Published online Jan 10, 2012. doi: 10.5306/wjco.v3.i1.1
Revised: December 19, 2011
Accepted: January 7, 2012
Published online: January 10, 2012
It was found that the discovery of 5.8% (84/1437) of all drugs on the market involved serendipity. Of these drugs, 31 (2.2%) were discovered following an incident in the laboratory and 53 (3.7%) were discovered in a clinical setting. In addition, 263 (18.3%) of the pharmaceuticals in clinical use today are chemical derivatives of the drugs discovered with the aid of serendipity. Therefore, in total, 24.1% (347/1437) of marketed drugs can be directly traced to serendipitous events confirming the importance of this elusive phenomenon. In the case of anticancer drugs, 35.2% (31/88) can be attributed to a serendipitous event, which is somewhat larger than for all drugs. The therapeutic field that has benefited the most from serendipity are central nervous system active drugs reflecting the difficulty in designing compounds to pass the blood-brain-barrier and the lack of laboratory-based assays for many of the diseases of the mind.
- Citation: Hargrave-Thomas E, Yu B, Reynisson J. Serendipity in anticancer drug discovery. World J Clin Oncol 2012; 3(1): 1-6
- URL: https://www.wjgnet.com/2218-4333/full/v3/i1/1.htm
- DOI: https://dx.doi.org/10.5306/wjco.v3.i1.1
It is well know that serendipity has played a pivotal role in the discovery of many drugs used today[1-3]. Indeed two major classes of anticancer drugs were discovered with the aid of serendipity, i.e., Barnett Rosenberg’s discovery of cisplatin and the breakthrough observation by Lieutenant Colonel Stewart F Alexander that the chemical warfare agent, nitrogen mustard, depleted white blood cell numbers; aiding in the development of alkylation agents[1-2]. The question therefore emerges of how important serendipity really is in drug discovery and development? The aim of this investigation is to identify all marketed drugs and their derivatives used in the clinic today in which discovery was in some way based on or aided by a serendipitous event. The numbers obtained will be compared to the total number of marketed drugs resulting in a quantitative measure of the impact of serendipity in the discovery of pharmaceuticals, and anticancer drugs in particular.
Three books were analysed: Laughing Gas, Viagra, and Lipitor: The Human Stories Behind the Drugs We Use[1], Happy Accidents: Serendipity in Modern Medical Breakthroughs[2] and Drug Discovery, a History[3]. Furthermore, one scientific paper was identified with a list of drugs discovered by the aid of serendipity[4]. The books and the paper are shown in Table 1. These resources were studied and the stories containing serendipitous events were recorded. The nature of the serendipitous findings were categorised as laboratory based or clinical. The drugs identified were reviewed in DrugBank[5-7] and only those that were approved, were small molecules, and in clinical use were included. Furthermore, drugs with similar chemical structures and with the same notation (i.e., used to treat the same condition) as the parent drug were considered to be their derivatives as identified by substructure and Tanimoto similarity searching in DrugBank[5-7]. A full list of the drugs found is given in Tables 2 and 3.
