Published online Sep 24, 2025. doi: 10.5306/wjco.v16.i9.111379
Revised: July 14, 2025
Accepted: August 21, 2025
Published online: September 24, 2025
Processing time: 87 Days and 11.9 Hours
Identifying the factors that contribute to individual susceptibility to cancer is essential for both prevention and treatment. The advancement of biotechnologies, particularly next-generation sequencing, has accelerated the discovery of genetic variants linked to cancer susceptibility. While hundreds of cancer-susceptibility genes have been identified, they only explain a small fraction of the overall cancer risk, a phenomenon known as "missing heritability". Despite progress, even con
Core Tip: Understanding individual susceptibility to cancer is critical for effective prevention and treatment strategies. While genetic studies have identified numerous cancer-susceptibility genes, much of the heritable risk remains unexplained. Emerging evidence indicates the microbiome as a key contributor to this "missing heritability". Microbiomes influence cancer risk through immune modulation, metabolic activity, and interaction with environmental exposures. This review discusses the complex gene-microbiome-environment interplay in cancer susceptibility and emphasizes the value of integrative models and tools, such as collaborative cross mice and artificial intelligence, for uncovering microbial determinants of cancer risk.
- Citation: Chang H, Perez-Losada J, Mao JH. Emerging multifaceted roles of the microbiome in cancer susceptibility. World J Clin Oncol 2025; 16(9): 111379
- URL: https://www.wjgnet.com/2218-4333/full/v16/i9/111379.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i9.111379
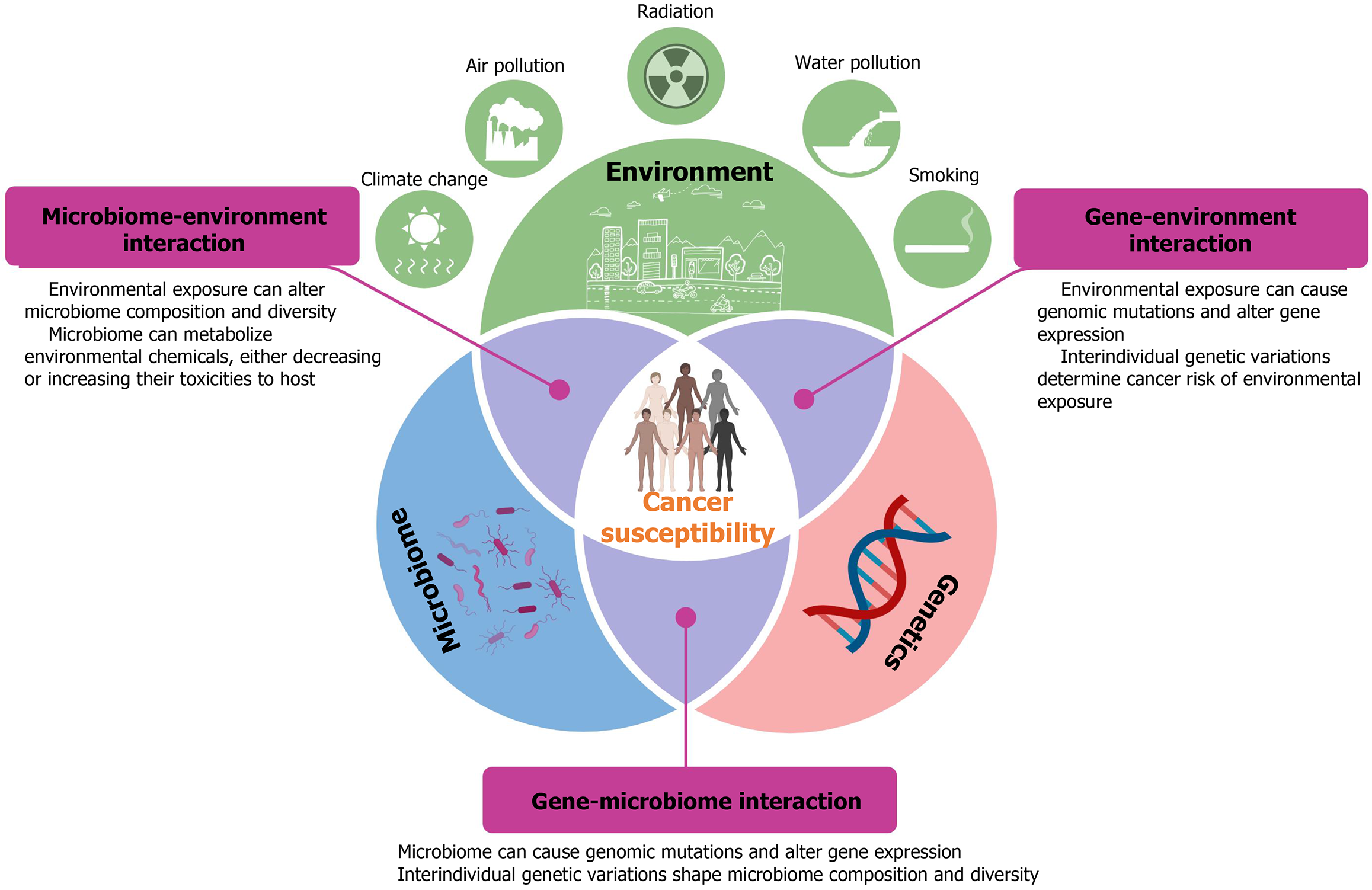
Cancer susceptibility, a term used to describe an individual’s increased risk of developing certain cancers, is a complex genetic trait. The genome-wide association study (GWAS) approach combined with the advent of biotechnologies has successfully identified genetic variants contributing to cancer risk[1-11]. However, it is strikingly found that these genetic variants identified so far account for only a small fraction of overall cancer risk, implying that much of the genetic risk remains to be missing, known as “missing heritability”[1,2,8,12,13]. Therefore, continuing to explore the genetic basis of cancer susceptibility and to better understand the molecular mechanisms by which genetic variants influence cancer risk is still one of the holy grails of cancer research.
