Published online Aug 10, 2015. doi: 10.5306/wjco.v6.i4.73
Peer-review started: February 14, 2015
First decision: March 6, 2015
Revised: April 8, 2015
Accepted: May 16, 2015
Article in press: May 18, 2015
Published online: August 10, 2015
Processing time: 186 Days and 10.2 Hours
AIM: To study the effectiveness of second-line treatments for advancer gastric cancer by application of Bayesian network meta-analysis.
METHODS: Our search covered the literature up to February 2015. The following 6 treatments were evaluated: (1) irinotecan (camptothecins); (2) paclitaxel (taxanes class); (3) docetaxel (taxanes); (4) everolimus (mammalian target of rapamycin inhibitors); (5) ramucirumab (vascular endothelial growth factor receptor 2 inhibitors); (6) ramucirumab + paclitaxel. Our methodology was based on standard models of Bayesian network meta-analysis. The reference treatment was best supportive care (BSC). The end-point was overall survival. Median survival was the outcome measure along with 95% credible intervals.
RESULTS: Our search identified a total of 7 randomized controlled trials. These trials included 2298 patients (in 15 treatment arms) in whom a total of 6 active treatments were evaluated as well as BSC. There were 21 head-to-head comparisons (6 direct, 15 indirect). The difference in survival between each of two active treatments (paclitaxel and ramucirumab + paclitaxel) vs BSC was statistically significant, while the other 4 showed no statistical difference. In the 6 head-to-head comparisons between active treatments, no significant survival difference was demonstrated.
CONCLUSION: Our results indicate that both paclitaxel monotherapy and ramucirumab + paclitaxel determine a significant prolongation in survival as compared with BSC.
Core tip: We carried out a Bayesian network meta-analysis to evaluate second-line treatments for advancer gastric cancer. After scanning the literature up to February 2015, 7 randomized controlled trials were included in our meta-analysis in which the treatments for this disease condition and best supportive care (BSC) were evaluated according to overall survival (OS). Our meta-analysis investigated 21 direct or indirect comparisons. The difference in OS between paclitaxel vs BSC and ramucirumab + paclitaxel vs BSC was statistically significant, while the other comparisons showed no statistical difference. In conclusion, our results indicate that both paclitaxel and ramucirumab + paclitaxel determine a significant prolongation in survival in comparison with BSC.
- Citation: Badiani B, Maratea D, Messori A. Second-line treatments for advanced gastric cancer: Interpreting outcomes by network meta-analysis. World J Clin Oncol 2015; 6(4): 73-79
- URL: https://www.wjgnet.com/2218-4333/full/v6/i4/73.htm
- DOI: https://dx.doi.org/10.5306/wjco.v6.i4.73
Gastric cancer is one of the most common malignancies and the third leading cause of cancer mortality worldwide[1-3]. This disease condition represents 3.4% of all cancers in both sexes, and ranks sixth among all cancers in incidence and fifth as mortality. The incidence varies with age and reaches its peak in the seventh decade of life.
The standard first-line chemotherapy for advanced gastric cancer (AGC) is the association of fluoropyrimidine and platinum complexes with or without anthracyclines[1-5]. However, more than half of patients with AGC do not respond to chemotherapy and even if patients show a response, its duration is only a few months. For this reason, a second-line therapy is needed in most patients.
While several pharmacological options have been proposed as second-line treatment [e.g., taxanes, camptothecins, selective mammalian target of rapamycin (mTOR) inhibitors, and more recently the R2 (VEGF-R2) antagonists of endothelial growth factor VEGF such as ramucirumab], there is currently no standard of care.
In the present study, we performed an updated meta-analysis of second-line treatments for AGC including the data from the most recent randomized controlled trials (RCTs).
