INTRODUCTION
Circulating tumor cells (CTCs) have long been known to exist in the peripheral blood of some patients with cancer, particularly those with metastatic disease. The clinical impact of this phenomenon has long been debated. CTCs have been measured in patients with cancer to analyze metastatic mechanisms or in the hope of developing clinical applications for diagnosis and therapy; various CTC-related studies have been performed. We review the historical development of research on CTCs and discuss possibilities for future clinical applications.
METASTATIC MECHANISMS AND CTCS
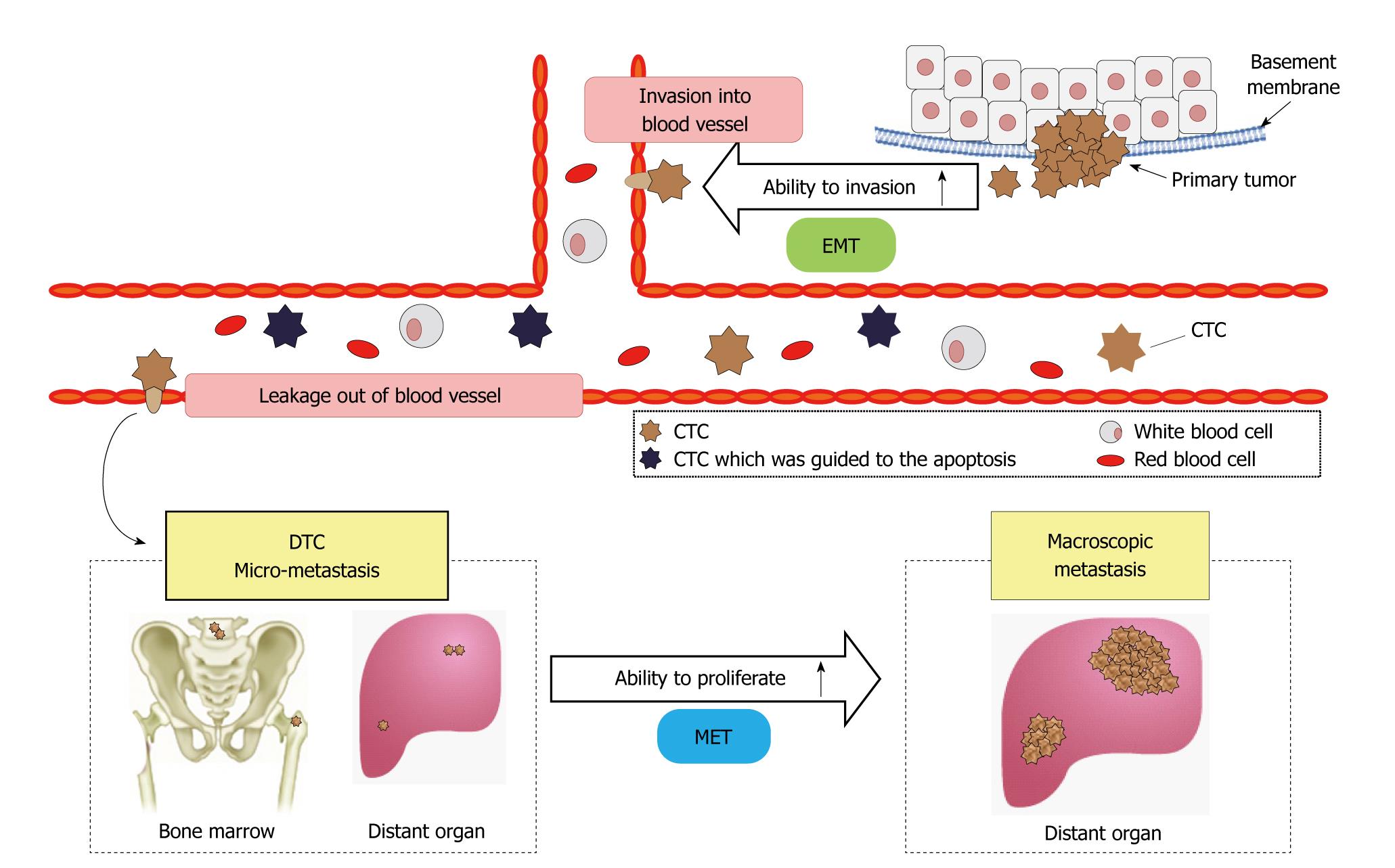
Intravascular infiltration of tumor cells and the development of distant metastases were previously considered late events in tumor progression. However, recent research has demonstrated that tumor cell infiltration and invasion of the surrounding stroma and blood vessels are early events in tumorigenesis. Subsequently, such metastatic tumor cells remain dormant[1-3]. Tumor cells invade blood vessels via fragile tumor vessels. Tumor-cell proliferation reduces the oxygen supply to cells and activates various types of interstitial and inflammatory reactions, such as angiogenesis[4]. Decreased expression of the cell adhesion factor, E-cadherin, reduces adhesive strength between tumor cells, promoting infiltration of surrounding tissue[5].
Infiltrated cells undergo epithelial-mesenchymal transition (EMT) and assume the characteristics of interstitial cells, including migratory ability[6]. Tumor cells that have undergone EMT invade blood vessels and enter the systemic circulation. Some circulating CTCs undergo apoptosis, whereas others continue to exist as dormant tumor cells.
