Published online Mar 10, 2011. doi: 10.5306/wjco.v2.i3.135
Revised: November 20, 2010
Accepted: November 27, 2010
Published online: March 10, 2011
Upper gastrointestinal (GI) tumors, including adenocarcinoma of the esophagus, stomach, pancreas, and biliary tree, have traditionally been difficult to treat with cytotoxic chemotherapeutic agents. There has been little drug development success in treating these cancers over the last 20 years, perhaps a reflection of a combination of the aggressive biology of these tumors, the void in effective and specific drug development for these varied tumors, and the lack of properly designed, biologically-based clinical trials. Recently, so called “targeted agents” have risen to the forefront in the care of cancer patients and have made strong impacts in many areas of oncology, particularly gastrointestinal stromal tumors (GIST), colon, breast, and lung cancers. Unfortunately, slow progress has been made using such agents in upper GI tumors. However, more recently, trials in some tumor types have demonstrated gains in progression free survival and overall survival. In this review, we discuss the drugs and pathways that have been most successful in the treatment of upper GI tumors and present the relevant data supporting their use for each tumor site. Additionally, we will explore a few novel pathways that may prove effective in the treatment of upper GI malignancies in the near future.
- Citation: Spratlin JL, Chu Q, Koski S, King K, Mulder K. Targeting metastatic upper gastrointestinal adenocarcinomas. World J Clin Oncol 2011; 2(3): 135-149
- URL: https://www.wjgnet.com/2218-4333/full/v2/i3/135.htm
- DOI: https://dx.doi.org/10.5306/wjco.v2.i3.135
Metastatic or locally advanced tumors of the stomach, liver, biliary tree, and pancreas have some of the worst prognoses of any cancer. Usually found at a stage when curative surgical resection is not possible, these tumors have incidence rates that approach mortality rates. Until recently there were no systemic therapy options for hepatocellular or biliary cancers. Cytotoxic therapies for gastric and pancreatic adenocarcinoma have limited benefit and there has been little advancement in the drug or drug combinations available to treat these diseases. In recent years, efforts to improve the outcomes for patients with metastatic gastrointestinal (GI) malignancies have focused on agents targeting one or more pathways involved in cell growth, proliferation, and/or metastases. Below, we explore these pathways and targets as well as evaluate several of the key areas that have been investigated using novel agents in advanced upper GI malignancies.
Human epidermal growth factor receptor (ErbB/HER) family cellular growth is a complex process regulated by a network of growth factors, growth factor receptors, and signal transduction pathways allowing essential communication between the outer and inner cellular environments[1]. The ErbB/HER family is comprised of four related tyrosine receptors: epidermal growth factor receptor (EGFR, ERBB1, Her-1), human EGFR-2 (HER-2, ERBB2), HER-3 (ERBB3), and HER-4 (ERBB4), each with a ligand binding extracellular, transmembrane, and intracellular tyrosine kinase (TK) domain[2,3]. Activation of the extracellular domain by a growth factor, leads to homo- or hetero-dimerization with another ErbB/HER family member, causing phosphorylation of intracellular TK residues and thereby downstream signaling[4,5]. ErbB/HER signal transduction is responsible for many normal cellular growth activities but constitutive or aberrant activation has been implicated in tumor progression via promotion of cell survival, proliferation, angiogenesis, anti-apoptosis, and metastases[4-7] (Figure 1). Inhibition of EGFR-1, HER-2, or both has been successful in the treatment of several upper GI malignancies. To date, monoclonal antibodies directed at EGFR or HER-2 and tyrosine kinase inhibitors (TKI) blocking downstream signal transduction pathways have had some success. Drugs targeting this pathway which have shown activity in upper GI adenocarcinomas are listed in Table 1.
| Drug | Mechanism of action | Applicable tumor site(s) |
| Cetuximab | Intravenous IgG1 monoclonal antibody inhibiting the extracellular domain of EGFR thereby preventing receptor activation | Gastric |
| Biliary tract | ||
| Pancreas | ||
| Erlotinib | Oral intracellular small molecule selective EGFR TKI | Biliary tract |
| Pancreas | ||
| Trastuzumab | Intravenous recombinant humanized anti-HER2 monoclonal antibody directed against the HER-2 extracellular domain | Gastric |
| Lapatinib | Oral TKI targeting EGFR and HER-2 | Gastric |
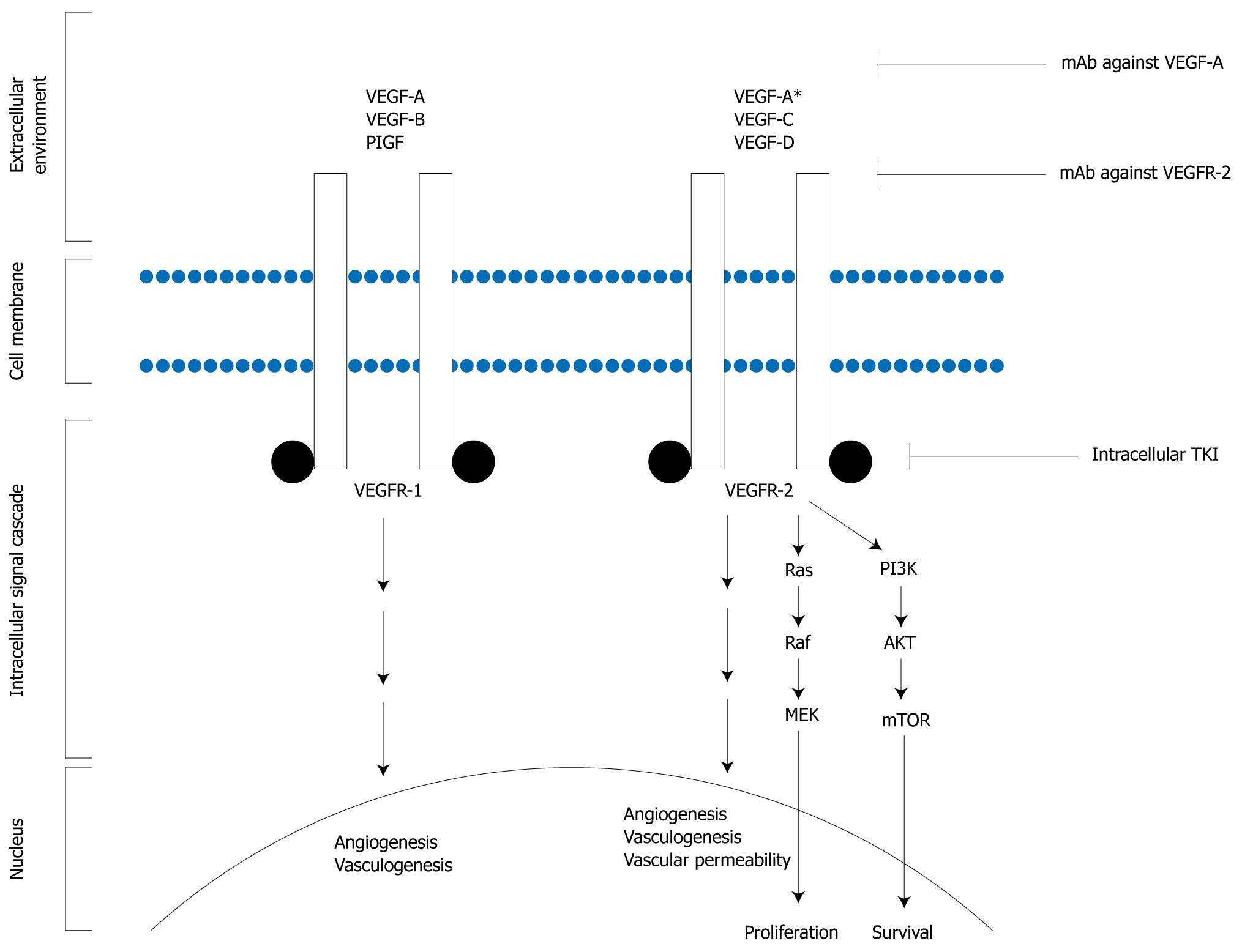
Angiogenesis is the process of new blood vessel formation from pre-existing vascular structures and is modulated by various inhibitors and inducers. Persistent up-regulation of this process is an important factor in development and maintenance of malignancy and is required for tumor growth and progression[8,9]. The vascular endothelial growth factor (VEGF) family of ligands and receptors are the most essential components in tumor angiogenesis. VEGF ligands include VEGF-A (VEGF), VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placental growth factor. Of these, VEGF is considered the critical regulator of endothelial proliferation, permeability, and survival. VEGF binds to VEGF receptor-1 and -2 (VEGFR-1, -2), expression of which is up-regulated in endothelial cells of the tumor vasculature. VEGF/VEGFR binding triggers a large spectrum of cellular changes including proliferation, vascular cell differentiation, changes in vascular permeability, and cellular migration[10-17]. Similarly to activation of EGFR, extracellular activation of VEGFRs induces receptor dimerization. Autophosphorylation of the receptor then results in activation of downstream proteins and effector molecules (Figure 2).
