Published online Dec 10, 2014. doi: 10.5306/wjco.v5.i5.1097
Revised: June 20, 2014
Accepted: July 25, 2014
Published online: December 10, 2014
Processing time: 247 Days and 2 Hours
AIM: To investigate the age differences in the risk factors, clinicopathological characteristics and patterns of treatment of female breast cancer patients.
METHODS: Seven thousand one hundred and fifty-two women with primary breast cancer from the Hong Kong Breast Cancer Registry were recruited after receiving patients’ consent, they were asked to complete standardized questionnaires which captured their sociodemographic characteristics and risk factors associated with breast cancer development. Among them, clinicopathological data and patterns of treatment were further collected from medical records of 5523 patients with invasive breast cancers. Patients were divided into two groups according to the age at diagnosis: younger (< 40 years old) vs older patients (≥ 40 years old) for subsequent analyses.
RESULTS: Analysis on the sociodemographic characteristics and exposure to risk factors were performed on 7152 women with primary breast cancer and the results revealed that younger patients were more likely to have unhealthy lifestyles; these include a lack of exercise (85.4% vs 73.2%, P < 0.001), having high stress in life (46.1% vs 35.5%, P < 0.001), having dairy/meat-rich diets (20.2% vs 12.9%, P < 0.001), having alcohol drinking habit (7.7% vs 5.2%, P = 0.002). Younger patients were also more likely to have hormone-related risk factors including nulliparity (43.3% vs 17.8%, P < 0.001) and an early age at menarche (20.7% vs 13.2%, P < 0.001). Analyses on clinicopathological characteristics and patterns of treatment were performed on 5523 women diagnosed with invasive breast cancer. The invasive tumours in younger patients showed more aggressive pathological features such as having a higher percentage of grade 3 histology (45.7% vs 36.5%, P < 0.001), having a higher proportion of tumours with lymphovascular invasion (39.6% vs 33.2%, P = 0.003), and having multifocal disease (15.7% vs 10.3%, P < 0.001); they received different patterns of treatment than their older counterparts.
CONCLUSION: Younger patients in Hong Kong are more likely to encounter risk factors associated with breast cancer development and have more aggressive tumours than their older counterparts.
Core tip: We conducted this study to investigate the age differences in the risk factors associated with breast cancer development of female breast cancer patients in Hong Kong. Further, among patients with invasive cancers, we compared the clinicopathological characteristics and the treatments received between these two groups of patients. Younger patients in Hong Kong were found to be more likely to encounter risk factors associated with breast cancer development and have more aggressive tumours than their older counterparts. Based on the current findings, we will conduct further research to evaluate the impact of age at diagnosis on the outcomes of the disease.
- Citation: Yeo W, Lee HM, Chan A, Chan EY, Chan MC, Chan KW, Chan SW, Cheung FY, Cheung PS, Choi PH, Chor JS, Foo WW, Kwan WH, Law SC, Li LP, Tsang JW, Tung Y, Wong LL, Wong TT, Yau CC, Yau TK, Zee BC. Risk factors and natural history of breast cancer in younger Chinese women. World J Clin Oncol 2014; 5(5): 1097-1106
- URL: https://www.wjgnet.com/2218-4333/full/v5/i5/1097.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i5.1097
Breast cancer has been the most frequent cancer affecting women in Hong Kong since 1993. Female breast cancer cases diagnosed in Hong Kong has more than doubled from 1152 in 1993 to 3014 in 2010. In 2010, breast cancer comprised of 24.1% of all cancers among women and was the third leading cause of cancer death in women[1]. On average, about 8 women in Hong Kong were diagnosed with breast cancer every day. The median age at diagnosis among breast cancer patients was 53, compared to that of age of 61 in the United States[2], 61 in Australia[3] and 53 in Singapore[4].
Breast cancer is an age-related disease and increasing age is the single most important risk factor after female gender[5]. In Hong Kong, the age-specific incidence rates for female with invasive breast cancer increased drastically after the age of 40. However, 8.9% of the cases in Hong Kong were diagnosed before the age of 40 in 2010[1], which was higher than that in Australia (aged below 40) (5.6%)[6] and the United Kingdom (aged below 40) (4.2%)[7], while the reported figure for United States patients diagnosed younger before age 45 was 11.4%[8].
It has been reported that risk factors for early onset breast cancer differ from those for postmenopausal breast cancer. Risk factors identified for premenopausal breast cancer include positive family history, high energy (caloric) intake, sedentary lifestyle, early age at menarche, heavy alcohol consumption and a high intake of red meat, while for postmenopausal breast cancer, high body mass index is identified as a risk factor. On the other hand, intense physical activity has been associated with a decreased breast cancer risk in premenopausal women[9].
