Published online Feb 10, 2017. doi: 10.5306/wjco.v8.i1.75
Peer-review started: August 23, 2016
First decision: October 21, 2016
Revised: December 1, 2016
Accepted: December 13, 2016
Article in press: December 14, 2016
Published online: February 10, 2017
Processing time: 171 Days and 12.1 Hours
To identify unique clusters of patients based on their concerns in using analgesia for cancer pain and predictors of the cluster membership.
This was a 3-mo prospective observational study (n = 207). Patients were included if they were adults (≥ 18 years), diagnosed with solid tumors or multiple myelomas, and had at least one prescription of around-the-clock pain medication for cancer or cancer-treatment-related pain. Patients were recruited from two outpatient medical oncology clinics within a large health system in Philadelphia. A choice-based conjoint (CBC) analysis experiment was used to elicit analgesic treatment preferences (utilities). Patients employed trade-offs based on five analgesic attributes (percent relief from analgesics, type of analgesic, type of side-effects, severity of side-effects, out of pocket cost). Patients were clustered based on CBC utilities using novel adaptive statistical methods. Multiple logistic regression was used to identify predictors of cluster membership.
The analyses found 4 unique clusters: Most patients made trade-offs based on the expectation of pain relief (cluster 1, 41%). For a subset, the main underlying concern was type of analgesic prescribed, i.e., opioid vs non-opioid (cluster 2, 11%) and type of analgesic side effects (cluster 4, 21%), respectively. About one in four made trade-offs based on multiple concerns simultaneously including pain relief, type of side effects, and severity of side effects (cluster 3, 28%). In multivariable analysis, to identify predictors of cluster membership, clinical and socioeconomic factors (education, health literacy, income, social support) rather than analgesic attitudes and beliefs were found important; only the belief, i.e., pain medications can mask changes in health or keep you from knowing what is going on in your body was found significant in predicting two of the four clusters [cluster 1 (-); cluster 4 (+)].
Most patients appear to be driven by a single salient concern in using analgesia for cancer pain. Addressing these concerns, perhaps through real time clinical assessments, may improve patients’ analgesic adherence patterns and cancer pain outcomes.
Core tip: Lack of adherence to analgesia for cancer pain is a prevalent clinical problem. The 2016 Centers for Disease Control and Prevention guidelines provide recommendations to clinicians for opioid prescription. However, this focus will be incomplete without understanding what concerns anchor patients’ decisions to use analgesia for cancer pain. We used a trade-off analysis technique and novel adaptive methods to first show that unique clusters of patients exist based on the main concerns that anchor their preferences for analgesia for cancer pain. We then identified factors that predict membership in each preference cluster. We found that socioeconomic factors, including education, health literacy, income (rather than attitudes and beliefs about analgesics) played a role in predicting three out of four clusters. Most analgesic beliefs and concerns, including the widely indicated addiction concerns, did not predict cluster membership.
- Citation: Meghani SH, Knafl GJ. Salient concerns in using analgesia for cancer pain among outpatients: A cluster analysis study. World J Clin Oncol 2017; 8(1): 75-85
- URL: https://www.wjgnet.com/2218-4333/full/v8/i1/75.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i1.75
In the early part of 2016, the Centers for Disease Control and Prevention (CDC) released guidelines for prescribing opioids in chronic pain, including cancer pain beyond active cancer treatment[1]. While the guidelines are shaping a conversation and debate among professionals and policy makers on opioid prescription[2-4], little is known about the other side of the coin-patients’ preferences that shape their analgesic taking behaviors. Cancer pain in the United States is mainly managed using analgesics[5]. Non-pharmacological pain treatment approaches are either not consistently offered to patients by their clinicians/covered by health insurance or lack data on clinical effectiveness[6-10]. For the treatments that have demonstrated clinical effectiveness, the cost burden for the patients may be excessive[11,12]. Thus, clinicians and oncologists rely on analgesics as well as opioid medications to help patients whose daily lives and function are affected by significant pain[11]. Unfortunately, patients with unrelieved chronic pain have some of the lowest quality of life observed for any medical condition[13].
Despite widespread use of analgesics in managing cancer pain, there is serious paucity of literature to understand the heuristics cancer patients may employ in making decisions to use analgesics. The few extant studies had methodological aims, that is to investigate the predictive validity of a trade-off analysis technique in eliciting analgesic preferences with diverse subgroups of patients with cancer pain[14]. Others investigating analgesic trade-offs included patients with cancer as part of the broader category of chronic pain sufferers[15]. Also, to our knowledge, no studies have investigated the sociodemographic and clinical predictors of patients’ analgesic preferences. Thus, the purpose of this study was to investigate if unique clusters exist with regard to cancer patients’ preference to use analgesics for cancer pain and factors predicting cluster membership.
This was a prospective study conducted with a cohort of adult (18 years or older) patients who were diagnosed with solid tumors or multiple myelomas and had at least one prescription of around-the-clock pain medication for cancer or cancer-treatment-related pain. Patients were self-identified African-Americans and Whites and were recruited from two outpatient medical oncology clinics within a large health system in Philadelphia, United States. Data were collected at baseline and at 3-mo. This study was approved by the Institutional Review Board of the University of Pennsylvania. All patients provided written informed consent.
Analgesic concern: Analgesic preferences (utilities) for cancer pain was derived from a choice-based conjoint (CBC) analysis experiment, which is a valuation technique based on the Random Utility Theory[16] and mathematical psychology[17]. The goal of CBC is to elicit what people value and what really drives them to choose one set of alternatives over another when facing competing choices[18]. CBC proposes that the overall utility or desirability of any good can be described based on the value of its separate, but, conjoined parts[19], which are termed “attributes”. Each attribute may have multiple levels. Individuals are asked to make trade-offs between attributes and attribute levels generating a unique set of values called part-worth utilities. A higher part-worth utility represents a higher level of value or importance individuals assign to that attribute. The design of CBC experiments is tailored based on the needs of an individual study.
