Published online May 10, 2014. doi: 10.5306/wjco.v5.i2.164
Revised: February 18, 2014
Accepted: March 3, 2014
Published online: May 10, 2014
Processing time: 181 Days and 15.8 Hours
AIM: To analyze the costs of cancer drugs administered in a Portuguese Hospital compared with the Karolinska Institute study.
METHODS: To evaluate spending on cancer drugs, we retrospectively analyzed data on the overall costs of cancer drugs, obtained at the Department of Medical Oncology of the Centro Hospitalar de Entre Douro e Vouga, between 2004 and 2010. In this comparative study we selected only drugs belonging to the following groups: chemotherapy, targeted therapy, immunotherapy and endocrine therapy. The selected drugs were further grouped according to their market placement year: ≤ 1998, 1999 to 2002, 2003 to 2005, and 2006 to 2010. Drugs used as supportive therapy and bisphosphonates were excluded.
RESULTS: The overall costs of cancer drugs increased gradually between 2004 and 2008 (from €1911947 to €3666284), with an increase in the number of patients treated during this period. The expenditure decreased in 2009 (€3438155) and increased again in 2010 (€3673116), but the costs increment was not the same as in previous years. Chemotherapy and targeted therapy were responsible for most of the expenditure. Drugs placed on the national market before 1999 accounted for more than 50% of the expenditure up to 2007. From 2008, these drugs represented less than 50% of the total expenditure. Cancer drugs placed between 1999 and 2002 accounted for 25%-35% of the costs in all the years studied, while drugs placed between 2003 and 2005 accounted for less than 30%. Drugs placed between 2006 and 2010 were responsible for less than 10% of the expenditure.
CONCLUSION: In this study, older drugs were responsible for most of the expenditure up to 2007, which is in agreement with the Karolinska study.
Core tip: In the last decade costs related to cancer drugs have increased significantly. This growth seems to be explained by the increase in cancer incidence, new indications for treatment with previously approved cancer drugs and to placement of new drugs on the market, which are frequently more expensive than those already on sale. The results of the Karolinska Institute study demonstrated a substantial increase in available cancer drugs and costs between 1998 and 2007. The cost increment was not only related to the introduction of new drugs, but 68% of the costs in 2007 were due to drugs approved before 1999.
- Citation: Peixoto V, Faria AL, Gonçalves M, Macedo J, Rego S, Macías E, Magano A, Loureiro M, Araújo A. Evolution of costs of cancer drugs in a Portuguese hospital. World J Clin Oncol 2014; 5(2): 164-169
- URL: https://www.wjgnet.com/2218-4333/full/v5/i2/164.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i2.164
In Portugal, the number of inhabitants and the average life expectancy at birth have increased in the last century[1,2]. The most recent data revealed that the average life expectancy in Portugal is 80.8 years, similar to the European average[3]. This increase reflects improvements in the population’s socioeconomic conditions and in the resources dedicated to health care[1,4,5].
In the aging population, among other aspects, an increase in the incidence in chronic and incurable diseases has been observed[4,6,7]. Of these diseases, the incidence of cancer dominates[4,6,7]. Cancer incidence has increased over the last decades and in 2008 there was an estimated 12.7 million new cancer cases worldwide[4,6,7]. In Portugal, the cancer incidence rate standardized by age is 428:100000 in men and 289:100000 in women[1]. Globally, cancer is the second most common cause of death after cardiovascular diseases[1,8]. In recent years, there has been a slight decrease in the mortality rate related to cancer[1,4,6-8]. However, this rate is still high[1,3-5]. In 2008, 7.6 million cancer deaths occurred worldwide[7].
The burden of cancer to society can be measured by direct and indirect costs[4,6,9,10]. Direct costs are related to prevention and treatment, while indirect costs include loss of production due to inability to work caused by disease, disability and death[4,6,9,10]. Drugs are one of the most investigated components of Oncology, consuming most of its economic resources[4,8,11]. In the past few years, direct costs related to cancer treatment have increased significantly[4,12-15]. This increment in costs can be explained by the increase in cancer incidence, new indications for treatment with previously approved cancer drugs and to placement of new drugs on the market, which are frequently more expensive those already on sale[4,12,16-19]. Despite the continuous growth in expenditure due to cancer drugs, this growth is not expected to be the same as in the last decade[4].
