Published online Nov 10, 2013. doi: 10.5306/wjco.v4.i4.85
Revised: June 26, 2013
Accepted: July 17, 2013
Published online: November 10, 2013
Processing time: 241 Days and 16.5 Hours
The incidence of non-melanoma skin cancers (NMSC) is rising worldwide resulting in demand for clinically useful prognostic biomarkers for these malignant tumors, especially for invasive and metastatic cutaneous squamous cell carcinoma (cSCC). Important risk factors for the development and progression of cSCC include ultraviolet radiation, chronic skin ulcers and immunosuppression. Due to the role of cumulative long-term sun exposure, cSCC is usually a disease of the elderly, but the incidence is also growing in younger individuals due to increased recreational exposure to sunlight. Although clinical diagnosis of cSCC is usually easy and treatment with surgical excision curable, it is responsible for the majority of NMSC related deaths. Clinicians treating skin cancer patients are aware that certain cSCCs grow rapidly and metastasize, but the underlying molecular mechanisms responsible for the aggressive progression of a subpopulation of cSCCs remain incompletely understood. Recently, new molecular markers for progression of cSCC have been identified.
Core tip: Several molecular markers for progression of cutaneous squamous cell carcinoma (cSCC) have been identified, but a clinically useful panel of biomarkers is still not available. Further studies are required to determine whether prognostic cSCC biomarker panel can be incorporated into clinical practice. In the meantime, while waiting for novel diagnostic and prognostic tools, clinicians must actively advocate public awareness on skin protection against excessive sun exposure in order to lower the increasing incidence of cSCC.
- Citation: Kivisaari A, Kähäri VM. Squamous cell carcinoma of the skin: Emerging need for novel biomarkers. World J Clin Oncol 2013; 4(4): 85-90
- URL: https://www.wjgnet.com/2218-4333/full/v4/i4/85.htm
- DOI: https://dx.doi.org/10.5306/wjco.v4.i4.85
The incidence of cutaneous squamous cell carcinoma (cSCC) is increasing worldwide[1,2]. SCC of the skin is responsible for the majority of non-melanoma skin cancer (NMSC) deaths, as invasive cSCC displays a potential for recurrence and metastasis[3]. At present, cSCC is primarily a disease of the elderly, but the incidence is also increasing in younger individuals due to excessive recreational exposure to sunlight[4]. The rising incidence of cSCC is also a reason for increased demand for medical care related to skin cancer which is estimated to grow 5% annually in the Central Europe[5]. Public awareness of skin cancer as a potentially lethal disease should therefore be fostered, and the growing economical burden of rising skin cancer incidence to the societies should be taken into consideration in healthcare planning[6,7]. In addition to promoting avoidance of excessive sun-exposure, early lesional skin biopsy and treatment of premalignant lesions are essential in prevention of cSCC[8]. To ensure early diagnosis, dermatologic expertise is obviously needed, and this should be taken into account in planning of medical education[9].
The treatment of choice for primary cSCC is surgical excision, whereas Mohs micrographic surgery is recommended for high-risk cases[1,10]. Unfortunately, excision is not always curative, as cSCCs have an overall 5-year recurrence rate of approximately 5%[11]. Here, the challenge faced by clinicians is to identify the high-risk cases before the recurrence and metastasis of the tumor. There is an obvious demand for predictive molecular markers that could be used at the time of excision of the primary tumor for evaluation of the risk of recurrence and metastasis. Furthermore, if dissemination of the tumor has already taken place, novel targeted therapies are needed.
In this editorial, we discuss the molecular pathways involved in the development of invasive cSCC and the risk factors for cSCC progression, recurrence and metastasis. Finally, recent progress in the search for cSCC progression biomarkers will be discussed.
NMSC, including cSCC, are among the most commonly diagnosed cancers in Europe, United States and Australia[12-14]. Moreover, the incidence of cSCC is steadily increasing worldwide, especially among the white population living in the proximity of the equator[1]. Interestingly, the incidence of cSCC is also rising in the less sun exposed regions of the globe, as approximately 4% annual increase in the incidence has been registered in Finland during the past decades[15]. The growing number of new cSCC cases, as well higher incidence of recurrent tumors makes cSCC one of the costliest cancers in many countries[7,16,17]. It is conceivable, that the cost of NMSC to the societies will continue to grow due to the extension of individual lifespan and aging of the population.
