordlinger B, Adam R, Köhne CH, Pozzo C, Poston G, Ychou M, Rougier P. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42:2212-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 441] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 11. | Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher J, Weitz J. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 722] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 12. | Nordlinger B, Van Cutsem E, Rougier P, Köhne CH, Ychou M, Sobrero A, Adam R, Arvidsson D, Carrato A, Georgoulias V. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer. 2007;43:2037-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Van Cutsem E, Nordlinger B, Cervantes A. Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Ann Oncol. 2010;21 Suppl 5:v93-v97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 364] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 14. | Isoniemi H, Osterlund P. Surgery combined with oncological treatments in liver metastases from colorectal cancer. Scand J Surg. 2011;100:35-41. [PubMed] |
| 15. | Chua TC, Saxena A, Liauw W, Kokandi A, Morris DL. Systematic review of randomized and nonrandomized trials of the clinical response and outcomes of neoadjuvant systemic chemotherapy for resectable colorectal liver metastases. Ann Surg Oncol. 2010;17:492-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Schroeder RA, Marroquin CE, Bute BP, Khuri S, Henderson WG, Kuo PC. Predictive indices of morbidity and mortality after liver resection. Ann Surg. 2006;243:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 251] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 17. | Dinant S, de Graaf W, Verwer BJ, Bennink RJ, van Lienden KP, Gouma DJ, van Vliet AK, van Gulik TM. Risk assessment of posthepatectomy liver failure using hepatobiliary scintigraphy and CT volumetry. J Nucl Med. 2007;48:685-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Erdogan D, Heijnen BH, Bennink RJ, Kok M, Dinant S, Straatsburg IH, Gouma DJ, van Gulik TM. Preoperative assessment of liver function: a comparison of 99mTc-Mebrofenin scintigraphy with indocyanine green clearance test. Liver Int. 2004;24:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 378] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 20. | Fernandez FG, Ritter J, Goodwin JW, Linehan DC, Hawkins WG, Strasberg SM. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 357] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 21. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1225] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 22. | Ikeda Y, Matsumata T, Takenaka K, Sasaki O, Soejima K, Sugimachi K. Preliminary report of tumor metastasis during liver regeneration after hepatic resection in rats. Eur J Surg Oncol. 1995;21:188-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Mizutani J, Hiraoka T, Yamashita R, Miyauchi Y. Promotion of hepatic metastases by liver resection in the rat. Br J Cancer. 1992;65:794-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257:674-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 402] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 25. | Glover C, Douse P, Kane P, Karani J, Meire H, Mohammadtaghi S, Allen-Mersh TG. Accuracy of investigations for asymptomatic colorectal liver metastases. Dis Colon Rectum. 2002;45:476-484. [PubMed] |
| 26. | Choi DJ, Kwak JM, Kim J, Woo SU, Kim SH. Preoperative chest computerized tomography in patients with locally advanced mid or lower rectal cancer: its role in staging and impact on treatment strategy. J Surg Oncol. 2010;102:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Lucchese AM, Kalil AN, Schwengber A, Suwa E, Rolim de Moura GG. Usefulness of intraoperative ultrasonography in liver resections due to colon cancer metastasis. Int J Surg. 2015;20:140-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Mattar RE, Al-Alem F, Simoneau E, Hassanain M. Preoperative selection of patients with colorectal cancer liver metastasis for hepatic resection. World J Gastroenterol. 2016;22:567-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Vauthey JN, Choti MA, Helton WS. AHPBA/SSO/SSAT Consensus Conference on hepatic colorectal metastases: rationale and overview of the conference. January 25, 2006. Ann Surg Oncol. 2006;13:1259-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Ksienski D, Woods R, Speers C, Kennecke H. Patterns of referral and resection among patients with liver-only metastatic colorectal cancer (MCRC). Ann Surg Oncol. 2010;17:3085-3093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | House MG, Ito H, Gönen M, Fong Y, Allen PJ, DeMatteo RP, Brennan MF, Blumgart LH, Jarnagin WR, D’Angelica MI. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744-752, 752-755. [PubMed] |