| Serendipitous drugs | Derivatives | No. of derivatives |
| Acetanilide | Acetaminophen | 1 |
| Acetohexamide | Tolbutamide, glimepiride, glibenclamide, glipizide, chlorpropamide, gliquidone, tolazamide, gliclazide | 8 |
| Captopril | Ramipril, fosinopril, lisinopril, trandolapril, enalapril, perindopril, spirapril, quinapril | 8 |
| Cisplatin | Oxaliplatin, carboplatin | 2 |
| Diethylstilbestrol | Dienestrol | 1 |
| Digoxin | Digitoxin, deslanoside, acetyldigitoxin, ouabain | 4 |
| Ergotamine | Dihydroergotamine, dihydroergotoxine, ergoloidmesylate, methysergide, methylergonovine, ergonovine | 6 |
| Ephedrine | Pseudoephedrine, ritodrine, metaraminol, phenylephrine, isoetharine, fenoterol, epinephrine, orciprenaline, terbutaline | 9 |
| Griseofulvin | NA | 0 |
| Heparin | Pentosan, polysulfate, enoxaparin, ardeparin, fondaparinux sodium | 4 |
| lsoniazid | NA | 0 |
| Lidocaine | Prilocaine, tocainide, mepivacaine, bupivacaine, levobupivacaine, ropivacaine | 6 |
| Lithium | NA | 0 |
| Marinol | Nabilone | 1 |
| Mechlorethamine | Chlorambucil, cyclophosphamide, melphalan, uracil mustard, estramustine | 5 |
| Mecillinam | Pivmecillinam | 1 |
| Methotrexate | Leucovorin | 1 |
| Nalidixic acid | Rosoxacin, enoxacin, pefloxacin, norfloxacin, lomefloxacin, ciprofloxacin, levofloxacin, ofloxacin | 8 |
| Nitroglycerine | Erythrityl tetranitrate, Isosorbide dinitrate | 2 |
| Penicillin | Ampicillin, amoxicillin, azidocillin, azlocillin, bacampicillin, carbenicillin, cloxacillin, cyclacillin, dicloxacillin, flucloxacillin, hetacillin, meticillin, mezlocillin, nafcillin, oxacillin, penicillin g, penicillin V, piperacillin, pivampicillin, tazobactam, ticarcillin | 21 |
| Pentamidine | NA | 0 |
| Physostigmine | NA | 0 |
| Quinine | Quinidine | 1 |
| Sorafenib | NA | 0 |
| Streptomycin | Framycetin, neomycin, josamycin, tobramycin, kanamycin, candicidin, spectinomycin | 7 |
| Sulfanilamide | Silver sulfadiazine, sulfacetamide, sulfacytine, sulfadiazine, sulfadimethoxine, sulfadoxine, sulfamerazine, sulfamethizole, sulfametopyrazine, sulfamethoxazole, sulfamoxole, sulfapyridine, sulfisoxazole | 13 |
| Valproic acid | Divalproex sodium | 1 |
| Vinblastine | Vincristine, vindesine, vinorelbine | 3 |
| Dicoumarol | NA | 0 |
| Warfarin | Acenocoumarol, phenprocoumon, dicumarol | 3 |
| Zinc Sulfate | NA | 0 |
| Off-label drugs | Derivatives | No. of derivatives |
| Aminoglutethimide | NA | 0 |
| Alprostadil | Dinoprostone, carboprost, tromethamine, dinoprost, tromethamine, misoprostol | 4 |
| Amphetamine | Phentermine, methamphetamine, dextroamphetamine, alverine, selegiline, mephentermine, tranylcypromine, phenelzine, benzphetamine, diethylpropion | 10 |
| Aspirin | NA | 0 |
| Auranofin | NA | 0 |
| Carbamazepine | Oxcarbazepine | 1 |
| Celecoxib | NA | 0 |
| Chlordiazepoxide | Diazepam, temazepam, oxazepam, fludiazepam, clorazepate, halazepam, prazepam, flurazepam, lorazepam, cinolazepam, clonazepam, nitrazepam, bromazepam, flunitrazepam, quazepam, clotiazepam, alprazolam, estazolam, adinazolam, midazolam | 20 |
| Chlorothiazide | Benzthiazide, diazoxide, hydrochlorothiazide, hydroflumethiazide, bendroflumethiazide, cyclothiazide, polythiazide, trichlormethiazide, methyclothiazide, furosemide, bumetanide | 11 |
| Clofibrate | Fenofibrate | 1 |
| Dactinomycin | NA | 0 |
| Diisopropylfluorophosphate | NA | 0 |
| Diltiazem | NA | 0 |
| Dimenhydrinate | NA | 0 |
| Diphenhydramine | Bromodiphenhydramine, diphenylpyraline | 2 |
| Diphenoxylate | Loperamide | 1 |
| Dipyridamole | NA | 0 |
| Disulfiram | NA | 0 |
| Doxorubicin | Epirubicin, daunorubicin, idarubicin, valrubicin, plicamycin | 5 |