Although breast cancer in young patients is relatively uncommon, it represents a substantial clinical problem. Extensive researches have been carried out to study the differences in the pathological features of the tumours between younger and older patients. When compared to older patients, it has been suggested that invasive breast cancer in young women has a higher proportion with more aggressive features; these include presence of lymphovascular invasion, Grade 3 histology, extensive intraductal component, presence of necrosis, over-expression of the human epidermalgrowth factor receptor-2 (HER-2) oncogene and absence of estrogen receptor. Irrespective of the pathological differences, younger patients were also found to have poorer prognosis, with different treatment outcome and survival pattern, when compared with their older counterparts[10]. Younger patients were more likely to recur both locoregionally and distantly[11]. Survival rates were also found to be relatively lower for younger women when adjusted for the histologic subtypes and stages[9].
Although the differences in the sociodemographic characteristics, risk factors associated with breast cancer development, clinicopathological characteristics and patterns of treatment among breast cancer women in different ages have been studied extensively worldwide, only two studies were conducted to study the differences in the clinicopathological characteristics and patterns of treatment between the younger and older breast cancer patients in Hong Kong[12,13]. These latter two studies have reported that younger patients were more likely to be diagnosed at more advanced cancer stage, had higher pathological grade, more nodal involvement and presence of lymphovascular permeation; at the same time, a higher proportion of them underwent breast conservation surgery and reconstructive surgery. However, neither has focused on the differences in the sociodemographic characteristics and the risk factors associated with breast cancer development[12,13]. Further, these studies recruited patients at a single hospital only, and as such the data might not reflect the overall clinical pattern of breast cancer in Hong Kong.
Thus, using the data from Hong Kong Breast Cancer Registry, we conducted this study to investigate the differences in the sociodemographic characteristics and risk factors associated with breast cancer development between younger female breast cancer patients and their older counterparts in Hong Kong. Further, among patients with invasive cancers, we compared the clinicopathological characteristics and the treatments received between these two groups of patients.
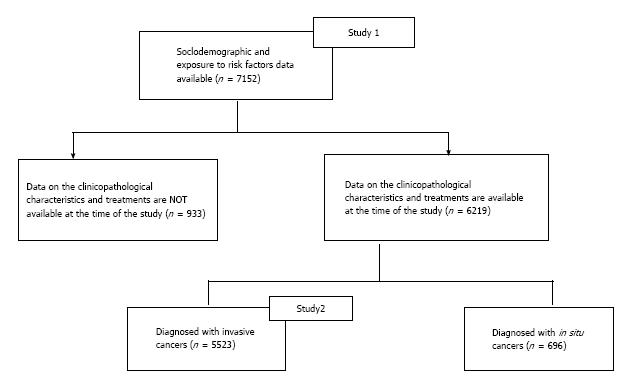
Seven thousand one hundred and fifty-two consecutive Chinese women with primary breast cancer, recruited during the period of February 2008 to April 2012, from the Hong Kong Breast Cancer Registry (HKBCR) were studied. The HKBCR recruited breast cancer patients, regardless of the year of their diagnosis, from 35 centres including both private clinics/hospitals and public hospitals in Hong Kong. Among these women, 4320 (60.4%) were recruited from public hospitals. Ethics approvals for collecting patients’ data for the HKBCR were obtained from the respective institutional review boards of each participating hospital. The overall study consists of 2 parts: Study 1 and Study 2 (Figure 1). All registrants participated in Study 1 upon voluntary written consent; they were asked to complete standardized questionnaires which captured their sociodemographic characteristics data and the risk factors associated with breast cancer development. Among these 7152 patients, 5523 patients with confirmed invasive breast cancers participated in Study 2. The clinicopathological data, including method of first detection, clinical presentation for self-detected cancers, cancer stage, tumour histological type, grade, tumour size, presence of lymphovascular invasion, nodal status, breast cancer biological markers including estrogen receptor (ER), progesterone receptor (PR) and the HER-2 receptor statuses, and the treatments received were further collected for these 5523 patients from their medical records. Patients were arbitrarily divided into two groups according to age at diagnosis: younger (< 40 years old) vs older patients (≥ 40 years old) for subsequent analyses.
The Chi-square test was used to evaluate differences in categorical variables between the different age groups. T-test was used to evaluate the differences in the continuous variable between the two groups of patients. Median test, a non-parametric test, was used where assumptions for parametric tests were not met. A P-value < 0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS software version 19.0 (SPSS Inc., Chicago, IL, United States).
Table 1 summarizes the sociodemographic characteristics of the patient cohort. Although population-based breast screening is not conducted in Hong Kong, Table 1 also provides information on patients’ self-initiated breast screening habits. The age at diagnosis ranged from 18.8 to 101.4 years. Majority (71.0%) of the women was diagnosed in the age group of 40-59 years old, with a mean age ± SD of 50.1 ± 10.4 years and a median age of 48.8 years. Around half (40.5%) of the patients were premenopausal women. 1008 (14.1%) of the patients were categorized as younger patients while 6144 (85.9%) were categorized as older patients. Younger patients were found to be more educated (education level at matriculation or above) (45.5% vs 19.4%, P < 0.001). As expected, more younger patients were engaged in an occupation before cancer diagnosis (80.8% vs 56.0%, P < 0.001) and thus had higher monthly household income (≥ US$3, 750: 50.5% vs 34.6%, P < 0.001) than their older counterparts. In addition, younger patients were also found to have higher rates of breast self-examination (66.2% vs 60.3%, P = 0.001) and clinical breast examination (61.6% vs 56.0%, P = 0.004).