One of the main reasons for the rising incidence of cSCC is popularity of recreational sun-exposure despite growing public awareness of the harmful effects of solar ultraviolet (UV)-light[4]. On the other hand, the number of individuals receiving long-term immunosuppressive medication after organ transplantation has increased. As organ transplant recipients have a 65-fold higher risk of developing cSCC, regular follow-up of these individuals is mandatory and early diagnosis of cSCC, preferably at pre-malignant stage, is essential[18]. Even if the skin malignancies are diagnosed at premalignant stage, the treatment may be costly due to the field cancerization of the skin and the relatively high-cost of topical therapies available. Interestingly, kidney transplant recipients with a history of cSCC also have a higher risk for internal malignancies[19]. It has been proposed that switching the immunosuppressive medication from calcineurin inhibitors to inhibitors of the mammalian target of rapamycin, such as sirolimus could have an antitumoral effect among kidney-transplant recipients with previous cSCC[20] but this observation has been challenged[21].
For dermatologists, diagnosis of cSCC and its precursor, actinic keratosis and cSCC in situ (Bowen’s disease), is often easy by visual inspection[22]. However, clinical diagnosis may sometimes be challenging, especially in patients suffering from severe generalized form of recessive dystrophic epidermolysis bullosa (gs-RDEB), with multiple chronic ulcers, mimicking malignant lesions[23].
Sunlight has a vital role on the Earth as the primary source of energy, but it is also the primary cause of skin cancer[24]. Theoretically, UV-radiation as the major risk factor for cSCC could be avoided, but avoiding sun exposure is a challenge in daily life[4]. UVB (wavelength 280-320 nm) radiation can damage both DNA and RNA directly leading to the generation of mutagenic photoproducts such as pyrimidine-pyrimidine adducts and cyclopyrimidine dimers[25]. UVA (wavelength 320-400 nm) radiation damages DNA indirectly via a photo-oxidative-stress- mediated mechanism which results in DNA double-strand breaks[26,27]. As both UVB and UVA are carcinogenic, the sunscreen used should block both UVB and UVA rays[28]. The risk factors for the development of cSCC are summarized in Table 1.
| Exposure to ultraviolet (UV) radiation (UVA, UVB) |
| Therapy with methoxalen and UVA |
| Fair skin type |
| Ionizing radiation |
| Genodermatosis (albinism, xeroderma pigmentosum, epidermolysis bullosa) |
| Chronic inflammation of skin (lupus erythematosus, epidermolysis bullosa) |
| Chronically injured skin with ulcers (burn scars, leg ulcers, epidermolysis bullosa) |
| Human papilloma virus infection |
| Exposure to chemical carcinogens |
| Immunosuppression |
| Immunosuppressive medications |
| Organ transplantation |
| Osteomyelitis |
| Sinus tracts |
| Precursor lesions (actinic and arsenical keratoses) |
| In situ squamous cell carcinoma (Bowen’s disease and Erythro plasia Queyrat) |
| Tobacco smoking |
| Leukemia and lymphoma |
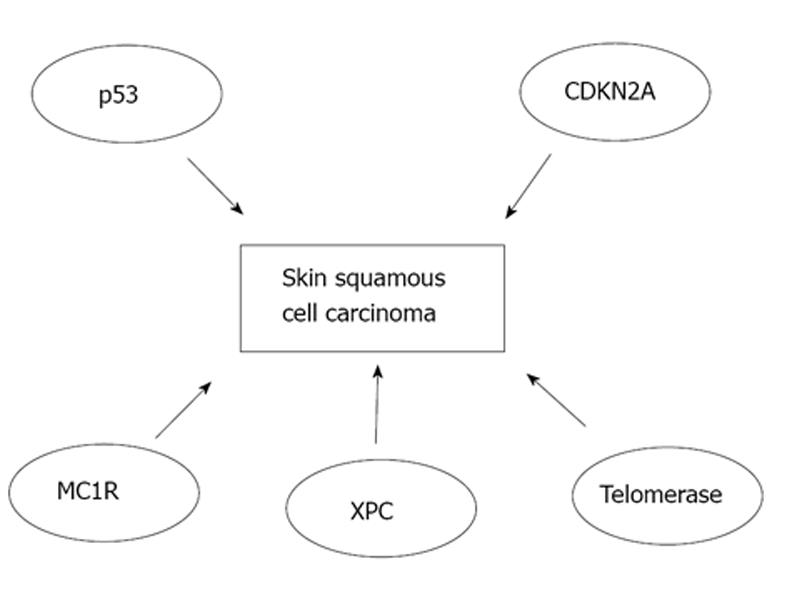