| Etomidate | NA | 0 |
| Finasteride | Dutasteride | 1 |
| Guanethidine | Debrisoquin, guanidine | 2 |
| Haloperidol | Droperidol | 1 |
| Imatinib | NA | 0 |
| Imipramine | Trimipramine, desipramine, clomipramine, protriptyline, amitriptyline, nortriptyline, cyclobenzaprine, maprotiline, doxepin, amoxapine | 10 |
| Iproniazid | Isocarboxazid | 1 |
| Linezolid | NA | 0 |
| Lysergic acid diethylamide | Cabergoline, lisuride, bromocriptine, nicergoline, pergolide | 5 |
| Meprobamate | Carisoprodol | 1 |
| Mercaptopurine | Thioguanine, azathioprine | 2 |
| Metronidazole | Tinidazole | 1 |
| Mifepristone | NA | 0 |
| Minoxidil | NA | 0 |
| Mycophenolic acid | Mycophenolatemofetil | 1 |
| Naloxone | Naltrexone | 1 |
| Norethindrone | Levonorgestrel, norgestrel, etonogestrel, gestodene,desogestrel, medroxyprogesterone, megestrol, progesterone, drospirenone, norelgestromin, ethynodioldiacetate | 11 |
| Pethidine | Anileridine | 1 |
| Phenobarbital | Methylphenobarbital, secobarbital, metharbital, aprobarbital, primidone, methsuximide | 6 |
| Prednisone | Medrysone, methylprednisolone, prednisolone, rimexolone, fluocortolone, desoximetasone | 6 |
| Probenecid | NA | 0 |
| Procarbazine | NA | 0 |
| Promethazine | Acepromazine, aceprometazine, acetophenazine, arphenazine, chlorpromazine, ethopropazine, fluphenazine, mesoridazine, methotrimeprazine, perphenazine, pipotiazine, prochlorperazine, promazine, propericiazine, propiomazine, thioproperazine, thioridazine, trifluoperazine, triflupromazine, trimeprazine | 20 |
| Quinacrine | Chloroquine, primaquine, hydroxychloroquine, amodiaquine | 4 |
| Reserpine | Deserpidine, rescinnamine | 2 |
| Salicyclic acid | Salsalate, olsalazine, diflunisal, mesalazine | 4 |
| Sildenafil | Tadalafil, vardenafil | 2 |
| Sirolimus | Everolimus | 1 |
| Tamoxifen | Toremifene | 1 |
| Terfenadine | Fexofenadine | 1 |
| Thalidomide | Lenalidomide | 1 |
| Tolazoline | NA | 0 |
| Trimethadione | Paramethadione | 1 |
| Zidovudine | Trifluridine, telbivudine, idoxuridine, zalcitabine, stavudine | 5 |
Serendipity refers to chance discoveries that have been exploited with sagacity[3]. This requires both a chance event and the mental ability to understand the occurrence and realise its potential. In this work, only stories that fit both requirements for serendipity were recorded. The serendipitous events were divided into two categories; laboratory based and clinical. A classic example of the former is Barnett Rosenberg’s discovery of cisplatin, and for the latter dimenhydrinate (Dramamine), which was developed as an antihistamine but is now sold as a travel sickness medication due to a chance observation/realisation by one of the participants in the clinical trials. The division of the drugs into these two categories is not always obvious, but we believe that it helps in the analysis of the results. In his book, Serendipity, Roberts[8] coined the term pseudoserendipity to describe accidental discoveries of ways to achieve an end sought for in contrast to the meaning of true serendipity, which describes accidental discoveries of things not sought for. Certainly all of the drugs discovered in the clinic can be described as pseudoserendipitous according to this definition and many of the drugs found in the laboratory.
To calculate the proportion of drugs with a serendipitous background, the total number of small molecule drugs on the market (FDA approved) is taken to be 1437 according to DrugBank[5-7]. Overington et al[9] reported 1204 small molecule drugs in clinical use, which is a somewhat smaller number.