| < 40 yr (n = 1008) n (%) | ≥40 yr (n = 6144) n (%) | P value | |||
| Age | |||||
| < 20 | 1 | (0) | -- | ||
| 20-29 | 84 | (1.2) | |||
| 30-39 | 923 | (12.9) | |||
| 40-49 | 2 978 | (41.6)- | |||
| 50-59 | 2 102 | (29.4) | |||
| 60-69 | 698 | (9.8) | |||
| 70-79 | 268 | (3.7) | |||
| 80+ | 98 | (1.4) | |||
| Education level | |||||
| No schooling | 4 | (0.4) | 344 | (5.7) | χ2 = 473.730, bP < 0.001 |
| Primary | 47 | (4.7) | 1 544 | (25.5) | |
| Secondary | 488 | (49.3) | 2 986 | (49.4) | |
| Matriculation | 133 | (13.4) | 425 | (7) | |
| Undergraduate/postgraduate | 318 | (32.1) | 748 | (12.4) | |
| Occupation | |||||
| Professional/Clerical | 608 | (63) | 1 845 | (31) | χ2 = 412.754, bP < 0.001 |
| Non-clerical/Labor | 126 | (13.1) | 1 298 | (21.8) | |
| Self-employed | 45 | (4.7) | 190 | (3.2) | |
| Housewife | 170 | (17.6) | 2 043 | (34.3) | |
| Retired/Unemployed | 16 | (1.7) | 585 | (9.8) | |
| Monthly household income | |||||
| < HK$10000 | 77 | (10.4) | 837 | (22.5) | χ2 = 88.503, bP < 0.001 |
| HK$10000-29999 | 290 | (39.1) | 1 591 | (42.8) | |
| ≥ HK$30000 | 375 | (50.5) | 1 287 | (34.6) | |
| Breast screening habits | |||||
| Breast self examination | |||||
| Never | 332 | (33.8) | 2 359 | (39.7) | χ2 = 13.136, bP = 0.001 |
| Occasional | 428 | (43.6) | 2 295 | (38.6) | |
| Monthly | 222 | (22.6) | 1 288 | (21.7) | |
| Clinical breast examination | |||||
| Never | 380 | (38.5) | 2 625 | (44.1) | χ2 = 10.934, bP = 0.004 |
| Occasional | 132 | (13.4) | 718 | (12.1) | |
| Regular1 | 476 | (48.2) | 2 611 | (43.9) | |
| Mammogram | |||||
| Never | -- | 3 929 | (66.1) | -- | |
| Occasional | -- | 554 | (9.3) | ||
| Regular1 | -- | 1 463 | (24.6) | ||
| Ultrasound | |||||
| Never | -- | 4 029 | (70.3) | -- | |
| Occasional | -- | 520 | (9.1) | ||
| Regular1 | -- | 1 179 | (20.6) | ||
The associated risk factors within the patient cohort are summarized in Table 2. The proportions having positive family history was significantly higher in younger patients (17.7% vs 14.5%, P = 0.010). A significantly higher proportion of younger patients did not have enough exercise (< 3 h/wk) (85.4% vs 73.2%, P < 0.001). Compared to their older counterparts, they also had a significantly higher proportion of having high level of stress (> 50% of time) (46.1% vs 35.5%, P < 0.001), having diets rich in meat/dairy products (20.2% vs 12.9%, P<0.001), and having alcohol drinking habit (7.7% vs 5.2%, P=0.002). In addition, a significantly higher proportion of younger patients had an early age at menarche (< 12 years old) (20.7% vs 13.2%, P < 0.001) and were nulliparious (43.3% vs 17.8%, P < 0.001). On the other hand, a significantly higher proportion of younger patients who bore children had breastfed their children (46.3% vs 40.9%, P = 0.023). Overweight or obesity was more common in older patients (23.9% vs 38.2%, P < 0.001).