In solitary tumor cells, the cell cycle is thought to be dormant, resulting in the lack of proliferation. However, tumor cells may spread to bone marrow and distant organs and exist as disseminated tumor cells (DTCs). DTCs maintain a state of dormancy and do not proliferate, but can lead to micrometastases or metastatic foci. CTCs may also undergo mesenchymal-epithelial transition, leading to restoration of proliferative ability causing tumor-cell proliferation, angiogenesis, and further metastases in distant organs[6,7] (Figure 1).
Figure 1 Circulating tumor cell and metastatic process.
EMT: Epithelial-mesenchymal transition; CTC: Circulating tumor cell; DTC: Disseminated tumor cell; MET: Mesenchymal-epithelial transition.
Metastatic models have suggested that about 1 × 106 tumor cells per 1 g of tumor enter the bloodstream daily[8]. However, CTCs have very low survival rates in peripheral blood, and 85% of CTCs disappear within 5 min[9,10]. Experiments in animal models have reported that 2.5% of CTCs cause micrometastases, and 0.01% of CTCs proliferate and form macroscopic metastases[11]. Because most CTCs that enter the circulation undergo apoptosis, and angiogenesis is not promoted by micrometastases in distant organs, the proportion of CTCs that cause macroscopic metastases is not necessarily high. In fact, tumor cells may remain dormant for several years because of cessation of the cell cycle in micrometastases[12].
Tumor cell infiltration and entry into blood vessels thus leads to the presence of CTCs in peripheral blood. Measuring CTCs in patients with cancer has thus been expected to contribute to the analysis of metastatic mechanisms and the development of new clinical applications for diagnosis and therapy. Various CTC-related studies have been performed, however, the clinical significance of CTCs remains to be established due to factors such as the extremely small number of CTCs in peripheral blood as compared with the number of blood cells, and technical problems in the detection of CTCs (e.g. reproducibility and reliability).
PRINCIPLES FOR THE IDENTIFICATION OF CTCS
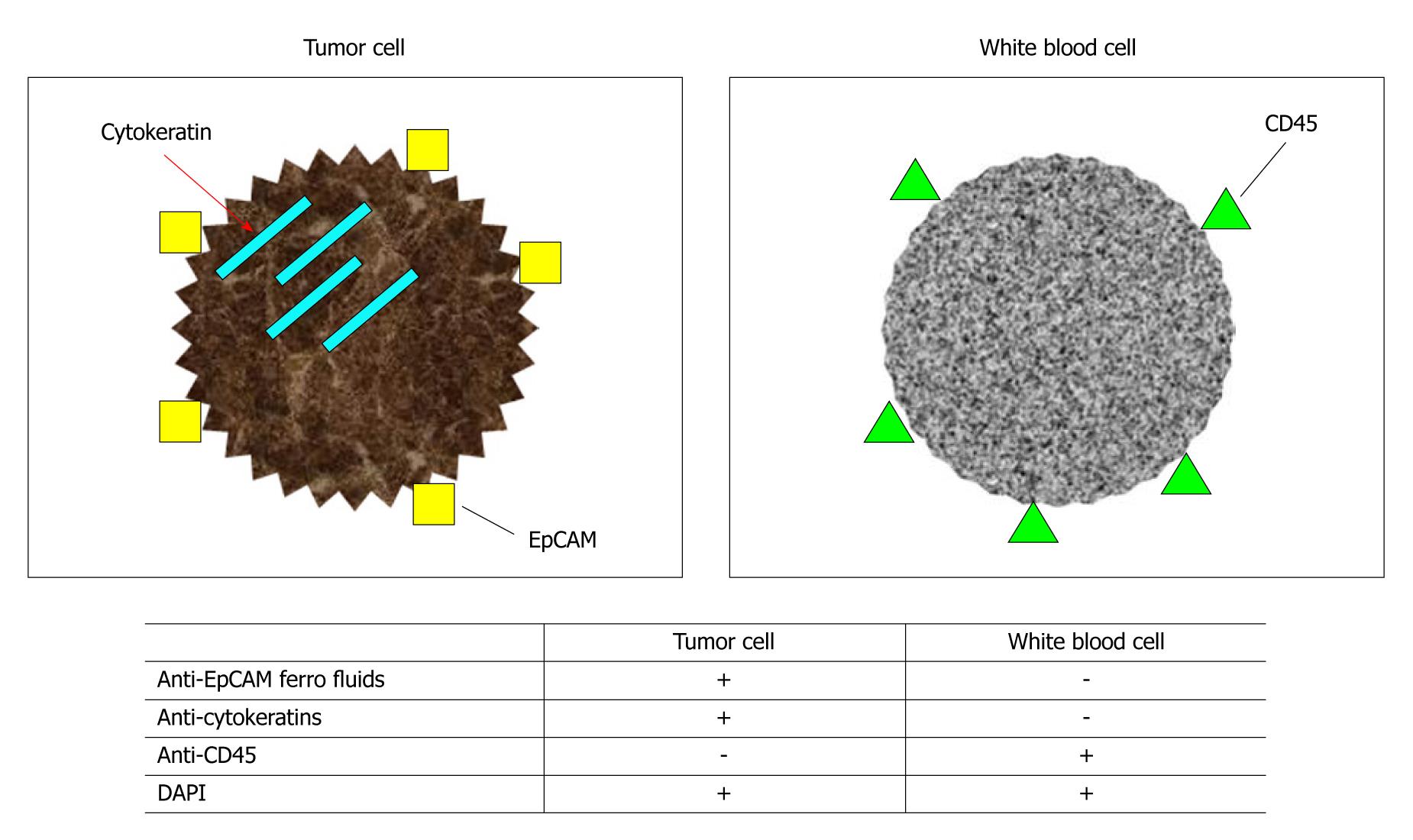
Epithelial-cell adhesion molecule (EpCAM) and cytokeratins (CK) are most commonly used to distinguish between epithelial cells and non-epithelial cells. EpCAM is widely expressed by epithelial cells and cancer cells[13] and is also referred to as Trop1, epithelial surface antigen, or cancer-related antigen. Monoclonal antibodies against EpCAM include HEA125[13], Ber-EP4[14], and KSI/4[15]. Various CK subfractions have been identified, some of which are specifically expressed by certain epithelial cells and epithelial tissue. Studies by Bártek et al[16], using various types of anti-CK antibodies, showed that CK subfractions CK4 to CK8, CK10, CK13, and CK18 are expressed in nearly all monolayer cultures of epithelial cells. As for breast cancer, Taylor-Papadimitriou et al[17] and Bratthauer et al[18] reported that CK7, CK8, CK18, and CK19 are expressed in luminal epithelial cells of terminal duct lobular units, lobular intraepithelial tumors, and ductal intraepithelial tumors.