According to the Karolinska Institute study, in 2007 the cost increment was not only related to the introduction of new drugs, but 68% of the costs were due to drugs approved before 1999 in Europe[4]. The increased cost of these drugs, from €4.3 to €26.3 per capita, was the major cause of the rise in costs related to cancer drugs[4]. In this study, cancer drugs (chemotherapy, targeted therapy, endocrine therapy and immunotherapy) were grouped according to their market placement year: ≤ 1998; 1999 to 2002; 2003 to 2005; 2006 to 2007. Supportive drug treatments were excluded[4].
In Portugal, the growth in public spending on cancer drugs has been the subject of great debate[1,9]. However, data related to cancer treatment, particularly the direct costs of drug treatments are scarce[9]. For this reason, we conducted the current study to better understand the costs involved in cancer drug therapies in Portugal.
The aims of the current study were the analysis the cost evolution of cancer drugs from data collected from 2004 to 2010 at the Department of Medical Oncology of the Centro Hospitalar de Entre Douro e Vouga. An analysis of costs according to the type of drug and the date of its placement on the national market was also performed and compared with the results obtained in the Karolinska Institute study.
After obtaining the necessary authorization from our Administration Board, we conducted a retrospective observational study to analyze the evolution of costs of cancer drugs from 2004 to 2010 in the Department of Medical Oncology of the Centro Hospitalar de Entre Douro e Vouga. The first year studied was 2004 because there was difficulty in obtaining data relating to previous years, and the last year studied was 2010 as data collection was conducted in 2011.
The Centro Hospitalar de Entre Douro e Vouga, Portugal, is a medium-sized hospital, established in 1999, with 409 beds and is responsible for the health care of 350000 inhabitants. Until 2010, the Department of Medical Oncology treated solid (unless sarcomas, melanomas and tumors of the central nervous system) and hematologic malignancies, mostly in outpatient settings. Thereafter, the Department only treats solid malignancies.
The patients selected were treated in the Department of Medical Oncology from 2004 to 2010. Due to incomplete records on the number of patients treated by the various types of treatment in the first four years analyzed, it was not possible to calculate the average cost per patient and the cost per type of treatment.
According to data provided by the Pharmacy Department, we selected all the cancer drugs used during the study period in both inpatients and outpatients, and divided this by the type of drug: chemotherapy (cytostatics), targeted therapy (monoclonal antibodies, tyrosine kinase inhibitors, mammalian target of rapamycin inhibitors), immunotherapy and endocrine therapy. To analyze the costs, we considered the absolute global cost (purchasing cost to the hospital) for each drug, which was provided by the Pharmacy Department.
To compare our results with the Karolinska Institute study, drugs used in the Department of Medical Oncology were grouped according to their market placement year: ≤ 1998; 1999 to 2002; 2003 to 2005; 2006 to 2010. To access dates of placement on the national market, we consulted the Portuguese National Authority for Medication and Healthcare Products database. As supportive drugs were not analyzed in the Karolinska study, these drugs were excluded in the present study. Other drug costs not used in cancer treatment were also excluded.
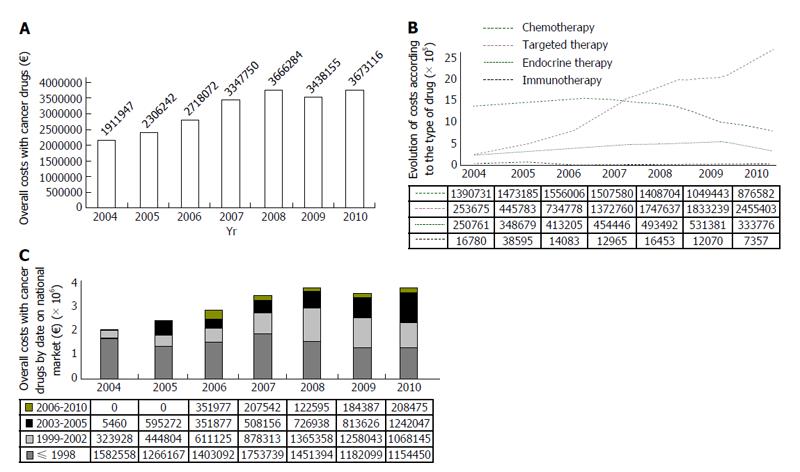
From 2004 and 2010 there was a gradual increase in the number of patients treated in the Department of Medical Oncology (924 patients in year 2004, 1111 in year 2005, 1222 in 2006, 1376 in 2007, 1550 in 2008, 1589 in 2009 and 1560 patients in year 2010). According to data provided by the Pharmacy Department and specifically for the period under analysis, this increase was followed by an increment in the overall costs of cancer drugs up to 2008 (Figure 1A). In 2009, the global expenditure decreased, and in 2010 there was an increase in expenditure to values similar to those in 2008 (Figure 1A).
