Published online Dec 10, 2014. doi: 10.5306/wjco.v5.i5.1020
Revised: June 23, 2014
Accepted: September 6, 2014
Published online: December 10, 2014
Processing time: 209 Days and 0.5 Hours
Although a wide range of studies have addressed the relationship between estrogen receptor (ER) expression and prognosis in non-small cell lung cancer (NSCLC), that relationship remains controversial. This is in large part because there is no consensus on the rate of ER expression in NSCLC or on the intracellular distribution of ER expression. This suggests that establishing the relationship between ER expression and prognosis will require standardization of the antibodies used as well as the definition of a positive response. For example, it is supposed from previous studies that ERs in the cytoplasm and nucleus have different relationships to prognosis than ERs in the cytoplasm. Moreover, ER signaling in NSCLC is known to be affected by aromatase, progesterone receptor and epidermal growth factor receptor mutation. However, there has been little functional analysis these mutants and subtypes. This review will focus on what is known about the role of ERs in NSCLC and whether ER can be a useful prognostic marker or therapeutic target in NSCLC.
Core tip: Although there were many studies regarding the role of estrogen receptor (ER) in non-small cell lung cancer (NSCLC), the rate of ER expression or the intracellular distribution of ER remains controversial. This suggests that establishing the relationship between ER expression and prognosis will require standardization of the antibodies used as well as the definition of a positive response. Furthermore, there has been little functional analysis for ER variants. This review will focus on what is known about the role of ERs in NSCLC and whether ER can be a useful prognostic marker or therapeutic target in NSCLC.
- Citation: Kawai H. Estrogen receptors as the novel therapeutic biomarker in non-small cell lung cancer. World J Clin Oncol 2014; 5(5): 1020-1027
- URL: https://www.wjgnet.com/2218-4333/full/v5/i5/1020.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i5.1020
The estrogen receptor (ER) is one of the main targets of breast cancer therapy, and hormone therapy is generally administered to ER-positive breast cancer patients. There are two known ER subtypes, α and β. ER-α is the conventional receptor and is a useful prognostic marker in breast cancer. On the other hand, ER-β is a recently identified ER subtype, widely expressed in various organs including mammary gland and uterus. ER has also been detected in lung cancer cells. The first report of ER expression in lung cancer appeared in 1982[1]. At that time, ERs were detected using radioimmunoassays, and the detection rate was relatively low. The first use of immunohistochemical staining to detect the ER in lung cancer tissue was reported by Canver et al[2] in 1994. Since then, several different anti-ER antibodies have been employed to detect the receptor, and it has become apparent that there are differences in the detection rates and localization of the antigen, depending upon which antibody is used or the definition of a positive result[3-30]. Furthermore, Stabile et al[31] demonstrated that both NSCLC and normal lung express ERs and show biological responses to estrogen. Consequently, the role of ER in lung cancer remains controversial.
It is now known that ER affects other signals, such as that mediated via epidermal growth factor receptor (EGFR)[32]. Based on this finding, a clinical trial of the target plus hormone therapy is now ongoing[33]. However, many questions remain unanswered. This is in part because the reasons for differences in the ER detection rate and the apparently different functions of ERs at different sites remain unclear. This review will focus on previous findings to assess the potential utility of ER as a prognostic factor and as the basis for novel therapeutic strategies for NSCLC, as well as the challenges that will need to be overcome in the future.
In breast cancer cells, the intracellular localization of ER-α is generally performed using clone 1D5 antibody, the epitope for which is in the N-terminus of ER-α. Using this antibody, ER-α is detected in the nucleus. Nuclear ER-α has also been detected using clone 6F11 antibody, which was raised against the full-length form of the receptor molecule[7,8,15,17-20,25]. On the other hand, the rate of ER-α detection in NSCLC using clone 1D5 is very low, from 0%-7%[5-8,10,17,19,23,25]. Moreover, ER-α is reportedly located not only in the nucleus, but also in the cytoplasm and in the plasma membrane[9,11-14,16,17,20-22,24]. Cytoplasmic and plasma membrane ER-α is mainly detected using clone HC-20 antibody, the epitope for which is in the C-terminus. The detection rate with this antibody is 70%-80%, much higher than with clone 1D5[5-14,16,17,19-25]; indeed, we confirmed that ER-α detected using clone HC-20 is nearly always missed by clone 1D5. This suggests that ER-α detected by clone HC-20 may have an N-terminal deletion mutation that prevents its translocation to the nucleus[9,31].