Major pathways involved in the pathogenesis of SCC of the skin are shown in Figure 1. Early inactivation of both alleles for tumor protein 53 gene (TP53) has an important role in the development of cSCC[29,30]. TP53 mutations are observed in roughly 90% of all cSCCs and these mutations occur mainly due to UV radiation. Following inactivation of both TP53 alleles, a marked expansion in simple mutations takes place making cSCCs the human cancer with highest mutation rate known[31]. Subsequently, epidermal keratinocytes undergo malignant transformation and clonal expansion will occur which is clinically manifested as the development of early in situ SCC, actinic keratosis[30,32]. In patients with xeroderma pigmentosum, mutations in xeroderma pigmentosum complementation group C, that lead to failure to repair DNA, are the key event of cSCC development[33]. Melanocortin-1 receptor variants are associated with fair skin, red hair and increased risk of developing melanoma, and they are also an independent risk factor for development of SCC of the skin[34]. In addition, telomerase activity may be elevated in cSCC leading to immortalization of tumor cells[35]. Moreover, inactivation of cyclin-dependent kinase inhibitor 2A locus has been detected in SCC of the skin[36]. Moreover, loss of function mutations of NOTCH-1 and NOTCH-2 genes have been noted in 75% of cSCCs emphasizing the importance of NOTCH genes as tumor suppressors in these epithelial malignancies[37].
Chronic skin exposure to UV light results in DNA damage and mutations in the genes mentioned above leading to malignant transformation of keratinocytes. Moreover, UV light can promote cSCC tumorigenesis and progression also via other mechanisms, such as immunosuppression and inhibition of macrophage migration[28,38].
SCC of the skin is often relatively rapidly growing, locally invasive malignant tumor that has potential for recurrence and metastasis, with an overall 5-year recurrence rate of 8% and a 5-year rate of metastasis of approximately 5%[11]. The location and the size of the primary tumor are relevant in the assessment of the risk of recurrence and metastasis of a given tumor. Typical high-risk anatomical areas are lips and ears, as cSCC in these areas recur and metastasize at a rate of 10% to 25%. The relatively low general risk of recurrence and metastatic spread is markedly higher if the primary lesion is large, as tumors with a diameter > 2 cm show a recurrence rate of 15% and metastasis rate of 30%[11]. In certain cSCC subtypes, such as as in tumors arising in chronic ulcers or chronically injured skin, the risk of metastasis may be as high as 40%[39,40]. In addition, certain histological features of cSCC are known to be related to poor prognosis[1,41]. These features are shown in the Table 2.
| Variable | Approximate relative risk1 | |
| Recurrence | Metastasis | |
| Rapid tumor growth | - | - |
| Tumor size > 2 cm | 2 | 2 |
| Tumor location (lip/ear) | 2 | 3 |
| Immunosuppression | - | 2 |
| Previous radiotherapy | - | - |
| Previously treated cSCC | 3 | 4 |
| RDEB -associated cSCC | - | - |
| Tumor depth > 4 mm | 2 | 5 |
| Poor differentiation | 2 | 3 |
| Acantholytic features | - | - |
| Spindle-cell features | - | - |
| Perineural invasion | 5 | 5 |
Although these clinical and histological risk factors have been established, clinicians treating patients with cSCC still do not have access to molecular tools to assess the risk of recurrence and metastasis in a given patient. Thus, novel molecular biomarkers would be of great value in the risk assessment.