| < 40 yr (n = 1008) n (%) | ≥40 yr (n = 6144) n (%) | P value | |||
| Family history of breast cancer | |||||
| Yes | 178 | (17.7) | 891 | (14.5) | χ2 = 6.788, aP = 0.010 |
| No | 830 | (82.3) | 5 253 | (85.5) | |
| Lifestyle-related risk factors | |||||
| Lack of exercise (< 3h/wk) | |||||
| Yes | 861 | (85.4) | 4 497 | (73.2) | χ2 = 68.848, bP < 0.001 |
| No | 147 | (14.6) | 1 647 | (26.8) | |
| High level of stress (> 50% of time) | |||||
| Yes | 465 | (46.1) | 2 179 | (35.5) | χ2 = 42.272, aP < 0.001 |
| No | 543 | (53.9) | 3 965 | (64.5) | |
| Being overweight/obese (BMI ≥ 23.0) | |||||
| Yes | 241 | (23.9) | 2 344 | (38.2) | χ2 = 76.104, bP < 0.001 |
| No | 767 | (76.1) | 3 800 | (61.8) | |
| Diet rich in meat/dairy products | |||||
| Yes | 204 | (20.2) | 791 | (12.9) | χ2 = 39.205, bP < 0.001 |
| No | 804 | (79.8) | 5 353 | (87.1) | |
| Frequently night shifts | |||||
| Yes | 81 | (11.4) | 285 | (9.3) | χ2 = 2.958, P = 0.091 |
| No | 632 | (88.6) | 2 796 | (90.7) | |
| Alcohol drinking habit | |||||
| Yes | 77 | (7.7) | 316 | (5.2) | χ2 = 10.008, bP = 0.002 |
| No | 925 | (92.3) | 5 747 | (94.8) | |
| Hormone-related risk factors | |||||
| Breastfeeding | |||||
| Yes | 226 | (46.3) | 1 933 | (40.9) | χ2 = 5.249, aP = 0.023 |
| No | 262 | (53.7) | 2 788 | (59.1) | |
| Nulliparity | |||||
| Yes | 381 | (43.3) | 1 042 | (17.8) | χ2 = 297.601, bP < 0.001 |
| No | 499 | (56.7) | 4 805 | (82.2) | |
| First live birth age after 35 | |||||
| Yes | 21 | (4.3) | 238 | (5.1) | χ2 = 0.630, P = 0.514 |
| No | 469 | (95.7) | 4 420 | (94.9) | |
| Early menarche (< 12 yr old) | |||||
| Yes | 197 | (20.7) | 759 | (13.2) | χ2 = 37.243, bP < 0.001 |
| No | 755 | (79.3) | 4 982 | (86.8) | |
| Use of hormone replacement therapy | |||||
| Yes | 5 | (8.1) | 376 | (11.7) | χ2 = 0.778, P = 0.546 |
| No | 57 | (91.9) | 2 841 | (88.3) | |
Table 3 summarizes the differences in the clinicopathological characteristics of the invasive tumours between the two groups of patients. Most (90.5%) of the patients self-detected their cancers but a higher proportion of younger patients self-detected their cancers than the older patients (94.1% vs 90.3%, P = 0.001). Except for a higher proportion of younger patients having nipple discharge as the presenting symptom (5.1% vs 3.0%, P = 0.012), younger and older patients did not show any differences in their presentation symptoms and the duration of symptoms prior to the first medical consultation. A significantly higher proportion of younger patients were diagnosed with early stage cancer (Stage I-IIB) (88.2% vs 83.0%, P = 0.001). Although younger patients tended to have smaller tumour sizes (median tumour size: 1.90 cm vs 2.00 cm, P = 0.065) and negative axillary nodal status (59.4% vs 61.9%, P = 0.075), these tumours usually exhibited more aggressive features. Younger patients had a higher percentage of grade 3 histology (45.7% vs 36.5%, P < 0.001) and a higher proportion of tumours with lymphovascular invasion (39.6% vs 33.2%, P = 0.003). A higher proportion of younger patients had presence of multifocal disease (15.7% vs 10.3%, P < 0.001). On the other hand, the two groups of patients did not show any significant differences in hormone receptor status (ER and PR), as well as the HER2 status. The proportions of patients having triple negative biological subtype (ER-PR-HER2-) were also not significantly different between the two groups of patients.
| < 40 yr (n = 706) n(%) | ≥40 yr (n = 4 817) n(%) | P value | |
| Method of detection | |||
| Self-detected | 589 (94.1) | 3962 (90.3) | χ2 = 9.513, bP = 0.001 |
| Screen-detected1 | 37 (5.9) | 427 (9.7) | |
| Presenting symptoms (for self-detected cancers) | |||
| Lump | 553 (93.9) | 3729 (94.1) | χ2 = 0.049, P = 0.780 |
| Pain | 29 (4.9) | 173 (4.4) | χ2 = 0.375, P = 0.521 |
| Nipple discharge | 30 (5.1) | 118 (3) | χ2 = 7.291, aP = 0.012 |
| Nipple retraction | 5 (0.8) | 75 (1.9) | χ2 = 3.237, P = 0.090 |
| Skin change | 3 (0.5) | 37 (0.9) | χ2 = 1.061, P = 0.475 |
| Axillary node | 5 (0.8) | 23 (0.6) | NA2 |
| Asymmetry | 2 (0.3) | 11 (0.3) | NA2 |
| Duration of onset of symptoms (for self-detected cancers) | |||
| < 1 mo | 93 (39.9) | 536 (39.6) | χ2 = 0.466, P = 0.926 |
| 1-3 mo | 90 (38.6) | 515 (38.1) | |