The reports published to date on the immunohistochemical detection of ER-α expression in NSCLC are listed in Table 1. It is noteworthy that the positivity rates vary depending on the definition of “positive” used and on the antibody. To establish ER-α as a prognostic marker in NSCLC, it will necessary to standardize the definition of “positive” based on the use of a particular antibody.
| Ref. | Antibody clone | Location | Detection rates |
| Canver et al[2] | NS | Nucleus | 97% |
| Ollayos et al[3] | NS | Nucleus | 7% |
| Su et al[4] | NS | Nucleus | 6% |
| Di Nunno et al[5] | 1D5 | None | 0 |
| Omoto et al[6] | 1D5 | None | 0 |
| Dabbs et al[7] | 1D5/6F11 | None/nucleus | 0/67% |
| Radzikowska et al[8] | 1D5/6F11 | Nucleus | 3%/3% |
| Kawai et al[9] | HC-20 | Cytoplasm | 73% |
| Schwartz et al[10] | 1D5/6F11 | None | 0/0 |
| Wu et al[11] | NS | Cytoplasm | 3% |
| Schwartz et al[12] | HC-20 | Cytoplasm | 66% |
| Márquez-Garbán et al[13] | HC-20 | Nucleus/cytoplasm | 45%/75% |
| Skov et al[14] | 1D5 | Nucleus/cytoplasm | 3%/55% |
| Niikawa et al[15] | 6F11 | Nucleus | 54% |
| Nose et al[16] | HC-20 | Cytoplasm | 84% |
| Raso et al[17] | 6F11 | Nucleus | 36% |
| HC-20 | Nucleus/cytoplasm | 5%/42% | |
| 1D5 | Nucleus/cytoplasm | 34%/18% | |
| Abe et al[18] | 6F11 | Nucleus | 1% |
| Gomez-Fernandez et al[19] | 1D5/6F11/SP-1 | Nucleus | 8%/14%/27% |
| Mauro et al[20] | 6F11+HC-20 | Nucleus/cytoplasm | 38%/71% |
| Stabile et al[21] | HC-20 | Nucleus/cytoplasm | 39%/54% |
| Sun et al[22] | HC-20 | Cytoplasm | 36% |
| Rades et al[23] | 1D5 | NS | 19% |
| Shimizu et al[24] | HC-20 | Cytoplasm | 47% |
| Rouquette et al[25] | 1D5 | Nucleus | 9% |
| F10 | Nucleus | 8% |
ER-β was first identified in 1996[34], and the first report of ER-β expression in NSCLC was from Omoto et al[6] in 2001. They observed that ER-β is expressed in lung carcinomas as well as in normal lung tissue. They also showed that adenocarcinomas expressed significantly more ER-β than squamous cell carcinomas. Unlike ER-α, strong expression of ER-β is observed in the cytoplasm as well as the nucleus of NSCLC cells. The reports published to date on immunohistochemical detection of ER-β expression in NSCLC are listed in Table 2. Three antibody clones were mainly used in those studies. The epitopes for clones H-150 and 14C8 are in the N-terminus of ER-β, and their detection rates in the nucleus are 51%-74% and 42%-71%, respectively[9,16-18,27,28]. The epitope for the third clone, PPG5/10, is in the C-terminus, and the detection rate in the nucleus is 61%-84%[10,14,26,29]. In recent years, expression of ER-β in NSCLC has been the focus of study more frequently than ER-α, including immunohistochemical analysis of ER-β variants[29]. However, further study will be needed to determine which ER-β variant has the most impact in NSCLC. In immunohistochemical study, it should be made clear which ER expression (i.e., type or location) is more responsible for the NSCLC progression. In addition, it should be also evaluated which antibody is reliable for detecting ER as the biomarker for NSCLC therapy.