The missing heritability of cancer is a notable unsolved problem for genetic association studies. Since few cancers result in mutations in a single gene, cancer susceptibility is usually highly polygenic, i.e., influenced by a multitude of common genes[14-17]. The effect sizes of each common gene often fell below significance thresholds that can be identified by GWAS, which may account for most missing heritability[1]. A combination of the methods to sequence genomes and the advanced computational tools allows us to detect subtle differences in the genetic background of individuals and to discover rare variations that mitigate cancer risk[18,19], but the gaps are still not filled. Several other mechanisms have also been proposed for missing heritability, such as epistasis and epigenetics[12,13,20-22]. Also, the gene-environment (GxE) interactions are known to contribute to cancer susceptibility[23]. Moreover, recent studies provide evidence that analysis of GxE interactions holds promise for identifying additional genetic and environmental determinants. Therefore, GxE interactions can provide valuable insights into missing heritability[24]. However, taking all counts, the proportion of missing heritability still has not been satisfactorily explained. Other strategies have been proposed to identify the missing heritability[12]. Recently, researchers suggested that the human microbiome must be considered in order to improve our estimates of the heritability of phenotypes[25,26].
There is a growing understanding and appreciation for the functional significance of the human microbiome in health and disease such as cancer[27-32]. A sharply increasing number of studies have revealed the roles of microbiomes in cancer development and progression, diagnosis, prognosis, and treatment. Some of these aspects have been extensively reviewed[27-31]. While sufficient evidence shows that the microbiome composition differs greatly between individuals[33-36], a critical question remains open as to whether inter-individual variation in the microbial community contributes to cancer susceptibility. This review will first overview the links between microbiome and cancer susceptibility. Su
Our body harbors 10-100 trillion microbial cells, including bacteria, viruses, and fungi, the majority of which live in the gut[37-39]. All these microbial cells are defined as human microbiota, which develops to reach a climax stage during the first 3 years of life. In healthy adults, the commensal microbial communities typically maintain a stable composition, although the relative abundance of each microbe oscillates over time. As evidenced by an increasing number of epidemiological and experimental studies, disrupting the infant microbiota is significantly associated with immune and metabolic disorders and other diseases, such as cancer, in later life, indicating that the early human gut microbiota plays a critical role in modulating disease susceptibility, including cancer susceptibility (see below).
The genetic material of these microbial cells is collectively defined as the human microbiome, which is also referred to as the human “second genome”[40,41]. We humans have 20000-25000 genes in each of our cells, which are inherited and largely static, whereas the human microbiome contains approximately 500 times more, which is acquired and dynamic through life[37,42]. In 2007, the National Institutes of Health initiated The Human Microbiome Project to characterize the human microbiome and to understand how it contributes to normal physiology and predisposition to disease[42]. The findings from this initiative and numerous other studies have shown that the human microbiome exhibits huge both intra- and inter-individual variability in composition and function[35,36,42]. In contrast to the human genome, which is about 99.9% identical among individual humans, they are completely different in their microbiome[37]. The 50% of all genes in the human microbiome are individual-specific[37,39,42]. Therefore, the human microbiome is a great source of genetic diversity, which can be a modifier of disease, such as cancer.
It is well known that many factors can influence gut microbiota, including host genetics and environmental factors[43-45]. The 5%-45% of inter-individual variation in microbiome composition and diversity can be explained by genetics[33]. Hundreds of genetic variants have been discovered to be significantly associated with the abundance of specific gut microbes by large GWASs[33]. In addition to genetic influences, environmental factors including diet, pharmacological use, and lifestyle have been shown to shape microbial composition, which is associated with human cancer[43]. These collective findings indicate that the microbiome might be the missing link among genes, environments, and cancer. The following sections discuss how bidirectional interactions between genetics and microbiota, as well as between environmental factors and microbiota, impact cancer susceptibility.