In our own studies, we have searched for novel biomarkers for progression of cSCC. We have utilized a diverse range of research methods to identify relevant candidate genes and tumor proteins. The first step has been genome-wide expression profile analysis of cSCC cell lines vs normal human epidermal keratinocytes[42]. Secondly, we have validated the expression profiling data at the mRNA level with quantitative real-time polymerase chain reaction[43]. Then, we have confirmed the findings at the protein level with Western blotting[44]. As cultured tumor cells represent only a selected portion of a given tumor, we have collected a large panel of in vivo tissue samples containing normal human skin, actinic keratoses, cSCCs in situ (Bowen’s diseases) and cSCCs[42-44]. These formalin fixed, paraffin embedded samples were used for immunohistochemical studies as tissue microarrays[42-44]. In addition, we have used chemically induced mouse skin carcinogenesis model for validation of our human data[42].
Matrix metalloproteinases (MMP) contribute to the homeostasis of a variety of tissues and participate in many physiological processes, such as proteolysis of extracellular matrix in skin[45]. Upregulation of MMP expression has been seen in many different types of cancers, including cSCC[46,47].
In recent studies, we showed that the expression and production of MMP-7 is specifically elevated in cSCCs[43,44]. Interestingly, MMP-7 expression was even more abundant in the gs-RDEB-associated cSCCs representing an aggressive subtype of SCC of the skin[43]. Immunohistochemical studies revealed elevated MMP-7 expression especially in the invasive edge of the cSCC tumors[43].
Furthermore, we studied the mechanistic role of MMP-7 in cSCC and noted that MMP-7 activates heparin binding epidermal growth factor-like growth factor (HB-EGF) in cSCC cells[44]. In functional studies, proliferation of cSCC cells was suppressed when the activation of HB-EGF by MMP-7 was inhibited[44]. These findings provide mechanistic evidence for proposed therapeutic effect of epidermal growth factor receptor antagonists in treatment of advanced cSCCs.
Serine peptidase inhibitors (Serpins) constitute the largest and most broadly distributed superfamily of peptidase inhibitors described in humans[48,49]. We studied the gene expression levels of entire serpin family in cSCC cell lines vs normal keratinocytes and found that expression of SerpinA1, also known as 1-antitrypsin, was markedly elevated[42]. Furthermore, elevated SerpinA1 expression correlated with the tumorigenic potential of transformed keratinocytes[42]. Moreover, SerpinA1 expression in SCC tumor cells in vivo correlated with tumor progression[42]. Furthermore, SerpinA1 expression was clearly more abundant in gs-RDEB-associated cSCCs representing an aggressive subtype of cSCC[42]. To further verify the role of SerpinA1 in the progression of cSCC, we used chemically induced mouse skin carcinogenesis model that showed correlation with SerpinA1 expression and progression of mouse skin SCC[42]. Our findings clearly demonstrate that SerpinA1 may serve as a useful biomarker for progression of cSCC.
Although patients with cSCC in general do not have as poor prognosis as those with melanoma, the impact of cSCC to the quality of life of the patients, as well as to the societies in general will be greater in the near future due to the increased incidence of this malignant tumor and the longer life-span of the population. To improve the accuracy of diagnosis and the assessment of individual prognosis, there is a demand for novel biomarkers for progression of cSCC. We have identified potential biomarkers for this purpose, but further research is required to validate their feasibility in clinical practice. As cSCC is not a uniform disease but rather a heterogenous group of tumors, we assume that a single biomarker probably will not be sufficient, but a panel of biomarkers is needed for making clinical decisions. Finally, the power of preventive measures against skin cancer should not be underestimated. For this purpose, physicians, together with other healthcare professionals, must actively promote public awareness of skin protection against excessive sun exposure.