| 4-12 mo | 33 (14.2) | 212 (15.7) | |
| > 12 mo | 17 (7.3) | 89 (6.6) | |
| Cancer stage | |||
| Early stage (Stage I-IIB) | 603 (88.2) | 3900(83) | χ2 = 11.490, bP = 0.001 |
| Advanced stage (Stage IIIA –IV) | 81 (11.8) | 797(17) | |
| Tumour size4 | |||
| Mean ± SD (cm) | 2.15 ± 1.30 | 2.24 ± 1.49 | T = -1.514, P = 0.130 |
| Median (cm)3 | 1.9 | 2 | P = 0.065 |
| IQR (cm) | 1.40-2.70 | 1.40-2.80 | -- |
| ≤ 2.0 | 361 (55.4) | 2283 (51.5) | χ2 = 3.407, P = 0.071 |
| > 2.0 | 291 (44.6) | 2150 (48.5) | |
| Bloom and Richardson grade4 | |||
| 1 | 80 (12.9) | 806 (19.2) | χ2 = 24.682, bP < 0.001 |
| 2 | 258 (41.5) | 1865 (44.3) | |
| 3 | 284 (45.7) | 1537 (36.5) | |
| Lymphovascular invasion4 | 229 (39.6) | 1336 (33.2) | χ2 = 8.972, bP = 0.003 |
| Disease4 | |||
| Multifocality | 104 (15.7) | 463 (10.3) | χ2 = 17.472, bP < 0.001 |
| Multicentricity | 21 (3.2) | 120 (2.7) | χ2 = 0.597, P = 0.442 |
| Estrogen receptor + ve (ER+)4 | 481 (75) | 3389 (75.6) | χ2 = 0.100, P = 0.768 |
| Progesterone receptor +ve (PR+)4 | 400 (62.7) | 2836 (63.5) | χ2 = 0.162, P = 0.693 |
| Human Epidermal Growth Factor Receptor 2 +ve (HER2+)4 | 151 (28.5) | 970 (26.8) | χ2 = 0.728, P = 0.402 |
| Triple negative subtype4 | 74 (14.0) | 450 (12.5) | χ2 = 1.000, P = 0.327 |
| Nodal status (no. of positive nodes)4 | |||
| 0 | 409 (61.9) | 2680 (59.4) | χ2 = 6.908, P = 0.075 |
| 1-3 | 184 (27.8) | 1198 (26.6) | |
| 4-9 | 44 (6.7) | 420 (9.3) | |
| 10+ | 24 (3.6) | 212 (4.7) |
Table 4 summarizes the treatments received by the patients. Younger patients were significantly more likely to undergo breast conserving surgery (46.1% vs 34.3%, P < 0.001), reconstruction after mastectomy (35.7% vs 11.7%, P < 0.001), and sentinel node biopsy (32.2% vs 28.7%, P = 0.014). Younger patients were significantly more likely to receive radiotherapy (72.0% vs 66.2%, P = 0.002), however, such difference was not observed after adjusted for the type of surgery they received. A higher proportion of younger patients received chemotherapy; specifically, statistical significance were observed in stages I and IIB patients (Stage I: 59.2% vs 39.7%, P < 0.001; Stage IIB: 100.0% vs 91.1%, P < 0.001). However, younger patients were less likely to receive endocrine therapy (68.7% vs 74.2%, P = 0.003).
| < 40 yr (n = 706) n (%) | ≥40 yr (n = 4817) n (%) | P value | |
| Surgery | |||
| Breast conserving surgery | 324 (46.1) | 1638 (34.3) | χ2 = 36.921, bP < 0.001 |
| Mastectomy | 379 (53.9) | 3135 (65.7) | |
| Reconstruction | |||
| Yes | 135 (35.7) | 367 (11.7) | χ2 = 158.189, bP < 0.001 |
| No | 243 (64.3) | 2762 (88.3) | |
| Nodal Surgery | |||
| Sentinel node biopsy (SNB) | 222 (32.2) | 1351 (28.7) | χ2 = 8.496, aP = 0.014 |
| Axillary dissection (AD) | 336 (48.7) | 2572 (54.6) | |
| SNB+AD | 132 (19.1) | 787 (16.7) | |
| Chemotherapy | |||
| Stage I | 151 (59.2) | 633 (39.7) | χ2 = 34.433, bP < 0.001 |
| Stage IIA | 210 (88.6) | 1291 (83.8) | χ2 = 3.572, P = 0.067 |
| Stage IIB | 97 (100) | 631 (91.1) | χ2 = 9.417, bP < 0.001 |
| Stage III | 72 (98.6) | 664 (93.7) | NA1 |
| Stage IV | 8 (100) | 69(89.6) | NA1 |
| Radiotherapy (overall) | |||
| All patients | 496 (72) | 3106 (66.2) | χ2 = 9.189, bP = 0.002 |
| BCS patients | 306 (96.2) | 1522 (95.4) | χ2 = 0.460, P = 0.556 |
| MTX patients | 190 (51.6) | 1564 (51.2) | χ2 = 0.027, P = 0.912 |
| Irradiated regions (overall) | |||
| Breast ± regional lymph nodes | 187 (60.1) | 998 (46) | χ2 = 21.859, bP < 0.001 |
| Chest wall ± regional lymph nodes | 124 (39.9) | 1173 (54) | |
| Endocrine therapy | 477 (68.7) | 3496 (74.2) | χ2 = 9.206, bP = 0.003 |
| Targeted therapy | 59 (8.6) | 339 (7.2) | χ2 = 1.682, P = 0.211 |
This is the first comprehensive study to describe the differences in the sociodemographic characteristics, risk factors associated with breast cancer development, cancer characteristics and patterns of treatment among female breast cancer patients of different ages in Hong Kong. Around one-seventh (14.1%) of the patients were of younger age (aged < 40 years), which was higher than 8.7% recorded by the Hong Kong Cancer Registry[1], reflecting a higher percentage of younger patients having participated in this voluntary study. This figure is also relatively higher than that reported from other areas of the world[6-8].