Our literature search was conducted in PubMed (http://www.pubmed.org) and in Scopus (http://www.scopus.com) and covered the period from 1 January 1990 to present time (last query on 28 February 2015). A single search term (“advanced gastric cancer”) was employed (in combination with the filter “randomized controlled trials”). Since the number of citations retrieved through these keywords was small (less than 400 with PubMed), we analyzed all of these articles by examining the abstract or, when necessary, their full text, and we identified the RCTs that met our inclusion criteria. These criteria included: (1) metastatic or non-resectable, locally advanced gastric or gastro-esophageal junction adenocarcinoma; (2) age from 18 to 75 years; (3) adequate organ function (bone marrow function, liver function, kidney function); (4) Eastern Cooperative Oncology Group performance status (PS) of 0, 1 or 2; and (5) first-line chemotherapy with fluoropyrimidine plus platinum with or without anthracycline. The end-point of our analysis was overall survival (OS), which was handled as a continuous endpoint.
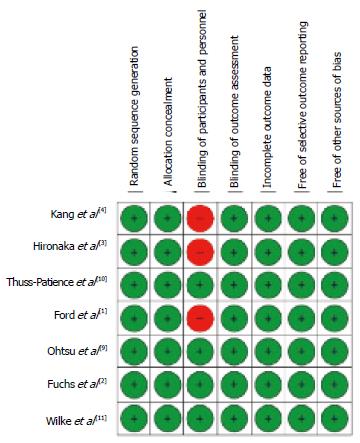
For each trial, we extracted the basic information needed for our analysis and the information on the primary end-point, i.e., OS. Data on OS (median value of OS with lower and upper extremes of the 95%CI) were meant to reflect the intention-to-treat population; however, there were some occasional post-randomization exclusions in some trials, and so our clinical material in some cases reflected the so-called modified intention-to-treat population[6]. As regards the assessment of methodological quality, two reviewers (BB and DM) applied the Cochrane Collaboration’s tool[7] to evaluate the risk of bias in the studies included in our analysis. This tool assesses six domains (namely: random sequence generation, concealment of allocation, blinding of participants and personnel, incomplete data, selective outcome reporting of outcomes, and other sources of bias). Studies with adequate procedures in all domains were considered to have a low risk of bias.
For our statistical analysis, we employed a Bayesian model of network meta-analysis[8]. This approach is advantageous because all treatments under comparison are incorporated into a single model; another advantage is that the Bayesian technique enables rank ordering of each treatment. This Bayesian model is available as fixed-effect model and random-effect model. For the purposes of our analysis, these two versions of the model (i.e., fixed-effect and random-effect) were run separately using the same data set of primary data (median and 95%CI of OS). Thereafter, the Deviance Information Criterion (DIC) was used to choose the model that yielded the better performance. Only the results generated by the better model were presented, while those generated by the worse model were not reported.
In running our analysis, the following second-line chemotherapy treatments were evaluated: (1) irinotecan (class of camptothecin); (2) paclitaxel (class of taxanes); (3) docetaxel (class of taxanes); (4) everolimus (m-TOR inhibitor); (5) ramucirumab (VEGF-R2 inhibitor); and (6) ramucirumab + paclitaxel. Firstly, we analyzed the data of included trials to determine if the OS for each active treatment was significantly different from that of best supportive care (BSC). Next, we estimated the statistics for all pairwise comparisons (6 direct comparisons and 15 indirect comparisons) by determining the difference in OS [with 95% credible interval (CrI)]. The rank order was calculated for each treatment according to the endpoint of OS. In summary, the main output of our analysis consisted of the meta-analytic survival difference with CrIs along with ranking statistics.
Finally, to evaluate the reproducibility of our results, we changed the initial parameter estimates from which the Markov chain Monte Carlo simulation begins according to a verification that is customary employed in these Bayesian analyses. All of our analyses were conducted by using the software package WinBUGS 1.4.3 (Cambridge, United Kingdom) and by running the meta-analysis code for continuous end-points made available by the NICE Support Unit (United Kingdom)[8]. The statistical methods of our study were reviewed by AM according to his role of Lecturer in Medical Statistics at the Faculty of Pharmacy of the University of Firenze, Italy.