The evolution of costs, according to the type of drug, is presented in Figure 1B. Chemotherapy and targeted therapy accounted for most of the expenditure, followed by endocrine therapy. The drugs studied according to the year of placement on the national market are described in Table 1.
| Drugs on the national market before 1999 | Market placement yr | Drugs on the national market before 1999 | Market placement yr | Drugs on the national market between 1999 and 2010 | Market placement yr |
| Aldesleukin | 1992 | Ifosfamide | 1979 | Bleomycin | 2001 |
| Amifostine | 1995 | Interferon Alfa-2A | 1998 | Capecitabine | 2001 |
| Anastrozole | 1996 | Intravenous vinorelbine | 1993 | Exemestane | 1999 |
| Bicalutamide | 1998 | Irinotecan | 1997 | Hydroxyurea | 2001 |
| Bleomycin | 1998 | Letrozole | 1997 | Imatinib | 2001 |
| Carboplatin | 1989 | Megestrol | 1987 | Ketoconazole | 2002 |
| Carmustine | 1983 | Melphalan | 1966 | Leuprorelin | 1999 |
| Chlorambucil | 1966 | Mercaptopurine | 1997 | Liposomal Doxorubicin | 2000 |
| Cisplatin | 1980 | Methotrexate | 1993 | Oral vinorelbine | 2001 |
| Cyclophosphamide | 1960 | Mitomycin | 1984 | Raltitrexed | 2001 |
| Cyclosporine | 1990 | Mitoxantrone | 1998 | Temozolomide | 1999 |
| Cyproterone | 1994 | Octreotide | 1989 | Trastuzumab | 2000 |
| Cytarabine | 1996 | Oxaliplatin | 1993 | Anagrelide | 2004 |
| Dacarbazine | 2000 | Paclitaxel | 1997 | Bevacizumab | 2005 |
| Dactinomycin | 1980 | Pegylated liposomal doxorubicin | 1996 | Bortezomib | 2004 |
| Docetaxel | 1995 | Procarbazine | 1998 | Cetuximab | 2004 |
| Doxorubicin | 1998 | Rituximab | 1998 | Erlotinib | 2005 |
| Epirubicin | 1992 | Tamoxifen | 1984 | Fulvestrant | 2004 |
| Etoposide | 1998 | Tegafur | 1985 | Interleukin 2 | 2005 |
| Estramustine | 1982 | Thalidomide | 1961 | Pemetrexed | 2004 |
| Fludarabine | 1995 | Topotecan | 1996 | Azacitidine | 2008 |
| Fluorouracil | 1997 | Vaccine, Bacillus Calmette-Guerin | 1992 | Lapatinib | 2008 |
| Flutamide | 1998 | Vinblastine | 1991 | Sorafenib | 2006 |
| Gemcitabine | 1996 | Vincristine | 1993 | Sunitinib | 2006 |
| Goserelin | 1998 | Temsirolimus | 2007 | ||
| Idarubicin | 1995 | Trabectedin | 2008 |
The global distribution of the drugs used by the Department, according to placement on the national market, is shown in Figure 1C.