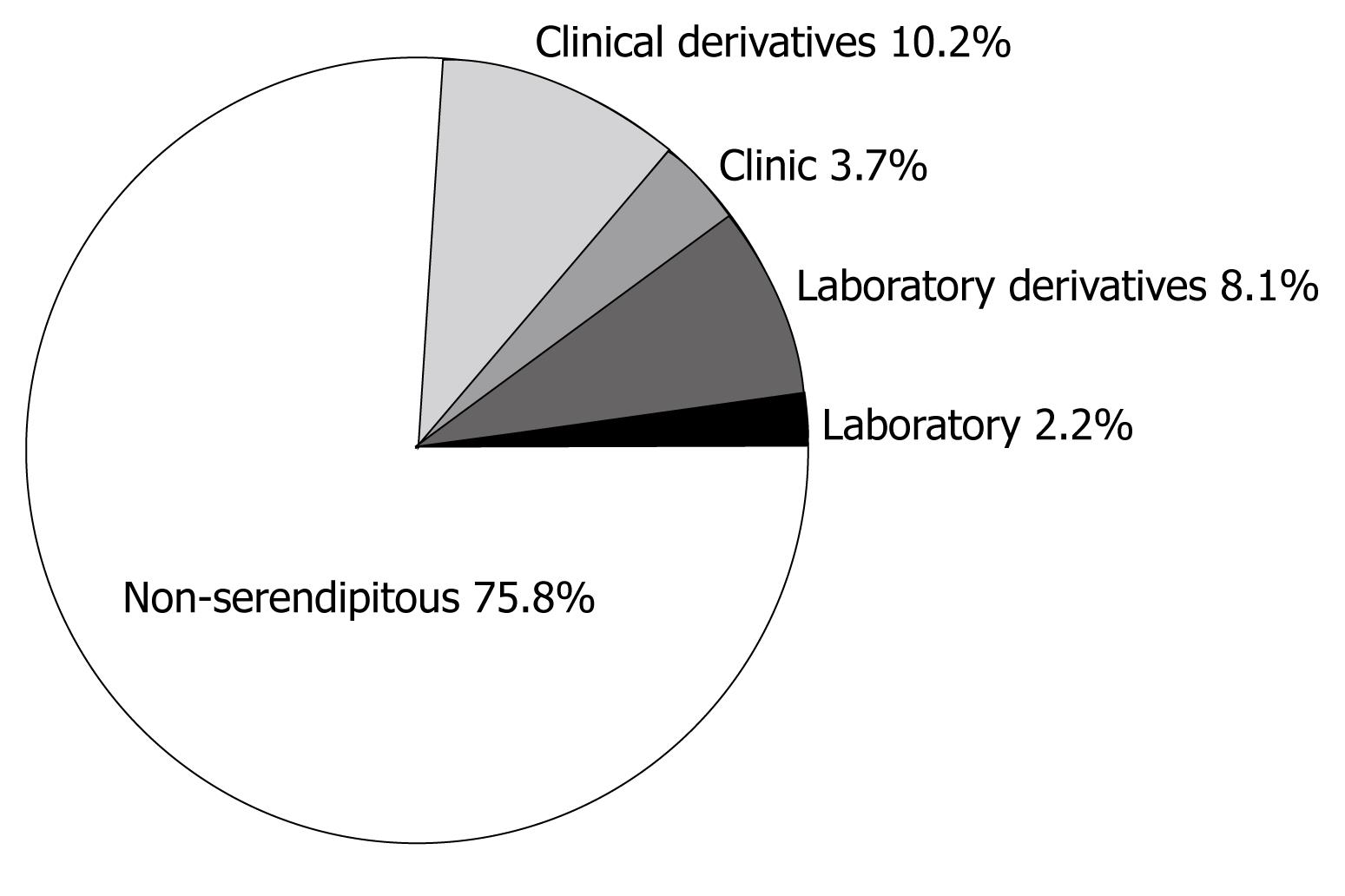
In this analysis, 84 drugs were identified to have serendipitous events aiding their discovery, which is 5.8% of all drugs currently in use. Thirty-one drugs (2.2%) were identified in the laboratory and 116 derivatives (8.1%) of these drugs were identified as shown in Table 4. Fifty-three pharmaceuticals (3.7%) were discovered in clinical settings and 147 derivatives (10.2%) of these were identified (Table 4). Therefore, in total there are 347 drugs currently on the market, in which discovery was aided by a serendipitous event, representing a staggering 24.1% of all drugs currently on the market. A graphical representation of these results is shown in Figure 1.