| Ref. | Antibody clone | Location | Detection rates |
| Omoto et al[6] | NS | Nucleus | 67% |
| Kawai et al[9] | H-150 | Nucleus | 51% |
| Schwartz et al[10] | PPG5/10 | Nucleus | 61% |
| Wu et al[11] | NS | Nucleus | 46% |
| Márquez-Garbán et al[13] | Polyclonal | Nucleus | 52% |
| Cytoplasm | 69% | ||
| Skov et al[14] | PPG5/10 | Nucleus | 84% |
| Niikawa et al[15] | MS-ER β13-PX1 | Nucleus | 90% |
| Alì et al[26] | PPG5/10 | Nucleus | 75% |
| Nose et al[16] | H-150 | Nucleus | 74% |
| Raso et al[17] | H-150 | Nucleus | 56% |
| Cytoplasm | 98% | ||
| 14C8 | Nucleus | 42% | |
| Cytoplasm | 19% | ||
| Abe et al[18] | 14C8 | Nucleus | 71% |
| Mauro et al[20] | NS | Nucleus | 40% |
| Cytoplasm | 64% | ||
| Navaratnam et al[27] | 14C8 | Nucleus | 49% |
| Rouquette et al[25] | Polyclonal | Nucleus | 38% |
| Karlsson et al[28] | 14C8 | Nucleus | 86% |
| Liu et al[29] | PPG5/10 | Nucleus | 45% |
| Cytoplasm | 59% |
On the other hand, there were some new studies on RNA expression of ERs in NSCLC[35,36]. Brueckl et al[35] reported that ER-α high expression was of significant positive prognostic value and patients with ER-α high tumors did not have any benefit from adjuvant chemotherapy. Atmaca et al[36] demonstrated that ER-α mRNA expression was an independent prognostic factor in metastatic NSCLC. These studies were interesting because of different approach from immunohistochemistry, however, there were small size and needed to be more studied in the future.
It is well known that ER-α expression is a useful prognostic marker in breast cancer[37,38]. In 2005, we first proposed that ERs could potentially serve as prognostic factors in NSCLC[9]. In that study, we observed that cytoplasmic ER-α was predictive of a survival rate in NSCLC. The reports published to date on the relationship between ER expression and prognosis in NSCLC are listed in Table 3. Unlike in breast cancer, expression of ER-α in NSCLC cells is mainly observed in the cytoplasm, and its detection is associated with a poorer prognosis. Among those studies, only one reported that ER-α is predictive of a better prognosis in NSCLC[25]. In that paper, unlike the others, ER-α was detected in the nucleus. Recently, Mauro et al[20] reported that nuclear immunostaining for ER-α expression declines with age. However, it is unclear whether the age-related reduction in nuclear ER-α is associated with prognosis in NSCLC. The cytoplasmic ER-α in NSCLC is a variant type and may be associated with non-genomic signaling. However, it is still unclear whether or not cytoplasmic ER-α affects wild type nuclear ER-α. Thus, many questions remain unanswered about the role of ER-α in NSCLC.
| Ref. | ER subtype | Methods | Location | Prognosis |
| Kawai et al[9] | α | IHC | Cytoplasm | Worse |
| β | IHC | Nucleus | Better | |
| Wu et al[11] | β | IHC | Nucleus | Better |
| Schwartz et al[10] | β | IHC | Nucleus | Better (male) |
| Skov et al[14] | β | IHC | Nucleus | Better (male) |
| Nose et al[16] | α | IHC | Cytoplasm | Worse |
| Abe et al[18] | β | IHC | Nucleus | Better |
| Mauro et al[20] | β | IHC | Nucleus | Better |
| Olivo-Marston et al[30] | α | RT-PCR | NS | Worse |
| Stabile et al[21] | β | IHC | Cytoplasm | Worse |
| Rouquette et al[25] | α | IHC | Nucleus | Better |
| Rades et al[23] | α | IHC | NS | Worse |
| Karlsson et al[28] | β | IHC | Nucleus | Better (ADCA) |
| Liu et al[29] | β2,5 | IHC | Cytoplasm | Better |
In the time since we first reported that ER-β expression was an independent factor associated with a better prognosis of NSCLC[9], there have been several other studies on the relation between ER-β and prognosis in NSCLC (Table 3). Most found that nuclear ER-β was predictive of a better prognosis in NSCLC. When nuclear ER-β is high in NSCLC, nuclear ER-α is low; i.e., wild type ER-α is low. It is therefore thought that ER-β dominates estrogen signaling in NSCLC. On the contrary, a study by Stabile et al[21] found that cytoplasmic ER-β is associated with a poorer prognosis. Interestingly, both ER-α and ER-β are associated with a poor prognosis in NSCLC when they are detected in the cytoplasm. This might be indicative of the non-genomic actions of ER variants.
The role of ER variants in cancer cells has attracted much interest[39-43]. It now appears that the oncogenic potential of ER variants derives from their ability to suppress the action of the normal hormone receptor, thereby acting as a dominant negative oncogene, or from their ability to activate hormone-responsive genes in a hormone-independent manner[44]. ER has two functional domains: AF-1, which associates with a hormone-independent signaling pathway, and AF-2, which associates with a hormone-dependent pathway. Consequently, the oncogenic activity of an ER variant likely depends on specific site of its mutation.