| 1. | Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:975-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 913] [Cited by in RCA: 873] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 2. | Madan V, Lear JT, Szeimies RM. Non-melanoma skin cancer. Lancet. 2010;375:673-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 595] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 3. | Weinberg AS, Ogle CA, Shim EK. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007;33:885-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Lin JS, Eder M, Weinmann S, Zuber SP, Beil TL, Plaut D, Lutz K. Behavioral Counseling to Prevent Skin Cancer: Systematic Evidence Review to Update the 2003 U.S. Preventive Services Task Force Recommendation [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US). . [PubMed] |
| 5. | de Vries E, van de Poll-Franse LV, Louwman WJ, de Gruijl FR, Coebergh JW. Predictions of skin cancer incidence in the Netherlands up to 2015. Br J Dermatol. 2005;152:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 178] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Geller AC, Swetter SM. Reporting and registering nonmelanoma skin cancers: a compelling public health need. Br J Dermatol. 2012;166:913-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Tinghög G, Carlsson P, Synnerstad I, Rosdahl I. Societal cost of skin cancer in Sweden in 2005. Acta Derm Venereol. 2008;88:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Berman B, Cohen DE, Amini S. What is the role of field-directed therapy in the treatment of actinic keratosis? Part 1: overview and investigational topical agents. Cutis. 2012;89:241-250. [PubMed] |
| 9. | Oliveria SA, Heneghan MK, Cushman LF, Ughetta EA, Halpern AC. Skin cancer screening by dermatologists, family practitioners, and internists: barriers and facilitating factors. Arch Dermatol. 2011;147:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Belkin D, Carucci JA. Mohs surgery for squamous cell carcinoma. Dermatol Clin. 2011;29:161-74, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Rowe DE, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976-990. [PubMed] |
| 12. | Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, Coldiron BM. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 822] [Cited by in RCA: 832] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 13. | Cancer Council Australia. Skin cancer facts and figures. Available from: http: //www.cancer.org.au/cancersmartlifestyle/Sunsmart/Skincancerfactsandfigures.htm. |
| 14. | International Agency for Research on Cancer. Cancer Incidence, Mortality and Prevalence Worldwide in 2008. Available from: http: //globocan.iarc.fr/. |
| 16. | Morris S, Cox B, Bosanquet N. Cost of skin cancer in England. Eur J Health Econ. 2009;10:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Guy GP, Ekwueme DU. Years of potential life lost and indirect costs of melanoma and non-melanoma skin cancer: a systematic review of the literature. Pharmacoeconomics. 2011;29:863-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Wisgerhof HC, Wolterbeek R, de Fijter JW, Willemze R, Bouwes Bavinck JN. Kidney transplant recipients with cutaneous squamous cell carcinoma have an increased risk of internal malignancy. J Invest Dermatol. 2012;132:2176-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Euvrard S, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, Broeders N, del Marmol V, Chatelet V, Dompmartin A. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 415] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 21. | Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, Proby CM, Wolterbeek R, Bouwes Bavinck JN, de Fijter JW. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013;31:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Lehmann P. Sun exposed skin disease. Clin Dermatol. 2011;29:180-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Reed WB, College J, Francis MJ, Zachariae H, Mohs F, Sher MA, Sneddon IB. Epidermolysis bullosa dystrophica with epidermal neoplasms. Arch Dermatol. 1974;110:894-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Preston DS, Stern RS. Nonmelanoma cancers of the skin. N Engl J Med. 1992;327:1649-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 364] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 25. | Rünger TM. How different wavelengths of the ultraviolet spectrum contribute to skin carcinogenesis: the role of cellular damage responses. J Invest Dermatol. 2007;127:2103-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Ridley AJ, Whiteside JR, McMillan TJ, Allinson SL. Cellular and sub-cellular responses to UVA in relation to carcinogenesis. Int J Radiat Biol. 2009;85:177-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Greinert R, Volkmer B, Henning S, Breitbart EW, Greulich KO, Cardoso MC, Rapp A. UVA-induced DNA double-strand breaks result from the repair of clustered oxidative DNA damages. Nucleic Acids Res. 2012;40:10263-10273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Moyal DD, Fourtanier AM. Broad-spectrum sunscreens provide better protection from solar ultraviolet-simulated radiation and natural sunlight-induced immunosuppression in human beings. J Am Acad Dermatol. 