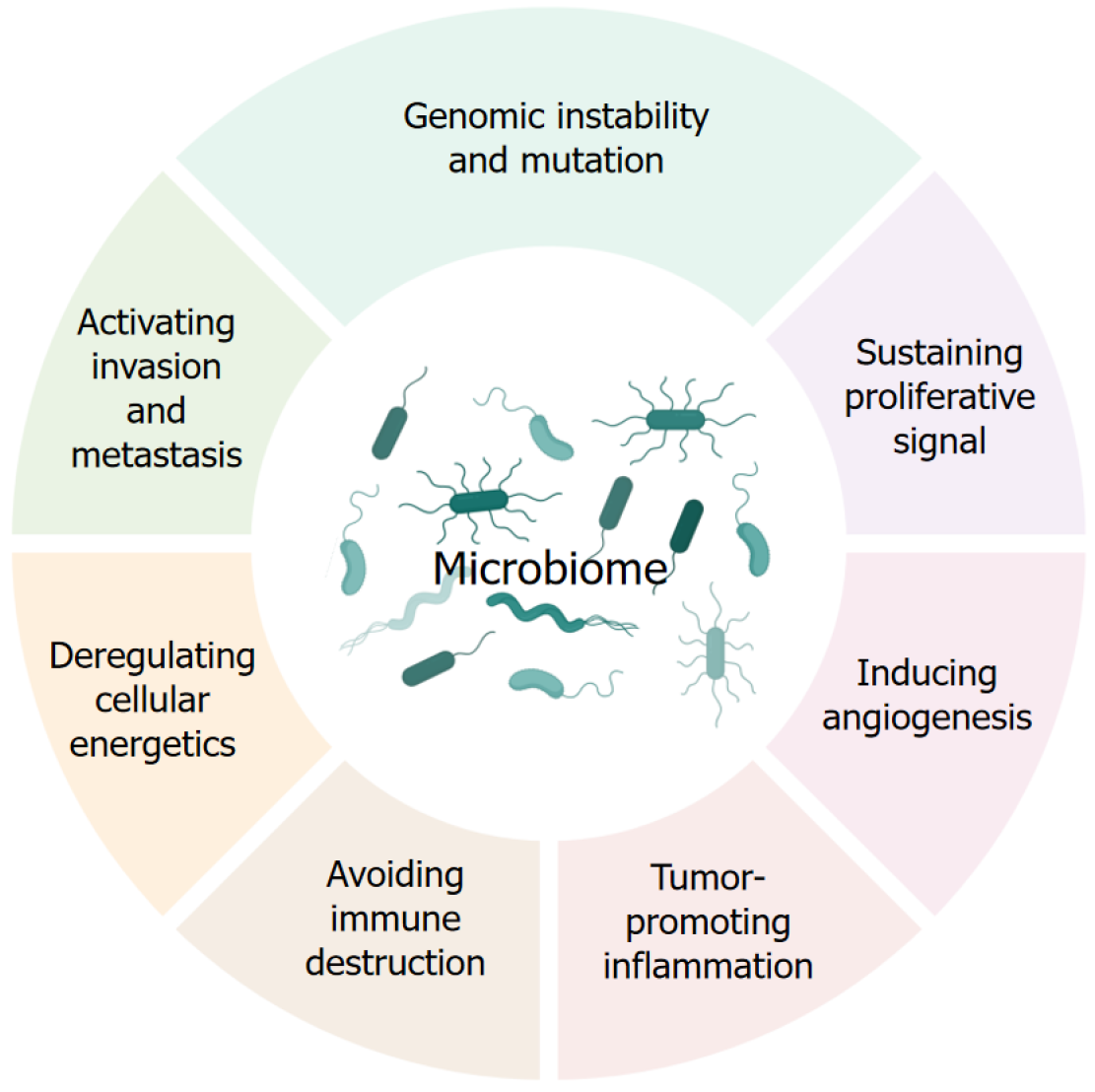
Accumulating evidence has shown that the human microbiome contributes to all stages of cancer development by modulating the hallmarks of cancer (Figure 2). For example, some pathogens directly target genes that regulate cell growth; others cause chronic inflammation that can eventually lead to cancer development. Some infections weaken the people’s immune system, reducing the body’s ability to combat malignancy. Different mechanisms have been reviewed for microbiota to cause cancer[27,30,32]. The tremendous interindividual variation in microbiome diversity and function, coupled with the critical roles of the human microbiome in cancer, suggests that the human microbiome may also be an important factor driving individual cancer susceptibility.