Braun et al[19] used an antibody cocktail of anti-CK8, anti-CK18, and anti-CK20 and the non-cancer-cell marker, anti-CD45 antibody, to identify breast cancer cells. Lymph nodes obtained from patients with breast cancer were stained with these antibodies. They found that only cancer cells entering lymph nodes were positive for CK8, CK18, and CK19 and negative for CD45. Other mesenchymal cells were negative for CK subfractions and positive for CD45.
Combinations of these markers have been used to identify cancer cells entering bone marrow[15,20-24], lymph nodes[19,24-26], or peripheral blood[15]. Although anti-EpCAM antibody and anti-CK antibody target a single antigen molecule, the possibility of false-positive results caused by nonspecific reactions cannot be ruled out. However, the use of flow cytometric analysis, which allows the cancer-cell markers, anti-EpCAM antibody and anti-CK antibody, to be combined with the non-cancer-cell marker, anti-CD45 antibody, has enhanced specificity for cancer-cell detection[27] (Figure 2).
Figure 2 Identification of tumor cells and white blood cells using monoclonal antibodies.
EpCAM: Epithelial-cell adhesion molecule; DAPI: 4’,6-diamidino-2-phenylindole.
CELLSEARCH SYSTEM®
The CellSearch System® is an automated detection system for CTCs that uses anti-EpCAM antibodies, anti-CK antibodies, and anti-CD45 antibodies. It was developed by Veridex Co., Ltd. (Raritan, NJ, USA). The CellSearch System® is based on a combination of immunomagnetic labeling and automated digital microscopy. The principles of measurement are described below: (1) Cancer cells are labeled with anti-EpCAM antibody magnetic beads that target EpCAM, which is expressed by the cell membrane of tumor cells; (2) Tumor cells are immunomagnetically captured and concentrated; and (3) Tumor cells are stained with phycoerythrin (PE)-labeled anti-CK antibodies (CK-PE). Cell nuclei are then immunofluorescently stained with 4’,6-diamino-2-phenylindole. Leukocytes are stained with allophycocyanin-labeled anti-CD45 antibodies (CD45-APC), and cellular fixation is performed; and (4) Concentrated, stained tumor cells are examined by fluorescence microscopy to assess labeling by PE, 4’,6-diamidino-2-phenylindole (DAPI), and allophycocyanin (APC) and thereby distinguish tumor cells from leukocytes (Figure 3).
Figure 3 Measurement of circulating tumor cells by the CellSearch® System.
CTC: Circulating tumor cell; DAPI: 4’,6-diamidino-2-phenylindole; CK: Cytokeratins; PE: Phycoerythrin.
The CellSearch System® is a semiautomated system for the detection and enumeration of CTCs. The main advantages of this system are its ease of use and high reproducibility. A validation study conducted by Riethdorf et al[28] showed that CTC counts remained stable for 72 h after blood sample collection, even at room temperature. CTC counts did not differ among hospitals, and reproducibility was high.
The CellSearch System® can detect 1 CTC per 7.5 mL of peripheral blood, with high reproducibility. This system has been confirmed to accurately identify and enumerate CTCs. In the United States, clinical trials of the CellSearch System® have been performed in patients with metastatic breast cancer, metastatic colorectal cancer, and metastatic prostate cancer, confirming that CTC counts are a useful prognostic factor. In metastatic breast cancer, the CellSearch System® has been suggested to be useful for monitoring treatment response. This system was approved by the United States Food and Drug Administration in 2004.