In breast cancer, ER variants are often co-expressed with the wild type receptor, which can affect disease sensitivity to hormone therapy[40,42]. For example, expression of the variant ER-α36 affects estrogen signaling and is associated with tamoxifen resistance in breast cancer[45]. In addition, the localization of ER variants in breast cancer cells reportedly differs from that of wild type ER[42,46].
There has been very little study of ER-α variants in NSCLC, although they appear to be present, given the observed antibody-dependent variation in the ER positivity rate and intracellular localization. There have also been reports that the ER-β1, 2 and 5 variants have distinct distributions within NCLSC cells and distinct effects on prognosis[29]. However, there has been no analysis of the relationship between wild type ER-β and its variants, or their influence on estrogen signaling.
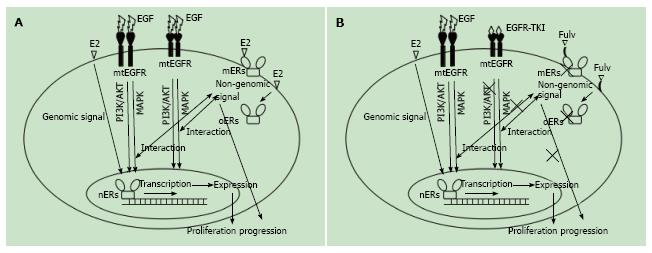
EGFR is a receptor tyrosine kinase involved in pathways leading to DNA synthesis and cell proliferation[47]. Evidence suggests that ER interacts with one or more of the downstream mediators of EGFR signaling in NSCLC[32,48-51], and that mutation of EGFR makes it susceptible to EGFR-tyrosine kinase inhibitor (EGFR-TKI), and thus a therapeutic target[52,53]. The scheme of the interaction of between ER and EGFR signaling in NSCLC is shown in Figure 1A. It is well known that cancers usually become resistant to EGFR-TKI[54], which raises the possibility that this drug resistance is related to the interaction between the EGFR and ER signals. For example, Stabile et al[32] showed that ER signaling is activated by EGFR-TKI in lung cancer cells, while EGFR signaling is activated by anti-estrogen drugs.
Other ER-related mediators include progesterone receptor (PR), androgen receptor (AR) and aromatase. The functional importance of PR and AR in NSCLC remains unknown because expression levels are very low. Aromatase catalyzes the synthesis of estrogen in adipose tissue, and is associated with endogenous estrogen expression in NSCLC[15]. In addition, BRCA1 is a regulator of ER signaling in breast cancer[55-57], and was also recently detected in NSCLC[58-60]. Rosell et al[61] suggested that expression of BRCA1 and an EGFR variant carrying a T790M mutation (known as the EGFR-TKI resistant gene) is predictive of outcome and could provide the basis for alternative individualized treatment to patients with NSCLC. Although it is not yet clear whether BRCA1 is associated with ER in NSCLC, such an association could be a critical determinant of ER signaling in NSCLC.
Clinical trials of lung cancer treatments targeting ER and EGFR are currently ongoing[62]. In these trials, fulvestrant, an ER antagonist, is used in nearly all tests. Fulvestrant acts on ER, blocking its signal, irrespective of whether the receptor is localized in the nucleus, cytoplasm or cell membrane (Figure 1B)[63]. It is therefore thought that fulvestrant would be effective, even in tamoxifen-resistant breast cancers[64]. However, fulvestrant is a selective ER-α antagonist, and in one report fulvestrant acted to stabilize, and thus enhance, ER-β signaling[63]. In NSCLC, extranuclear ER-α is a variant type thought to be involved in non-genomic signaling[31], whereas ER-β is localized in the nucleus, where it exerts genomic effects and associates with a better prognosis[9-11,14,18,20]. It is possible that the genomic signal mediated by ER-β is enhanced by fulvestrant’s blocking of the non-genomic signal of the ER-α variant. Further studies will be needed to resolve the mechanism underlying the therapeutic effects by fulvestrant in NSCLC.