| 32. | Chua TC, Saxena A, Liauw W, Chu F, Morris DL. Hepatectomy and resection of concomitant extrahepatic disease for colorectal liver metastases--a systematic review. Eur J Cancer. 2012;48:1757-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Ruiz-Tovar J, López Hervás P. Repeated liver resection for recurrence of colorectal cancer metastases. Clin Transl Oncol. 2010;12:634-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309-318; discussion 318-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2861] [Article Influence: 106.0] [Reference Citation Analysis (1)] |
| 35. | Mann CD, Metcalfe MS, Leopardi LN, Maddern GJ. The clinical risk score: emerging as a reliable preoperative prognostic index in hepatectomy for colorectal metastases. Arch Surg. 2004;139:1168-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Nagashima I, Takada T, Adachi M, Nagawa H, Muto T, Okinaga K. Proposal of criteria to select candidates with colorectal liver metastases for hepatic resection: comparison of our scoring system to the positive number of risk factors. World J Gastroenterol. 2006;12:6305-6309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Ferrero A, Viganò L, Polastri R, Muratore A, Eminefendic H, Regge D, Capussotti L. Postoperative liver dysfunction and future remnant liver: where is the limit? Results of a prospective study. World J Surg. 2007;31:1643-1651. [PubMed] |
| 38. | Lykoudis PM, O’Reilly D, Nastos K, Fusai G. Systematic review of surgical management of synchronous colorectal liver metastases. Br J Surg. 2014;101:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 39. | Waisberg J, Ivankovics IG. Liver-first approach of colorectal cancer with synchronous hepatic metastases: A reverse strategy. World J Hepatol. 2015;7:1444-1449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Feng Q, Wei Y, Zhu D, Ye L, Lin Q, Li W, Qin X, Lyu M, Xu J. Timing of hepatectomy for resectable synchronous colorectal liver metastases: for whom simultaneous resection is more suitable--a meta-analysis. PLoS One. 2014;9:e104348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Ruers TJ, Hagendoorn J. Treatment dilemmas in patients with synchronous colorectal liver metastases. Recent Results Cancer Res. 2012;196:37-49. [PubMed] |
| 42. | Bischof DA, Clary BM, Maithel SK, Pawlik TM. Surgical management of disappearing colorectal liver metastases. Br J Surg. 2013;100:1414-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 972] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 44. | Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, Rougier P, Nordlinger B. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1-7. [PubMed] |
| 45. | Mitry E, Fields AL, Bleiberg H, Labianca R, Portier G, Tu D, Nitti D, Torri V, Elias D, O’Callaghan C. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26:4906-4911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 414] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 46. | Power DG, Kemeny NE. Role of adjuvant therapy after resection of colorectal cancer liver metastases. J Clin Oncol. 2010;28:2300-2309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 47. | Brandi G, De Lorenzo S, Nannini M, Curti S, Ottone M, Dall’Olio FG, Barbera MA, Pantaleo MA, Biasco G. Adjuvant chemotherapy for resected colorectal cancer metastases: Literature review and meta-analysis. World J Gastroenterol. 2016;22:519-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 48. | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Colon Cancer (version 2. 2016). [accessed 2016 Jan 24]. Available from: http://www.nccn.org. |
| 49. | Ychou M, Hohenberger W, Thezenas S, Navarro M, Maurel J, Bokemeyer C, Shacham-Shmueli E, Rivera F, Kwok-Keung Choi C, Santoro A. A randomized phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Ann Oncol. 2009;20:1964-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 197] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 50. | Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, Sobrero A. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev. 2015;41:729-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 429] [Article Influence: 39.0] [Reference Citation Analysis (2)] |
| 51. | Masi G, Loupakis F, Pollina L, Vasile E, Cupini S, Ricci S, Brunetti IM, Ferraldeschi R, Naso G, Filipponi F. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann Surg. 2009;249:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 52. | Giacchetti S, Itzhaki M, Gruia G, Adam R, Zidani R, Kunstlinger F, Brienza S, Alafaci E, Bertheault-Cvitkovic F, Jasmin C. Long-term survival of patients with unresectable colorectal cancer liver metastases following infusional chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin and surgery. Ann Oncol. 1999;10:663-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 421] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 53. | Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 904] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 54. | Ychou M, Rivoire M, Thezenas S, Quenet F, Delpero JR, Rebischung C, Letoublon C, Guimbaud R, Francois E, Ducreux M. A randomized phase II trial of three intensified chemotherapy regimens in first-line treatment of colorectal cancer patients with initially unresectable or not optimally resectable liver metastases. The METHEP trial. Ann Surg Oncol. 2013;20:4289-4297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 55. | Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi R. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 824] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 56. | Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, Mass R, Perrou B, Nelson B, Novotny WF. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697-3705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 663] [Article Influence: 31.6] [Reference Citation Analysis (1)] |