Inhibition of angiogenesis is considered a promising area of anti-cancer research and therapy. The first approved indication for the use of an antiangiogenic agent in cancer therapy was the use of bevacizumab in metastatic colorectal cancer which demonstrated an almost 5 mo benefit in survival in the bevacizumab arm[18]. Since then, multiple avenues have been used in attempts to inhibit angiogenesis in other GI tumors, including inhibition of the ligand VEGF with bevacizumab, inhibition of the VEGFRs, and inhibition of intracellular tyrosine kinase pathways. Antiangiogenic drugs which have shown activity in upper GI adenocarcinomas, including bevacizumab, sunitinib and sorafenib, are discussed below. Mechanisms of action of these drugs are described in Table 2.
| Drug | Mechanism of action | Applicable tumor sites |
| Bevacizumab | Intravenous recombinant humanized monoclonal antibody against VEGF | Gastric |
| Hepatocellular | ||
| Biliary tract | ||
| Pancreas | ||
| Sunitinib | Oral multitargeted TKI inhibiting VEGFR-1, VEGFR-2, PDGFR-β, c-KIT, FLT3, and RET | Gastric |
| Hepatocellular | ||
| Sorafenib | Oral multitargeted TKI inhibiting VEGFR-1, VEGFR-2, PDGFR-β, Raf-1, B-Raf, and intracellular serine-threonine kinases | Gastric |
| Hepatocellular | ||
| Pancreas |
Rapamycin, an immunosuppressant and anti-fungal, was the first drug to implicate mammalian target of rapamycin (mTOR) as a possible target for anti-cancer therapy[19]. As a member of the phosphatidylinositol 3-kinase (PI3K) and protein kinase B (Akt) pathways, mTOR plays an important role in ribosomal synthesis and protein translation required for cell cycle progression, cell growth, proliferation, and survival. Additionally, the mTOR pathway is affected by other growth factors and nutrition[20-22] (Figure 1). The activity of mTOR is orchestrated through two complexes, mTOR complexes 1 and 2 (TORC1 and TORC2), whose interaction and signaling systems are still incompletely understood[23-25].
Mammalian target of rapamycin inhibitors have demonstrated in vitro and in vivo growth inhibition against a number of different cancers, the most successful of which has been renal cell carcinoma (RCC) with phase III study data establishing mTOR inhibition has survival advantage for poor prognosis RCC patients[26]. Limited mature data exists for the use of mTOR inhibitors in upper GI malignancies. The exception is a phase III study evaluating everolimus in gemcitabine refractory pancreatic cancer which showed limited clinical benefit[27].
The tumor microenvironment is increasingly being investigated to determine its role in cancer growth and spread. Included within this microenvironment is a complex interplay between the cancer cell and surrounding stroma including non-malignant cells, vasculature, and enzymes. Matrix metalloproteinases (MMPs), found within the cellular microenvironment, are a family of endopeptidases with proteolytic activity having critical roles in inflammation, tissue remodeling, and tumorigenesis[28-31]. There are 23 known MMPs, the activity of which is tightly regulated by their requirement for activation by proteolytic enzymes and the presence or absence of MMP inhibitors[31,32]. Physiologic MMP inhibitors exist and are found at sites of cancer[33]. Synthetic inhibitors have been tested alone and in combination with chemotherapeutics in clinical trials with manageable toxicities. Unfortunately, the effectiveness of MMPs in cancer patients on clinical trials has been disappointing despite their proven roles in the development of malignant proliferation and metastases.
Gastric and esophageal cancers are the second and sixth leading causes, respectively, of cancer-related death worldwide[34]. Advanced esophageal adenocarcinomas are usually treated akin to advanced gastric cancer adenocarcinoma as it is often difficult to determine if the cancer originates in the gastroesophageal junction (GEJ) or distal esophagus. Most patient with esophagogastric cancer (EGC) present with advanced, inoperable, or metastatic disease; 5 year survival rates are approximately 10%-15%. Palliative cytotoxic chemotherapy improves survival compared to best supportive care[35-37]. There is no internationally accepted standard of care despite a large number of chemotherapy regimens being tested in randomized trials. The best survival rates are achieved with three drug regimens compared to doublet therapy[38]. Capecitabine and oxaliplatin are as effective as 5-fluorouracil (FU) and cisplatin, respectively, when combined with epirubicin[39]. The addition of docetaxel to cisplatin and FU (DCF) showed a small survival benefit over FU/cisplatin but increased toxicity limits its widespread use[40]. DCF has not been compared to a FU/anthracycline/platinum regimen. As the benefits of palliative chemotherapy remain modest, novel target agents are being tested in EGC.
Phase II studies of bevacizumab combined with chemotherapy (irinotecan + cisplatin; oxaliplatin + docetaxel or FU; DCF) showed promising results in previously treated and untreated patients (response rate (RR) 63%-71%)[41-44]. AVAGAST, a Phase III study of bevacizumab versus placebo combined with capecitabine and cisplatin showed a significant improvement in overall RR (ORR 38% vs 29.5%) and progression free survival (PFS 6.7 mo vs 5.3 mo)[45]. However, the addition of bevacizumab failed to improve overall survival (OS), the primary endpoint of this study.
Several small molecule multitargeted TKIs to VEGFRs have been tested in phase II studies. Sorafenib in combination with docetaxel and cisplatin in treatment naive patients with metastatic EGC demonstrated 41% partial response (PR), median PFS of 5.8 mo and median OS of 13.6 mo[46]. Sunitinib as a second-line single agent treatment for advanced EGC demonstrated a disease control rate (DCR) of 35%[47]. Further randomized trials are required to assess the benefit of these agents.
Ramucirumab, a monoclonal antibody directed against VEGFR-2, is currently being tested in the second-line setting of EGC in a randomized phase III study (NCT00917384) after a heavily pre-treated gastric cancer patient had prolonged response to the drug in the phase I dose finding study[48].
In pretreated EGC patients, single agent cetuximab has poor RR (5%)[49]. However, in previously untreated patients in combination with FU and oxaliplatin or irinotecan, an RR of 45%-65% was observed[50,51]. As a second line treatment, cetuximab combined with docetaxel resulted in 43% stable disease (SD)[52]. A randomized phase III trial (EXPAND) comparing capecitabine and cisplatin with or without cetuximab in advanced EGC is ongoing (NCT00678535). Most phase II clinical trials using EGFR TKIs as single agents in EGC have shown minimal efficacy. Erlotinib has a 10% RR in previously untreated patients[53]; gefitinib an 18% SD rate in previously treated patients[54] and lapatinib showed a 5% RR and 20% of patients had SD in untreated patients[55]. In the phase I trial of matuzumab in combination with epirubicin, cisplatin and capecitabine the DCR was 43%-57% which looked very promising[56]. The subsequent phase II trial failed to show a significant benefit[57].
Reported rates of over-expression and amplification of ERBB2/HER-2 in EGC varies widely due to sample sizes and methodological differences. The largest data set of advanced EGC samples had an HER-2 positivity rate of 22.9%[58]. Differences were found based on tumor location with higher HER-2 positivity in GEJ tumors compared to gastric tumors (33.2% vs 20.9%) as well as increased rates in intestinal versus diffuse/mixed cancers (32.2% vs 6.1%).
A small phase II study in advanced EGC with HER -2 overexpression/amplification (n = 21) receiving trastuzumab in combination with cisplatin observed a RR of 35%and SD of 17%[59]. The first randomized controlled phase III study, ToGA, comparing combination chemotherapy with a fluoropyrimidine (5-FU or capecitabine) plus cisplatin with or without trastuzumab in HER2 positive ECG patients showed a statistically significant improvement in median OS with the addition of trastuzumab (13.5 mo vs 11.1 mo, P = 0.0048) and a 26% reduction in the risk of death[60]. Furthermore, the addition of trastuzumab improved PFS (6.7 mo vs 5.5 mo, P = 0.0002) and DCR (47.3% vs 34.5%, P = 0.0017). Safety profiles were similar in both groups, including cardiotoxicity. In a pre-planned analysis, patients with high immunohistochemistry (IHC) positivity for HER-2 had a trend for better survival; furthermore, those patients with HER-2 IHC2+/FISH + or IHC3+ had a longer survival (16 mo) with trastuzumab compared to chemotherapy alone (11.8 mo).
Lapatinib, an oral TKI, which targets EGFR1 and 2 (HER-2), is currently being tested in a phase III study, LOGiC (NCT00680901). Patients with HER2 amplified EGC will receive capecitabine and oxaliplatin with lapatinib or placebo with the primary endpoint being PFS.
Despite advances in the treatment of locally advanced or metastatic EGC, prognosis remains poor; novel treatment options and predictors of treatment response are needed. Trastuzumab in combination with cisplatin and a fluoropyrimidine, is the only targeted therapy to date to have modest but clinically significant improvement in OS compared to chemotherapy alone in patients with HER2 positive gastric cancer. Unfortunately, only about 20% of patients would be potential candidates for this treatment. Furthermore, it is not clear if this benefit would be observed if compared to proven triplet regimens.