This study revealed that among patients in Hong Kong, younger patients were more likely to be exposed to risk factors associated with breast cancer development. These included unhealthy lifestyles, such as a lack of exercise, having high stress in life, taking dairy/meat-rich diets and having alcohol drinking habit. They were also more likely to be associated with hormone-related risk factors such as nulliparity, not having breastfeeding experience and having an early age at menarche. Younger patients also had higher proportions with positive family history and these results were in line with those found in studies from other areas of the world[9,14-23]. Combinations of these risk factors might have increased the risks of early onset breast cancer in Hong Kong. Differences in the clinicopathological characteristics and patterns of treatment were also found between younger and older patients.
This study uses the age of 40 as the cut-offs for younger patients. Although most studies categorized women age less than 35 in the “younger” age group, Zhou et al[10] found that women “35 to 40 years of age or younger” defined a group of patients in which age was an independent risk factor associated with high rates of recurrence. The age cut-off in this study is arbitrarily set at 40 years because it has been shown that in Hong Kong women, the risk of having breast cancer after the age of 40 years is significantly increased[1].
Although no population-based breast cancer screening policy exists, younger females in Hong Kong are more aware of breast health than their older counterparts, which can be reflected by their regular self-initiated breast screening habits (both breast self-examination and clinical breast examination) and this might explain why younger patients were more likely to be diagnosed at earlier stages. Despite that, invasive tumours in younger patients showed more aggressive features with a higher proportion having higher grade tumours, presence of lymphovascular invasion, and tumours being multifocal; these are similar to previous report on local Chinese women which included a smaller number of patients[13]. Between the younger and older patient population, there was no difference in the hormone receptor status, and these results were slightly different from those found in other reports from western and Asian populations[18,24-33]. More frequently, younger women were found to have breast cancer with negative endocrine receptors than the older women[18,19,24,25,28,30-32]. In China, a study conducted in 2012 revealed that younger patients were found to have larger tumours, higher metastatic lymph node rates and higher positivity rates for HER-2 overexpression than older patients, while older patients were less likely to be negative for estrogen and progesterone receptors[34].
In the present study, due to the young age at diagnosis and the relatively smaller tumour size, younger patients were more likely to undergo breast conserving surgery. This is associated with a higher rate of breast radiotherapy. Previous reports have described a more aggressive therapeutic strategy with the use of chemotherapy for younger patients in an attempt to optimize the outcome[11,29]. This study concurs with these reports and shows that younger patients with early stage breast cancer had a significantly higher percentage receiving chemotherapy. For patients with more advanced stage disease (stages III and IV), the differences in the rate of using chemotherapy did not reach statistical significance. It has to be noted that chemotherapy can cause age-specific problems such as infertility, bone loss and changes in sexual function and physical appearance, which are of great concerns to younger patients. Thus, whilst aiming to improve the prognosis of these patients with various anticancer therapies, considerations have to be made on treatment-related long term morbidities[35,36].
There have been conflicting reports on young age at diagnosis in breast cancer patients in relation to associated risk factors for breast cancer development, cancer characteristics and prognosis[11,37]. Gene expression profile was suggested to be a powerful predictor of disease outcome in young patients with breast cancer, but age per se was not an independent prognostic factor[38]. However, gene expression profile and outcomes of the disease were not studied in this study, and further research has to be carried out to evaluate the effects of age at diagnosis on the survival outcomes in this group of patients.
Although data from a large number of breast cancer patients are described in this study, several limitations do exist. One of the limitations is that breast cancer in Hong Kong is not a statutory notifiable disease. Although the Hong Kong Cancer Registry captures the incidence and mortality rates for all types of cancers every year, detailed information on breast cancer patients, such as the exposure of risk factors, clinicopathological data etc, were not fully captured. The HKBCR provides a more comprehensive data collection system capturing detailed information on breast cancer cases with patients’ consent. Nevertheless, the respondents of HKBCR are usually a self-selected group, patients who agreed to participate in the registry are likely to be more health conscious when compared to the non-respondents. In addition, since the HKBCR collects registrants’ data by asking them to fill out standardized questionnaires, it is likely that the proportions of older patients and those with difficulty in filling out questionnaires (such as those with advanced disease or recurrent disease) maybe under-represented in the HKBCR. As a result, data on patients who had worse prognosis may have been under-estimated. Another limitation is that since questionnaires were used, data on patients’ stress and nutritional aspects before cancer diagnosis could not be objectively assessed and were only based on patients’ subjective impression.