| Drugs | Ref. | Derivatives | Notation |
| Acetanilide | [3,4] | 1 | Antipyretic |
| Acetohexamide | [1,3,4] | 8 | Diabetes II |
| Captopril | [1,3] | 8 | Cardiovascular |
| Cisplatin | [1-4] | 2 | Cancer |
| Diethylstilbestrol | [3,4] | 1 | Hormonal |
| Digoxin | [1,3] | 4 | Cardiovascular |
| Ergotamine | [1-3] | 6 | Cardiovascular |
| Ephedrine | [3] | 9 | CNS |
| Griseofulvin | [3,4] | 0 | Antifungal |
| Heparin | [2-4] | 4 | Cardiovascular |
| Isoniazid | [3,4] | 0 | Antibiotic |
| Lidocaine | [3] | 6 | CNS |
| Lithium | [1-4] | 0 | CNS |
| Marinol | [3] | 1 | CNS |
| Mechlorethamine | [1-4] | 5 | Cancer |
| Mecillinam | [3] | 1 | Antibiotic |
| Methotrexate | [1,3] | 1 | Cancer |
| Nalidixic acid | [1,3] | 8 | Antibiotic |
| Nitroglycerine | [1,3,4] | 2 | Cardiovascular |
| Penicillin | [1-4] | 21 | Antibiotic |
| Pentamidine | [3] | 0 | Antiprotozoal |
| Physostigmine | [3] | 0 | Ocular |
| Quinine | [3] | 1 | Antiprotozoal |
| Sorafenib | [1] | 0 | Cancer |
| Streptomycin | [1,2] | 7 | Antibiotic |
| Sulfanilamide | [1-3] | 13 | Antibiotic |
| Valproic acid | [3,4] | 1 | CNS |
| Vinblastine | [1-3] | 3 | Cancer |
| Dicoumarol | [2-4] | 0 | Cardiovascular |
| Warfarin | [2-4] | 3 | Cardiovascular |
| Zinc sulfate | [3] | 0 | Wilson’s disease |
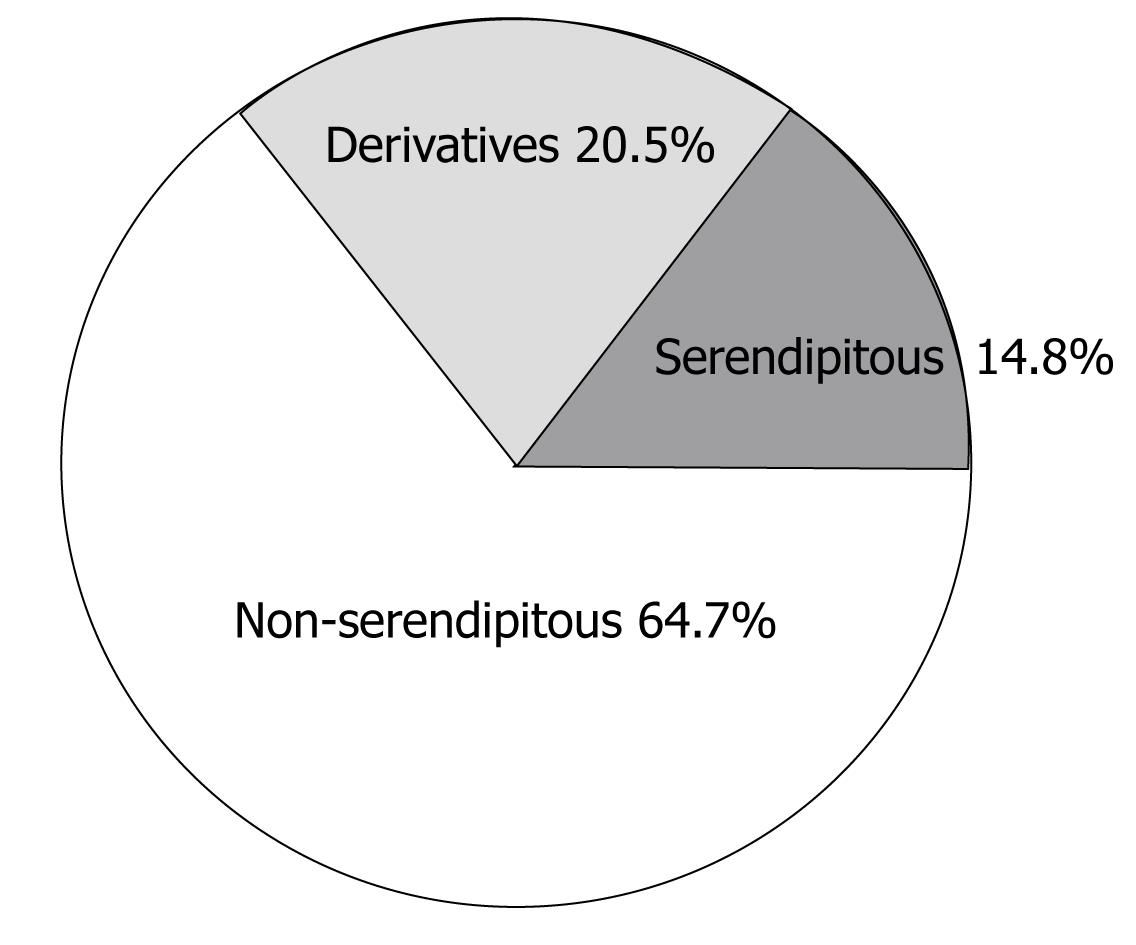
According to DrugBank[5-7] there are 88 anticancer drugs in clinical use today. Of the drugs identified with serendipitous origin, 13 are used to treat cancer and 18 are their chemical derivatives. This means that 35.2% of all anticancer drugs in clinical use involved serendipity of some kind. The statistical distribution is shown in Figure 2. This represents a larger portion of serendipitous effects than for pharmaceuticals in general.
Of the primary serendipitous events, anticancer drugs represent 15.5% (13/84), i.e., a sizeable portion. However, relatively few derivatives were found for anticancer drugs (6.8% of the derivatives). This highlights the difficulty in developing effective anticancer drugs.
When the primary serendipitous events are investigated, it is clear that antibiotic, anticancer, cardiovascular and central nervous system (CNS) drugs are the most common notations with about 10 events for each (Tables 5). Other therapeutic fields such as antiprotozoal and antifungal are also reported. Less common treatments for conditions such as gout and alcoholism are reported. A high frequency of CNS discoveries is seen in the clinical settings in Table 5, i.e., 17 out of a total of 53. This reflects the difficulty in developing drugs that need to pass the Blood-Brain-Barrier (e.g., reference[10] and references therein), and the dearth of biochemical assays modelling the diseases of the mind and pain.