STUDIES OF CTCS AS A PROGNOSTIC FACTOR IN BREAST CANCER
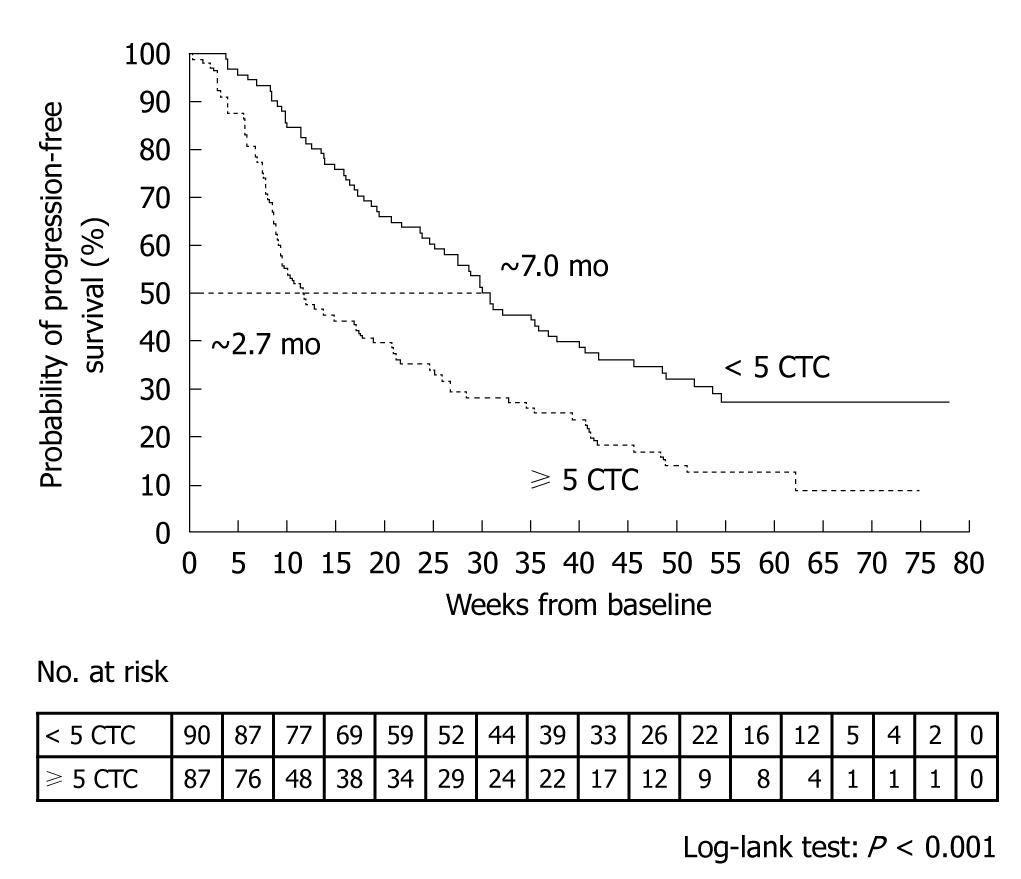
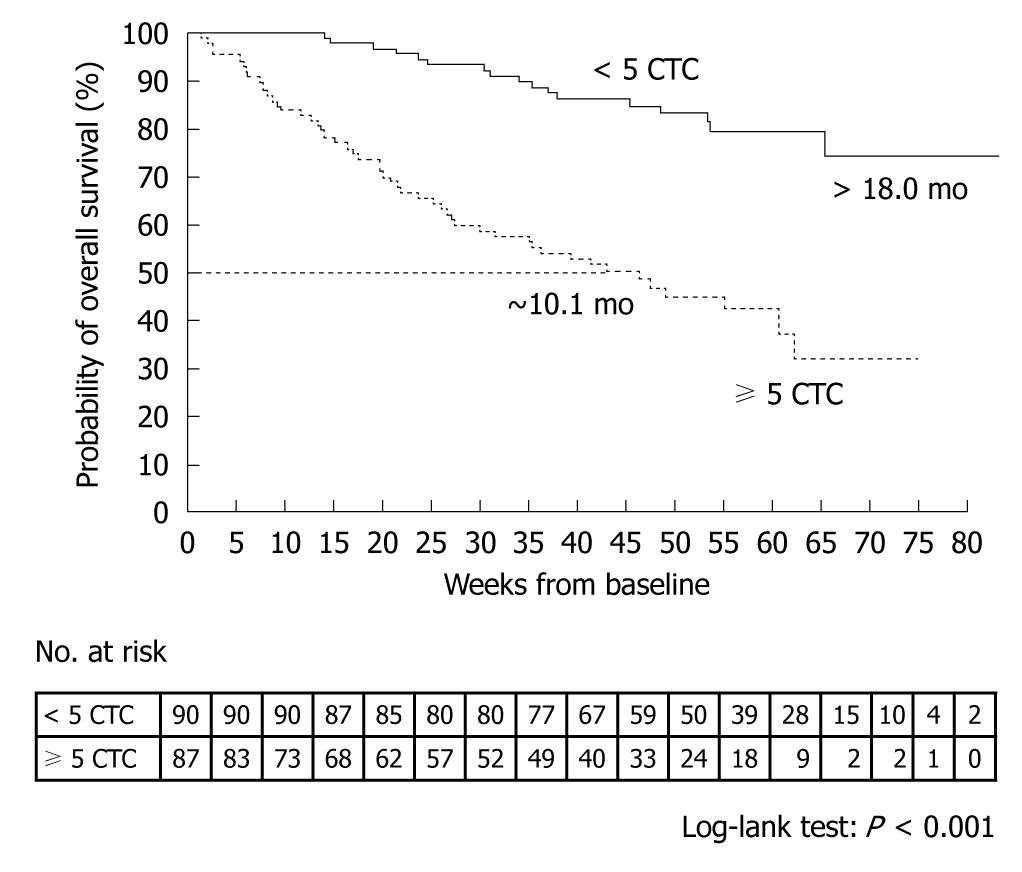
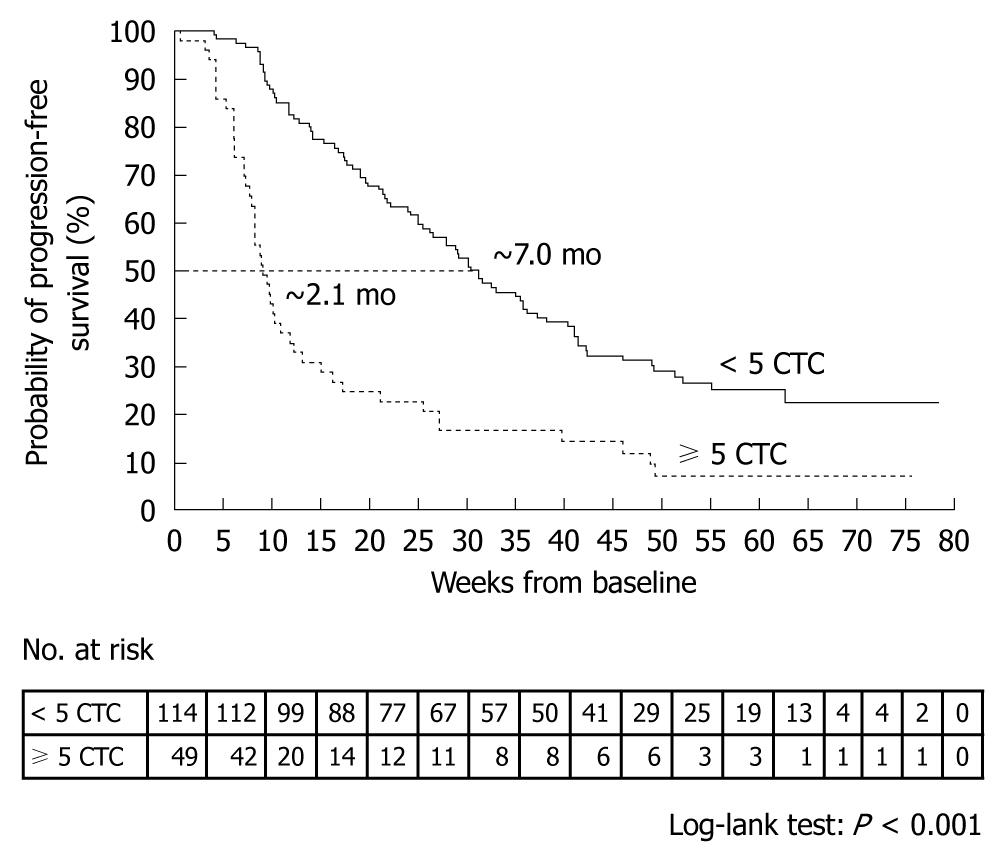
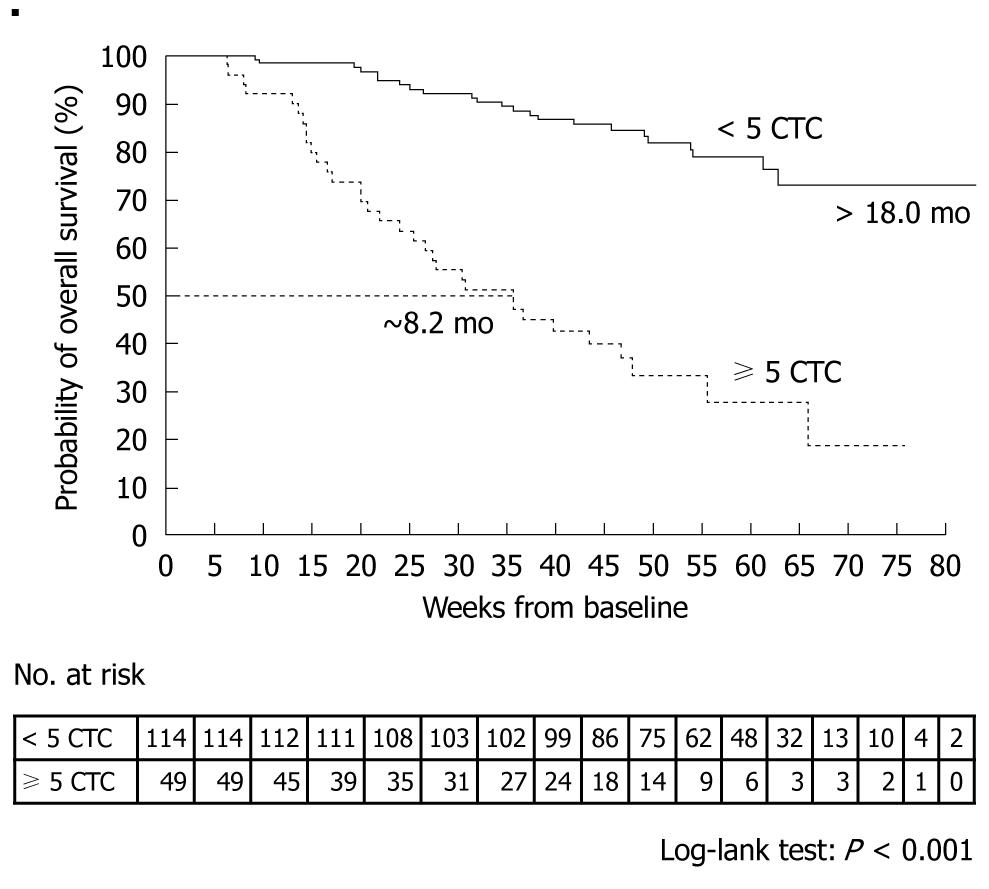
A prospective, multicenter clinical trial performed by Cristofanilli et al[29] counted the number of CTCs in 7.5-mL peripheral blood samples obtained from 145 healthy subjects, 200 patients with benign breast tumor, and 177 patients with metastatic breast cancer. The mean CTC count in the patients with metastatic breast cancer was 54 ± 138. When outcomes were assessed according to the CTC counts before the start of treatment, median progression-free survival was found to be 7.0 mo in patients with less than 5 CTCs per 7.5 mL of blood, as compared with only 2.7 mo in those with 5 or more CTCs (P < 0.001) (Figure 4). Moreover, median overall survival was significantly longer in patients with less than 5 CTCs (> 18 mo) than in those with 5 or more CTCs (10.1 mo, P < 0.001) (Figure 5)[29].