Drugs placed on the national market before 1999 accounted for more than 50% of the expenditure on drugs up to 2007 (in 2004 these accounted for 83%; in 2005 for 55%; in 2006 and 2007 for 52% of the expenditure, respectively). After 2008, these drugs represented less than 50% of the total expenditure, and in the last two years of the study expenditure overlapped in drugs placed between 2003 and 2005 (in 2008 these represented 40%; in 2009 34% and in 2010 31% of the costs). Drugs placed between 1999 and 2002 accounted for 25%-35% of the expenditure on drugs in each year analyzed. When the drugs placed between 2003 and 2005 were analyzed, there was a progressive increase in the costs (in 2004 these accounted for 0.3% of the expenditure; in 2005 for 26%; in 2006 for 13%; in 2007 for 15%; in 2008 for 20%; in 2009 for 24% and in 2010 for 34%). The most recent drugs (from 2006 to 2010) accounted for less than 10% of the expenditure, with higher spending in 2006 and increased expenditure again after 2008.
Expenditure on chemotherapy, targeted therapy and endocrine therapy according to the date of placement on the national market is shown in Figure 2, respectively. The expenditure on immunotherapy is not represented here as it was found to have the lowest cost.
With regard to the expenditure on chemotherapy (Figures 1B and 2A), the costs of cytostatics decreased after 2006 and these drugs were no longer responsible for the main costs related to cancer treatment after 2007. The drugs placed on the market before 1999 accounted for the largest expenditure in all the years analyzed, while drugs placed between 1999 and 2002 accounted for less than 25% of the expenditure. The drugs placed between 2003 and 2005 accounted for about 20% of costs, showing a progressive decrease. More recent cytostatics accounted for less than 10% of the expenditure.
The expenditure for targeted therapy increased over the years, and was responsible for most costs after 2007 (Figures 1B and 2B). Drugs placed on the market between 1999 and 2002 cost most in each year studied, except in 2010 where most of the expenditure was on more recent drugs (placed between 2003 and 2005). When analyzing the drugs placed before 1999, there was an increase in expenditure due to these drugs up to 2006, after which a decrease in costs was observed, with a new increment in the last year studied. The cost of drugs placed between 2003 and 2005 increased gradually during the study period. The most recent drugs (placed between 2006 and 2010) used in cancer treatment cost least and this cost remained stable.
When analyzing the expenditure on drugs for endocrine therapy (Figure 2C), it was observed that drugs placed before 1999 represented the biggest share of the expenditure. Drugs placed between 1999 and 2002 accounted for 25% to 33% of the expenditure in each year and drugs placed between 2003 and 2005 showed a progressive increase in costs, and was more prominent in 2010.
Oncology has registered important progress in available treatments, especially cancer drugs, resulting in a significant improvement in healthcare in recent years, both in terms of overall survival and quality of care[20-24].
In Europe, between 1998 and 2007, there was an important increase in direct costs related to cancer drugs[4,16,25]. The primary reasons for this were new indications for already approved drugs and the introduction of new drugs which cost significantly more than most of the older cancer drugs[4,6,7]. According to the Karolinska study, the increase in costs was mainly due to the growth in sales of drugs already on the market[4]. In 2007, drugs placed on the market before 1999 accounted for 68% of the total costs of drugs for cancer; drugs placed between 1999 and 2002 accounted for 17%; drugs placed between 2003 and 2005 accounted for 11%; and drugs placed between 2006 and 2007 accounted for 3%[4].
In our study, chemotherapy was mainly responsible for the costs of cancer drugs up to 2007, after which targeted therapy was responsible for most of the expenditure. In this work, we observed an increase in global costs between 2004 and 2008. Explanations for this increment may be related to the increased number of patients treated, to more drug administration cycles per patient (data not shown) in part due to better overall survival, and to the placement of new drugs on the market, which are frequently more expensive those already on sale. The expenditure decreased in 2009 and increased again in 2010, but the costs increment was not the same as in previous years. Possible reasons for the cost stability over the last 3 years of the study may be at the local level. The Department of Medical Oncology has adopted certain strategies which may explain this cost stability, such as the acquisition of drugs in smaller doses, a study of cytostatics stability to determine the time allowed to administer a drug after its reconstitution, implementation of treatment guidelines at the Department for the most frequent cancer pathologies, and specific scheduling during weekdays to administer certain drugs such as monoclonal antibodies, and reducing waste. Other reasons for this observed stability in expenditure may be more general, but of great importance, and related to patent expiration, greater use of generics, increased competition and optimization on the negotiation of prices between the Healthcare Centers Board and Pharmaceutical Industries.