The first evidence for the contribution of specific bacterial species to cancer susceptibility is the link between Helicobacter pylori (H. pylori) and gastric cancer[27,46]. In addition to the oncogenic bacterium H. pylori, many other cancer-related pathogens have been identified, including seven oncogenic viruses: Hepatitis virus B and C, human papillomavirus, human T-cell lymphoma virus 1, Epstein-Barr virus, Merkel cell polyomavirus, and Kaposi’s sarcoma virus (or HHV8), and three oncogenic parasites: Schistosoma haematobium, Opisthorchis viverrini, and Clonorchis sinensis[27,46]. Some studies have reported that an estimated 12%-20% of cancers are caused by viruses or bacteria[46-48]. It is believed that this figure will significantly increase since advanced tools and methods allow for the detection of more microbes controlling cancer susceptibility. For example, microbiome-wide association studies (MWAS), together with next-generation sequencing technologies, will provide unprecedented views into the association of the human microbiome with cancer risk. MWAS has successfully identified microbial taxa or functions associated with human cancer[49]. These findings reveal that the higher abundance of some bacteria decreases while others increase cancer risk, consistent with the concept that our body harbors both “good” and “bad” bacteria. Identification of microbial determinants of cancer susceptibility will provide new avenues for cancer prevention. However, we still face many challenges in human studies. The most challenge is that the human microbiome is sensitive to many environmental factors that are almost impossible to control.
Increasing attention has focused on bidirectional interactions between host genetics and microbiome. On the one hand, host genetics determines the microbiome composition and diversity (Figure 1), which have been documented in both human and mouse studies[33,50-54]. Conversely, the microbiome exerts substantial influence on the host genome (Figure 1). Growing evidence suggests that bidirectional interactions between host genetics and microbiome are critical determinants of disease susceptibility, including cancer[55,56].
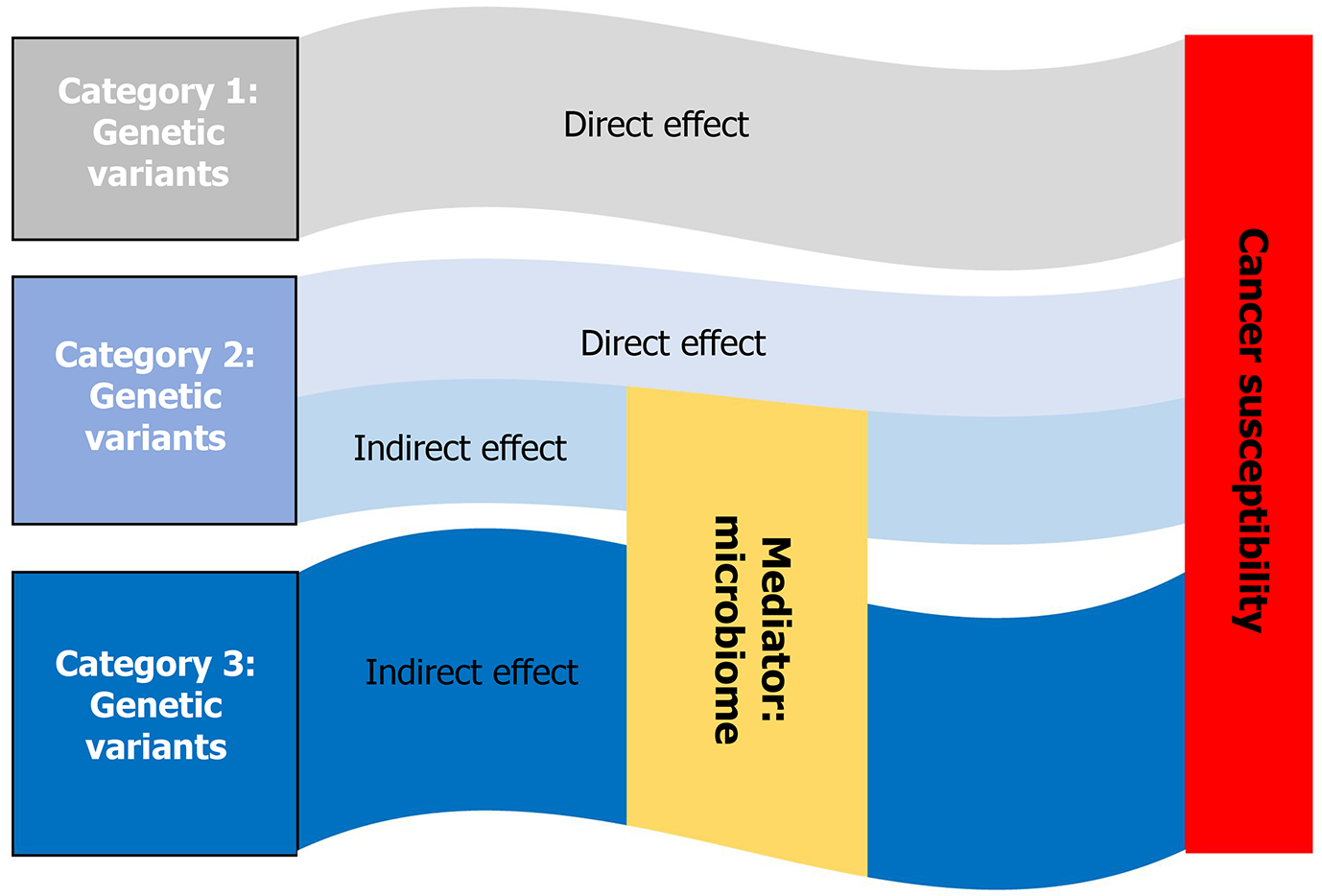
Although recent breakthroughs in cancer genetics have identified hundreds of genetic variations responsible for the increased cancer susceptibility, the missing heritability of cancer remains a challenge. Strong links between genetics and microbiome and between microbiome and cancer lead us to hypothesize the effect of some genetic variants on cancer susceptibility through the microbiome, which is defined as an indirect effect. Based on this hypothesis, we could divide pathogenic variants into three categories: (1) Genetic variants, which pose a direct effect; (2) Genetic variants that pose both direct and indirect effects; and (3) Genetic variants that pose an indirect effect on cancer susceptibility (Figure 3). The GWAS could hardly detect Category 3 and some Category 2 variants, which can possibly be identified by combining GWAS of the microbiome and MWAS of cancer with mediation analysis (details see below).