Hepatocellular carcinoma (HCC) is the third leading cause of death worldwide after lung and gastric cancers[61]. Although 5-year survival rates can exceed 70% with surgical management, < 30% of patients are eligible for surgery due to an advanced stage of disease at presentation. The treatment of advanced disease with cytotoxic chemotherapy has been disappointing with multiple studies failing to show an improvement in OS[62]. Several molecular pathways have been identified in the tumorigenesis of HCC including angiogenesis, the epidermal growth factor receptor pathway and the RAS/RAF/MAP kinase pathway[63].
HCCs are highly vascular tumors. With high microvessel density and levels of circulating VEGF being associated with poorer outcomes, the angiogenesis pathway is an attractive therapeutic target[63-68]. Sorafenib and sunitinib, both of which target VEGFR-1, -2 and -3, have shown clinical activity in Phase II and III clinical trials.
Sorafenib is the first targeted agent that has demonstrated an improvement in OS for patients with advanced HCC and is the first systemic therapy approved for this indication. An initial phase II study of 137 patients showed promising activity for sorafenib in patients with advanced HCC with a median OS of 9.2 mo and a median time to progression (TTP) of 5.5 mo[69]. Patients with Childs-Pugh Class B liver function had a similar incidence of drug-related adverse events but had more frequent worsening of liver disease than patients with Childs-Pugh A liver function. OS was also significantly shorter in Childs-Pugh B patients (14 wk vs 41 wk)[70]. Subsequently, two phase III, multicentre, randomized, placebo-controlled studies confirmed the activity of this agent[71,72]. Enrollment was limited to patients with Childs-Pugh A liver function. The SHARP study enrolled patients from Europe, North and South America and Australasia and had hepatitis C and alcohol as the predominant risk factors for HCC. The Asia-Pacific trial enrolled patients from China, South Korea and Taiwan and had hepatitis B as the predominant risk factor for HCC. Both studies demonstrated a significant improvement in OS (SHARP: 10.7 mo vs 7.9 mo, HR 0.69, P < 0.001; Asia-Pacific: 6.5 mo vs 4.2 mo, HR 0.68, P = 0.014) and DCR (SHARP: 43% vs 32%, P = 0.0002; Asia-Pacific: 35.5% vs 15.8%, P = 0.0019) for sorafenib compared to best supportive care.
Sunitinib has also demonstrated activity in the treatment of advanced HCC[73,74]. However, a phase III clinical trial comparing sunitinib to sorafenib was terminated in April 2010 due to increased toxicity in the sunitinib arm and because sunitinib did not meet the pre-defined criteria for superiority or non-inferiority (NCT00699374).
Two phase II studies examining the activity of bevacizumab in the treatment of advanced HCC both demonstrate promising antitumor activity (RR 12.5%-13%; PFS 6.9 mo) but toxicity, in particular GI bleeding, is concerning[75,76]. There have been three single arm phase II studies of bevacizumab in combination with a variety of chemotherapy regimens which show evidence of clinical activity but randomized comparisons are required[77-79].
EGFR is known to be expressed in HCCs and this pathway has been implicated in hepatocarcinogenesis[63]. However, the role of EGFR inhibitors in HCC is unclear. Minimal activity has been seen with the use of single agent lapatinib, gefitinib or cetuximab[80-85]. Modest activity is seen with the use of erlotinib but increased grade 3/4 toxicity was seen in a large proportion of patients in one of these studies, particularly those with Childs-Pugh Class B liver function[86,87]. A randomized phase III study of sorafenib plus erlotinib versus sorafenib is currently underway (NCT00901901). To date, there has been no correlation demonstrated between expression of EGFR and response to EGFR-directed therapies in HCC.
Interest has been raised by results seen with the combination of erlotinib and bevacizumab. In the initial report of a 40 patient phase II study there was a confirmed PR of 25% and 16 wk PFS of 62.5%[88]. Updated data with 58 patients reports a confirmed PR of 28%, SD 62% and 16 wk PFS of 72%. The median PFS is 7.9 mo and median OS 12.8 mo[89]. Due to a significant incidence of GI bleeding early in the study, a protocol amendment required all patients with portal hypertension undergo screening for varices prior to enrollment, and treatment thereof if detected. A preliminary report of this combination in Asian patients demonstrates 2 confirmed and 1 unconfirmed PR in 51 patients enrolled[90]. A randomized phase II trial of bevacizumab plus erlotinib vs sorafenib is currently underway (NCT00881751).
The use of targeted therapy in advanced/unresectable HCC has generated considerable interest. The greatest activity has been shown with dual blockage of both angiogenesis and EGFR mediated growth.
Biliary tract cancer (BTC), consisting of intra- and extra-hepatic cholangiocarcinoma as well as gallbladder malignancies, are rare tumors and only account for 3%-4% of gastrointestinal cancers. Surgery is the only curative option, but the majority of patients present with unresectable disease[91]. There are numerous phase II clinical trials of cytotoxic chemotherapy, with most activity seen with gemcitabine in combination with either a fluoropyrimidine or a platinum analogue. Only recently has treatment with gemcitabine and cisplatin demonstrated a clear improvement in OS[92]. With limited options for these patients, there is great interest in exploring new treatments with targeted agents.
In contrast to HCC, metastases from BTC tend to be hypovascular. However, VEGF expression has been detected in these tumors and correlates with advanced disease stage and poor prognosis[93,94]. A phase II clinical trial using gemcitabine + oxaliplatin (GEMOX) in combination with bevacizumab demonstrated modest activity with an ORR 40% and SD 29%, median PFS was 7.0 mo, and median OS was 12.7 mo. The 6-mo PFS of 63% did not meet the pre-specified endpoint of an improvement from 50% to 70% as compared to GEMOX alone[95]. Randomized comparisons are needed to evaluate the true added benefit of bevacizumab. TKI inhibition has been less fruitful with two phase II clinical trials of sorafenib failing to show significant clinical activity[96,97].
EGFR is overexpressed in the majority of cancers of the gallbladder and biliary tract, leading to a potential therapeutic target. Promising activity has been seen with the use of erlotinib. A phase II study of erlotinib as a first- or second-line treatment in 42 patients with advanced BTC demonstrated a DCR of 51% and 24 wk PFS of 17%, median TTP 2.6 mo and median OS 7.5 mo[98]. In contrast, dual targeting of EGFR-1 and -2 with lapatinib failed to demonstrate any significant clinical activity[81].
Two single arm studies of cetuximab in combination with chemotherapy have shown activity. In a first-line study of 22 patients, GEMOX + cetuximab demonstrated an ORR 58% [including 1 complete response (CR)], SD 32% and median PFS 9.0 mo. Six initially unresectable patients subsequently underwent curative resection following a major response[99]. A second smaller study of 9 patients with intrahepatic BTC, who had previously progressed on GEMOX, received cetuximab in addition to GEMOX demonstrating an ORR 33% (including 1 CR) with median PFS 4 mo and median OS 7 mo[100]. Randomized comparisons are needed to evaluate the added benefit of cetuximab over chemotherapy alone.
A preliminary report of a multicentre, phase II clinical trial of the combination of bevacizumab and erlotinib suggests favourable results. In the first 20 evaluable patients, there is a confirmed PR 20% and an additional 7 patients have SD > 4 mo. Further results are anticipated shortly[101].
The role of targeted therapy in the treatment of advanced BTC is still under development, with many clinical trials ongoing. Promising preliminary results have been reported for the combination of erlotinib and bevacizumab[101]. Impressive activity was seen with the combination of GEMOX plus cetuximab, both in the first-line and second-line setting, but randomized comparisons are needed[99,100].
Worldwide, pancreatic adenocarcinoma is the eighth leading cause of cancer death[102]. The prognosis for pancreatic cancer is poor, with one and five year survival rates for all stages of 23% and 5%, respectively[103]. Only 15%-20% of patients will present with surgically resectable disease, and of these, only 20% will survive 5 years[104]. The OS for patients with metastatic or locally advanced disease ranges from 4-9 mo. Single agent gemcitabine is considered the standard treatment with only modest improvements in median OS[105]. A clear benefit in OS when adding a second chemotherapeutic, such as FU, oxaliplatin, or capecitabine to gemcitabine has not been observed[106-108]. An increase in the understanding of the unique molecular and genetic alterations in the development of pancreatic carcinoma has allowed for rational design of treatment strategies with targeted agents. Since gemcitabine is considered the standard treatment, most clinical trials of targeted agents have been directed at combining the novel agent with gemcitabine.
Multiple anti-angiogenic agents have been tested in the pancreatic cancer population, including but not limited to bevacizumab, sorafenib, sunitinib, and axitinib and have failed to show a survival advantage[109-116].
A pivotal phase III trial randomized 569 unresectable, locally advanced, or metastatic patients to receive standard gemcitabine or gemcitabine + erlotinib (100 or 150 mg orally daily)[117]. Statistically significant improvement in OS (6.24 mo vs 5.91 mo, P = 0.038) was observed along with prolonged one year survival (23% vs 17%, P = 0.023) in the combination arm. Subgroup analysis suggested benefit from erlotinib regardless of EGFR status. Despite these positive results, there has been hesitancy in the general medical oncology community to recommend gemcitabine + erlotinib as the standard of care for these patients as results demonstrate limited OS benefit and questionable clinical benefit.