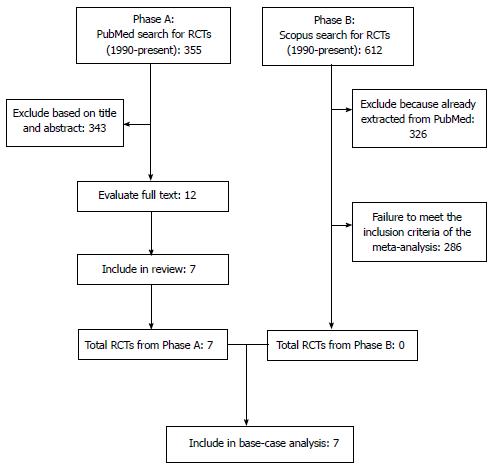
Our literature search is summarized in Figure 1 according to the PRISMA schematic. After the initial selection of 355 articles in PubMed and 612 in Scopus, we examined the full text of 12 articles and we finally identified 7 studies that met our inclusion criteria[1-4,9-11]. The treatments evaluated in these 7 studies are shown in Table 1 along with the information on OS extracted from their respective results. In 6 out of these 7 cases, the RCTs compared a second-line treatment with BSC. Overall, these 7 RCTs enrolled 2298 patients (in 15 treatment arms). As regards the methodological quality, the 7 RCTs showed a low risk of bias. As illustrated in Figure 2, the only source of potential risk of bias was the open-label design of three randomized studies, but all the other items of the scoring method were at low risk of bias.
| Ref. | Year ofpublication | Patients | Control arm | Experimental arm | P value | |||||||
| Age1(yr) | Race | Treatment | N | MedianOS(mo) | SE2(mo) | Treatment | N | MedianOS(mo) | SE2(mo) | |||
| 3Kang et al[4] | 2012 | 56 | Korean | BSC | 69 | 3.8 | 0.36 | Docetaxel | 66 | 5.2 | 0.71 | 0.07 |
| Hironaka et al[3] | 2011 | 65 | Japanese | Irinotecan | 111 | 8.4 | 0.56 | Paclitaxel | 108 | 9.4 | 0.59 | 0.22 |
| Thuss- Patience et al[10] | 2011 | 56 | - | BSC | 19 | 2.4 | 0.82 | Irinotecan | 21 | 4 | 0.99 | 0.22 |
| Ford et al[1] | 2014 | 65 (34-84) | English | BSC | 84 | 3.6 | 0.28 | Docetaxel | 84 | 5.2 | 0.46 | 0.003 |
| Ohtsu et al[9] | 2013 | 62 (22-86) | Various (white, Asian, black or other) | BSC | 217 | 4.3 | 0.43 | Everolimus | 439 | 5.4 | 0.31 | 0.039 |
| Fuchs et al[2] | 2014 | 60 (51-69) | Various (white, Asian, black or other) | BSC | 117 | 3.8 | 1.38 | Ramucirumab | 238 | 5.2 | 1.94 | 0.65 |
| Wilke et al[11] | 2014 | 61 (24-83) | Various (white, Asian, black or other) | Paclitaxel | 335 | 7.4 | 0.54 | Ramucirumab + paclitaxel | 330 | 9.6 | 0.59 | 0.006 |
In running our Bayesian analysis, the value of DIC was found to be more favourable for the fixed-effect model. For this reason, only the results generated by this model are presented below.