Erlotinib and gefitinib are two tyrosine kinase inhibitors used in the treatment of lung cancer. Erlotinib is used far more to target EGFR and, notably, the effects of both these medications may vary depending upon the race and/or gender of the patient. The intratumoral concentration of gefitinib reaches levels 40 times higher than that in blood[65]. In other words, gefitinib is well distributed to the target tissue. By contrast, intratumoral concentrations of erlotinib are generally lower than its blood concentration[65,66]. Nonetheless, erlotinib’s IC50 for EGFR is more than 10 times lower than that of gefitinib[67]. That is, the effective anti-EGFR dose of erlotinib substantially lower than that of gefitinib. Clinical findings show that both drugs produce an effective response against mutant EGFR, but are less effective against wild type EGFR. To establish a combination therapy targeting both ER and EGFR signaling, development of an antagonist effective against wild type EGFR will be necessary. For example, if the intratumoral concentration of erlotinib could be increased without raising its blood concentration, it may be possible to enhance its antitumor effects without worsening its side effects. With that aim, the use of erlotinib with the angiogenesis inhibitor bevacizumab is being considered[68]. In addition, because EGFR forms a heterodimer with HER-2, the use of the HER-2 inhibitor trastuzumab may be an effective approach to treatment. It may also be useful to consider the interaction or EGFR and/or ER with the ALK fusion protein, which is expressed exclusively with the variant receptors.
The role of ER in NSCLC is gradually becoming clearer. However, ER is involved in a complicated network through its interaction with a variety of mediators. In conventional immunohistochemical studies, wild type ER and its variants are handled similarly, but this may not be the best approach to future development of new strategies for treating NSCLC. Instead, it will be important to establish novel treatments based on the specific ER types dominant in the particular lung cancer being treated, and to select the most effective drugs in that context.
| 1. | Chaudhuri PK, Thomas PA, Walker MJ, Briele HA, Das Gupta TK, Beattie CW. Steroid receptors in human lung cancer cytosols. Cancer Lett. 1982;16:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Canver CC, Memoli VA, Vanderveer PL, Dingivan CA, Mentzer RM. Sex hormone receptors in non-small-cell lung cancer in human beings. J Thorac Cardiovasc Surg. 1994;108:153-157. [PubMed] |
| 3. | Ollayos CW, Riordan GP, Rushin JM. Estrogen receptor detection in paraffin sections of adenocarcinoma of the colon, pancreas, and lung. Arch Pathol Lab Med. 1994;118:630-632. [PubMed] |
| 4. | Su JM, Hsu HK, Chang H, Lin SL, Chang HC, Huang MS, Tseng HH. Expression of estrogen and progesterone receptors in non-small-cell lung cancer: immunohistochemical study. Anticancer Res. 1996;16:3803-3806. [PubMed] |
| 5. | Di Nunno L, Larsson LG, Rinehart JJ, Beissner RS. Estrogen and progesterone receptors in non-small cell lung cancer in 248 consecutive patients who underwent surgical resection. Arch Pathol Lab Med. 2000;124:1467-1470. [PubMed] |
| 6. | Omoto Y, Kobayashi Y, Nishida K, Tsuchiya E, Eguchi H, Nakagawa K, Ishikawa Y, Yamori T, Iwase H, Fujii Y. Expression, function, and clinical implications of the estrogen receptor beta in human lung cancers. Biochem Biophys Res Commun. 2001;285:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 157] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Dabbs DJ, Landreneau RJ, Liu Y, Raab SS, Maley RH, Tung MY, Silverman JF. Detection of estrogen receptor by immunohistochemistry in pulmonary adenocarcinoma. Ann Thorac Surg. 2002;73:403-405; discussion 406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Radzikowska E, Langfort R, Giedronowicz D. Estrogen and progesterone receptors in non small cell lung cancer patients. Ann Thorac Cardiovasc Surg. 2002;8:69-73. [PubMed] |
| 9. | Kawai H, Ishii A, Washiya K, Konno T, Kon H, Yamaya C, Ono I, Minamiya Y, Ogawa J. Estrogen receptor alpha and beta are prognostic factors in non-small cell lung cancer. Clin Cancer Res. 2005;11:5084-5089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 10. | Schwartz AG, Prysak GM, Murphy V, Lonardo F, Pass H, Schwartz J, Brooks S. Nuclear estrogen receptor beta in lung cancer: expression and survival differences by sex. Clin Cancer Res. 2005;11:7280-7287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Wu CT, Chang YL, Shih JY, Lee YC. The significance of estrogen receptor beta in 301 surgically treated non-small cell lung cancers. J Thorac Cardiovasc Surg. 2005;130:979-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 131] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Schwartz AG, Wenzlaff AS, Prysak GM, Murphy V, Cote ML, Brooks SC, Skafar DF, Lonardo F. Reproductive factors, hormone use, estrogen receptor expression and risk of non small-cell lung cancer in women. J Clin Oncol. 