| 57. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7792] [Article Influence: 354.2] [Reference Citation Analysis (8)] |
| 58. | Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2298] [Article Influence: 127.7] [Reference Citation Analysis (0)] |
| 59. | Gordon CR, Rojavin Y, Patel M, Zins JE, Grana G, Kann B, Simons R, Atabek U. A review on bevacizumab and surgical wound healing: an important warning to all surgeons. Ann Plast Surg. 2009;62:707-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 60. | Tamiya A, Yamazaki K, Boku N, Machida N, Kojima T, Taku K, Yasui H, Fukutomi A, Hironaka S, Onozawa Y. Safety of bevacizumab treatment in combination with standard chemotherapy for metastatic colorectal cancer: a retrospective review of 65 Japanese patients. Int J Clin Oncol. 2009;14:513-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Van Cutsem E, Rivera F, Berry S, Kretzschmar A, Michael M, DiBartolomeo M, Mazier MA, Canon JL, Georgoulias V, Peeters M. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20:1842-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 415] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 62. | Okines A, Puerto OD, Cunningham D, Chau I, Van Cutsem E, Saltz L, Cassidy J. Surgery with curative-intent in patients treated with first-line chemotherapy plus bevacizumab for metastatic colorectal cancer First BEAT and the randomised phase-III NO16966 trial. Br J Cancer. 2009;101:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 63. | Wong R, Cunningham D, Barbachano Y, Saffery C, Valle J, Hickish T, Mudan S, Brown G, Khan A, Wotherspoon A. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol. 2011;22:2042-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 64. | Martin RC, Scoggins CR, Schreeder M, Rilling WS, Laing CJ, Tatum CM, Kelly LR, Garcia-Monaco RD, Sharma VR, Crocenzi TS. Randomized controlled trial of irinotecan drug-eluting beads with simultaneous FOLFOX and bevacizumab for patients with unresectable colorectal liver-limited metastasis. Cancer. 2015;121:3649-3658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 65. | Karagkounis G, Torbenson MS, Daniel HD, Azad NS, Diaz LA, Donehower RC, Hirose K, Ahuja N, Pawlik TM, Choti MA. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer. 2013;119:4137-4144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 66. | Nash GM, Gimbel M, Shia J, Nathanson DR, Ndubuisi MI, Zeng ZS, Kemeny N, Paty PB. KRAS mutation correlates with accelerated metastatic progression in patients with colorectal liver metastases. Ann Surg Oncol. 2010;17:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 67. | Stremitzer S, Stift J, Gruenberger B, Tamandl D, Aschacher T, Wolf B, Wrba F, Gruenberger T. KRAS status and outcome of liver resection after neoadjuvant chemotherapy including bevacizumab. Br J Surg. 2012;99:1575-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 68. | Vauthey JN, Zimmitti G, Kopetz SE, Shindoh J, Chen SS, Andreou A, Curley SA, Aloia TA, Maru DM. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2013;258:619-626; discussion 626-627. [PubMed] |
| 69. | Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1610] [Cited by in RCA: 1770] [Article Influence: 136.2] [Reference Citation Analysis (0)] |
| 70. | Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1250] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 71. | Köhne CH, Poston G, Folprecht G, Ciardiello F, Ronga P, Beier F, Van Cutsem E. FOLFIRI plus cetuximab in patients with liver-limited or non-liver-limited RAS wild-type metastatic colorectal cancer: A retrospective subgroup analysis of the CRYSTAL study. Eur J Surg Oncol. 2016;42:1540-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Garufi C, Torsello A, Tumolo S, Ettorre GM, Zeuli M, Campanella C, Vennarecci G, Mottolese M, Sperduti I, Cognetti F. Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. Br J Cancer. 2010;103:1542-1547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 73. | Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, Ye QH, Yu Y, Xu B, Qin XY. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol. 2013;31:1931-1938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 318] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 74. | Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103-2114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 766] [Cited by in RCA: 768] [Article Influence: 51.2] [Reference Citation Analysis (2)] |