A phase II study of 41 patients with EGFR expressing pancreatic cancer receiving gemcitabine and cetuximab showed a promising median OS of 7.1 mo with a 12% PR and 63.4% SD[118]. The subsequent phase III clinical trial which randomized 735 patients between gemcitabine alone or gemcitabine + cetuximab failed to show a statistical advantage in OS or PFS in the patients exposed to cetuximab[119].
Early studies looking at the efficacy of combining HER1/EGFR and VEGF inhibition alone or in combination with chemotherapy in pancreatic carcinoma are underway or have been completed. In a phase III trial, 607 patients with metastatic pancreatic cancer were randomized to gemcitabine + erlotinib plus/minus bevacizumab[115]. The addition of bevacizumab did not prolong OS although there was an improvement in disease free survival (DFS). A phase II trial enrolled 139 patients who received gemcitabine, bevacizumab + erlotinib or gemcitabine, bevacizumab + cetuximab but did not show improvement in OS or PFS[110].
The PI3K/Akt/mTOR pathway is activated in the majority of pancreatic cancers and preclinical studies have shown that inhibition of this pathway has an antitumor effect. However, the oral mTOR inhibitor everolimus, had minimal clinical activity in gemcitabine refractory disease[27]. Furthermore, the MMPs marimastat and talomastat failed to show significant clinical activity[120,121].
Pancreatic cancer is a devastating disease. For more than 20 years, the standard of care for patients with advanced disease has been single agent gemcitabine. Erlotinib was the first targeted agent in pancreatic cancer to improve OS in a randomized phase III setting but despite a statistical benefit the medical community has been hesitant to adopt its use. Clearly, novel therapies, biomarkers and better clinical trial planning and development are needed for patients afflicted with this disease.
As described, novel anti-cancer agents targeting angiogenesis, the epidermal growth factor family of receptors and others, either alone or in combination with cytotoxic chemotherapy, have achieved modest success in upper GI malignancies. There is an urgent need to identify novel therapeutic options for these patients. We have elected to discuss two promising novel targets: the hedgehog (Hh) pathway and poly (ADP-ribose) polymerases (PARP) inhibition.
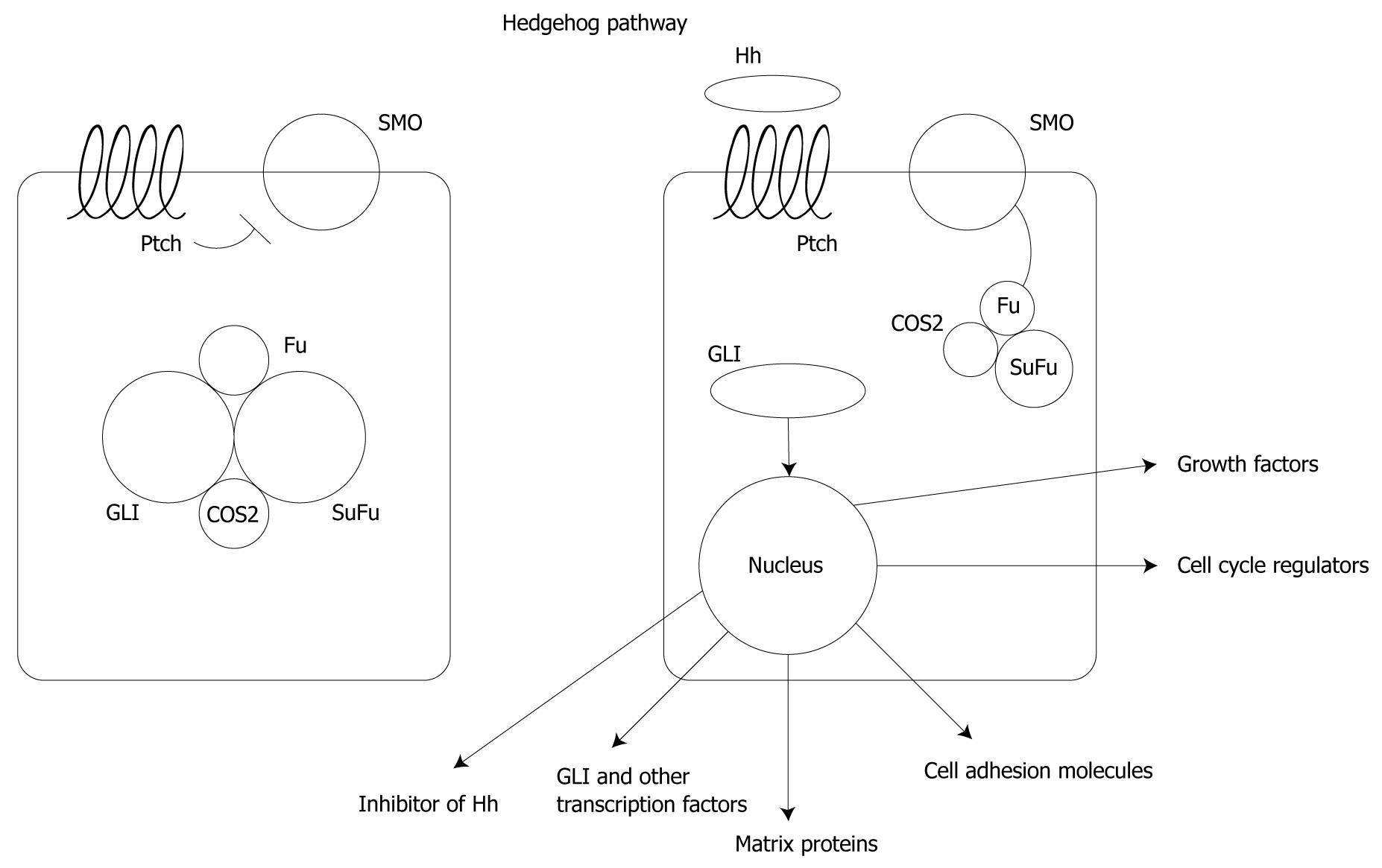
The Hh pathway was originally identified as a normal developmental pathway in Drosophila[122,123]. Three mammalian homologues, Sonic, Indian and Desert Hh, have been identified as being required for embryonic development among which Sonic Hh is essential for lung, skin, foregut, brain and limb development[124,125]. All three are extracellular proteins that bind to a 12-transmembrane hedgehog receptor, Patched (Ptch). In the absence of Hh, Ptch inhibits Smoothened (Smo) and the downstream pathway. Smo is de-repressed upon binding by Hh, leading to dissociation of Gli transcription factors from the inhibitory complex of serine/threonine protein kinase Fused and Suppressor of Fused (Sufu)[126]. Gli is then transported into the nucleus leading to regulation of the expression of multiple pathways including growth factors, cell cycle regulators, cell adhesion molecules, matrix proteins, other transcription factors, and inhibitors of the Hh pathway itself[127-133] (Figure 3).
Alterations of the Hh pathway have been identified in various malignancies, including: (1) somatic mutation of Ptch; (2) mutation of Smo; (3) autocrine or paracrine overexpression of Sonic Hh; (4) amplification or overexpression of Gli-1; and (5) dysregulation of HIP in a Sonic Hh independent fashion, most likely through methylation of HIP gene[134-145]. In GI malignancies, the Hh pathway is activated through overexpression of Sonic Hh[139,146-148]. In gastric cancer xenografts, blockade of the pathway led to tumor apoptosis and regression[139]. In pancreatic cancer, the Hh pathway is important in both the development and maintenance of the malignant phenotype[139,147]. In HBCs, decreased proliferation and cell cycle arrest has been demonstrated with Hh inhibition[149].
The first member of the Hh pathway being explored in the clinic is inhibition of Smo, with the first tested Smo inhibitor being GDC-0449[150]. Nineteen patients were treated over 3 dose levels with the recommended phase II dose being 150 mg daily. The drug was well tolerated with no dose-limiting toxicities observed. Common grade 1-2 toxicities included fatigue, dysguesia, and hyponatremia. Various single agent or combination phase I or II studies are ongoing with GDC-0449 in colorectal, ovarian, and advanced basal cell carcinoma. In 2010, preliminary results from two other agents inhibiting Hh were presented and further information should be forthcoming[151,152].
A number of other strategies against various parts of the Hh pathway are in preclinical or early clinical development, including Hh antagonist and Gli inhibitor.
Poly (ADP-ribose) polymerases (PARP) is a superfamily of 17 proteins which senses the presence of DNA damage and has conserved catalytic domains among which, the function and biology of the nuclear protein PARP1 is the best characterized[153-155]. PARP1 consists of three functional domains: a DNA binding fragment, an auto-modification domain, and a NAD+-binding C-terminal catalytic domain[156]. The presence of single strand DNA damage leads PARP1 to undergo an NAD+-dependent polymerization of ADP-ribose to base excision repair proteins (XRCC1, DNA polymerase beta and ligase III), histones H1 and H2B, and PARP1 itself[157,158]. These will in turn affect DNA replication, transcription, differentiation, gene regulation, protein degradation, and spindle maintenance. In knockout mouse models, PARP1 is only responsible for 90% of the DNA repair, the rest completed by PARP2, which is critical in the absence of PARP1[156,159]. PARP1 is also involved in the detection of double-strand DNA damage via the homologous recombination repair by BRCA1 and BRCA2 and nonhomologous recombination repair by XRCC1 and DNA ligase III[159-163].