| Clinical drugs | Ref. | Derivatives | Notation |
| Aminoglutethimide | [3,4] | 0 | Cancer |
| Alprostadil | [3] | 4 | Cardiovascular |
| Amphetamine | [1,3,4] | 10 | CNS |
| Aspirin | [1-3] | 0 | Cardiovascular/Cancer |
| Auranofin | [2,3] | 0 | Antirheumatic |
| Carbamazepine | [3] | 1 | CNS |
| Celecoxib | [2] | 0 | Cancer |
| Chlordiazepoxide | [1-4] | 20 | CNS |
| Chlorothiazide | [1,3,4] | 11 | Diuretic |
| Clofibrate | [3] | 1 | Cardiovascular |
| Dactinomycin | [3] | 0 | Cancer |
| Diisopropylfluorophosphate | [3] | 0 | Ocular |
| Diltiazem | [3] | 0 | Cardiovascular |
| Dimenhydrinate | [2-4] | 0 | CNS |
| Diphenhydramine | [3] | 2 | CNS |
| Diphenoxylate | [3,4] | 1 | Antidiarrheal |
| Dipyridamole | [3] | 0 | Cardiovascular |
| Disulfiram | [1,2,4] | 0 | Alcoholism treatment |
| Doxorubicin | [3] | 5 | Cancer |
| Etomidate | [3,4] | 0 | CNS |
| Finasteride | [2] | 1 | Baldness |
| Guanethidine | [3,4] | 2 | Cardiovascular |
| Haloperidol | [1,3,4] | 1 | CNS |
| Imatinib | [1] | 0 | Cancer |
| Imipramine | [1-4] | 10 | CNS |
| Iproniazid | [1-4] | 1 | CNS |
| Linezolid | [1] | 0 | Antibiotic |
| LSD | [1-4] | 5 | CNS |
| Meprobamate | [2,4] | 1 | CNS |
| Mercaptopurine | [1,3] | 2 | Immunosuppressive |
| Metronidazole | [3] | 1 | Antiprotozoal |
| Mifepristone | [3,4] | 0 | Hormonal |
| Minoxidil | [2] | 0 | Cardiovascular |
| Mycophenolic acid | [3] | 1 | Immunosuppressive |
| Naloxone | [3] | 1 | CNS |
| Norethindrone | [1,3,4] | 11 | Hormonal |
| Pethidine | [3,4] | 1 | CNS |
| Phenobarbital | [3] | 6 | CNS |
| Prednisone | [3,4] | 6 | Anti-inflammatory |
| Probenecid | [2] | 0 | Gout |
| Procarbazine | [3] | 0 | CNS |
| Promethazine | [1-3] | 20 | Antihistamine |
| Quinacrine | [3] | 4 | Antiprotozoal |
| Reserpine | [1-3] | 2 | CNS |
| Salicyclic acid | [3] | 4 | Antirheumatic |
| Sildenafil | [1-3] | 2 | Erectile dysfunction |
| Sirolimus | [3] | 1 | Immunosuppressive |
| Tamoxifen | [1-4] | 1 | Cancer |
| Terfenadine | [3] | 1 | Antihistamine |
| Thalidomide | [1,2] | 1 | Cancer |
| Tolazoline | [3] | 0 | Cardiovascular |
| Trimethadione | [3] | 1 | CNS |
| Zidovudine | [1,2] | 5 | Antiviral |
Recently a new concept of Known Drug Space (KDS) has been developed to help drug designers to navigate chemical space based on the analysis of drugs in clinical use[11-13]. It is known that 10% of KDS are unaltered natural products and 29% are their derivatives (semi-synthetics)[14]. With this fact and the results presented in this paper it can be stated that KDS is, to a large extent, populated by chance rather than design. Therefore, the analysis of the physicochemical properties of known drugs gives a region of property space that really works for successful pharmaceuticals.