Figure 4 Kaplan-Meier estimates of probabilities of progression-free survival in patients with metastatic breast cancer who had less than 5 circulating tumor cells and those who had 5 or more circulating tumor cells (before initiation of a new line of therapy)[29].
CTC: Circulating tumor cell.
Figure 5 Kaplan-Meier estimates of probabilities of overall survival in patients with metastatic breast cancer who had less than 5 circulating tumor cells and those who had 5 or more circulating tumor cells (before initiation of a new line of therapy)[29].
CTC: Circulating tumor cell.
In Japan, a multicenter trial has been conducted in 38 patients with metastatic breast cancer. Overall survival (P = 0.04) and progression-free survival (P = 0.04) were both significantly shorter in the 11 patients who had 5 or more CTCs than in those who had less than 5 CTCs. These results were consistent with those obtained in the United States[30].
ROLE OF CTC COUNTS AS A PROGNOSTIC FACTOR FOR BREAST CANCER
Cristofanilli et al[29] counted CTCs at baseline and at the first follow-up visit (mean ± SD, 4.5 ± 2.4 wk) after the initiation of therapy in 163 patients. Patients who had 5 or more CTCs per 7.5 mL blood at the first follow-up visit, as compared with those who had less than 5 CTCs per 7.5 mL blood, had a shorter median progression-free survival (2.1 mo vs 7.0 mo, P < 0.001, Figure 6) and shorter median overall survival (8.2 mo vs > 18 mo, P < 0.001, Figure 7). A similar difference was seen between the groups on the basis of the baseline values.
Figure 6 Kaplan-Meier estimates of probabilities of progression-free survival in patients with metastatic breast cancer who had less than 5 circulating tumor cells and those who had 5 or more circulating tumor cells (at the first follow-up visit after initiation of a new line of therapy)[29].
CTC: Circulating tumor cell.
Figure 7 Kaplan-Meier estimates of probabilities of overall survival in patients with metastatic breast cancer who had less than 5 circulating tumor cells and those who had 5 or more circulating tumor cells (at the first follow-up visit after initiation of a new line of therapy)[29].
CTC: Circulating tumor cell.
The median progression-free survival and median overall survival in the 33 patients with 5 or more CTCs at baseline, but less than 5 CTCs at follow-up differed significantly from the respective values in the 25 patients who had a decrease in CTCs from baseline, but had 5 or more CTCs at follow-up (progression-free survival, 7.6 mo vs 2.1 mo, P = 0.002; overall survival, 14.6 mo vs 9.2 mo, P = 0.006)[29].
Hayes et al[31] studied the prognostic implications of CTC counts before treatment and after each treatment cycle. Even if the CTC count was 5 or more before treatment, patients who had CTC counts of less than 5 at any follow-up time point during treatment had good outcomes. In contrast, patients with CTC counts of 5 or higher at any time point during treatment had poor outcomes. These studies showed that blood CTC counts at initial follow-up after chemotherapy can be used to evaluate the response to treatment and suggest that the results can contribute to reducing stress on patients caused by long-term treatment with ineffective anticancer drugs.
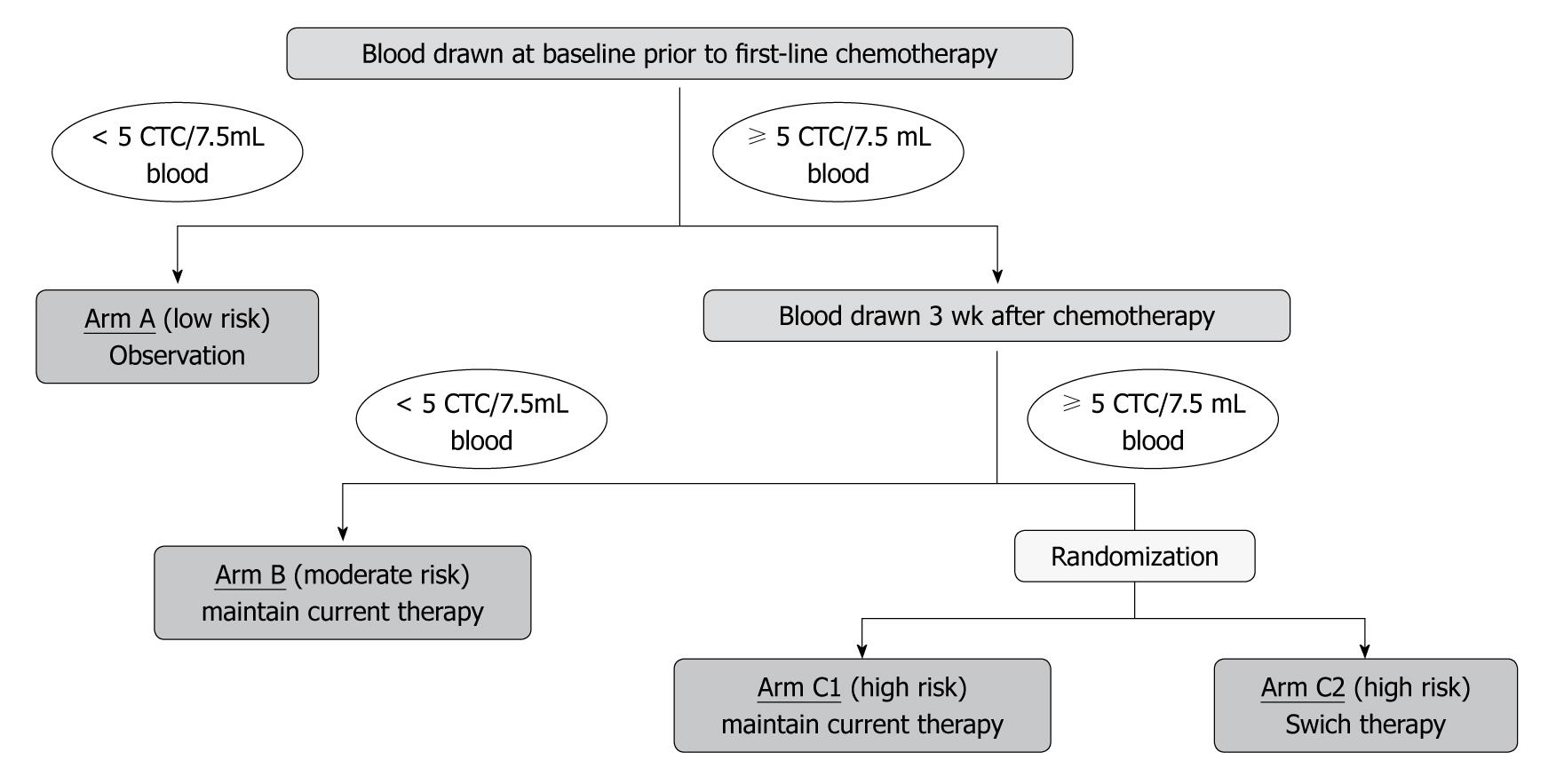