Cell lines and xenografts that have homozygous deletion of BRCA1 or BRCA2 gene are very sensitive to PARP1 inhibition[161,162]. It is postulated that PARP1 inhibition in BRCA deficient cells cannot undergo the most effective DNA repair by homologous recombination repair after single strand breaks, leading to double strand breaks and thus apoptosis. Germline loss of BRCA1/2 is commonly associated with breast and ovarian cancer; pancreatic cancer represents the third most common malignancy associated with this syndrome and thus PARP inhibition may be efficacious[164].
PTEN exerts transcriptional control of RAD51 gene expression, a gene involved in repair of double stranded DNA breaks. PTEN deficient astrocytes are sensitive to PARP1 inhibition[165]. Additionally, truncated PTEN mutation but not point mutations is the biomarker for sensitivity to PARP1 inhibition[166]. Homozygous loss of PTEN has been observed in a number of cancers including colorectal cancer and HCC. Furthermore, methylation of PTEN genes have been observed in gastric cancer and 50% of pancreatic cancers harbour K-ras mutations which lead to increase in transforming growth factor-beta expression which in turn decreases PTEN expression[167,168]. Finally, treatment of HCC cell lines with a PARP inhibitor leads to a decrease in tumor size, mitosis, angiogenesis and an increase in apoptosis through decrease in VEGFR-1, EGFR, HIF-2 and HGF expression[169].
With the above noted pre-clinical findings, multiple early phase clinical trials are underway with the use of various PARP inhibitors. As of yet, limited data is available as to their use and efficacy and tolerability in upper GI malignancies though a number of proof of concept phase I and II studies in GI malignancies are currently ongoing in microsatellite unstable colorectal cancer, locally advanced or metastatic colon cancer and gastric cancer (NCT00912743, NCT01063517, NCT00535353). Further investigations will look into the benefit of PARP inhibition in pancreatic cancer.
Upper GI malignancies are aggressive tumors and often present with poor prognoses at an incurable stage. To date, cytotoxic chemotherapies have been the mainstay of treatment, unfortunately with less than desirable benefits in PFS, OS, or clinical benefit.
Though there has been some advancement in the treatment of these diseases with targeted therapies, most notably with sorafenib in HCC and trastuzumab in gastric cancers expressing HER-2, many studies have failed. Those drugs or drug combinations that have shown promise in phase II clinical trial require validation in randomized phase III studies in order to prove efficacy. Over the next decade it is hoped that further advances will be made in the treatment of upper GI malignancies.
| 1. | Schlessinger J, Lemmon MA. Nuclear signaling by receptor tyrosine kinases: the first robin of spring. Cell. 2006;127:45-48. |
| 2. | Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787-2799. |
| 3. | Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006;12:5268-5272. |
| 4. | Giaccone G. HER1/EGFR-targeted agents: predicting the future for patients with unpredictable outcomes to therapy. Ann Oncol. 2005;16:538-548. |
| 5. | Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160-1174. |
| 6. | Hemming AW, Davis NL, Kluftinger A, Robinson B, Quenville NF, Liseman B, LeRiche J. Prognostic markers of colorectal cancer: an evaluation of DNA content, epidermal growth factor receptor, and Ki-67. J Surg Oncol. 1992;51:147-152. |
| 7. | Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183-232. |
| 10. | Klagsbrun M, D'Amore PA. Vascular endothelial growth factor and its receptors. Cytokine Growth Factor Rev. 1996;7:259-270. |
| 11. | Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306-1309. |
| 12. | Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9-22. |
| 13. | Inoue M, Hager JH, Ferrara N, Gerber HP, Hanahan D. VEGF-A has a critical, nonredundant role in angiogenic switching and pancreatic beta cell carcinogenesis. Cancer Cell. 2002;1:193-202. |
| 14. | Gille H, Kowalski J, Li B, LeCouter J, Moffat B, Zioncheck TF, Pelletier N, Ferrara N. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem. 2001;276:3222-3230. |
| 15. | de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989-991. |
| 16. | Plate KH, Breier G, Millauer B, Ullrich A, Risau W. Up-regulation of vascular endothelial growth factor and its cognate receptors in a rat glioma model of tumor angiogenesis. Cancer Res. 1993;53:5822-5827. |
| 17. | Plate KH, Breier G, Weich HA, Mennel HD, Risau W. Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer. 1994;59:520-529. |
| 18. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. |
| 19. | Edinger AL, Linardic CM, Chiang GG, Thompson CB, Abraham RT. Differential effects of rapamycin on mammalian target of rapamycin signaling functions in mammalian cells. Cancer Res. 2003;63:8451-8460. |
| 21. | Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151-3171. |
| 22. | Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596-603. |
| 23. | Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335-348. |
| 24. | Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471-484. |
| 25. | Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem. 2010;285:14071-14077. |
| 26. | Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, O'Toole T, Park Y, Moore L. A phase 3, randomized, 3-arm study of temsirolimus (TEMSR) or interferon-alpha (IFN) or the combination of TEMSR + IFN in the treatment of first-line, poor-risk patients with advanced renal cell carcinoma (adv RCC). J Clin Oncol. 2006;ASCO Annual Meeting Proceedings Part I. Vol 24, No. 18S:LBA4 2006. |
| 27. | Wolpin BM, Hezel AF, Abrams T, Blaszkowsky LS, Meyerhardt JA, Chan JA, Enzinger PC, Allen B, Clark JW, Ryan DP. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27:193-198. |
| 28. | Parks WC, Wilson CL, López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617-629. |
| 29. | Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221-233. |
| 30. | Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161-174. |
| 31. | Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52-67. |
| 32. | Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463-516. |
| 33. | Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9-34. |
| 34. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. |
| 35. | Glimelius B, Ekström K, Hoffman K, Graf W, Sjödén PO, Haglund U, Svensson C, Enander LK, Linné T, Sellström H. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163-168. |
| 36. | Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72:37-41. |
| 37. | Pyrhönen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995;71:587-591. |
| 38. | Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903-2909. |
| 39. | Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36-46. |
| 40. | Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991-4997. |
| 41. | Shah MA, Ramanathan RK, Ilson DH, Levnor A, D'Adamo D, O'Reilly E, Tse A, Trocola R, Schwartz L, Capanu M. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201-5206. |
| 42. | Enzinger PC, Fidias P, Meyerhardt J, Stuart K, Fuchs C, Huberman M, Goldstein R, Attawia M, Lawrence C, Zhu . Phase II study of bevacizumab and docetaxel in metastatic esophageal and gastric cancer. 2006 Gastrointestinal Cancers Symposium General Poster Session B Abstract No: 68, 2006. . |
| 43. | Cohenuram MK, Lacy J. FOLFOX6 and bevacizumab (FOLFOX6/B) for metastatic esophageal (E), gastroesophageal (GE), and gastric (G) adenocarcinoma: A single institution's initial clinical experience. 2008 Gastrointestinal Cancers Symposium General Poster Session A. Abstract No: 74, 2008. . |
| 44. | Hammad N, Philip PA, Shields AF, Heilbrun LK, El-Rayes BF. A phase II study of bevacizumab, docetaxel, and oxaliplatin in gastric and gastroesophageal junction (GEJ) cancer. 2008 Gastrointestinal Cancers Symposium General Poster Session A. Abstract No: 30, 2008. . |
| 45. | Kang Y, Ohtsu A, Cutsem EV, Rha SY, Sawaki A, Park S, Lim H, Wu J, Langer B, Shah MA. AVAGAST: A randomized, double-blind, placebo-controlled, phase III study of first-line capecitabine and cisplatin plus bevacizumab or placebo in patients with advanced gastric cancer (AGC). J Clin Oncol. 2010;28 suppl 18:abstr LBA4007. |