| 75. | Tveit KM, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S, Sigurdsson F, Kure E, Ikdahl T, Skovlund E. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol. 2012;30:1755-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 425] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 76. | Köhne CH, Hofheinz R, Mineur L, Letocha H, Greil R, Thaler J, Fernebro E, Gamelin E, Decosta L, Karthaus M. First-line panitumumab plus irinotecan/5-fluorouracil/leucovorin treatment in patients with metastatic colorectal cancer. J Cancer Res Clin Oncol. 2012;138:65-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 77. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-4705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1413] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 78. | Peeters M, Tabernero J. Resection Rates and Survival in Patients with Wild-Type KRAS/NRAS Metastatic Colorectal Cancer and Liver Metastases: Data from the PRIME Study. 2013;. |
| 79. | Gruenberger T, Bridgewater J, Chau I, García Alfonso P, Rivoire M, Mudan S, Lasserre S, Hermann F, Waterkamp D, Adam R. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26:702-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 80. | Adam R, de Haas RJ, Wicherts DA, Aloia TA, Delvart V, Azoulay D, Bismuth H, Castaing D. Is hepatic resection justified after chemotherapy in patients with colorectal liver metastases and lymph node involvement? J Clin Oncol. 2008;26:3672-3680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 81. | Hwang M, Jayakrishnan TT, Green DE, George B, Thomas JP, Groeschl RT, Erickson B, Pappas SG, Gamblin TC, Turaga KK. Systematic review of outcomes of patients undergoing resection for colorectal liver metastases in the setting of extra hepatic disease. Eur J Cancer. 2014;50:1747-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 82. | Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, De Wever I, Michel L. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 497] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 83. | Stang A, Fischbach R, Teichmann W, Bokemeyer C, Braumann D. A systematic review on the clinical benefit and role of radiofrequency ablation as treatment of colorectal liver metastases. Eur J Cancer. 2009;45:1748-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 84. | Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158-171. [PubMed] |
| 85. | Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 236] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 86. | Abdalla EK, Bauer TW, Chun YS, D’Angelica M, Kooby DA, Jarnagin WR. Locoregional surgical and interventional therapies for advanced colorectal cancer liver metastases: expert consensus statements. HPB (Oxford). 2013;15:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 87. | Swaminath A, Dawson LA. Emerging role of radiotherapy in the management of liver metastases. Cancer J. 2010;16:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 88. | Rule W, Timmerman R, Tong L, Abdulrahman R, Meyer J, Boike T, Schwarz RE, Weatherall P, Chinsoo Cho L. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol. 2011;18:1081-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 89. | Kingham TP, D’Angelica M, Kemeny NE. Role of intra-arterial hepatic chemotherapy in the treatment of colorectal cancer metastases. J Surg Oncol. 2010;102:988-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 90. | Allen PJ, Nissan A, Picon AI, Kemeny N, Dudrick P, Ben-Porat L, Espat J, Stojadinovic A, Cohen AM, Fong Y. Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: an institutional experience of 544 consecutive cases. J Am Coll Surg. 2005;201:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 91. | Karanicolas PJ, Metrakos P, Chan K, Asmis T, Chen E, Kingham TP, Kemeny N, Porter G, Fields RC, Pingpank J. Hepatic arterial infusion pump chemotherapy in the management of colorectal liver metastases: expert consensus statement. Curr Oncol. 2014;21:e129-e136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 92. | Boige V, Malka D, Elias D, Castaing M, De Baere T, Goere D, Dromain C, Pocard M, Ducreux M. Hepatic arterial infusion of oxaliplatin and intravenous LV5FU2 in unresectable liver metastases from colorectal cancer after systemic chemotherapy failure. Ann Surg Oncol. 2008;15:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 93. | Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, Weeks JC, Sigurdson ER, Herndon JE, Zhang C. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol. 2006;24:1395-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 296] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 94. | Mocellin S, Pilati P, Lise M, Nitti D. Meta-analysis of hepatic arterial infusion for unresectable liver metastases from colorectal cancer: the end of an era? J Clin Oncol. 2007;25:5649-5654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 95. | Fiorentini G, Aliberti C, Tilli M, Mulazzani L, Graziano F, Giordani P, Mambrini A, Montagnani F, Alessandroni P, Catalano V. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a phase III study. Anticancer Res. 2012;32:1387-1395. [PubMed] |