2007;25:5785-5792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Márquez-Garbán DC, Chen HW, Fishbein MC, Goodglick L, Pietras RJ. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids. 2007;72:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Skov BG, Fischer BM, Pappot H. Oestrogen receptor beta over expression in males with non-small cell lung cancer is associated with better survival. Lung Cancer. 2008;59:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Niikawa H, Suzuki T, Miki Y, Suzuki S, Nagasaki S, Akahira J, Honma S, Evans DB, Hayashi S, Kondo T. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin Cancer Res. 2008;14:4417-4426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Nose N, Sugio K, Oyama T, Nozoe T, Uramoto H, Iwata T, Onitsuka T, Yasumoto K. Association between estrogen receptor-beta expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J Clin Oncol. 2009;27:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Raso MG, Behrens C, Herynk MH, Liu S, Prudkin L, Ozburn NC, Woods DM, Tang X, Mehran RJ, Moran C. Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clin Cancer Res. 2009;15:5359-5368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Abe K, Miki Y, Ono K, Mori M, Kakinuma H, Kou Y, Kudo N, Koguchi M, Niikawa H, Suzuki S. Highly concordant coexpression of aromatase and estrogen receptor beta in non-small cell lung cancer. Hum Pathol. 2010;41:190-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Gomez-Fernandez C, Mejias A, Walker G, Nadji M. Immunohistochemical expression of estrogen receptor in adenocarcinomas of the lung: the antibody factor. Appl Immunohistochem Mol Morphol. 2010;18:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Mauro LV, Dalurzo M, Carlini MJ, Smith D, Nuñez M, Simian M, Lastiri J, Vasallo B, Bal de Kier Joffé E, Pallotta MG. Estrogen receptor β and epidermal growth factor receptor as early-stage prognostic biomarkers of non-small cell lung cancer. Oncol Rep. 2010;24:1331-1338. [PubMed] |
| 21. | Stabile LP, Dacic S, Land SR, Lenzner DE, Dhir R, Acquafondata M, Landreneau RJ, Grandis JR, Siegfried JM. Combined analysis of estrogen receptor beta-1 and progesterone receptor expression identifies lung cancer patients with poor outcome. Clin Cancer Res. 2011;17:154-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 22. | Sun HB, Zheng Y, Ou W, Fang Q, Li P, Ye X, Zhang BB, Yang H, Wang SY. Association between hormone receptor expression and epidermal growth factor receptor mutation in patients operated on for non-small cell lung cancer. Ann Thorac Surg. 2011;91:1562-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Rades D, Setter C, Dahl O, Schild SE, Noack F. The prognostic impact of tumor cell expression of estrogen receptor-α, progesterone receptor, and androgen receptor in patients irradiated for nonsmall cell lung cancer. Cancer. 2012;118:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Shimizu K, Hirami Y, Saisho S, Yukawa T, Maeda A, Yasuda K, Nakata M. Membrane-bound estrogen receptor-α expression and epidermal growth factor receptor mutation are associated with a poor prognosis in lung adenocarcinoma patients. World J Surg Oncol. 2012;10:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Rouquette I, Lauwers-Cances V, Allera C, Brouchet L, Milia J, Nicaise Y, Laurent J, Delisle MB, Favre G, Didier A. Characteristics of lung cancer in women: importance of hormonal and growth factors. Lung Cancer. 2012;76:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Alì G, Donati V, Loggini B, Servadio A, Dell’Omodarme M, Prati MC, Camacci T, Lucchi M, Melfi F, Mussi A. Different estrogen receptor beta expression in distinct histologic subtypes of lung adenocarcinoma. Hum Pathol. 2008;39:1465-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Navaratnam S, Skliris G, Qing G, Banerji S, Badiani K, Tu D, Bradbury PA, Leighl NB, Shepherd FA, Nowatzki J. Differential role of estrogen receptor beta in early versus metastatic non-small cell lung cancer. Horm Cancer. 2012;3:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Karlsson C, Helenius G, Fernandes O, Karlsson MG. Oestrogen receptor β in NSCLC - prevalence, proliferative influence, prognostic impact and smoking. APMIS. 2012;120:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Liu Z, Liao Y, Tang H, Chen G. The expression of estrogen receptors β2, 5 identifies and is associated with prognosis in non-small cell lung cancer. Endocrine. 2013;44:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Olivo-Marston SE, Mechanic LE, Mollerup S, Bowman ED, Remaley AT, Forman MR, Skaug V, Zheng YL, Haugen A, Harris CC. Serum estrogen and tumor-positive estrogen receptor-alpha are strong prognostic classifiers of non-small-cell lung cancer survival in both men and women. Carcinogenesis. 2010;31:1778-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, Finkelstein S, Siegfried JM. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62:2141-2150. [PubMed] |