| 46. | Sun W, Powell M, O'Dwyer PJ, Catalano P, Ansari RH, Benson AB 3rd. Phase II study of sorafenib in combination with docetaxel and cisplatin in the treatment of metastatic or advanced gastric and gastroesophageal junction adenocarcinoma: ECOG 5203. J Clin Oncol. 2010;28:2947-2951. |
| 47. | Bang YJ, Kang YK, Kang WK, Boku N, Chung HC, Chen JS, Doi T, Sun Y, Shen L, Qin S. Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Invest New Drugs. 2010;Epub ahead of print. |
| 48. | Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, Leong S, O'Bryant C, Chow LQ, Serkova NJ. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28:780-787. |
| 49. | Gold PJ, Goldman B, Iqbal S, Leichman LP, Lenz HJ, Blanke CD. Cetuximab as second-line therapy in patients with metastatic esophageal cancer: A phase II Southwest Oncology Group study. 2008 Gastrointestinal Cancers Symposium General Poster Session B. Abstract No: 96, 2008. . |
| 50. | Pinto C, Di Fabio F, Siena S, Cascinu S, Rojas Llimpe FL, Ceccarelli C, Mutri V, Giannetta L, Giaquinta S, Funaioli C. Phase II study of cetuximab in combination with FOLFIRI in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma (FOLCETUX study). Ann Oncol. 2007;18:510-517. |
| 51. | Lordick F, Lorenzen S, Hegewisch-Becker S, Folprecht G, Wöll E, Decker T, Endlicher E, Röthling N, Fend F, Pesche C. Cetuximab plus weekly oxaliplatin/5FU/FA (FUFOX) in 1st line metastatic gastric cancer. Final results from a multicenter phase II study of the AIO upper GI study group. J Clin Oncol 2007; ASCO Annual Meeting Proceedings Part I. Vol 25: 4526, 2007. . |
| 52. | Tebbutt NC, Sourjina T, Strickland AH, Hazel GAV, Pavlakis N, Ganju V, Murone C, MacGregor D, Gebski V, Cummins M. ATTAX2: Docetaxel plus cetuximab as second-line treatment for docetaxel-refractory oesophago-gastric cancer--Final results of a multicentre phase II trial by the AGITG. J Clin Oncol. 2008;26 suppl:A15554. |
| 53. | Dragovich T, McCoy S, Fenoglio-Preiser CM, Wang J, Benedetti JK, Baker AF, Hackett CB, Urba SG, Zaner KS, Blanke CD. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol. 2006;24:4922-4927. |
| 54. | Doi T, Koizumi W, Siena S, Cascinu S, Ohtsu A, Michael M, Takiuchi H, Swaisland H, Gallagher N, Cutsem EV. Efficacy, tolerability and pharmacokinetics of gefitinib (ZD1839) in pretreated patients with metastatic gastric cancer. Proc Am Soc Clin Oncol. 2003;22:A1036. |
| 55. | Iqbal S, Goldman B, Lenz HJ, Fenoglio-Preiser CM, Blanke CD. S0413: A phase II SWOG study of GW572016 (lapatinib) as first line therapy in patients (pts) with advanced or metastatic gastric cancer. J Clin Oncol. 2007;ASCO Annual Meeting Proceedings Part I., Vol 25, No. 18S:4621, 2007. |
| 56. | Rao S, Starling N, Cunningham D, Benson M, Wotherspoon A, Lüpfert C, Kurek R, Oates J, Baselga J, Hill A. Phase I study of epirubicin, cisplatin and capecitabine plus matuzumab in previously untreated patients with advanced oesophagogastric cancer. Br J Cancer. 2008;99:868-874. |
| 57. | Rao S, Starling N, Cunningham D, Sumpter K, Gilligan D, Ruhstaller T, Valladares-Ayerbes M, Wilke H, Archer C, Kurek R. Matuzumab plus epirubicin, cisplatin and capecitabine (ECX) compared with epirubicin, cisplatin and capecitabine alone as first-line treatment in patients with advanced oesophago-gastric cancer: a randomised, multicentre open-label phase II study. Ann Oncol. 2010;21:2213-2219. |
| 58. | Bang Y, Chung H, Xu J, Lordick F, Sawaki A, Lipatov O, Al-Sakaff N, See C, Rueschoff J, Cutsem EV. Pathological features of advanced gastric cancer (GC): Relationship to human epidermal growth factor receptor 2 (HER2) positivity in the global screening programme of the ToGA trial. J Clin Oncol. 2009;27 suppl 15:A4556. |
| 59. | Cortés-Funes H, Rivera F, Alés I, Márquez A, Velasco A, Colomer R, García-Carbonero R, Sastre J, Guerra J, Grávalos C. Phase II of trastuzumab and cisplatin in patients (pts) with advanced gastric cancer (AGC) with HER2/neu overexpression/amplification. J Clin Oncol 2007; ASCO Annual Meeting Proceedings Part I., Vol 25, No. 18S (June 20 Supplement), 2007: 4613. . |
| 60. | Cutsem EV, Kang Y, Chung H, Shen L, Sawaki A, Lordick F, Hill J, Lehle M, Feyereislova A, Bang Y. Efficacy results from the ToGA trial: A phase III study of trastuzumab added to standard chemotherapy (CT) in first-line human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer (GC). J Clin Oncol. 2009;27 suppl 18:abstr LBA4509. |
| 61. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CanceBase No.10. Vol. 2010. International Agency for Research on Cancer, 2010. . |
| 62. | Lopez PM, Villanueva A, Llovet JM. Systematic review: evidence-based management of hepatocellular carcinoma--an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23:1535-1547. |
| 63. | Finn RS. Development of molecularly targeted therapies in hepatocellular carcinoma: where do we go now? Clin Cancer Res. 2010;16:390-397. |
| 64. | Poon RT, Lau C, Pang R, Ng KK, Yuen J, Fan ST. High serum vascular endothelial growth factor levels predict poor prognosis after radiofrequency ablation of hepatocellular carcinoma: importance of tumor biomarker in ablative therapies. Ann Surg Oncol. 2007;14:1835-1845. |
| 65. | Poon RT, Lau CP, Cheung ST, Yu WC, Fan ST. Quantitative correlation of serum levels and tumor expression of vascular endothelial growth factor in patients with hepatocellular carcinoma. Cancer Res. 2003;63:3121-3126. |
| 66. | Ng IO, Poon RT, Lee JM, Fan ST, Ng M, Tso WK. Microvessel density, vascular endothelial growth factor and its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma. Am J Clin Pathol. 2001;116:838-845. |
| 67. | Jeng KS, Sheen IS, Wang YC, Gu SL, Chu CM, Shih SC, Wang PC, Chang WH, Wang HY. Prognostic significance of preoperative circulating vascular endothelial growth factor messenger RNA expression in resectable hepatocellular carcinoma: a prospective study. World J Gastroenterol. 2004;10:643-648. |
| 68. | Mise M, Arii S, Higashituji H, Furutani M, Niwano M, Harada T, Ishigami S, Toda Y, Nakayama H, Fukumoto M. Clinical significance of vascular endothelial growth factor and basic fibroblast growth factor gene expression in liver tumor. Hepatology. 1996;23:455-464. |
| 69. | Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293-4300. |
| 70. | Abou-Alfa GK, Amadori D, Santoro A, Figer A, Greve JD, Lathia C, Voliotis D, Anderson S, Moscovici M, Ricci S. Is sorafenib (S) safe and effective in patients (pts) with hepatocellular carcinoma (HCC) and Child-Pugh B (CPB) cirrhosis? J Clin Oncol. 2008;26 suppl:A4518. |
| 71. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:3783-3790. |
| 72. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. |
| 73. | Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, Sindhwani V, Blaszkowsky LS, Yoon SS, Lahdenranta J. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27:3027-3035. |
| 74. | Faivre S, Raymond E, Boucher E, Douillard J, Lim HY, Kim JS, Zappa M, Lanzalone S, Lin X, Deprimo S. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol. 2009;10:794-800. |
| 75. | Siegel AB, Cohen EI, Ocean A, Lehrer D, Goldenberg A, Knox JJ, Chen H, Clark-Garvey S, Weinberg A, Mandeli J. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26:2992-2998. |
| 76. | Malka D, Dromain C, Farace F, Horn S, Pignon J, Ducreux M, Boige V. Bevacizumab in patients (pts) with advanced hepatocellular carcinoma (HCC): Preliminary results of a phase II study with circulating endothelial cell (CEC) monitoring. J Clin Oncol. 2007;ASCO Annual Meeting Proceedings Part I., Vol 25, No. 18S:4570, 2007. |
| 77. | Hsu CH, Yang TS, Hsu C, Toh HC, Epstein RJ, Hsiao LT, Chen PJ, Lin ZZ, Chao TY, Cheng AL. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br J Cancer. 2010;102:981-986. |
| 78. | Hsu CH, Yang TS, Hsu C, Toh HC, Epstein RJ, Hsiao LT, Chen PJ, Lin ZZ, Chao TY, Cheng AL. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br J Cancer. 2010;102:981-986. |
| 79. | Sun W, Haller DG, Mykulowycz K, Rosen M, Soulen M, Capparo M, Faust T, Giantonia B, Olthoff K. Combination of capecitabine, oxaliplatin with bevacizumab in treatment of advanced hepatocellular carcinoma (HCC): A phase II study. J Clin Oncol 2007; ASCO Annual Meeting Proceedings Part I., Vol 25, No. 18S (June 20 Supplement), 2007: 4574, 2007. . |
| 80. | Bekaii-Saab T, Markowitz J, Prescott N, Sadee W, Heerema N, Wei L, Dai Z, Papp A, Campbell A, Culler K. A multi-institutional phase II study of the efficacy and tolerability of lapatinib in patients with advanced hepatocellular carcinomas. Clin Cancer Res. 2009;15:5895-5901. |