The overall results of our study were in accordance with those published by the Karolinska Institute[4]. Up to 2007, drugs placed on the national market before 1999 were responsible for most of the expenditure. Reasons for this may include new treatment indications for these drugs and the loss of some drug patents leading to a reduction in their price. Drugs available between 1999 and 2002 were the second leading cause of expenditure, followed by drugs placed on the market between 2003 and 2005. Recently placed drugs (from 2006) accounted for a smaller percentage of the costs. However, after 2008 there was a reduction in costs for previously available drugs followed by a gradual increase in the expenditure for new drugs.
Nevertheless, these results may be affected by several confounding factors which influence the price of pharmaceuticals, such as reimbursement and pricing policy, and the Portuguese financial system. Other limitations in our study are related to the retrospective analysis and the period studied that may have resulted in selection bias with many confounding factors.
In summary, in this study older drugs were responsible for most of the expenditure in cancer treatments up to 2007, after which we observed an increase in expenditure related to new drugs. Despite this increase in expenditure on new drugs in the last 3 years analyzed, the increase in costs for cancer drugs was not the same as in the previous years. However, more studies must be undertaken to fully understand this situation in Portugal.
Drugs are one of the most investigated components of Oncology, consuming most of its economic resources. Over the past few years, direct costs related to cancer treatment have increased significantly. This growth seems to be explained by the increase in cancer incidence, new indications for treatment with previously approved cancer drugs and to the placement of new drugs on the market, which are frequently more expensive than those already on sale. However, data related to cancer treatment cost is scarce in Portugal.
The results of the Karolinska Institute study demonstrated a substantial increase in available cancer drugs and costs between 1998 and 2007. This cost increment was not only related to the introduction of new drugs, as 68% of the costs in 2007 were from drugs approved before 1999. In 2007, drugs placed on the market between 1999 and 2002 accounted for 17% of the total costs; drugs placed between 2003 and 2005 accounted for 11%; and drugs placed between 2006 and 2007 accounted for 3%.
In this work the authors observed an increase in the global costs of cancer drugs which may be explained by the increased number of patients treated, new indications for treatment with previously approved drugs, and the placement of new drugs on the market. These results were in accordance to those published by the Karolinska Institute. Up to 2007, drugs placed on the national market before 1999 were responsible for cancer drug expenditure. Reasons for this may be new treatment indications for these drugs and loss of some drug patents leading to a reduction in their price. Drugs available between 1999 and 2002 were second regarding expenditure, followed by drugs placed on the market between 2003 and 2005. Recently placed drugs (from 2006) accounted for a smaller percentage of the costs. However, after 2008 there was a reduction in costs for previously available drugs followed by a gradual increase in expenditure for new drugs.
The study results suggest that older drugs were responsible for most of the expenditure in cancer treatment, but costs of new cancer drugs are increasing.
The burden of cancer to society can be measured by direct and indirect costs. Direct costs are related to prevention and treatment, while indirect costs include loss of production due to inability to work caused by disease, disability and death. The costs of chemotherapy drugs are related to expenditure for cytostatics, and costs for targeted therapy such as monoclonal antibodies, tyrosine kinase inhibitors and mammalian target of rapamycin inhibitors.
The paper is interesting. It will benefit readers in the field of hospital economic.