We used a systematic approach to designing the CBC study to elicit analgesic utilities reported in the present study. The procedures are detailed in a previously published manuscript[14]. Trade-offs were elicited on five analgesic attributes: (1) type of analgesic, (2) percentage pain relief with analgesics; (3) type of side-effects; (4) severity of side-effects; and (5) out-of-pocket cost of analgesics. In addition to the design components, we also investigated the internal, external predictive validity and temporal stability of the CBC experiment over the study period[14].
Analgesic attitudes and barriers: Barriers Questionnaire-II[20] was used to assess patients’ attitudes and beliefs about the management of cancer pain. It is a 27-item measure which elicits patients’ pain management concerns in eight domains: (1) fear of addiction; (2) fear of tolerance; (3) fear of side effects; (4) fatalism about cancer pain; (5) desire to be a good patient; (6) fear of distracting health provider from treating cancer; (7) fear that the analgesics impair the immune system; and (8) concern that analgesics may mask ability to monitor illness symptoms. The response range is from 0 (do not agree) to 5 (agree very much). The scores are based on sums for items for the total scale and four subscales (physiological, fatalism, communication, and harmful effects). The internal consistency reliability of the scale is excellent at 0.89[20].
Analgesic side-effects: Side-effects resulting from taking analgesics were assessed using the Medication Side-effects Checklist (MSEC). MSEC elicits information on the presence and severity of eight common analgesic side-effects (i.e., constipation, drowsiness, nausea, vomiting, confusion, dry mouth, stomach irritation, itching) on a scale of 0-10 (no severity-extreme severity). The internal consistency reliability is 0.80[21].
Pain severity and pain-related function: The Brief Pain Inventory (BPI) was used to assess pain severity. The BPI has two subscales; pain intensity (4-items) and pain-related functional interference (7-items: General activity, mood, walking ability, normal work, relationships, sleep and enjoyment of life)[22]. Each item is scored on a 0-10 scale (0 = no pain and 10 = pain as bad as you can imagine; and 0 = no interference and 10). The psychometric properties of the BPI are well-established with cancer patients with a Cronbach’s alpha that ranges from 0.77 to 0.91[23,24].
Pain management index: Pain management index (PMI) is a measure of adequacy of pain treatment based on the World Health Organization’s (WHO) guidelines for managing cancer-related pain[25,26]. The measure takes into account the most potent analgesic prescribed to patients relative to the level of their reported pain. PMI is calculated by subtracting patient’s “pain worst” score (from BPI coded as mild, moderate, or severe) from the most potent analgesia prescribed based on the 3-step WHO analgesic ladder. A negative PMI means inadequate analgesic prescription relative to the pain level.
Social support questionnaire: A 6-item instrument was used to measure participants’ perceptions of social support and satisfaction with social support[27]. The first part of the question asks participants to list individuals who provide social support and the second part asks them to indicate the level of satisfaction with this support. This questionnaire is an abridged version of the original 27-item Social Support Questionnaire[27].
Prescribed analgesics: Prescribed analgesics were coded according to the WHO analgesic ladder[25,26]. This included step 1 (non-opioid analgesics); step 2 (weak opioid analgesics such as codeine); and step 3 (strong opioids such as morphine, oxycodone, methadone).
Sociodemographic and clinical variables: Sociodemographic data were gathered on age, gender, self-identified race, marital status, education, health insurance, household income, job status and health literacy. Health literacy was assessed using three brief screening questions that were previously validated[28] and performs well against the widely used Test of Functional Health Literacy in Adults[28]. The brief questions were also found to be effective in identifying inadequate health literacy (areas under the receiver operating characteristic curve of 0.87, 0.80 and 0.76, respectively for the three questions).
Clinical variables (collected from patients’ medical records) included stage of cancer, time since cancer diagnosis, past history of drug or substance abuse, comorbidities to compute the Charlson Comorbidity Index[29], presence of chronic kidney disease, and presence of depression. Pain and treatment related variables included total number and types of analgesics and co-analgesics, most potent analgesic prescribed, hours pain medications are effective, and pain relief with analgesics.
Descriptive statistics were generated for available baseline variables. A wide variety of variables were considered within the four categories of sociodemographic; illness; pain, function and pain treatment; and analgesic attitudes and barriers. Patients were clustered on their responses to the five analgesic attributes determined by the CBC analysis using the adaptive statistical methods of Knafl et al[30]. A variety of clustering procedures and numbers of clusters were considered, but restricted to alternatives with each cluster containing at least 10% of the patients, thereby avoiding sparse clusters. A clustering alternative was selected using likelihood cross-validation (LCV) scores with likelihoods based on mixtures of multivariate normal distributions as commonly used in cluster analysis.
Models were evaluated and compared using 10-fold LCV scores. These were computed by first randomly partitioning the data into 10 disjoint subsets, called folds. Likelihoods were then computed for the data in each fold using parameter estimates computed from the data in the other folds. These deleted fold likelihoods were combined over all the folds into a LCV score.
A larger LCV score indicates a better model for the data but not necessarily a distinctly better model. This issue was addressed using LCV ratio tests, based on the χ2 distribution (and so analogous to standard likelihood ratio tests). These tests were expressed in terms of a threshold for a distinct (or substantial or significant) percent change in the LCV scores. A percent decrease larger than the threshold indicates that the model with the larger LCV score provides a distinct improvement over the model with the smaller score. Otherwise, the model with the smaller score is a competitive alternative, and if also simpler then preferable as a parsimonious, competitive alternative. The threshold changes with the sample size.