| 32. | Stabile LP, Lyker JS, Gubish CT, Zhang W, Grandis JR, Siegfried JM. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res. 2005;65:1459-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 250] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 33. | Traynor AM, Schiller JH, Stabile LP, Kolesar JM, Eickhoff JC, Dacic S, Hoang T, Dubey S, Marcotte SM, Siegfried JM. Pilot study of gefitinib and fulvestrant in the treatment of post-menopausal women with advanced non-small cell lung cancer. Lung Cancer. 2009;64:51-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1630] [Cited by in RCA: 1531] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 35. | Brueckl WM, Al-Batran SE, Ficker JH, Claas S, Atmaca A, Hartmann A, Rieker RJ, Wirtz RM. Prognostic and predictive value of estrogen receptor 1 expression in completely resected non-small cell lung cancer. Int J Cancer. 2013;133:1825-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Atmaca A, Al-Batran SE, Wirtz RM, Werner D, Zirlik S, Wiest G, Eschbach C, Claas S, Hartmann A, Ficker JH. The validation of estrogen receptor 1 mRNA expression as a predictor of outcome in patients with metastatic non-small cell lung cancer. Int J Cancer. 2014;134:2314-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Goldhirsch A, Glick JH, Gelber RD, Senn HJ. Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. J Natl Cancer Inst. 1998;90:1601-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Surowiak P, Materna V, Györffy B, Matkowski R, Wojnar A, Maciejczyk A, Paluchowski P, Dziegiel P, Pudełko M, Kornafel J. Multivariate analysis of oestrogen receptor alpha, pS2, metallothionein and CD24 expression in invasive breast cancers. Br J Cancer. 2006;95:339-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Castles CG, Fuqua SA, Klotz DM, Hill SM. Expression of a constitutively active estrogen receptor variant in the estrogen receptor-negative BT-20 human breast cancer cell line. Cancer Res. 1993;53:5934-5939. [PubMed] |
| 40. | Fuqua SA, Allred DC, Auchus RJ. Expression of estrogen receptor variants. J Cell Biochem Suppl. 1993;17G:194-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Zhang QX, Hilsenbeck SG, Fuqua SA, Borg A. Multiple splicing variants of the estrogen receptor are present in individual human breast tumors. J Steroid Biochem Mol Biol. 1996;59:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Bollig A, Miksicek RJ. An estrogen receptor-alpha splicing variant mediates both positive and negative effects on gene transcription. Mol Endocrinol. 2000;14:634-649. [PubMed] |
| 43. | Herynk MH, Fuqua SA. Estrogen receptor mutations in human disease. Endocr Rev. 2004;25:869-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 280] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 44. | Sluyser M. Nuclear hormone receptor variants: their role in malignancy and progression to hormone resistance in cancer. Acta Endocrinol (Copenh). 1991;125 Suppl 1:48-53. [PubMed] |
| 45. | Shi L, Dong B, Li Z, Lu Y, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B. Expression of ER-{alpha}36, a novel variant of estrogen receptor {alpha}, and resistance to tamoxifen treatment in breast cancer. J Clin Oncol. 2009;27:3423-3429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 46. | Chaudhri RA, Olivares-Navarrete R, Cuenca N, Hadadi A, Boyan BD, Schwartz Z. Membrane estrogen signaling enhances tumorigenesis and metastatic potential of breast cancer cells via estrogen receptor-α36 (ERα36). J Biol Chem. 2012;287:7169-7181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 47. | Gullick WJ, Downward J, Parker PJ, Whittle N, Kris R, Schlessinger J, Ullrich A, Waterfield MD. The structure and function of the epidermal growth factor receptor studied by using antisynthetic peptide antibodies. Proc R Soc Lond B Biol Sci. 1985;226:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, Osborne CK. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res. 2004;10:331S-336S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 352] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 49. | Lichtner RB. Estrogen/EGF receptor interactions in breast cancer: rationale for new therapeutic combination strategies. Biomed Pharmacother. 2003;57:447-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Levin ER. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol. 2003;17:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 238] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 51. | Kawai H, Ishii A, Washiya K, Konno T, Kon H, Yamaya C, Ono I, Ogawa J. Combined overexpression of EGFR and estrogen receptor alpha correlates with a poor outcome in lung cancer. Anticancer Res. 2005;25:4693-4698. [PubMed] |