Serendipity in drug discovery has not been investigated to a great extent, however, some papers were found in the literature and the opinions expressed vary greatly, which is not surprising due to the ambiguous nature of this phenomenon. For instance, Jeste et al[15] downplay the importance of serendipity arguing that few if any discoveries in their field of psychiatry were truly serendipitous. Conversely, Lombardino and Lowe state that “the role of serendipity, chemical intuition and creativity in thoughtfully selecting a chemical target to synthesize in order to discover the best-quality drug has not diminished” irrespective of the introduction of new technologies[16]. Furthermore, Klein strongly believes that a loss of chance observations and unexpected clinical benefits are due to recent changes in the process of drug discovery[17]. He criticises cost-control measures which remove a creative environment in hospitals that fosters serendipity[17]. Finally, Kubinyi[4] suggests that researchers should not be manipulated by short-term business cycles; drug discoveries require good science, enlightened management, and freedom for researchers to act, challenge dogma and take risks.
This investigation provides a limited scope of serendipitous drug discovery since only four sources were analysed. It is certain that not all serendipitous events are recorded; researchers may choose not to report them in favour of standard scientific methods of inquiry. It can therefore be argued that the impact of serendipity is even larger than found in this investigation.
According to the results presented here, approximately 24% of all drugs currently on the market were discovered with the aid of serendipity and thus, may never have been discovered without the curiosity, observation, and sagacity of the researchers. This serves to highlight the unpredictability in drug research and the necessity to allow for and encourage freedom in research directions and promote the intellectual freedom of the scientists involved. Also, a sound education in science is indispensable and the promotion of critical thinking of our students is vital (for further discussion see Lenox[18]).
The term “drug repositioning” is sometimes used when a new notation is found for a drug molecule. A good example is the reintroduction of the infamous thalidomide in clinical use. This is obviously a very positive development since new drugs do not have to be developed from scratch with a large price tag. As shown in this work, serendipitous events in the clinic are important and have facilitated drug repositioning emphasising the need to educate clinicians about this phenomenon.