The indicators for being in each of the CBC clusters were modeled separately using logistic regression. This approach allows for identification of a different set of predictors for each cluster and so was considered preferable to multinomial regression modeling of membership in all four clusters combined since that would use the same predictors for all clusters. Each available baseline variable was used to adaptively identify an associated binary characteristic for predicting being in a CBC cluster by dichotomizing the associated variable’s values and choosing the dichotomization that maximized the LCV score (with likelihoods based on the Bernouilli distribution as appropriate for logistic regression). Only dichotomizations with both sets of values having at least 10% of the data were considered to avoid sparse cases. The binary characteristic was defined using the indicator variable with value 1 for the set of values generating an odds ratio (OR) > 1. This indicator was conservatively set to 0 for missing variable values if there were any. The total BQ-II along with each of its subscales and items were considered as predictors to provide a broad assessment of the impact of analgesic attributes and barriers on the analgesic preferences (CBC types or clusters).
Dichotomization can sometimes result in loss of predictive capability compared to using the associated variable as an unadjusted predictor. This can be assessed for ordinal and continuous variables by comparing LCV scores for models based on those variables to the models based on the associate binary characteristics, but only when there are no missing values. LCV ratio tests can be used to assess whether binary characteristics provide a distinct improvement or not by comparing their LCV scores to the score for the constant model (i.e., with only an intercept).
An adaptive multiple binary characteristics model was generated for each CBC-cluster indicator based on the binary characteristics that were individually significantly (P < 0.05) related to it in bivariate models using standard Wald χ2 tests. The adaptive modeling process[31] is based on a heuristic search guided by LCV scores through alternative models. First, the model is systematically expanded adding in predictors, in this case binary characteristics, to the model. The expanded model is then contracted to remove extraneous predictors. LCV ratio tests are used to decide when to stop the contraction, leaving the adaptively generated model. This modeling process is implemented in a SAS® (SAS Institute Inc., Cary, NC) macro available upon request from G. Knafl. All results were computed in SAS Version 9.4.
The statistical methods of this study were reviewed by Dr. George Knafl, Biostatistician and Professor in the School of Nursing at the University of North Carolina at Chapel Hill.
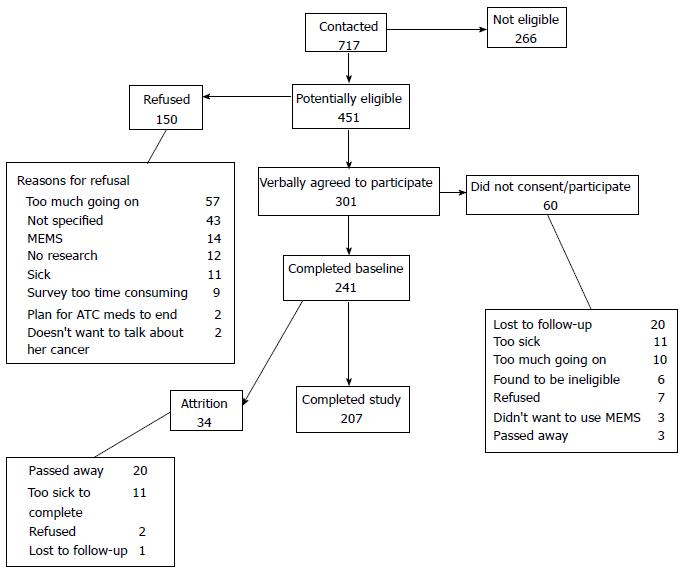
Complete data were available for 207 patients (Figure 1). The baseline demographic and illness related data are presented in Tables 1 and 2, respectively. The mean age of the respondents was 54 years (SD = 11). More than half were married (53%) and had college or more than college education (64%). About one-third (35%) reported a household income of less than $30000 year. None of the patients had any missing CBC analgesic attribute values. Only three of all these variables had any missing values. The threshold for a distinct percent change in LCV score for data with 207 observations is 0.92% (in contrast, the percent decrease is 2.00% for 95 observations and 1.00% for 190 observations).