In the United States, a phase III clinical trial (SWOG S0500) is ongoing to determine whether CTC counts can be used to facilitate decisions concerning whether to change or maintain therapy. The feasibility or using CTC counts to compare outcomes is also being assessed. In that study, the number of CTCs is counted before the first course of chemotherapy, and patients with 5 or more CTCs are given a specified regimen of chemotherapy. If the CTC count is less than 5 after the first course of chemotherapy (after 3 wk), current therapy is maintained. If the CTC count is 5 or more at this time, patients are randomly assigned to maintain current therapy or to switch to a new regimen of chemotherapy. This study is designed to determine whether CTC monitoring can be used to predict the response to treatment at an early time, and thereby promptly discontinue ineffective anticancer drugs and switch to another therapy if a response is unlikely. Another important objective is to determine whether outcomes can be improved by adhering to this procedure (Figure 8).
Figure 8 Study design of the Southwest Oncology Group Trial S0500.
CTC: Circulating tumor cell.
Epithelial growth factor receptor (EGFR) is overexpressed in many cancers. EGFR includes 4 families: EGFR (HER1), HER2, HER3, and HER4. Molecular targeted agents that target these receptors have been developed. HER2 gene amplification or protein overexpression is found in about 20% to 30% of patients with breast cancer. Treatment with trastuzumab, a humanized monoclonal antibody against HER2 receptor, has been shown to improve outcomes in such patients[32].
Meng et al[33] studied 24 patients with HER2-negative primary breast cancer who had recurrence. HER2 gene amplification on CTCs during tumor progression was confirmed in 9 (37.5%) of 24 patients. Trastuzumab was given to 4 of the 9 patients. One had a complete response, 2 had a partial response, and 1 had progressive disease. In these 4 patients, the primary tumor was HER2 negative, but HER2 gene amplification was confirmed on CTCs.
Trastuzumab may be effective in patients in whom resected or biopsy specimens are not available and those with HER2-negative primary breast cancer in whom HER2 on CTCs is positive. We believe that molecular expression analysis of CTCs combined with increased sensitivity for the detection and enumeration of CTCs will provide important biomarkers for molecular targeted agents.
CTCS AND IMAGING STUDIES IN BREAST CANCER
De Giorgi et al[34] retrospectively studied the relation between CTC counts and therapeutic response by performing[18] fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) in patients with metastatic breast cancer. In 115 patients with metastatic breast cancer who newly started therapy, CTCs in blood were counted and FDG-PET/CT was performed at the time of starting treatment and 9 to 12 wk after treatment had begun. The mean survival time in 102 patients with assessable lesions was 14 mo. In 68 patients (67%), CTC counts during treatment correlated with the tumor response on FDG-PET/CT. On univariate analysis, better overall survival was significantly associated with less than 5 CTCs (P < 0.001) and a treatment response as evaluated by FDG-PET/CT (P = 0.001). Multivariate analysis showed that the CTC count was an important factor for overall survival (P = 0.004). They concluded that prospective studies are needed to confirm the results and assess cost-effectiveness.