Mounting evidence has shown that gut microbiota modulates host gene expression[57-59]. Several mechanisms for microbial control of host gene regulation have been discovered, including disruption of the epigenetic landscape, interference with host signaling pathways, chromatin remodeling, altered RNA splicing, and modulations by miRNAs[60-63]. In addition, gut microbiota can produce genotoxic metabolites that cause DNA damage, subsequently leading to genomic instability, chromosomal aberrations, and gene mutations[60-63]. Three bacterial genotoxins have been identified: Cytolethal distending toxin, typhoid toxin, and colibactin[64-67]. The most well-studied genotoxic bacteria are Escherichia coli, which produce colibactin[68,69]; enterotoxigenic Bacteroides fragilis, which produce a toxin[70,71]; and Campylobacter jejuni strains, which produce the cytolethal distending toxin[68]. A recent study has identified more bacterial strains that show DNA-damaging activity[72]. It is possible that more genotoxic bacteria will be discovered in the future. The discovery of genotoxic bacteria and other microbial determinants of cancer susceptibility is critical for cancer prevention, diagnosis, and treatment.
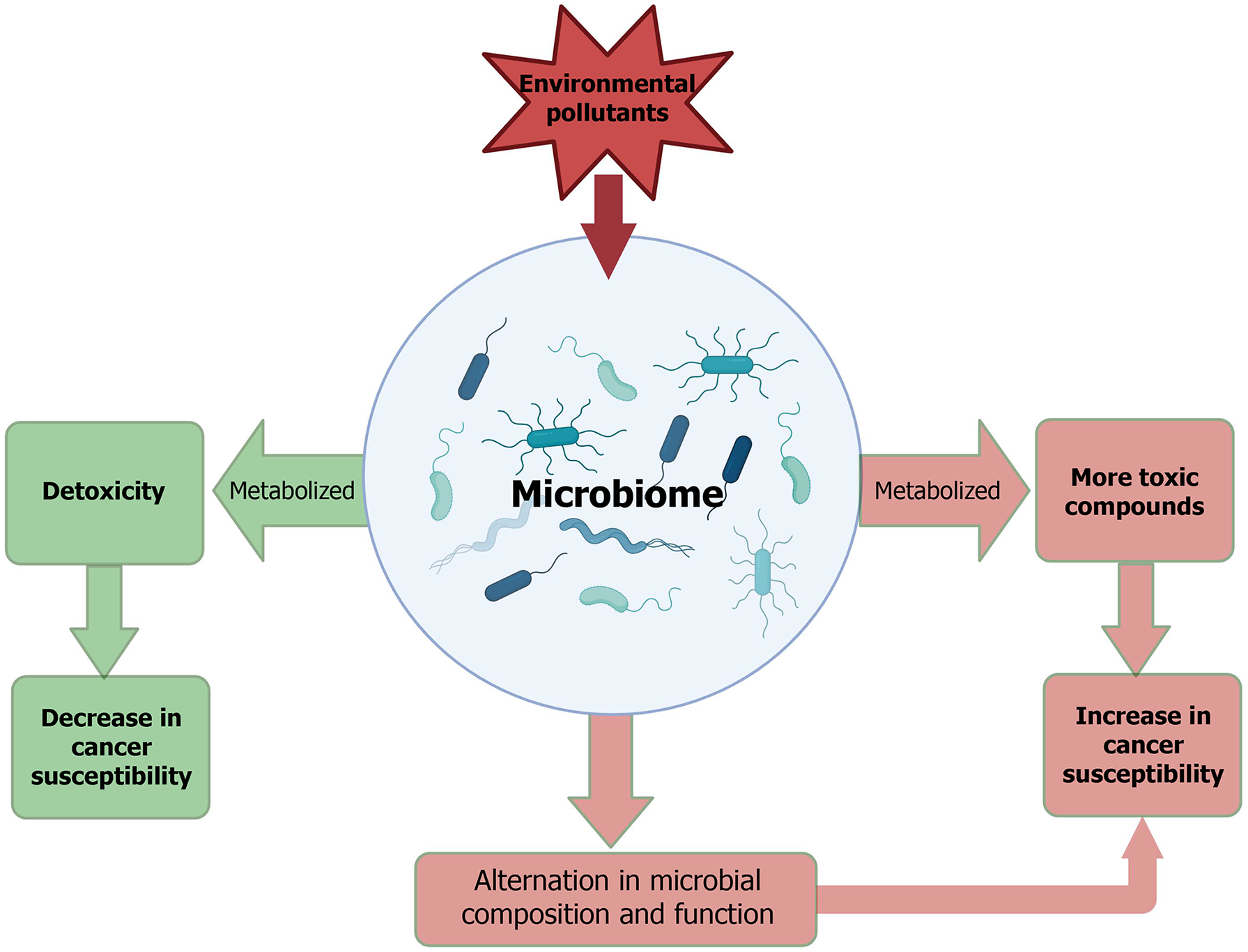
Scientific research has begun to elucidate the diverse mechanisms through which the human microbiome interacts with environmental exposures[73-75]. The gut microbiota plays a central role in modulating the toxicity of environmental pollutants, while, as discussed earlier, environmental exposures are key determinants of the composition and function of human microbial communities (Figure 4). Compelling evidence has indicated that the human microbiome can modulate exposure to environmental pollutants, which modifies disease susceptibility, such as cancer, to environmental exposures[76]. Microbial metabolism of environmental pollutants might affect the host favorably or unfavorably through metabolic byproducts or modulation of compound toxicity. Many studies have shown that the gut microbiome directly participates in the metabolic transformation of environmental chemicals, including metals, polycyclic aromatic hydrocarbons, nitrosamines, aromatic amines, and organochlorine pesticides[76,77]. Research also shows that gut microbiome indirectly involves metabolisms of environmental pollutants through deconjugation of host-generated metabolites, modulation of epithelial permeability to facilitate the transport or excretion of chemicals, and regulation of the expression or activity of key endogenous metabolic pathways[76,77]. While the roles of microbiomes in the metabolism of environmental pollutants are well established, substantial gaps remain in our understanding of the full range of metabolic pathways for environmental pollutants within individual microbiomes. Moreover, how substantial differences in microbiome composition among different individuals influence the metabolism and disposition of environmental pollutants has received little attention, which might play an important contribution to individual cancer susceptibility.