| Variable | Range | n (%)1 | Mean (SD) |
| Age | 23-75 | 53.8 (11.1) | |
| Education | Elementary | 3 (1.5) | |
| High school | 70 (33.8) | ||
| College/Trade school | 101 (48.8) | ||
| More than college | 33 (15.9) | ||
| Employment status | Employed outside home (full-time) | 43 (20.8) | |
| Employed outside home (part-time) | 12 (5.8) | ||
| Employed at home (full-time) | 4 (1.9) | ||
| Employed at home (part-time) | 4 (1.8) | ||
| Retired | 44 (21.3) | ||
| Unemployed | 25 (12.1) | ||
| Other | 75 (36.2) | ||
| Health literacy | 3-15 | 13.1 (2.6) | |
| Income | < $10000 | 28 (13.5) | |
| $10000-$20000 | 26 (12.6) | ||
| $20000-$30000 | 19 (9.2) | ||
| $30000-$50000 | 36 (17.4) | ||
| $50000-$70000 | 37 (17.9) | ||
| $70000-$90000 | 24 (11.6) | ||
| > $90000 | 37 (17.9) | ||
| Primary insurance (1 missing) | Private | 107 (51.9) | |
| Medicare | 41 (19.9) | ||
| Medicaid | 27 (13.1) | ||
| Multiple | 25 (12.1) | ||
| VA/other | 6 (2.9) | ||
| Marital status | Married | 110 (53.1) | |
| Separated/Divorced | 48 (23.2) | ||
| Widowed | 8 (3.9) | ||
| Never married | 41 (19.8) | ||
| Race | Black/African American | 86 (41.5) | |
| White/Caucasian | 121 (58.5) | ||
| Social support | 0.17-9.00 | 3.7 (2.1) |
| Variable | Range | n (%)1 | Mean (SD) |
| Cancer stage | I | 20 (9.7) | |
| II | 33 (15.9) | ||
| III | 37 (17.9) | ||
| IV | 64 (30.9) | ||
| Unknown or unsure | 53 (25.6) | ||
| Time since cancer diagnosis | 1-120 mo | 36.7 (35.5) | |
| Charlson comorbidity index | 0-13 | 4.3 (2.6) | |
| General health | Excellent | 9 (4.3) | |
| Very good | 23 (11.1) | ||
| Good | 63 (30.4) | ||
| Fair | 77 (37.2) | ||
| Poor | 35 (16.9) | ||
| Physical health not good (number of days within last 30 d) | 0-30 | 14.7 (10.7) | |
| Mental health not good (number of days within last 30 d) | 0-30 | 9.5 (10.7) | |
| Past history of substance abuse | No | 172 (83.1) | |
| Yes | 35 (16.9) | ||
| Presence of depression | No | 120 (58.0) | |
| Yes | 87 (42.0) | ||
| Worst pain (last week) | 0-10 (no pain - pain as bad as you can imagine) | 6.9 (2.4) | |
| Average pain (last week) | 0-10 (no pain - pain as bad as you can imagine) | 4.9 (2.1) | |
| Least pain (last week) | 0-10 (no pain - pain as bad as you can imagine) | 3.4 (2.0) | |
| Pain-related functional interference score | 7-70 (does not interfere-completely interferes) | 35.2 (15.9) | |
| Pain relief with medications (last week) | 1-10 (10%-100%) | 7.2 (2.1) | |
| Pain management index | -2 | 5 (2.4) | |
| -1 | 13 (6.3) | ||
| 0 | 92 (44.4) | ||
| 1 | 63 (30.4) | ||
| 2 | 31 (15.0) | ||
| 3 | 3 (1.4) | ||
| Number of analgesic side effects (MSEC) | 0-8 | 3.8 (2.4) | |
| Severity of analgesic side effects (MSEC) | 8-80 (not severe-extremely severe) | 25.2 (15.0) | |
| BQ-II analgesic barriers (total) | 0-96 | 39.8 (20.1) | |
| No. of complementary alternative modalities used | 0-8 | 2.1 (1.7) |
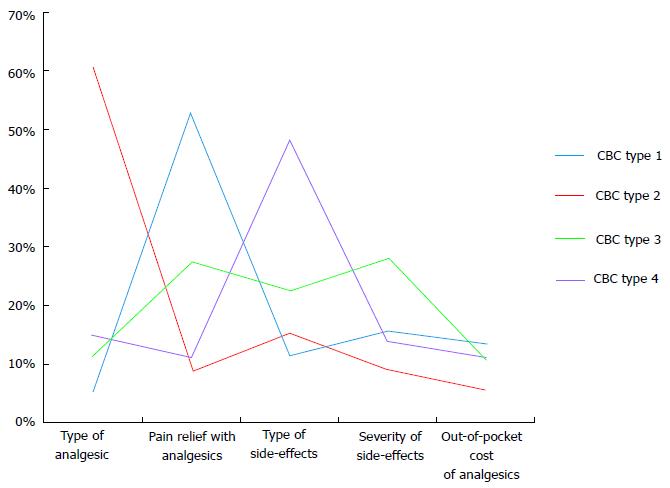
Using methods described (see data analysis), a 4-cluster solution was chosen. Figure 2 contains plots of the four cluster centroids, that is, the vectors with entries equal to averages of the five CBC analgesic attributes for patients in the clusters. Based on these plots, the clusters were characterized in terms of the more strongly rated analgesic attributes (Table 3).
| Cluster | n (%) | Salient concern(s) |
| 1 | 84 (40.6) | Pain relief |
| 2 | 23 (11.1) | Type of analgesic |
| 3 | 57 (27.5) | Pain relief, type of side-effects and severity of side-effects |
| 4 | 43 (20.8) | Type of side-effects |
For less than half the patients (41%) in this study, expectation of pain relief was the main anchor in making analgesic related trade-offs for cancer pain. A total of 16 individually significant binary characteristics were identified for patients in this cluster (Supplemental Table 1). Patients in cluster 1 were more likely be White/Caucasians, carried a private health insurance, had higher education and health literacy, and reported less analgesic-related barriers in general. The strongest of these predictors, that is, the one generating the best (largest) LCV score, was lower endorsement of the belief that pain medicine can mask changes in your health with LCV score 0.51908 (LCV scores not reported).
The individually significant binary characteristics were adaptively combined into a multiple logistic regression model (Table 4). The three factors that remained in the multiple risk factor model and predicted membership in cluster 1 included, higher education, poor physical health and a lower endorsement of the belief that pain medications can mask changes in health. The most important of these (i.e., the one whose removal generated the lowest LCV score) was BQ-II item, pain medicine can mask changes in your health. The LCV score was 0.53503, and so this model provided a distinct improvement over the best individual binary characteristic model with percent decrease 2.98% (since this was larger than the threshold of 0.92%).
| Variable domain | Variable | Characteristic | n (% out of 207) | P value | OR | 95%CI |
| Sociodemographic | Education | College/trade school or more than college vs Elementary or High school | 134 (64.7) | 0.001 | 3.88 | 1.75-8.59 |
| Illness | Physical health not good (number of days within last 30 d) | ≥ 22 vs < 22 | 59 (28.5) | 0.002 | 2.81 | 1.47-5.38 |
| Pain, function and pain treatment | NS | |||||
| Analgesic attitudes and barriers | BQ-II item - pain medicine can mask changes in your health | ≤ 3 vs > 3 | 158 (76.3) | 0.016 | 2.26 | 1.17-4.36 |
For only 11% of patients in this study, the main anchor for analgesic trade-offs was “type of analgesic”. A total of 15 individually significant binary characteristics were identified for patients in cluster type 2 (Supplemental Table 2). Patients in cluster 2 were more likely to have lower income, lower social support, greater burden of comorbidities and pain, and lower relief from taking pain medications. Patients in this cluster were more likely to hold beliefs such as pain medications can harm immune system, or make you addicted. However, the strongest of these predictors was lower (≤ $50000) income with LCV score 0.71212 (LCV scores not reported).