| 96. | Aliberti C, Tilli M, Benea G, Fiorentini G. Trans-arterial chemoembolization (TACE) of liver metastases from colorectal cancer using irinotecan-eluting beads: preliminary results. Anticancer Res. 2006;26:3793-3795. [PubMed] |
| 97. | Fiorentini G, Aliberti C, Turrisi G, Del Conte A, Rossi S, Benea G, Giovanis P. Intraarterial hepatic chemoembolization of liver metastases from colorectal cancer adopting irinotecan-eluting beads: results of a phase II clinical study. In Vivo. 2007;21:1085-1091. [PubMed] |
| 98. | Aliberti C, Fiorentini G, Muzzio PC, Pomerri F, Tilli M, Dallara S, Benea G. Trans-arterial chemoembolization of metastatic colorectal carcinoma to the liver adopting DC Bead®, drug-eluting bead loaded with irinotecan: results of a phase II clinical study. Anticancer Res. 2011;31:4581-4587. [PubMed] |
| 99. | Narayanan G, Barbery K, Suthar R, Guerrero G, Arora G. Transarterial chemoembolization using DEBIRI for treatment of hepatic metastases from colorectal cancer. Anticancer Res. 2013;33:2077-2083. [PubMed] |
| 100. | Lewis AL, Holden RR. DC Bead embolic drug-eluting bead: clinical application in the locoregional treatment of tumours. Expert Opin Drug Deliv. 2011;8:153-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 101. | Fiorentini G, Aliberti C, Mulazzani L, Coschiera P, Catalano V, Rossi D, Giordani P, Ricci S. Chemoembolization in colorectal liver metastases: the rebirth. Anticancer Res. 2014;34:575-584. [PubMed] |
| 102. | Fiorentini G, Aliberti C, Sarti D, Coschiera P, Tilli M, Mulazzani L, Giordani P, Graziano F, Gonzalez AM, Marcos RG. Locoregional therapy and systemic cetuximab to treat colorectal liver metastases. World J Gastrointest Oncol. 2015;7:47-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 103. | Aliberti C, Carandina R, Sarti D, Mulazzani L, Catalano V, Felicioli A, Coschiera P, Fiorentini G. Hepatic Arterial Infusion of Polyethylene Glycol Drug-eluting Beads for Primary and Metastatic Liver Cancer Therapy. Anticancer Res. 2016;36:3515-3521. [PubMed] |
| 104. | Damm R, Seidensticker R, Ulrich G, Breier L, Steffen IG, Seidensticker M, Garlipp B, Mohnike K, Pech M, Amthauer H. Y90 Radioembolization in chemo-refractory metastastic, liver dominant colorectal cancer patients: outcome assessment applying a predictive scoring system. BMC Cancer. 2016;16:509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 105. | Lim L, Gibbs P, Yip D, Shapiro JD, Dowling R, Smith D, Little A, Bailey W, Liechtenstein M. A prospective evaluation of treatment with Selective Internal Radiation Therapy (SIR-spheres) in patients with unresectable liver metastases from colorectal cancer previously treated with 5-FU based chemotherapy. BMC Cancer. 2005;5:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 106. | Bester L, Meteling B, Pocock N, Saxena A, Chua TC, Morris DL. Radioembolisation with Yttrium-90 microspheres: an effective treatment modality for unresectable liver metastases. J Med Imaging Radiat Oncol. 2013;57:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 107. | Cosimelli M, Golfieri R, Cagol PP, Carpanese L, Sciuto R, Maini CL, Mancini R, Sperduti I, Pizzi G, Diodoro MG. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer. 2010;103:324-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 108. | Hendlisz A, Van den Eynde M, Peeters M, Maleux G, Lambert B, Vannoote J, De Keukeleire K, Verslype C, Defreyne L, Van Cutsem E. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol. 2010;28:3687-3694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 277] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 109. | Seidensticker R, Denecke T, Kraus P, Seidensticker M, Mohnike K, Fahlke J, Kettner E, Hildebrandt B, Dudeck O, Pech M. Matched-pair comparison of radioembolization plus best supportive care versus best supportive care alone for chemotherapy refractory liver-dominant colorectal metastases. Cardiovasc Intervent Radiol. 2012;35:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 110. | Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2286] [Cited by in RCA: 2539] [Article Influence: 253.9] [Reference Citation Analysis (41)] |
| 111. | Lordan JT, Karanjia ND, Quiney N, Fawcett WJ, Worthington TR. A 10-year study of outcome following hepatic resection for colorectal liver metastases - The effect of evaluation in a multidisciplinary team setting. Eur J Surg Oncol. 2009;35:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 112. | Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J, Primrose JN, Parks RW. Guidelines for resection of colorectal cancer liver metastases. Gut. 2006;55 Suppl 3:iii1-iii8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 113. | Ruiz-Tovar J, López Hervás P. Value of third metastasectomy of colorectal adenocarcinoma. Clin Transl Oncol. 2007;9:56-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E, E
P- Reviewer: Kai K, Ooi LLPJ, Rege RV, Zhong JH S- Editor: Song XX L- Editor: A E- Editor: Lu YJ