| 52. | Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7278] [Cited by in RCA: 7565] [Article Influence: 343.9] [Reference Citation Analysis (0)] |
| 53. | Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8739] [Cited by in RCA: 8883] [Article Influence: 403.8] [Reference Citation Analysis (0)] |
| 54. | Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3081] [Cited by in RCA: 3244] [Article Influence: 154.5] [Reference Citation Analysis (0)] |
| 55. | Fan S, Wang J, Yuan R, Ma Y, Meng Q, Erdos MR, Pestell RG, Yuan F, Auborn KJ, Goldberg ID. BRCA1 inhibition of estrogen receptor signaling in transfected cells. Science. 1999;284:1354-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 353] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 56. | Fan S, Ma YX, Wang C, Yuan RQ, Meng Q, Wang JA, Erdos M, Goldberg ID, Webb P, Kushner PJ. Role of direct interaction in BRCA1 inhibition of estrogen receptor activity. Oncogene. 2001;20:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 197] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 57. | Kawai H, Li H, Chun P, Avraham S, Avraham HK. Direct interaction between BRCA1 and the estrogen receptor regulates vascular endothelial growth factor (VEGF) transcription and secretion in breast cancer cells. Oncogene. 2002;21:7730-7739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Taron M, Rosell R, Felip E, Mendez P, Souglakos J, Ronco MS, Queralt C, Majo J, Sanchez JM, Sanchez JJ. BRCA1 mRNA expression levels as an indicator of chemoresistance in lung cancer. Hum Mol Genet. 2004;13:2443-2449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 237] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 59. | Rosell R, Skrzypski M, Jassem E, Taron M, Bartolucci R, Sanchez JJ, Mendez P, Chaib I, Perez-Roca L, Szymanowska A. BRCA1: a novel prognostic factor in resected non-small-cell lung cancer. PLoS One. 2007;2:e1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 60. | Gachechiladze M, Skarda J. The role of BRCA1 in non-small cell lung cancer. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2012;156:200-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Rosell R, Molina MA, Costa C, Simonetti S, Gimenez-Capitan A, Bertran-Alamillo J, Mayo C, Moran T, Mendez P, Cardenal F. Pretreatment EGFR T790M mutation and BRCA1 mRNA expression in erlotinib-treated advanced non-small-cell lung cancer patients with EGFR mutations. Clin Cancer Res. 2011;17:1160-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 250] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 62. | Baik CS, Eaton KD. Estrogen signaling in lung cancer: an opportunity for novel therapy. Cancers (Basel). 2012;4:969-988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Peekhaus NT, Chang T, Hayes EC, Wilkinson HA, Mitra SW, Schaeffer JM, Rohrer SP. Distinct effects of the antiestrogen Faslodex on the stability of estrogen receptors-alpha and -beta in the breast cancer cell line MCF-7. J Mol Endocrinol. 2004;32:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Brünner N, Frandsen TL, Holst-Hansen C, Bei M, Thompson EW, Wakeling AE, Lippman ME, Clarke R. MCF7/LCC2: a 4-hydroxytamoxifen resistant human breast cancer variant that retains sensitivity to the steroidal antiestrogen ICI 182,780. Cancer Res. 1993;53:3229-3232. [PubMed] |
| 65. | McKillop D, Partridge EA, Kemp JV, Spence MP, Kendrew J, Barnett S, Wood PG, Giles PB, Patterson AB, Bichat F. Tumor penetration of gefitinib (Iressa), an epidermal growth factor receptor tyrosine kinase inhibitor. Mol Cancer Ther. 2005;4:641-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 66. | Pollack VA, Savage DM, Baker DA, Tsaparikos KE, Sloan DE, Moyer JD, Barbacci EG, Pustilnik LR, Smolarek TA, Davis JA. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and antitumor effects in athymic mice. J Pharmacol Exp Ther. 1999;291:739-748. [PubMed] |
| 67. | Moyer JD, Barbacci EG, Iwata KK, Arnold L, Boman B, Cunningham A, DiOrio C, Doty J, Morin MJ, Moyer MP. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57:4838-4848. [PubMed] |
| 68. | Herbst RS, Johnson DH, Mininberg E, Carbone DP, Henderson T, Kim ES, Blumenschein G, Lee JJ, Liu DD, Truong MT. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23:2544-2555. [PubMed] |
P- Reviewer: Cebi N, Garfield D, Georgoulias V S- Editor: Song XX L- Editor: A E- Editor: Lu YJ