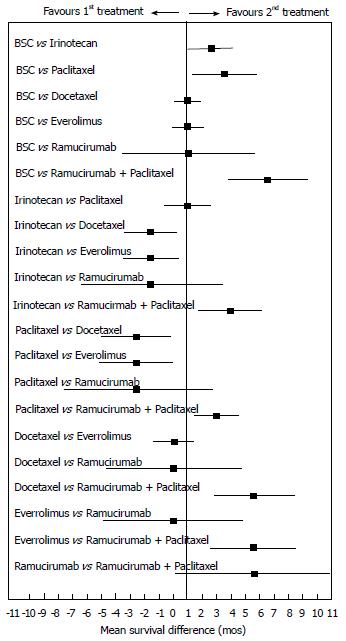
Our results (Figure 3) revealed a statistically significant difference in the direct comparisons between two second-line active treatments vs BSC (namely, paclitaxel monotherapy and ramucirumab + paclitaxel). Furthermore, 4 indirect head-to-head comparisons reached the threshold of statistical significance (namely, the comparisons of ramucirumab + paclitaxel with irinotecan or docetaxel or paclitaxel or everolimus).
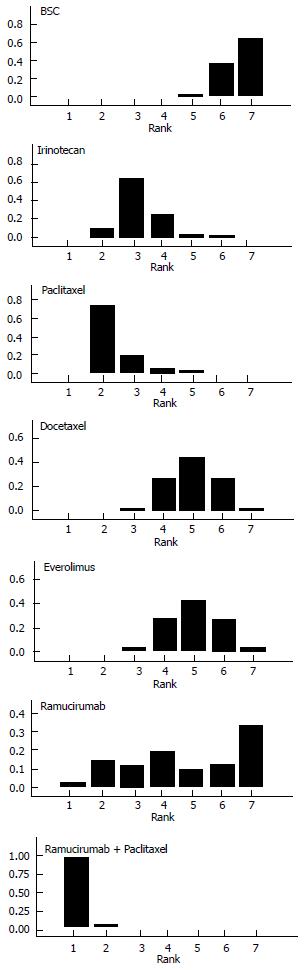
Figure 4 illustrates the ranking histograms generated by the Bayesian probabilistic analysis. Individual rankings for the 6 second-line treatments and BSC were the following (lowest rank = highest effectiveness, highest rank = lowest effectiveness; 95%CrI in parenthesis): ramucirumab+paclitaxel, 1 (1 to 2); paclitaxel, 2 (2 to 5); irinotecan, 3 (2 to 6); docetaxel, 4 (3 to 7); everolimus, 4 (3 to 7); ramucirumab, 6 (1 to 7); BSC, 7 (5 to 7).
Finally, our sensitivity analysis showed that using different initial parameter estimates did not affect the results.
The results of our Bayesian meta-analysis provided a summary of the effectiveness data concerning the main second-line treatments for AGC and were successful in evaluating the statistical significance of differences between active treatments and in defining the ranking in effectiveness for each treatment.
Overall, our results are of interest under several viewpoints. The information on rankings is, in our view, the most interesting result of our analysis. Among the 6 active treatments, ramucirumab + paclitaxel and paclitaxel monotherapy had the two best rankings, while ramucirumab monotherapy had a quite variable ranking.
As shown in Figure 3, our choice of employing an absolute outcome measure (i.e., OS) was advantageous in comparison with the approaches based on relative outcome measures (e.g., relative risk, odds-ratio or hazard ratio) that are commonly employed in meta-analysis[12]. In fact, absolute outcome measures allow us to better interpret the clinical relevance of the differences; for example, the differences shown in Figure 3 that proved to be statistically significant were mostly around 2 or 3 mo, but those involving ramucirumab in association were remarkably around 6 mo.
As confirmed by the present analysis, the Bayesian approach for evaluating direct and indirect comparisons according to a network of treatments shows a number of important advantages, mainly because a single programming language (i.e., Winbugs) has been adopted worldwide for conducting this type of research. This translates into a very high degree of standardization in doing these analyses. For example, if one examines a random sample of Bayesian meta-analyses published over the past months[14-25], it is impressive to see the extraordinary homogeneity of the models adopted by different researchers and also the important scientific impact that this type of research determines as demonstrated by the authoritativeness of the journals where these studies have been published.