On the other hand, environmental pollutants can, directly and indirectly, alter the microbiome. Many environmental pollutants have been identified to disrupt the microbiome composition and diversity[78,79]. Animal studies have demonstrated that exposure to a variety of pesticides, metals, artificial sweeteners, and drugs can induce microbiome changes[78,79]. Alterations in the microbiome by environmental pollutants can be characterized using multi-omics approaches. It should be noted that the effects of environmental pollutants on the microbiome composition and diversity can be modulated by host genetics. However, the mechanisms by which environmental pollutants induce gut microbiota dysbiosis and the subsequent impact on cancer susceptibility remain to be elucidated in future studies.
Environmental exposures are difficult to quantify and control for human populations, which severely hinders the identification of microbial determinants of cancer susceptibility in humans. In contrast, mice offer many advantages for the study of the role of microbiome in cancer susceptibility. These advantages include well-designed populations tailored to specific research questions, standardized husbandry conditions, comprehensive phenotypic analyses, and the ability to manipulate the ecosystem to investigate the role of the microbiome in tumor progression and metastasis. Moreover, mouse models have been instrumental in developing our knowledge of the microbiome in vivo and of the aberrations in microbiome that are causally associated with disease. Human and mouse gut microbiota exhibit approximately 90% similarity at the phylum level and 89% at the genus level[80-83]. Therefore, mouse models will be pivotal in laying the foundation for microbial oncogenesis by providing information on the crosstalk between cancer and the microbial community.
The CC is a large panel of novel inbred mouse strains derived from eight genetically diverse founders (A/J, C57BL/6J, 129S1/SvImJ, NOD/LtJ, NZO/HlLtJ, CAST/EiJ, PWK/PhJ, and WSB/EiJ) through a targeted funneling breeding strategy designed to randomize the genetic composition of each inbred line. The CC captures nearly 90% of the known genetic variation found in laboratory mice, making it a powerful tool to model the genetic diversity of human po
A major driver of advances in microbiome research has been the use of gnotobiotic (or germ-free) mice, which are devoid of all microorganisms and maintained under sterile conditions[96,97]. Gnotobiotic mice effectively allow us to transfer selective bacterial species or whole fecal microbiota[98-100]. Especially, establishing a humanized gnotobiotic mouse model through the fecal microbiota transplantation (FMT) of humans into germ-free mice offers an innovative and powerful approach for recapitulating the human microbial system[81,101-103]. Microbial transplantation into germ-free mice can be used to explore the complex relationship between the specific microbe or microbiome and cancer etiology and progression, to characterize the impact of specific microbe or microbiome on the toxicity of environmental pollutants, and to evaluate potential microbiome-based prevention and therapeutics in validated in vivo cancer model paradigms[98,104-109].