2008;58:S149-S154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Brash DE. Roles of the transcription factor p53 in keratinocyte carcinomas. Br J Dermatol. 2006;154 Suppl 1:8-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Ratushny V, Gober MD, Hick R, Ridky TW, Seykora JT. From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma. J Clin Invest. 2012;122:464-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 406] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 31. | Durinck S, Ho C, Wang NJ, Liao W, Jakkula LR, Collisson EA, Pons J, Chan SW, Lam ET, Chu C. Temporal dissection of tumorigenesis in primary cancers. Cancer Discov. 2011;1:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 32. | Feldman SR, Fleischer AB. Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis. 2011;87:201-207. [PubMed] |
| 33. | Bradford PT, Goldstein AM, Tamura D, Khan SG, Ueda T, Boyle J, Oh KS, Imoto K, Inui H, Moriwaki S. Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterises the role of DNA repair. J Med Genet. 2011;48:168-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 383] [Cited by in RCA: 348] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 34. | Bastiaens MT, ter Huurne JA, Kielich C, Gruis NA, Westendorp RG, Vermeer BJ, Bavinck JN. Melanocortin-1 receptor gene variants determine the risk of nonmelanoma skin cancer independently of fair skin and red hair. Am J Hum Genet. 2001;68:884-894. [PubMed] |
| 35. | Parris CN, Jezzard S, Silver A, MacKie R, McGregor JM, Newbold RF. Telomerase activity in melanoma and non-melanoma skin cancer. Br J Cancer. 1999;79:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Pacifico A, Goldberg LH, Peris K, Chimenti S, Leone G, Ananthaswamy HN. Loss of CDKN2A and p14ARF expression occurs frequently in human nonmelanoma skin cancers. Br J Dermatol. 2008;158:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Wang NJ, Sanborn Z, Arnett KL, Bayston LJ, Liao W, Proby CM, Leigh IM, Collisson EA, Gordon PB, Jakkula L. Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proc Natl Acad Sci USA. 2011;108:17761-17766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 366] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 38. | Heise R, Vetter-Kauczok CS, Skazik C, Czaja K, Marquardt Y, Lue H, Merk HF, Bernhagen J, Baron JM. Expression and function of macrophage migration inhibitory factor in the pathogenesis of UV-induced cutaneous nonmelanoma skin cancer. Photochem Photobiol. 2012;88:1157-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Königová R, Rychterová V. Marjolin’s ulcer. Acta Chir Plast. 2000;42:91-94. [PubMed] |
| 40. | Novick M, Gard DA, Hardy SB, Spira M. Burn scar carcinoma: a review and analysis of 46 cases. J Trauma. 1977;17:809-817. [PubMed] |
| 41. | Lohmann CM, Solomon AR. Clinicopathologic variants of cutaneous squamous cell carcinoma. Adv Anat Pathol. 2001;8:27-36. [PubMed] |
| 42. | Farshchian M, Kivisaari A, Ala-Aho R, Riihilä P, Kallajoki M, Grénman R, Peltonen J, Pihlajaniemi T, Heljasvaara R, Kähäri VM. Serpin peptidase inhibitor clade A member 1 (SerpinA1) is a novel biomarker for progression of cutaneous squamous cell carcinoma. Am J Pathol. 2011;179:1110-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 43. | Kivisaari AK, Kallajoki M, Mirtti T, McGrath JA, Bauer JW, Weber F, Königová R, Sawamura D, Sato-Matsumura KC, Shimizu H. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Kivisaari AK, Kallajoki M, Ala-aho R, McGrath JA, Bauer JW, Königová R, Medvecz M, Beckert W, Grénman R, Kähäri VM. Matrix metalloproteinase-7 activates heparin-binding epidermal growth factor-like growth factor in cutaneous squamous cell carcinoma. Br J Dermatol. 2010;163:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Toriseva M, Kähäri VM. Proteinases in cutaneous wound healing. Cell Mol Life Sci. 2009;66:203-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 46. | Kerkelä E, Saarialho-Kere U. Matrix metalloproteinases in tumor progression: focus on basal and squamous cell skin cancer. Exp Dermatol. 2003;12:109-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 244] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 48. | Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, Irving JA, Lomas DA, Luke CJ, Moyer RW. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001;276:33293-33296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 927] [Cited by in RCA: 928] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 49. | Law RH, Zhang Q, McGowan S, Buckle AM, Silverman GA, Wong W, Rosado CJ, Langendorf CG, Pike RN, Bird PI. An overview of the serpin superfamily. Genome Biol. 2006;7:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 442] [Cited by in RCA: 550] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
P- Reviewers: Aksoy B, Negosanti L, Streckfus CF S- Editor: Gou SX L- Editor: A E- Editor: Wu HL