In the multiple logistic regression model, lower social support, health literacy and income levels were predictive of membership in this cluster (Table 5). The most important of these was health literacy (LCV score was 0.72894), and so this model provided a distinct improvement over the best individual binary characteristic model with percent decrease 2.31%.
| Variable domain | Variable | Characteristic | n (% out of 207) | P value | OR | 95%CI |
| Sociodemographic | Health literacy | = 15 vs < 15 | 93 (44.9) | 0.006 | 3.86 | 1.46-10.2 |
| Income | ≤ $50000 vs < $50000 | 109 (52.7) | 0.017 | 3.64 | 1.26-10.5 | |
| Social support | ≤ 4.17 vs > 4.17 | 137 (66.2) | 0.027 | 4.25 | 1.18-15.4 | |
| Illness | NS | |||||
| Pain, function and pain treatment | NS | |||||
| Analgesic attitudes and barriers | NS |
More than one in four patients (28%) made trade-offs based on multiple factors including expectation of pain relief, type of side-effects, and severity of side-effects. A total of 18 individually significant binary characteristics were identified for patients in cluster 3 (Supplemental Table 3). Patients in this cluster were more likely to be married, had greater social support, reported lower pain and pain related functional impairment, and greater pain relief with analgesics. They were less likely to report analgesic side-effects and had lower endorsement for BQ items indicating lower attitudinal barriers. The strongest of these predictors was lower average pain (≤ 6) in the last week with LCV score 0.56530 (LCV scores not reported). In the multiple logistic regression model, being married, having greater social support, having lower average pain, lower side-effects predicted membership in cluster 3 (Table 6).
| Variable domain | Variable | Characteristic | n (% out of 207) | P value | OR | 95%CI |
| Sociodemographic | Marital status | Married vs Separated, Divorced, Widowed or Never married | 110 (53.1) | 0.023 | 2.26 | 1.12-4.56 |
| Social support | ≥ 1.83 vs < 1.83 | 177 (85.5) | 0.022 | 4.55 | 1.24-16.7 | |
| Illness | Mental health not good (number of days within last 30 d) | ≥ 2 vs < 2 | 140 (67.6) | 0.002 | 3.46 | 1.55-772 |
| Pain, function and pain treatment | Average pain (last week) | ≤ 6 vs > 6 | 163 (78.7) | 0.01 | 4.41 | 1.42-6.86 |
| Severity of analgesic side effects (MSEC) | ≤ 28 vs > 28 | 133 (64.3) | 0.005 | 3.11 | 1.41-6.86 | |
| Analgesic attitudes and barriers | NS |
For one in five patients (21%), type of side-effects experienced was the main factor driving analgesic trade-offs. A total of 21 individually significant binary characteristics were identified for patients in cluster type 4 (Supplemental Table 4). Patients in this cluster had lower education and health literacy, were more likely to be Blacks/African Americans, reported lower relief with medications and reported shorter duration of relief with pain medications. Patients in this cluster were more likely to report greater severity of analgesic side-effects and past history of substance abuse but fewer number of days when mental health was not good. Patients in this cluster had the highest number of BQ barriers than any other cluster.
In the multiple logistic regression model, four factors including, lower health literacy, mental health, more analgesic side effects, and belief that pain medications keep you from knowing what is going on in your body predicted membership in this cluster (Table 7).
| Variable domain | Variable | Characteristic | n (% out of 207) | P value | OR | 95%CI |
| Sociodemographic | Health literacy | ≤ 13 vs > 13 | 84 (40.6) | 0.004 | 3.11 | 1.43-6.76 |
| Illness | Mental health not good (number of days within last 30 d) | ≤ 12 vs > 12 | 144 (69.6) | 0.001 | 6.18 | 2.06-18.5 |
| Pain, function and pain treatment | Severity of analgesic side effects (MSEC) | ≥ 40 vs < 40 | 37 (17.9) | 0.002 | 4.19 | 1.68-10.5 |
| Analgesic attitudes and barriers | BQ-II item - pain medicine can keep you from knowing what’s going on in your body | ≥ 4 vs < 4 | 42 (20.3) | < 0.001 | 5.25 | 2.32-11.9 |
This is the first study to identify the sociodemographic and clinical predictors of unique clusters based on what may drive patients’ preference for analgesic treatment for cancer pain. Lack of adherence to analgesia for cancer pain is a prevalent clinical problem[32-35]. Studies in cancer[35] and non-cancer[36-43] pain settings suggest that patterns of analgesic adherence are consequential in explaining clinical and health services outcomes. The 2016 CDC guidelines provide recommendations to clinicians for opioid prescription[1]. However, this focus will be incomplete without an understanding of how patients take prescribed analgesics and what salient concerns anchor their decisions. Previous studies have documented correlates of non-adherence to analgesia for cancer pain[44-47]. These studies do not allow discerning how risk factors and predictors may be distributed dissimilarly across subgroups of cancer patients. Using a well-established trade-off analysis technique (CBC) and more novel adaptive methods, we first showed that unique clusters of patients exist based on the main concern(s) anchoring their preferences for analgesia for cancer pain. We then identified sociodemographic and clinical factors that predict membership in each preference cluster.