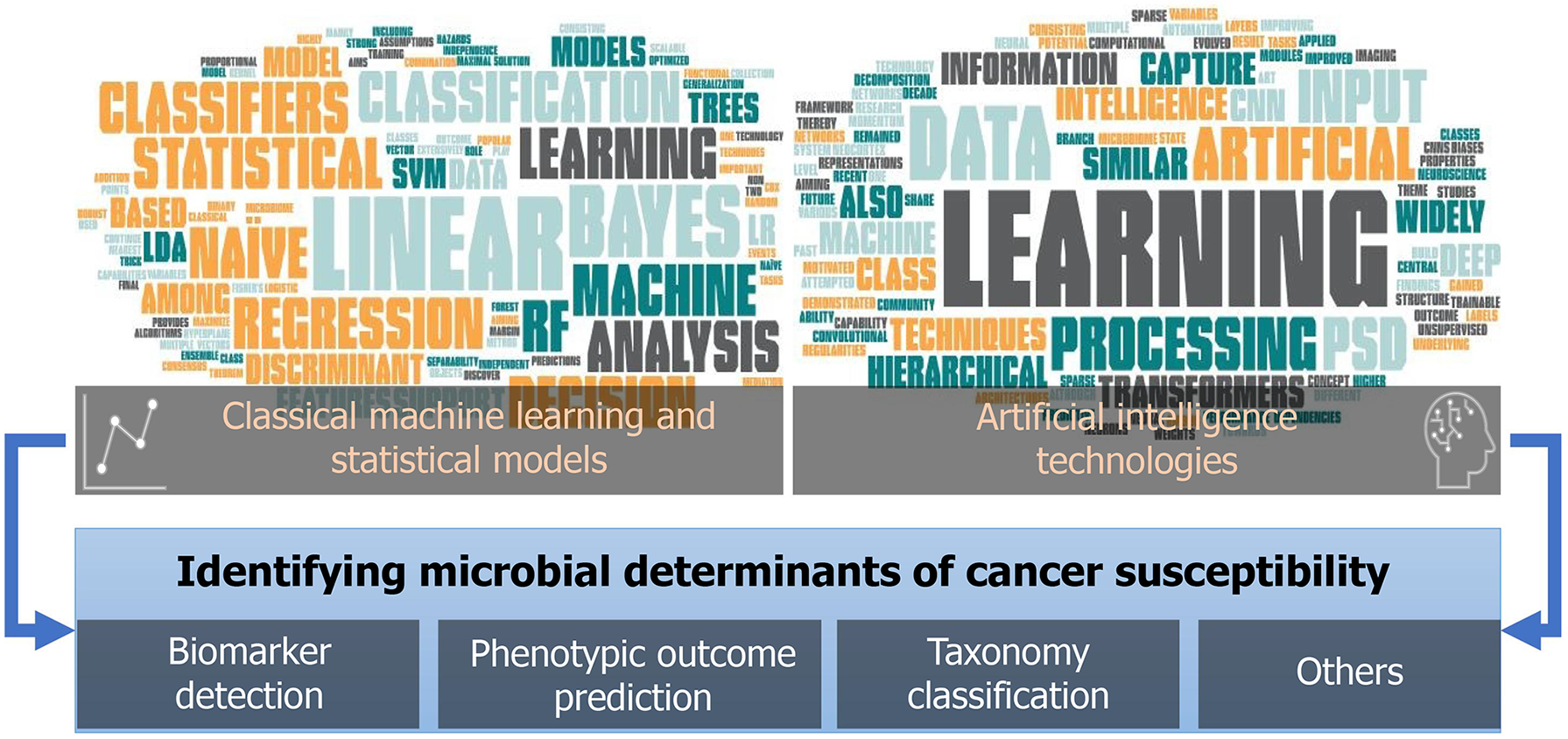
Microbiome-related studies on cancer development and treatment have notably increased recently[110-112], which provide both the challenges and opportunities for developing and deploying advanced analytical tools to explore the host-microbiome determinants or their intermediate role towards phenotypic outcomes, including cancer susceptibility. These analytical tools majorly fall into two categories: (1) Classical machine learning (ML) and statistical models; and (2) Artificial intelligence techniques, all of which play important roles in microbiome studies towards diagnostic and predictive biomarker detection and many other applications (Figure 5).
Classical ML and statistical models continue to play an essential role in microbiome data analysis, among which logistic regression (LR), linear discriminant analysis (LDA), support vector machine (SVM), naïve Bayes classifiers, and random forest (RF) are among the most popular techniques that have been extensively used.
LR: LR is a statistical model for binary outcome (Y) predictions based on one or more independent variables (X). LR and its variations have been used to predict cancer prognosis and drug response from the tumor microbiome[113]; to improve CRC diagnosis using gut microbiome data[114]; to identify gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma[115]; and to identify non-invasive diagnostic biomarkers in colorectal carcinogenesis using the metabolomics and/or metagenomics profiles from fecal samples[116].