In conclusion, this study shows that in Hong Kong, there are differences in the sociodemographics characteristics and associated risk factors for breast cancer development in younger vs older breast cancer patients. Further, among those with invasive breast cancers, the clinicopathological characteristics and patterns of treatment between younger and older breast cancer patients vary. A higher proportion of younger women is more likely to be exposed to various breast cancer risk factors and since breast cancer has become the most common cancer among Hong Kong women, efforts have to be made to educate women to undertake primary preventive measures against the development of breast cancer with respective to modifiable factors, for example, leading an active lifestyle and modifying diets. In addition, secondary preventive measures should be considered to enable early detection of breast cancer by increasing breast cancer awareness and conducting prompt breast assessments as clinically indicated. Younger patients were found to have more aggressive tumours than their older counterparts and further research will be conducted to evaluate the impact of age at diagnosis on the outcome of diseases.
We would like to express our gratitude to patients/survivors who participate in the HKBCR, institutions and individuals working with the HKBCR to facilitate data collection at clinics and hospitals throughout the territory, and the HKBCR Steering Committee.
Breast cancer has been the most frequent cancer affecting women in Hong Kong since 1993. It has been reported that risk factors for early onset breast cancer differ from those for postmenopausal breast cancer. To date, only two studies were conducted to address the differences in the clinicopathological characteristics and patterns of treatment between the younger and older breast cancer patients in Hong Kong, and the findings have been limited by small sample size.
Using the data from Hong Kong Breast Cancer Registry, the authors conducted this study to investigate the differences in the sociodemographic characteristics and risk factors associated with breast cancer development between younger female breast cancer patients and their older counterparts in Hong Kong. Further, among patients with invasive cancers, the authors compared the clinicopathological characteristics and the treatments received between these two groups of patients.
Analysis on the sociodemographic characteristics and exposure to risk factors were performed on 7152 women with primary breast cancer. Younger female breast cancer patients in Hong Kong were found to be more likely to encounter risk factors associated with breast cancer development and have more aggressive tumours than their older counterparts.
Based on the current findings, the authors decided to conduct further research to evaluate the impact of age at diagnosis on the outcomes of the disease.
An excellent review of the current status of breast cancer in Hong Kong. The data are used correctly, the methodology is sound and conclusions adequate. The paper represents significant contribution to this field.
| 1. | Hong Kong Cancer Stat 2010. Hong Kong Cancer Registry. Hong Kong: Hospital Authority 2012; . |
| 2. | Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z. SEER Cancer Statistics Review, 1975-2008, National Cancer Institute. Bethesda, MD. Available from: http: //seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011. Last accessed: 24 June 2013. |
| 3. | Australian Institute of Health and Welfare & Australasian Association of Cancer Registries 2012. Cancer in Australia: an overview, 2012. Cancer series no. 74. Cat. no. CAN 70. Canberra: AIHW 2012; . |
| 4. | Howlader N; Singapore. Health Factsheet: Trends of Female Breast Cancer in Singapore 2006-2010. USA: National Registry of Diseases Office 2012; . |
| 5. | Jatoi I, Anderson WF. Qualitative age interactions in breast cancer studies: a mini-review. Future Oncol. 2010;6:1781-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Australian Institute of Health and Welfare. Australian Cancer Incidence and Mortality (ACIM) books - Breast Cancer. Australian: Australian Government 2012; Available from: http: //www.aihw.gov.au/acim-books. Last accessed: 24 June 2013. |
| 7. | United Kindgom, Cancer Research UK. Breast cancer incidence statistics, 2013. Available from: http: //www.cancerresearchuk.org/cancer-info/cancerstats/types/breast/incidence/uk-breast-cancer-incidence-statistics#source1. Last accessed: 24 June 2013. |
| 8. | Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z. SEER Cancer Statistics Review, 1975-2010, National Cancer Institute. Bethesda, MD. Available from: http: //seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site, 2013. Last accessed: 24 June 2013. |
| 9. | Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009;36:237-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 519] [Cited by in RCA: 525] [Article Influence: 30.9] [Reference Citation Analysis (1)] |
| 10. | Zhou P, Recht A. Young age and outcome for women with early-stage invasive breast carcinoma. Cancer. 2004;101:1264-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Beadle BM, Woodward WA, Buchholz TA. The impact of age on outcome in early-stage breast cancer. Semin Radiat Oncol. 2011;21:26-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (2)] |