Importantly, for an overwhelming majority in this study, analgesic preference for cancer pain was driven by a single salient underlying concern (see cluster 1, 2 and 4). In multivariable analysis to identify predictors of these clusters, “clinical” and “socioeconomic factors” (rather than attitudes and beliefs) were found important. Of note, at least one socioeconomic factor (including education, health literacy, income) played a role in predicting three out of four preference clusters. Furthermore, most analgesic beliefs and concerns, including the widely implicated addiction concerns, did not play a role as predictors of cluster membership. Only the belief that pain medications can mask changes in health or keep you from knowing what is going on in your body was found significant in predicting two of the four clusters. This is a common clinical concern among cancer patients and relates to the fear of disease progression[48-50].
An interesting finding was the contrast between cluster 1 and 4. Unlike cluster 1 (pain relief), those in the side-effects cluster (cluster 4) had lower health literacy and greater analgesic barriers using BQ-II questionnaire. Patients in this cluster were more likely to report greater burden of analgesic side-effects. Of note, there is a stark difference in the identified correlates of these two clusters. The correlates of cluster 1 included being white/Caucasian and having higher education, income and health literacy and lower analgesic barriers. Cluster 4, however was predicted by being African Americans and having lower education, literacy, and more analgesic barriers. Another interesting noteworthy contrast between the two clusters (1 and 4) was that in the multiple logistic regression models, individuals in cluster 1 (pain relief) were less likely to believe that pain medications can mask changes in your health whereas patients in cluster 4 were more likely to endorse pain can keep you from knowing what is going on in your body. Thus, literacy and analgesic beliefs appear to be at play in different ways in the two clusters.
Previous studies have investigated and found racial and socioeconomic disparities in pain management in general, including cancer pain management[51-55]. Our findings indicate that analgesic side-effects are also poorly treated in cancer patients with lower health literacy. These patients will benefit from meticulous assessment of pain and symptoms and accessible interventions that promote self-advocacy and negotiation of pain and side-effects management with their clinicians and oncologists.
In the last few decades, significant resources have been devoted towards psychoeducational interventions that have a major focus on dismantling analgesic beliefs and barriers[56]. Unfortunately, a number of systematic reviews show that these interventions do not improve adherence to analgesia for cancer pain or cancer pain outcomes[57,58]. Our findings imply that meticulous assessment of clinical factors such as pain levels, analgesic side-effects, and addressing SES factors (such as health literacy) may play a role in improving cancer pain outcomes. Also, the finding that decision-making for most patients was driven by single salient underlying factor raises an exciting possibility of designing two-part interventions focused on eliciting real-time trade-offs and linking real-time preferences sequentially to brief, tailored, and patient-centered clinical interventions.
The clusters identified in this study are based on the CBC design. While CBC is a well-established method and we previously tested the validity of the CBC utilities used in this study, there is a notable consideration. About 1 in 3 patients used lexicographic decision rules (i.e., unwillingness to trade more or less of one attribute in favor or detriment of the other)[14]. These processes may represent patients’ actual preferences or mental shortcuts to get through the CBC exercise, potentially compromising the clinical validity of the data. Our confidence that the clusters represent actual preferences is enhanced by the study findings. For instance, patients in cluster 4 (side effects) were more likely to report greater burden of analgesic side-effects, which remained significant in the multivariable model. Similarly, patients in cluster 3 weighed multiple factors similarly (pain relief, type and severity of side-effects) possibly because of their experience of lower pain severity and lower burden of side-effects (e.g., MSEC < 28 in cluster 3 vs > 40 in cluster 4). These findings increase confidence that the clusters identified in this study represent actual preferences rather than mental shortcuts. Also, we restricted our analysis to those patients who completed the study to avoid having missing data that may have affected the conclusions of the study. Excluded patients were with advanced illness who died or were too sick to complete the study (Figure 1), thus we caution against generalizing the findings to those with advanced illness. Nevertheless, our findings inform a scarce body of literature on what anchors cancer patients’ preferences in using analgesia for cancer pain and a potential new path to brief, tailored, and accessible interventions to improve pain and functional outcomes among cancer patients.
The purpose of this study was to investigate if unique clusters exist with regard to patients’ concerns in using analgesics for cancer pain and factors predicting cluster membership.
The new Centers for Disease Control and Prevention opioid guidelines are shaping a national conversation among professionals and policy makers on opioid prescription. Little is known about the other side of the coin, i.e., cancer patients’ concerns in using analgesia and factors shaping these concerns and preferences that may relate to their analgesic taking patterns. This study fills this important gap.
The authors employed novel statistical methods to understand unique subgroups of patients based on their concerns in using analgesics for cancer pain and identified sociodemographic and clinical correlates of these unique clusters. In recent decades, significant resources have been committed to psychoeducational interventions that have a major focus on dismantling analgesic beliefs and barriers. However, recent systematic reviews show that psychoeducational interventions do not consistently improve adherence to analgesia for cancer pain or cancer pain outcomes. The authors’ findings suggest that careful assessment of clinical factors such as analgesic side-effects and addressing social determinants, such as patients’ health literacy, may play a role in improving cancer pain outcomes.
The authors’ study finding that decision-making for most patients was driven by single salient underlying factor raise an exciting possibility of designing two-part interventions focused on eliciting real-time trade-offs and linking real-time preferences sequentially to brief, tailored, and patient-centered clinical interventions.
Analgesic concerns and preferences in this study were elicited using choice-based conjoint (CBC) analysis, which is a trade-off analysis technique. Individuals are asked to make trade-offs between attributes (e.g., pain relief, side-effects) and attribute levels (e.g., percent pain relief, severity of side-effects) generating a unique set of values called part-worth utilities. A higher part-worth utility represents a higher level of value or importance an individual assign to that attribute.