| 81. | Ramanathan RK, Belani CP, Singh DA, Tanaka M, Lenz HJ, Yen Y, Kindler HL, Iqbal S, Longmate J, Mack PC. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol. 2009;64:777-783. |
| 82. | O'Dwyer PJ, Giantonio BJ, Levy DE, Kauh JS, Fitzgerald DB, Benson AB. Gefitinib in advanced unresectable hepatocellular carcinoma: Results from the Eastern Cooperative Oncology Group's Study E1203. J Clin Oncol. 2006;ASCO Annual Meeting Proceedings Part I., Vol 24, No. 18S (June 20 Supplement), abstract 4143, 2006. |
| 83. | Zhu AX, Stuart K, Blaszkowsky LS, Muzikansky A, Reitberg DP, Clark JW, Enzinger PC, Bhargava P, Meyerhardt JA, Horgan K. Phase 2 study of cetuximab in patients with advanced hepatocellular carcinoma. Cancer. 2007;110:581-589. |
| 84. | Gruenwald V, Wilkens L, Gebel M, Greten TF, Kubicka S, Ganser A, Manns MP, Malek NP. A phase II open-label study of cetuximab in unresectable hepatocellular carcinoma: Final results. J Clin Oncol. 2007;ASCO Annual Meeting Proceedings Part I., Vol 25, No. 18S:4598, 2007. |
| 85. | Asnacios A, Fartoux L, Romano O, Tesmoingt C, Louafi S S, Mansoubakht T, Artru P, Poynard T, Rosmorduc O, Hebbar M. Gemcitabine plus oxaliplatin (GEMOX) combined with cetuximab in patients with progressive advanced stage hepatocellular carcinoma: results of a multicenter phase 2 study. Cancer. 2008;112:2733-2739. |
| 86. | Philip PA, Mahoney MR, Allmer C, Thomas J, Pitot HC, Kim G, Donehower RC, Fitch T, Picus J, Erlichman C. Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J Clin Oncol. 2005;23:6657-6663. |
| 87. | Thomas MB, Chadha R, Glover K, Wang X, Morris J, Brown T, Rashid A, Dancey J, Abbruzzese JL. Phase 2 study of erlotinib in patients with unresectable hepatocellular carcinoma. Cancer. 2007;110:1059-1067. |
| 88. | Thomas MB, Morris JS, Chadha R, Iwasaki M, Kaur H, Lin E, Kaseb A, Glover K, Davila M, Abbruzzese J. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol. 2009;27:843-850. |
| 89. | Kaseb AO, Iwasaki M, Javle M, Onicescu G, Garrett-Mayer E, Abbruzzese JL, Thomas MB. Biological activity of bevacizumab and erlotinib in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2009;27 suppl 15:A4522. |
| 90. | Hsu C, Kang Y, Yang T, Su W, Sandoval-Tan J, Chiou T, Jin K, Button P, Hsu C, Cheng A. A phase II study of bevacizumab (B) and erlotinib (E) in combination for Asian patients (pts) with advanced/metastatic hepatocellular carcinoma (HCC): An interim safety report. J Clin Oncol. 2009;27 suppl 15:A4585. |
| 91. | Leonard GD, O'Reilly EM. Biliary tract cancers: current concepts and controversies. Expert Opin Pharmacother. 2005;6:211-223. |
| 92. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. |
| 93. | Hida Y, Morita T, Fujita M, Miyasaka Y, Horita S, Fujioka Y, Nagashima K, Katoh H. Vascular endothelial growth factor expression is an independent negative predictor in extrahepatic biliary tract carcinomas. Anticancer Res. 1999;19:2257-2260. |
| 94. | Giatromanolaki A, Koukourakis MI, Simopoulos C, Polychronidis A, Sivridis E. Vascular endothelial growth factor (VEGF) expression in operable gallbladder carcinomas. Eur J Surg Oncol. 2003;29:879-883. |
| 95. | Zhu AX, Meyerhardt JA, Blaszkowsky LS, Kambadakone AR, Muzikansky A, Zheng H, Clark JW, Abrams TA, Chan JA, Enzinger PC. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol. 2010;11:48-54. |
| 96. | Bengala C, Bertolini F, Malavasi N, Boni C, Aitini E, Dealis C, Zironi S, Depenni R, Fontana A, Del Giovane C. Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial. Br J Cancer. 2010;102:68-72. |
| 97. | El-Khoueiry AB, Rankin C, Lenz HJ, Philip P, Rivkin SE, Blanke CD. SWOG 0514: A phase II study of sorafenib (BAY 43-9006) as single agent in patients (pts) with unresectable or metastatic gallbladder cancer or cholangiocarcinomas. J Clin Oncol 2007; ASCO Annual Meeting Proceedings Part I., Vol 25, No. 18S (June 20 Supplement): 4639, 2007. . |
| 98. | Philip PA, Mahoney MR, Allmer C, Thomas J, Pitot HC, Kim G, Donehower RC, Fitch T, Picus J, Erlichman C. Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol. 2006;24:3069-3074. |
| 99. | Gruenberger B, Schueller J, Kaczirek K, Bergmann M, Klose W, Bischof M, Schernthaner G, Gruenberger T. Efficacy results of cetuximab plus gemcitabine-oxaliplatin (GEMOX) in patients with advanced or metastatic cholangiocarcinoma: A single centre phase II study. J Clin Oncol. 2008;26 suppl 20; A4586. |
| 100. | Paule B, Herelle MO, Rage E, Ducreux M, Adam R, Guettier C, Bralet MP. Cetuximab plus gemcitabine-oxaliplatin (GEMOX) in patients with refractory advanced intrahepatic cholangiocarcinomas. Oncology. 2007;72:105-110. |
| 101. | Holen KD, Mahoney MR, LoConte NK, Szydlo DW, Picus J, Maples WJ, Kim GP, Pitot HC, Philip PA, Thomas JP. Efficacy report of a multicenter phase II trial testing a biologic-only combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer (BC): A Phase II Consortium (P2C) study. J Clin Oncol. 2008;26 suppl 20:A4522. |
| 102. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. |
| 103. | Society AC. Cancer Facts & Figures 2009, 2009. . |
| 105. | Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. |
| 106. | Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB 3rd. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270-3275. |
| 107. | Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, André T, Zaniboni A, Ducreux M, Aitini E, Taïeb J. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509-3516. |
| 108. | Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513-5518. |
| 109. | Kindler HL, Friberg G, Singh DA, Locker G, Nattam S, Kozloff M, Taber DA, Karrison T, Dachman A, Stadler WM. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033-8040. |
| 110. | Kindler HL, Gangadhar T, Karrison T, Hochster HS, Moore MJ, Micetich K, Sun W, Catenacci DV, Stadler WM, Vokes EE. Final analysis of a randomized phase II study of bevacizumab (B) and gemcitabine (G) plus cetuximab (C) or erlotinib (E) in patients (pts) with advanced pancreatic cancer (PC). J Clin Onco. 2008;26 suppl 20; A4502. |
| 111. | Siu LL, Awada A, Takimoto CH, Piccart M, Schwartz B, Giannaris T, Lathia C, Petrenciuc O, Moore MJ. Phase I trial of sorafenib and gemcitabine in advanced solid tumors with an expanded cohort in advanced pancreatic cancer. Clin Cancer Res. 2006;12:144-151. |
| 112. | Wallace JA, Locker G, Nattam S, Kasza K, Wade-Oliver K, Stadler WM, Vokes EE, Kindler HL. Sorafenib (S) plus gemcitabine (G) for advanced pancreatic cancer (PC): A phase II trial of the University of Chicago Phase II Consortium. J Clin Oncol 2007; ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (June 20 Supplement), 2007: 4608, 2007. . |
| 113. | O'Reilly EM, Niedzwiecki D, Hollis DR, Bekaii-Saab TS, Pluard T, Duffy A, Overcash F, Ivy SP, Goldberg RM. A phase II trial of sunitinib (S) in previously-treated pancreas adenocarcinoma (PAC), CALGB 80603. J Clin Oncol. 2008;26 suppl 20; A4515. |
| 114. | Spano J, Chodkiewicz C, Maurel J, Wong RP, Wasan HS, Pithavala YK, Bycott PW, Liau K, Kim S, Rixe . A randomized phase II study of axitinib (AG-013736) and gemcitabine versus gemcitabine in advanced pancreatic cancer, preceded by a phase I component. J Clin Oncol 2007; ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (June 20 Supplement), 2007: 4551, 2007. . |
| 115. | Vervenne W, Bennouna J, Humblet Y, Gill S, Moore MJ, Laethem JV, Shang A, Cosaert J, Verslype C, Cutsem EV. A randomized, double-blind, placebo (P) controlled, multicenter phase III trial to evaluate the efficacy and safety of adding bevacizumab (B) to erlotinib (E) and gemcitabine (G) in patients (pts) with metastatic pancreatic cancer. J Clin Oncol. 2008;26 suppl 20; A4507. |
| 116. | Kindler H, Niedzwiecki D, Hollis D, Oraefo E, Schrag D, Hurwitz H, McLeod H, Mulcahy M, Schilsky R, Goldberg R. A double-blind, placebo-controlled, randomized phase III trial of gemcitabine (G) plus bevacizumab (B) versus gemcitabine plus placebo (P) in patients (pts) with advanced pancreatic cancer (PC): A preliminary analysis of Cancer and Leukemia Group B (CALGB) 80303. 2007;Gastrointestinal Cancers Symposium Abstract 108, 2007. |
| 117. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. |
| 118. | Xiong HQ, Rosenberg A, LoBuglio A, Schmidt W, Wolff RA, Deutsch J, Needle M, Abbruzzese JL. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II Trial. J Clin Oncol. 2004;22:2610-2616. |