The points of strength of our study included the originality of the methodological approach because this is the first “all-in-one” Bayesian network meta-analysis carried out on this topic. Another advantage is that we evaluated the main second-line active treatments currently available for advanced gastric cancer, without focusing the analysis on a single agent (like in other published papers[26]).
In conclusion, our results convey an original information to establish the place in therapy of these 6 pharmacological second-line treatments for AGC.
In patients with advanced gastric cancer requiring second-line treatment, no meta-analysis for indirect comparisons between active treatments has been conducted. All data on effectiveness essentially refer to the comparison between an active treatment and best supportive care. In contrast, data on comparative effectiveness are needed to clarify which treatment is more effective in this disease condition.
Bayesian network meta-analysis is increasingly recognized to be the new standard for analyzing the effectiveness data from a series of randomized trials and for generate a ranking in effectiveness across the active treatments available.
The present study is the first meta-analysis in which a Bayesian network model has been used to synthetize the data of effectiveness and to generate the ranking histograms that are a typical output of this type of statistics.
After a standard literature search, the above Bayesian methodology was applied to a series of 7 randomized trials, that evaluated 5 active treatments in this disease condition. Twi of these trials were focused on ramucirumab, a new agent proposed for this clinical indication. Ramucirumab in association with paclitaxel rank first in comparative effectiveness across the 5 active treatment.
While standard pair-wise meta-analysis examines a single comparison, generally between a single active agent and a single reference treatment (or no treatment), network meta-analysis evaluates all head-to-head combinations across the therapeutic options evaluated in included trials. Network meta-analysis based on Bayesian methods has a further important advantage in that a single statistical analysis (i.e., an “all-in-one” statistical model) allows people to simultaneously evaluate the effectiveness of several treatment options.
In this network meta-analysis study, the author investigated 21 direct or indirect comparisons of overall survival of total of 2298 advanced gastric cancer patients. The result shows that there are statistically significant differences in overall survival between paclitaxel vs best supportive care (BSC) and ramucirumab + paclitaxel vs BSC groups, indicating that both paclitaxel and ramucirumab + paclitaxel determine a significant prolongation in survival in comparison with BSC. This has significance for the second-line drugs treatment of gastric cancer. The paper is about an interesting topic.
| 1. | Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, Mansoor W, Fyfe D, Madhusudan S, Middleton GW. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 457] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 2. | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1625] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 3. | Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, Sugimoto N, Shimodaira H, Tokunaga S, Moriwaki T. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31:4438-4444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 397] [Article Influence: 30.5] [Reference Citation Analysis (5)] |
| 4. | Kang JH, Lee SI, Lim do H, Park KW, Oh SY, Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30:1513-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 495] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 5. | Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. 2002;21:2313-2324. [PubMed] |
| 6. | Montedori A, Bonacini MI, Casazza G, Luchetta ML, Duca P, Cozzolino F, Abraha I; Modified versus standard intention-to-treat reporting: are there differences in methodological quality, sponsorship, and findings in randomized trials? Across-sectional study Trials. . [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [PubMed] |
| 8. | NICE Clinical Guidelines, No . 92 National Clinical Guideline Centre – Acute and Chronic Conditions (UK). London: Royal College of Physicians (UK) 2010; Available from: http://www.ncbi.nlm.nih.gov/books/NBK116530/. |