The paper contributes important information.
| 1. | Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1698] [Cited by in RCA: 2133] [Article Influence: 213.3] [Reference Citation Analysis (0)] |
| 2. | ASCO: American Society of Clinical Oncology. ASCO releases principles for balancing appropriate patient access to prescription opioids with curbing misuse, abuse of these drugs (2016). Available from: https://www.asco.org/advocacy-policy/asco-in-action/asco-releases-principles-balancing-appropriate-patient-access. |
| 3. | Paice JA, Lacchetti C, Bruera E. Management of Chronic Pain in Survivors of Adult Cancers: ASCO Clinical Practice Guideline Summary. J Oncol Pract. 2016;12:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Paice JA, Portenoy R, Lacchetti C, Campbell T, Cheville A, Citron M, Constine LS, Cooper A, Glare P, Keefe F. Management of Chronic Pain in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:3325-3345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 435] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 5. | Glare PA, Davies PS, Finlay E, Gulati A, Lemanne D, Moryl N, Oeffinger KC, Paice JA, Stubblefield MD, Syrjala KL. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 6. | Cheville AL, Basford JR. Role of rehabilitation medicine and physical agents in the treatment of cancer-associated pain. J Clin Oncol. 2014;32:1691-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Paley CA, Johnson MI, Tashani OA, Bagnall AM. Acupuncture for cancer pain in adults. Cochrane Database Syst Rev. 2011;CD007753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Paley CA, Tashani OA, Bagnall AM, Johnson MI. A Cochrane systematic review of acupuncture for cancer pain in adults. BMJ Support Palliat Care. 2011;1:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Bardia A, Barton DL, Prokop LJ, Bauer BA, Moynihan TJ. Efficacy of complementary and alternative medicine therapies in relieving cancer pain: a systematic review. J Clin Oncol. 2006;24:5457-5464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Meghani SH. Corporatization of pain medicine: implications for widening pain care disparities. Pain Med. 2011;12:634-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Fisch MJ, Chang VT. Striving for Safe, Effective, Affordable Care for Cancer Survivors With Chronic Pain: Another Kind of Moonshot. JAMA Oncol. 2016;2:862-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Meghani SH. Intended Target of the Centers for Disease Control and Prevention Opioid Guidelines. JAMA Oncol. 2016;2:1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Schmier JK, Palmer CS, Flood EM, Gourlay G. Utility assessments of opioid treatment for chronic pain. Pain Med. 2002;3:218-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Meghani SH, Chittams J, Hanlon AL, Curry J. Measuring preferences for analgesic treatment for cancer pain: how do African-Americans and Whites perform on choice-based conjoint (CBC) analysis experiments? BMC Med Inform Decis Mak. 2013;13:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Chancellor J, Martin M, Liedgens H, Baker MG, Müller-Schwefe GH. Stated preferences of physicians and chronic pain sufferers in the use of classic strong opioids. Value Health. 2012;15:106-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Thurstone LL. A law of comparative judgment. Psychol Review. 1927;34:278-286. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3059] [Cited by in RCA: 3151] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 17. | Luce D, Tukey J. Simultaneous conjoint measurement. J Math Psychol. 1964;1:1-27. [DOI] [Full Text] |
| 18. | Green P, Rao V. Conjoint measurement for quantifying judgmental data. J Mark Res. 1971;8:355-363. [DOI] [Full Text] |
| 19. | Green PE, Srinivasan V. Conjoint analysis in consumer research: issues and outlook. J Consum Res. 1978;5:103-152. [DOI] [Full Text] |
| 20. | Ward SE, Goldberg N, Miller-McCauley V, Mueller C, Nolan A, Pawlik-Plank D, Robbins A, Stormoen D, Weissman DE. Patient-related barriers to management of cancer pain. Pain. 1993;52:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 524] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 21. | Ward SE, Carlson-Dakes K, Hughes SH, Kwekkeboom KL, Donovan HS. The impact on quality of life of patient-related barriers to pain management. Res Nurs Health. 1998;21:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-138. [PubMed] |
| 23. | Anderson KO, Richman SP, Hurley J, Palos G, Valero V, Mendoza TR, Gning I, Cleeland CS. Cancer pain management among underserved minority outpatients: perceived needs and barriers to optimal control. Cancer. 2002;94:2295-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 175] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Cleeland CS, Gonin R, Hatfield AK, Edmonson JH, Blum RH, Stewart JA, Pandya KJ. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330:592-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1497] [Cited by in RCA: 1421] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 25. | World Health Organization. Cancer Pain Relief and Palliative Care. Switzerland: Geneva 1996; . |
| 26. | World Health Organization. Cancer Pain Relief. Switzerland: Geneva 1996; . |
| 27. | Sarason IG, Levine HM, Basham RB, Sarason BR. Assessing social support: the Social Support Questionnaire. J Pers Soc Psychol. 1983;44:127-139. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1533] [Cited by in RCA: 1590] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 28. | Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36:588-594. [PubMed] |
| 29. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32099] [Cited by in RCA: 39638] [Article Influence: 1016.4] [Reference Citation Analysis (0)] |
| 30. | Knafl GJ, Delucchi KL, Bova CA, Fennie KP, Ding K, Williams AB. A systematic approach for analyzing electronically monitored adherence data. Micro Electro Mechanical Systems (MEMS) technology, fabrication processes and applications. New York: Nova Science Publishers 2010; 1-66. |
| 31. | Knafl GJ, Ding K. Adaptive regression for modeling nonlinear relationships. Switzerland: Springer International Publishing, 2016, In press. |