| 119. | Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC, Rivkin SE. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605-3610. |
| 120. | Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161-167. |
| 121. | Moore MJ, Hamm J, Dancey J, Eisenberg PD, Dagenais M, Fields A, Hagan K, Greenberg B, Colwell B, Zee B. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:3296-3302. |
| 122. | Ingham PW. Signalling by hedgehog family proteins in Drosophila and vertebrate development. Curr Opin Genet Dev. 1995;5:492-498. |
| 124. | Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev. 2000;92:55-81. |
| 125. | Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58-61. |
| 126. | Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059-3087. |
| 127. | Ruizi Altaba A, Sánchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361-372. |
| 128. | Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003;3:903-911. |
| 129. | Cohen MM Jr. Hedgehog signaling update. Am J Med Genet A. 2010;152A:1875-1914. |
| 130. | Gill PS, Rosenblum ND. Control of murine kidney development by sonic hedgehog and its GLI effectors. Cell Cycle. 2006;5:1426-1430. |
| 131. | Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083-1086. |
| 132. | Marigo V, Tabin CJ. Regulation of patched by sonic hedgehog in the developing neural tube. Proc Natl Acad Sci USA. 1996;93:9346-9351. |
| 133. | Chuang PT, McMahon AP. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature. 1999;397:617-621. |
| 134. | Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden AB, Gillies S. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841-851. |
| 135. | Goodrich LV, Milenković L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109-1113. |
| 136. | Tostar U, Malm CJ, Meis-Kindblom JM, Kindblom LG, Toftgård R, Undén AB. Deregulation of the hedgehog signalling pathway: a possible role for the PTCH and SUFU genes in human rhabdomyoma and rhabdomyosarcoma development. J Pathol. 2006;208:17-25. |
| 137. | Aboulkassim TO, LaRue H, Lemieux P, Rousseau F, Fradet Y. Alteration of the PATCHED locus in superficial bladder cancer. Oncogene. 2003;22:2967-2971. |
| 138. | Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90-92. |
| 139. | Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846-851. |
| 140. | Ma X, Sheng T, Zhang Y, Zhang X, He J, Huang S, Chen K, Sultz J, Adegboyega PA, Zhang H. Hedgehog signaling is activated in subsets of esophageal cancers. Int J Cancer. 2006;118:139-148. |
| 141. | Ma X, Chen K, Huang S, Zhang X, Adegboyega PA, Evers BM, Zhang H, Xie J. Frequent activation of the hedgehog pathway in advanced gastric adenocarcinomas. Carcinogenesis. 2005;26:1698-1705. |
| 142. | Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313-317. |
| 143. | Sheng T, Li C, Zhang X, Chi S, He N, Chen K, McCormick F, Gatalica Z, Xie J. Activation of the hedgehog pathway in advanced prostate cancer. Mol Cancer. 2004;3:29. |
| 144. | Kinzler KW, Bigner SH, Bigner DD, Trent JM, Law ML, O'Brien SJ, Wong AJ, Vogelstein B. Identification of an amplified, highly expressed gene in a human glioma. Science. 1987;236:70-73. |
| 145. | Olsen CL, Hsu PP, Glienke J, Rubanyi GM, Brooks AR. Hedgehog-interacting protein is highly expressed in endothelial cells but down-regulated during angiogenesis and in several human tumors. BMC Cancer. 2004;4:43. |
| 146. | Thayer S. The emerging role of the hedgehog signaling pathway in gastrointestinal cancers. Clin Adv Hematol Oncol. 2004;2:17, 20, 63. |
| 147. | Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del Castillo C, Yajnik V. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851-856. |
| 148. | Osipo C, Miele L. Hedgehog signaling in hepatocellular carcinoma: novel therapeutic strategy targeting hedgehog signaling in HCC. Cancer Biol Ther. 2006;5:238-239. |
| 149. | Jinawath A, Akiyama Y, Sripa B, Yuasa Y. Dual blockade of the Hedgehog and ERK1/2 pathways coordinately decreases proliferation and survival of cholangiocarcinoma cells. J Cancer Res Clin Oncol. 2007;133:271-278. |
| 150. | LoRusso PM, Rudin CM, Borad MJ, Vernillet L, Darbonne WC, Mackey H, DiMartino JF, Sauvage FD, Low JA, Hoff DDV. A first-in-human, first-in-class, phase (ph) I study of systemic Hedgehog (Hh) pathway antagonist, GDC-0449, in patients (pts) with advanced solid tumors. J Clin Oncol. 2008;26 suppl 20:A3516. |
| 151. | Ahnert JR, Baselga J, Tawbi HA, Shou Y, Granvil C, Dey J, Mita MM, Thomas AL, Amakye DD, Mita AC. A phase I dose-escalation study of LDE225, a smoothened (Smo) antagonist, in patients with advanced solid tumors. J Clin Oncol. 2010;28 suppl 15: A2500. |
| 152. | Siu LL, Papadopoulos K, Alberts SR, Kirchoff-Ross R, Vakkalagadda B, Lang L, Ahlers CM, Bennett KL, Tornout JMV. A first-in-human, phase I study of an oral hedgehog (HH) pathway antagonist, BMS-833923 (XL139), in subjects with advanced or metastatic solid tumors. J Clin Oncol. 2010;28 suppl 15:A2501. |
| 153. | Otto H, Reche PA, Bazan F, Dittmar K, Haag F, Koch-Nolte F. In silico characterization of the family of PARP-like poly(ADP-ribosyl)transferases (pARTs). BMC Genomics. 2005;6:139. |
| 154. | Gagné JP, Hendzel MJ, Droit A, Poirier GG. The expanding role of poly(ADP-ribose) metabolism: current challenges and new perspectives. Curr Opin Cell Biol. 2006;18:145-151. |
| 155. | Yélamos J, Schreiber V, Dantzer F. Toward specific functions of poly(ADP-ribose) polymerase-2. Trends Mol Med. 2008;14:169-178. |
| 156. | de Murcia G, Ménissier de Murcia J. Poly(ADP-ribose) polymerase: a molecular nick-sensor. Trends Biochem Sci. 1994;19:172-176. |
| 157. | Dantzer F, Amé JC, Schreiber V, Nakamura J, Ménissier-de Murcia J, de Murcia G. Poly(ADP-ribose) polymerase-1 activation during DNA damage and repair. Methods Enzymol. 2006;409:493-510. |
| 158. | Schreiber V, Amé JC, Dollé P, Schultz I, Rinaldi B, Fraulob V, Ménissier-de Murcia J, de Murcia G. Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J Biol Chem. 2002;277:23028-23036. |
| 159. | Huber A, Bai P, de Murcia JM, de Murcia G. PARP-1, PARP-2 and ATM in the DNA damage response: functional synergy in mouse development. DNA Repair (Amst). 2004;3:1103-1108. |
| 160. | Yang YG, Cortes U, Patnaik S, Jasin M, Wang ZQ. Ablation of PARP-1 does not interfere with the repair of DNA double-strand breaks, but compromises the reactivation of stalled replication forks. Oncogene. 2004;23:3872-3882. |
| 161. | Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917-921. |
| 162. | Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913-917. |
| 163. | Audebert M, Salles B, Calsou P. Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J Biol Chem. 2004;279:55117-55126. |
| 164. | Greer JB, Whitcomb DC. Role of BRCA1 and BRCA2 mutations in pancreatic cancer. Gut. 2007;56:601-605. |
| 165. | McEllin B, Camacho CV, Mukherjee B, Hahm B, Tomimatsu N, Bachoo RM, Burma S. PTEN loss compromises homologous recombination repair in astrocytes: implications for glioblastoma therapy with temozolomide or poly(ADP-ribose) polymerase inhibitors. Cancer Res. 2010;70:5457-5464. |
| 166. | Mendes-Pereira AM, Martin SA, Brough R, McCarthy A, Taylor JR, Kim JS, Waldman T, Lord CJ, Ashworth A. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med. 2009;1:315-322. |
| 167. | Kang YH, Lee HS, Kim WH. Promoter methylation and silencing of PTEN in gastric carcinoma. Lab Invest. 2002;82:285-291. |
| 168. | Hettinger K, Vikhanskaya F, Poh MK, Lee MK, de Belle I, Zhang JT, Reddy SA, Sabapathy K. c-Jun promotes cellular survival by suppression of PTEN. Cell Death Differ. 2007;14:218-229. |
| 169. | Quiles-Perez R, Muñoz-Gámez JA, Ruiz-Extremera A, O’Valle F, Sanjuán-Nuñez L, Martín-Alvarez AB, Martín-Oliva D, Caballero T, Muñoz de Rueda P, León J. Inhibition of poly adenosine diphosphate-ribose polymerase decreases hepatocellular carcinoma growth by modulation of tumor-related gene expression. Hepatology. 2010;51:255-266. |
Peer reviewers: Toru Mukohara, MD, Associate Professor, Department of Medical Oncology/Hematology, Cancer Center, Kobe University Hospital, 7-5-2 Kusunoki-cho, Chuo-ku, Kobe 650-0017, Japan; Henry L Gomez, MD, Head, Division of Medicine, Instituto de Enfermedades Neoplasicas, Avenida Angamos este 2520, Surquillo, Lima 34, Peru; Simone Mocellin, MD, PhD, Department Oncological and Surgical Sciences, University of Padova, via Giustiniani 2, 35128 Padova, Italy
S- Editor Cheng JX L- Editor O’Neill M E- Editor Ma WH