Liu et al[35] conducted a prospective study to clarify the relation between CTC counts and radiographic disease progression. They serially measured CTC levels in patients starting a new treatment regimen for radiographically measurable metastatic breast cancer. Blood samples were collected before starting treatment and at 3- to 4-wk intervals after treatment began. The presence of 5 or more CTCs per 7.5 mL blood was defined as the cut-off value. Clinical outcomes were based on imaging studies performed at 9- to 12-wk intervals. Correlations between CTC counts and treatment response on imaging studies were assessable in 68 patients. Progression-free survival was assessable in 74 patients. Mean follow-up was 13.3 mo. In these patients, CTC counts were associated with radiographic disease progression. As compared with patients who had less than 5 CTCs, odds ratios for radiographic disease progression in patients who had 5 or more CTCs were 4.9 when CTCs were enumerated 7 to
9 wk before imaging, 3.1 when CTCs were enumerated 3 to 5 wk before imaging, and 6.3 when CTCs were enumerated at the time of imaging. As compared with patients who had less than 5 CTCs at each time point, progression-free survival was slightly, but not significantly shorter in patients who had 5 or more CTCs 3 to 5 wk after the start of treatment (5.1 mo vs 3.1 mo, P = 0.07) and was significantly shorter in patients who had 5 or more CTCs 7 to 9 wk after the start of treatment (6.7 mo vs 2.5 mo, P < 0.001). The study concluded that CTC monitoring combined with imaging studies provided a more accurate assessment of disease progression than imaging studies alone.
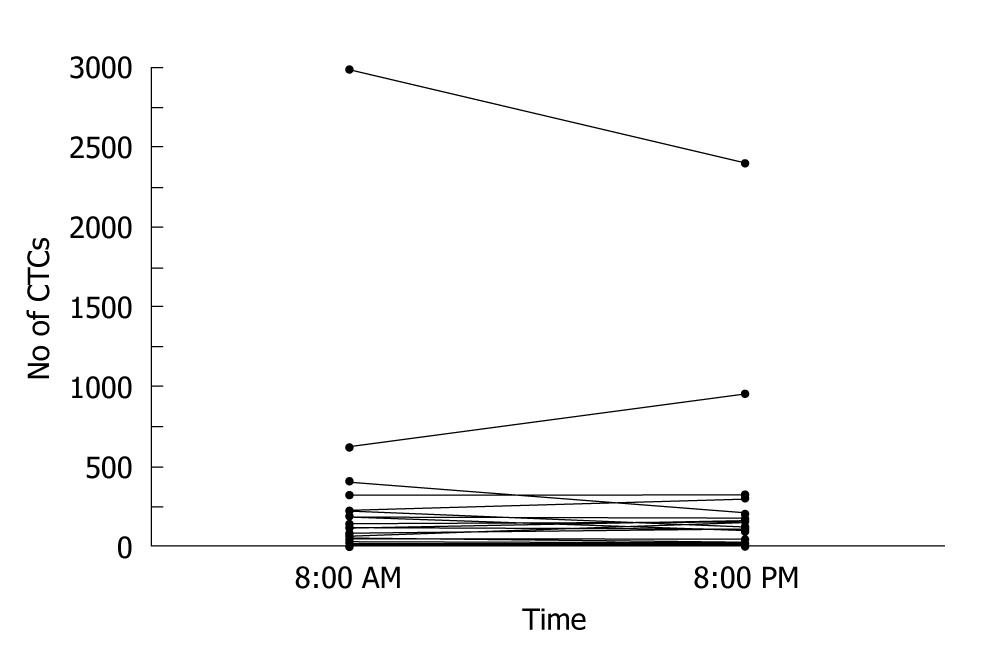
CTC counts combined with imaging studies can decrease the risk of radiation exposure, as well as facilitate the early assessment of treatment response. One study reported no significant circadian variations in CTC counts[36] (Figure 9). CTC counts are expected to be useful as a stable biomarker of disease progression and treatment response.
Figure 9 Circulating tumor cell values at 8:00 AM and at 8:00 PM in the 51 assessable patients[36].
CTC: Circulating tumor cell.
CONCLUSION
Breast cancer consists of diverse subtypes with different biologic characteristics. Individualized therapy matched to these characteristics is being developed. Appropriate treatment for patients requires a more accurate diagnosis. The enumeration of CTCs can help to predict response treatment at an early period and thereby avoid unnecessary therapy. When used as a biomarker for molecular targeted agents, CTC counts can facilitate the identification of patients most likely to respond to a given treatment and thereby provide the best-suited therapy to patients with breast cancer. Although CTC counts are associated with an extremely low false-positive rate in patients with metastatic breast cancer, the sensitivity has been reported to be only 26% to 49%[28,20,31,37-39]. The routine use of CTC monitoring as a diagnostic test would require the establishment of measurement techniques with improved sensitivity by, for example, the discovery of new detection targets, the establishment of quantitative and qualitative evaluation criteria to determine appropriate cut-off values, and further evidence clearly supporting the clinical significance of CTC counts. We expect that CTC counts will be established to be a new diagnostic and therapeutic index for patients with breast cancer.
ACKNOWLEDGMENTS
We are indebted to Mr. Satoshi Tsuchizawa for his help in preparing the manuscript.
Peer reviewers: Ali Syed Arbab, MD, PhD, Associate Scientist and Director, Cellular and Molecular Imaging Laboratory, Department of Radiology, Henry Ford Hospital, 1 Ford Place, 2F, Box 82, Detroit, MI 48202, United States; Yun Gong, MD, Associate Professor, Department of Pathology, Unit 53, The University of Texas, M D Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030, United States; Slimane Belbraouet, PhD, Professeur Titulaire, Directeur, École des sciences des aliments, de nutrition et d'études familiales, Université de Moncton, Moncton, NB, E1A 3E9, Canada; Songsak Petmitr, PhD, Associate Professor, Department of Tropical Nutrition and Food Science, Mahidol University, 420/6 Ratchawithi Road, Bangkok 10400, Thailand
S- Editor Tian L L- Editor Webster JR E- Editor Zheng XM