| 9. | Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol. 2013;31:3935-3943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 379] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 10. | Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, Dogan Y, Gebauer B, Schumacher G, Reichardt P. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer. 2011;47:2306-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 447] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 11. | Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1842] [Article Influence: 153.5] [Reference Citation Analysis (7)] |
| 12. | King NB, Harper S, Young ME. Use of relative and absolute effect measures in reporting health inequalities: structured review. BMJ. 2012;345:e5774. [PubMed] |
| 13. | Altman DG, Bland JM. How to obtain the P value from a confidence interval. BMJ. 2011;343:d2304. [PubMed] |
| 14. | Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 460] [Article Influence: 41.8] [Reference Citation Analysis (8)] |
| 15. | Messori A, Fadda V, Maratea D, Trippoli S. First-line treatments for chronic lymphocytic leukaemia: interpreting efficacy data by network meta-analysis. Ann Hematol. 2015;94:1003-1009. [PubMed] |
| 16. | Palmerini T, Benedetto U, Bacchi-Reggiani L, Della Riva D, Biondi-Zoccai G, Feres F, Abizaid A, Hong MK, Kim BK, Jang Y. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet. 2015;385:2371-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 318] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 17. | Messori A. Cardiovascular safety of new oral anticoagulants: re-analysis of 27 randomized trials based on Bayesian network meta-analysis. Br J Clin Pharmacol. 2015;80:168-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Singh S, Garg SK, Pardi DS, Wang Z, Murad MH, Loftus EV. Comparative efficacy of biologic therapy in biologic-naïve patients with Crohn disease: a systematic review and network meta-analysis. Mayo Clin Proc. 2014;89:1621-1635. [PubMed] |
| 19. | Chen YP, Wang ZX, Chen L, Liu X, Tang LL, Mao YP, Li WF, Lin AH, Sun Y, Ma J. A Bayesian network meta-analysis comparing concurrent chemoradiotherapy followed by adjuvant chemotherapy, concurrent chemoradiotherapy alone and radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2015;26:205-211. [PubMed] |
| 20. | Singh S, Garg SK, Pardi DS, Wang Z, Murad MH, Loftus EV. Comparative efficacy of pharmacologic interventions in preventing relapse of Crohn’s disease after surgery: a systematic review and network meta-analysis. Gastroenterology. 2015;148:64-76.e2; quiz e14. [PubMed] |
| 21. | Nagayama A, Hayashida T, Jinno H, Takahashi M, Seki T, Matsumoto A, Murata T, Ashrafian H, Athanasiou T, Okabayashi K. Comparative effectiveness of neoadjuvant therapy for HER2-positive breast cancer: a network meta-analysis. J Natl Cancer Inst. 2014;106:pii: dju203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Tu B, Rich B, Labos C, Brophy JM. Coronary revascularization in diabetic patients: a systematic review and Bayesian network meta-analysis. Ann Intern Med. 2014;161:724-732. [PubMed] |
| 23. | Messori A, Fadda V, Maratea D, Trippoli S. Indirect meta-analytical comparison of azathioprine and of beta interferon effectiveness in all forms of multiple sclerosis pooled together. J Neurol Sci. 2014;347:408-410. [PubMed] |
| 24. | Windecker S, Stortecky S, Stefanini GG, da Costa BR, Rutjes AW, Di Nisio M, Silletta MG, Maione A, Alfonso F, Clemmensen PM. Revascularisation versus medical treatment in patients with stable coronary artery disease: network meta-analysis. BMJ. 2014;348:g3859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 263] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 25. | Loymans RJ, Gemperli A, Cohen J, Rubinstein SM, Sterk PJ, Reddel HK, Jüni P, ter Riet G. Comparative effectiveness of long term drug treatment strategies to prevent asthma exacerbations: network meta-analysis. BMJ. 2014;348:g3009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Iacovelli R, Pietrantonio F, Farcomeni A, Maggi C, Palazzo A, Ricchini F, de Braud F, Di Bartolomeo M. Chemotherapy or targeted therapy as second-line treatment of advanced gastric cancer. A systematic review and meta-analysis of published studies. PLoS One. 2014;9:e108940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Bener A, Cao N, Grundmann RT, Herszenyi L, Mocellin S, Wang ZH S- Editor: Song XX L- Editor: A E- Editor: Wu HL