| 12. | Kwong A, Cheung P, Chan S, Lau S. Breast cancer in Chinese women younger than age 40: are they different from their older counterparts? World J Surg. 2008;32:2554-2561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Wei R, Lau SS, Cheung PS. Breast carcinoma in Chinese women: does age affect treatment choice and outcome? Asian J Surg. 2010;33:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Anderson WF, Chen BE, Brinton LA, Devesa SS. Qualitative age interactions (or effect modification) suggest different cancer pathways for early-onset and late-onset breast cancers. Cancer Causes Control. 2007;18:1187-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Carmichael AR, Bates T. Obesity and breast cancer: a review of the literature. Breast. 2004;13:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 236] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 16. | Chun J, Pocock B, Joseph KA, El-Tamer M, Klein L, Schnabel F. Breast cancer risk factors in younger and older women. Ann Surg Oncol. 2009;16:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Gajdos C, Tartter PI, Bleiweiss IJ, Bodian C, Brower ST. Stage 0 to stage III breast cancer in young women. J Am Coll Surg. 2000;190:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 132] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Kataoka A, Tokunaga E, Masuda N, Shien T, Kawabata K, Miyashita M. Clinicopathological features of young patients (< 35 years of age) with breast cancer in a Japanese Breast Cancer Society supported study. Breast Cancer. 2014;21:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Marchbanks PA, McDonald JA, Wilson HG, Folger SG, Mandel MG, Daling JR, Bernstein L, Malone KE, Ursin G, Strom BL. Oral contraceptives and the risk of breast cancer. N Engl J Med. 2002;346:2025-2032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 250] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 20. | Mavaddat N, Antoniou AC, Easton DF, Garcia-Closas M. Genetic susceptibility to breast cancer. Mol Oncol. 2010;4:174-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 250] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 21. | McCredie MR, Dite GS, Giles GG, Hopper JL. Breast cancer in Australian women under the age of 40. Cancer Causes Control. 1998;9:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Tavani A, Gallus S, La Vecchia C, Negri E, Montella M, Dal Maso L, Franceschi S. Risk factors for breast cancer in women under 40 years. Eur J Cancer. 1999;35:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Velentgas P, Daling JR. Risk factors for breast cancer in younger women. J Natl Cancer Inst Monogr. 1994;15-24. [PubMed] |
| 24. | Colleoni M, Rotmensz N, Robertson C, Orlando L, Viale G, Renne G, Luini A, Veronesi P, Intra M, Orecchia R. Very young women (& lt; 35 years) with operable breast cancer: features of disease at presentation. Ann Oncol. 2002;13:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 267] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 25. | Elkum N, Dermime S, Ajarim D, Al-Zahrani A, Alsayed A, Tulbah A, Al Malik O, Alshabanah M, Ezzat A, Al-Tweigeri T. Being 40 or younger is an independent risk factor for relapse in operable breast cancer patients: the Saudi Arabia experience. BMC Cancer. 2007;7:222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Foo CS, Su D, Chong CK, Chng HC, Tay KH, Low SC, Tan SM. Breast cancer in young Asian women: study on survival. ANZ J Surg. 2005;75:566-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Han W, Kim SW, Park IA, Kang D, Kim SW, Youn YK, Oh SK, Choe KJ, Noh DY. Young age: an independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer. 2004;4:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 179] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Kim JK, Kwak BS, Lee JS, Hong SJ, Kim HJ, Son BH, Ahn SH. Do very young Korean breast cancer patients have worse outcomes? Ann Surg Oncol. 2007;14:3385-3391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Kollias J, Elston CW, Ellis IO, Robertson JF, Blamey RW. Early-onset breast cancer--histopathological and prognostic considerations. Br J Cancer. 1997;75:1318-1323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 140] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Maggard MA, O’Connell JB, Lane KE, Liu JH, Etzioni DA, Ko CY. Do young breast cancer patients have worse outcomes? J Surg Res. 2003;113:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Nixon AJ, Neuberg D, Hayes DF, Gelman R, Connolly JL, Schnitt S, Abner A, Recht A, Vicini F, Harris JR. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol. 1994;12:888-894. [PubMed] |
| 32. | Walker RA, Lees E, Webb MB, Dearing SJ. Breast carcinomas occurring in young women (& lt; 35 years) are different. Br J Cancer. 1996;74:1796-1800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 156] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Winchester DP, Osteen RT, Menck HR. The National Cancer Data Base report on breast carcinoma characteristics and outcome in relation to age. Cancer. 1996;78:1838-1843. [PubMed] |
| 34. | Yang HJ, Yu XF, He XM, Fan JH, Li J, Xu F, Zhang BN, Tang ZH, Zheng S, Qiao YL. Age interactions in breast cancer: an analysis of a 10-year multicentre study in China. Int Med Res. 2012;40:1130-1140. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Goldhirsch A, Glick JH, Gelber RD, Senn HJ. Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. J Natl Cancer Inst. 1998;90:1601-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 328] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 36. | Early Breast Cancer Trialists' Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet. 1998;352:930-942. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1525] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 37. | Yoshida M, Shimizu C, Fukutomi T, Tsuda H, Kinoshita T, Akashi-Tanaka S, Ando M, Hojo T, Fujiwara Y. Prognostic factors in young Japanese women with breast cancer: prognostic value of age at diagnosis. Jpn J Clin Oncol. 2011;41:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4800] [Cited by in RCA: 4444] [Article Influence: 185.2] [Reference Citation Analysis (0)] |
P- Reviewer: Vetvicka V, Wang LS S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