Understanding the serendipity phenomenon is crucial so we can start to manipulate it to our advantage and we believe that quantifying the impact of serendipity facilitates our understanding of it. Finally, Pasteur’s comment on serendipity certainly still holds true: “Dans les champs de l’observation, le hasard ne favoriseque les esprits prepares.” (“In the field of observation, chance favours only the prepared mind.”)
It was found that 35.2% of all the anticancer drugs now in clinical use were discovered by serendipity. In general, 24% of all pharmaceuticals currently on the market were affected in a positive way during their development by this phenomenon with CNS active drugs being the most prominent. This leads to the conclusion that drug discovery is based on good science and where intuition, critical thinking, sagacity and open-mindedness play crucial roles.
| 1. | Li JJ. Laughing Gas, Viagra and Lipitor The human Stories Behind the Drugs We Use. Oxford: Oxford University Press 2006; . |
| 2. | Meyers MA. Happy Accidents Serendipity in Modern Medical Breakthroughs. New York: Arcade Publishing 2007; . |
| 3. | Sneader W. Drug Discovery a History. Chichester: John Wiley and Sons Ltd 2005; . |
| 4. | Kubinyi H. Chance favors the prepared mind--from serendipity to rational drug design. J Recept Signal Transduct Res. 1999;19:15-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 5. | Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008;36:D901-D906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1752] [Cited by in RCA: 1951] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 6. | Wishart DS, Knox C, Guo AC, Shrivastava S, Hassanali M, Stothard P, Chang Z, Woolsey J. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006;34:D668-D672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2270] [Cited by in RCA: 2653] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 7. | Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V. DrugBank 3.0: a comprehensive resource for ‘omics’ research on drugs. Nucleic Acids Res. 2011;39:D1035-D1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1292] [Cited by in RCA: 1342] [Article Influence: 83.9] [Reference Citation Analysis (0)] |
| 8. | Roberts RM. Serendipity Accidental Discoveries in Science. New York: Wiley Science Editions 1989; . |
| 9. | Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2616] [Cited by in RCA: 2638] [Article Influence: 138.8] [Reference Citation Analysis (0)] |
| 10. | King A. Breaking through the barrier. Chemistry World. 2011;36-39. |
| 11. | Ioakimidis L, Thoukydidis L, Naeem S, Mirza A, Reynisson J. Benchmarking the Reliability of QikProp. Correlation between Experimental and Predicted Values. QSAR Comb Sci. 2008;27:445-456. [RCA] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 12. | Axerio-Cilies P, Castañeda IP, Mirza A, Reynisson J. Investigation of the incidence of “undesirable” molecular moieties for high-throughput screening compound libraries in marketed drug compounds. Eur J Med Chem. 2009;44:1128-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Mirza A, Desai R, Reynisson J. Known drug space as a metric in exploring the boundaries of drug-like chemical space. Eur J Med Chem. 2009;44:5006-5011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Bade R, Chan HF, Reynisson J. Characteristics of known drug space. Natural products, their derivatives and synthetic drugs. Eur J Med Chem. 2010;45:5646-5652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Jeste DV, Gillin JC, Wyatt RJ. Serendipity in biological psychiatry--a myth? Arch Gen Psychiatry. 1979;36:1173-1178. [PubMed] |
| 16. | Lombardino JG, Lowe JA. The role of the medicinal chemist in drug discovery--then and now. Nat Rev Drug Discov. 2004;3:853-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 204] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Klein DF. The loss of serendipity in psychopharmacology. JAMA. 2008;299:1063-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Lenox RS. Educating for the Serendipitous Discovery. J Chem Edu. 1985;62:282-285. |
Peer reviewers: Arianna L Kim, PhD, Herbert Irving Assistant Professor of Dermatology, Department of Dermatology, Columbia Medical Center, 1130 St. Nicholas Ave 321B, New York, NY 10032, United States; Shufeng Zhou, MD, PhD, A/Professor, School of Health Sciences, RMIT University, Bundoora, Victoria 3083, Australia
S- Editor Yang XC L- Editor Webster JR E- Editor Li JY