| 32. | Simone CB, Vapiwala N, Hampshire MK, Metz JM. Cancer patient attitudes toward analgesic usage and pain intervention. Clin J Pain. 2012;28:157-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Rhee YO, Kim E, Kim B. Assessment of pain and analgesic use in African American cancer patients: factors related to adherence to analgesics. J Immigr Minor Health. 2012;14:1045-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Meghani SH, Thompson AM, Chittams J, Bruner DW, Riegel B. Adherence to Analgesics for Cancer Pain: A Comparative Study of African Americans and Whites Using an Electronic Monitoring Device. J Pain. 2015;16:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Meghani SH, Knafl GJ. Patterns of analgesic adherence predict health care utilization among outpatients with cancer pain. Patient Prefer Adherence. 2016;10:81-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Braden JB, Russo J, Fan MY, Edlund MJ, Martin BC, DeVries A, Sullivan MD. Emergency department visits among recipients of chronic opioid therapy. Arch Intern Med. 2010;170:1425-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 37. | Sjøgren P, Grønbæk M, Peuckmann V, Ekholm O. A population-based cohort study on chronic pain: the role of opioids. Clin J Pain. 2010;26:763-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | Jensen MK, Thomsen AB, Højsted J. 10-year follow-up of chronic non-malignant pain patients: opioid use, health related quality of life and health care utilization. Eur J Pain. 2006;10:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Sehgal N, Colson J, Smith HS. Chronic pain treatment with opioid analgesics: benefits versus harms of long-term therapy. Expert Rev Neurother. 2013;13:1201-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 984] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 41. | Kern DM, Zhou S, Chavoshi S, Tunceli O, Sostek M, Singer J, LoCasale RJ. Treatment patterns, healthcare utilization, and costs of chronic opioid treatment for non-cancer pain in the United States. Am J Manag Care. 2015;21:e222-e234. [PubMed] |
| 42. | Baser O, Xie L, Mardekian J, Schaaf D, Wang L, Joshi AV. Prevalence of diagnosed opioid abuse and its economic burden in the veterans health administration. Pain Pract. 2014;14:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 43. | Liu Y, Logan JE, Paulozzi LJ, Zhang K, Jones CM. Potential misuse and inappropriate prescription practices involving opioid analgesics. Am J Manag Care. 2013;19:648-665. [PubMed] |
| 44. | Miaskowski C, Dodd MJ, West C, Paul SM, Tripathy D, Koo P, Schumacher K. Lack of adherence with the analgesic regimen: a significant barrier to effective cancer pain management. J Clin Oncol. 2001;19:4275-4279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 168] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 45. | Liang SY, Yates P, Edwards H, Tsay SL. Factors influencing opioid-taking self-efficacy and analgesic adherence in Taiwanese outpatients with cancer. Psychooncology. 2008;17:1100-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Valeberg BT, Miaskowski C, Hanestad BR, Bjordal K, Moum T, Rustøen T. Prevalence rates for and predictors of self-reported adherence of oncology outpatients with analgesic medications. Clin J Pain. 2008;24:627-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 47. | Yoong J, Traeger LN, Gallagher ER, Pirl WF, Greer JA, Temel JS. A pilot study to investigate adherence to long-acting opioids among patients with advanced lung cancer. J Palliat Med. 2013;16:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Torresan MM, Garrino L, Borraccino A, Macchi G, De Luca A, Dimonte V. Adherence to treatment in patient with severe cancer pain: A qualitative enquiry through illness narratives. Eur J Oncol Nurs. 2015;19:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Meghani SH, Keane A. Preference for analgesic treatment for cancer pain among African Americans. J Pain Symptom Manage. 2007;34:136-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Lai YH, Keefe FJ, Sun WZ, Tsai LY, Cheng PL, Chiou JF, Wei LL. Relationship between pain-specific beliefs and adherence to analgesic regimens in Taiwanese cancer patients: a preliminary study. J Pain Symptom Manage. 2002;24:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Meghani SH, Byun E, Gallagher RM. Time to take stock: a meta-analysis and systematic review of analgesic treatment disparities for pain in the United States. Pain Med. 2012;13:150-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 355] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 52. | Meghani SH, Kang Y, Chittams J, McMenamin E, Mao JJ, Fudin J. African Americans with cancer pain are more likely to receive an analgesic with toxic metabolite despite clinical risks: a mediation analysis study. J Clin Oncol. 2014;32:2773-2779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 53. | Nguyen LM, Rhondali W, De la Cruz M, Hui D, Palmer L, Kang DH, Parsons HA, Bruera E. Frequency and predictors of patient deviation from prescribed opioids and barriers to opioid pain management in patients with advanced cancer. J Pain Symptom Manage. 2013;45:506-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 54. | Wieder R, Delarosa N, Bryan M, Hill AM, Amadio WJ. Prescription coverage in indigent patients affects the use of long-acting opioids in the management of cancer pain. Pain Med. 2014;15:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Meghani SH, Bruner DW. A pilot study to identify correlates of intentional versus unintentional nonadherence to analgesic treatment for cancer pain. Pain Manag Nurs. 2013;14:e22-e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. |
Cummings GG, Olivo SA, Biondo PD, Stiles CR, Yurtseven O, Fainsinger RL, Hagen NA.
Effectiveness of knowledge translation interventions to improve cancer pain management. |
| 57. | Oldenmenger WH, Sillevis Smitt PA, van Dooren S, Stoter G, van der Rijt CC. A systematic review on barriers hindering adequate cancer pain management and interventions to reduce them: a critical appraisal. Eur J Cancer. 2009;45:1370-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 58. | Bennett MI, Bagnall AM, José Closs S. How effective are patient-based educational interventions in the management of cancer pain? Systematic review and meta-analysis. Pain. 2009;143:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fassoulaki A, Noll-Hussong M S- Editor: Ji FF L- Editor: A E- Editor: Wu HL