| 1. | Araújo A, Barata F, Barroso S, Cortes P, Damasceno M, Parreira A, Espírito Santo J, Teixeira E, Pereira R. [Cost of cancer care in Portugal]. Acta Med Port. 2009;22:525-536. [PubMed] |
| 2. | Barros PP, Machado SR, Simões Jde A. Portugal. Health system review. Health Syst Transit. 2011;13:1-156. [PubMed] |
| 3. | Organisation for Economic Co-operation and Development (OECD) Health Data 2013. Available from: http: //www.oecd.org/els/health-systems/oecdhealthdata.htm. |
| 4. | Wilking N, Jonsson B, Högberg D, Justo N. Comparator report on patient access to cancer drugs in Europe. 2009;. |
| 5. | Lichtenberg FR. The impact of new drug launches on longevity: evidence from longitudinal, disease-level data from 52 countries, 1982-2001. Int J Health Care Finance Econ. 2005;5:47-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Jönsson B, Wilking N. The Burden and cost of cancer. Ann Oncol. 2007;18:iii8-iii22. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11889] [Article Influence: 792.6] [Reference Citation Analysis (6)] |
| 8. | Wilking N, Jönsson B, Institutet K, Stockholm H. A pan-European comparasion regarding patient acess to cancer drugs. 2005;. |
| 9. | Pinto CG, Silva LM. Manual de Farmacoeconomia. Estoril (Portugal): Manuel José Guedes da Silva, Lda 2010; 7-46. |
| 10. | Pinto CG. Gasta-se demais com a saúde em Portugal? Portugal e o Mundo. Lisboa: Universidade Autónoma de Lisboa e Jornal Público 2009; Available from: http://janusonline.pt/2009/2009_2_10.html#dados. |
| 11. | Jönsson B, Wilking N. A global comparison regarding patient access to cancer drugs. Ann Oncol. 2007;18 Suppl 3:iii1-iii77. [PubMed] |
| 12. | Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1722] [Cited by in RCA: 1924] [Article Influence: 128.3] [Reference Citation Analysis (0)] |
| 13. | Truffer CJ, Keehan S, Smith S, Cylus J, Sisko A, Poisal JA, Lizonitz J, Clemens MK. Health spending projections through 2019: the recession’s impact continues. Health Aff (Millwood). 2010;29:522-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Warren JL, Yabroff KR, Meekins A, Topor M, Lamont EB, Brown ML. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100:888-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 307] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 15. | Pinto CG, Pinheiro B. Os Medicamentos e o Sistema de Sáude: uma abordagem económica. In AC Fernandes (coord) O medicamento e o Sistema de Saúde. Cadernos Saúde e Sociedade. Loures: Diário de Bordo 2011; 17-38. |
| 16. | Schnipper LE, Meropol NJ, Brock DW. Value and cancer care: toward an equitable future. Clin Cancer Res. 2010;16:6004-6008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Amir E, Seruga B, Martinez-Lopez J, Kwong R, Pandiella A, Tannock IF, Ocaña A. Oncogenic targets, magnitude of benefit, and market pricing of antineoplastic drugs. J Clin Oncol. 2011;29:2543-2549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Soria JC, Blay JY, Spano JP, Pivot X, Coscas Y, Khayat D. Added value of molecular targeted agents in oncology. Ann Oncol. 2011;22:1703-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Fojo T, Grady C. How much is life worth: cetuximab, non-small cell lung cancer, and the $440 billion question. J Natl Cancer Inst. 2009;101:1044-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 255] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 20. | Smith S, Newhouse JP, Freeland MS. Income, insurance, and technology: why does health spending outpace economic growth? Health Aff (Millwood). 2009;28:1276-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 800] [Cited by in RCA: 799] [Article Influence: 36.3] [Reference Citation Analysis (1)] |
| 22. | Kalofonos HP, Aravantinos G, Kosmidis P, Papakostas P, Economopoulos T, Dimopoulos M, Skarlos D, Bamias A, Pectasides D, Chalkidou S. Irinotecan or oxaliplatin combined with leucovorin and 5-fluorouracil as first-line treatment in advanced colorectal cancer: a multicenter, randomized, phase II study. Ann Oncol. 2005;16:869-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Venook A. Critical evaluation of current treatments in metastatic colorectal cancer. Oncologist. 2005;10:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | McCabe C, Bergmann L, Bosanquet N, Ellis M, Enzmann H, von Euler M, Jönsson B, Kallen KJ, Newling D, Nüssler V. Market and patient access to new oncology products in Europe: a current, multidisciplinary perspective. Ann Oncol. 2009;20:403-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469-6487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 906] [Cited by in RCA: 877] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
P- Reviewers: Cheng G, Di Lorenzo G S- Editor: Gou SX L- Editor: Webster JR E- Editor: Liu SQ