LDA: LDA is a generalization of Fisher's linear discriminant, aiming to maximize the separability of two or more classes of objects or events through an optimized linear combination of features. LDA and its variations have been used to evaluate the microbial effects between tumor and normal groups[117]; to support high-dimensional metagenomic biomarker discovery and explanation between two or more biological conditions (or classes)[118]; and to assess and characterize differences in the gut microbiota among CRC patients according to the location of the tumor (i.e., left-sided CRC and right-sided CRC)[118].
SVM: SVM is a ML technology for both classification and regression analysis. It aims to discover a hyperplane with the maximal functional margin to the nearest training data points (i.e., support vectors) of any class. It provides a robust solution with both linear and non-linear capabilities through the kernel trick. SVM and its variations have been used to construct a phylogenetic approach for classifying oral microbiota, and to predict the clinical outcomes of oncology patients on immunotherapies using baseline gut microbiome features[119].
Naïve Bayes classifiers: Naïve Bayes classifiers are a collection of classification algorithms based on Bayes’ Theorem with strong (naïve) independence assumptions between the features. Naïve Bayes classifiers are highly scalable and have been used rapidly to assign rRNA sequences into the new bacterial taxonomy[120]; and to investigate the impact of training sets on taxonomy classification of high-throughput bacterial 16s rRNA gene sequences, where a selected region of target sequences helps improve the performance of naïve Bayes classifier[121].
RF: RF is an ensemble learning method consisting of multiple decision trees, mainly for classification and regression tasks, where the final decision of RF is the consensus of its decision trees. RF and its variations have been used to identify the association between gut microbes and CRC, to reveal host microbial determinants of clinical response to FMT therapy in type 2 diabetes patients, and to increase the prediction performance of CRC disease status based on metagenomic shotgun sequencing data.
In addition, other statistical models, including the mediation model and Cox proportional-hazards model, have also been widely used in microbiome studies to explore the intermediate role of the microbiome, together with other factors (e.g., genetics), towards phenotypic outcomes; or to construct microbiome signatures for time-to-event prediction (e.g., prognosis)[122].
Motivated by recent neuroscience findings[123,124], one area of ML has attempted to develop computational modules that emulate properties of the neocortex for constructing information representations. Although state-of-the-art ML architectures have evolved, the concept of the hierarchical structure for information processing has remained the central theme. Over the past decade, deep learning has gained momentum due to its demonstrated capability to enhance performance across diverse automation tasks and its promising potential for future research. Among different artificial intelligence techniques, convolutional neural networks (CNNs), predictive sparse decomposition (PSD), and transformers have been widely used in microbiome studies.
CNN: CNN is a class of artificial neural networks consisting of multiple layers of neurons with trainable weights and biases toward the outcome (e.g., classes/Labels of input data). Although CNNs have been mostly applied to imaging data, they have also been used to accurately classify host phenotypes from metagenomic data[125,126].
PSD: PSD is a class of unsupervised sparse learning technology with a hierarchical learning framework designed to capture higher-level dependencies of input variables, thereby improving the ability of the system to identify underlying regularities in the data. Similar to CNN, PSD has also been widely applied to imaging data. Until recently, it has been used to integrate tumor microbiome biomarkers with cellular morphometric biomarkers and gene biomarkers for improved multi-modal risk stratification in breast cancer patients[127].
Transformer: Transformer is a deep learning model originally designed to process sequential input data, such as natural language processing[128]. Transformers have now also been widely used in the fields of computer vision[129] and audio processing[130]. A recent study introduced transformer models to the field of microbiome analysis for the accurate identification of bacteriophages from metagenomic data[131], which opens a new opportunity for microbiome studies with large language models or even pretrained systems on microbiome data (microbGPT), similar to generative pre-trained transformers[132].
In summary, these analytical tools, empowered by classical ML and statistical models or more advanced artificial intelligence techniques, provide new avenues to microbiome data processing and knowledge mining with promising potential to further advance the discovery of microbial determinants of cancer susceptibility.
Mounting evidence highlights the microbiome as a vital and dynamic factor in cancer susceptibility. Research is increasingly revealing that the microbiome is not merely a passive passenger but an active modulator of host physiology, immunity, and even the efficacy of anticancer therapies. From influencing genetic stability and inflammation to modulating metabolic pathways and immune surveillance, the microbiome emerges as a critical determinant in both cancer initiation and progression. Despite these advances, much remains to be discovered about the full spectrum of microbes that individually or synergistically affect cancer risk, as well as the mechanisms behind their effects. Progress in this area will require sophisticated analytical tools, including artificial intelligence, to unravel these complexities. Notably, the context-specific roles of the microbiome, ranging from protective to pathogenic, highlight the complexity of its interactions with host and environmental factors. Certain microbial taxa have been implicated in carcinogenesis through genotoxic metabolite production or pro-inflammatory mechanisms, while others exhibit tumor-suppressive effects by enhancing immune responses or maintaining epithelial barrier integrity. This duality underscores the need for precision approaches in microbiome research, tailored to individual host genetics, lifestyle, and tumor microenvironments. Importantly, future research must aim to move beyond association studies and unravel causal mechanisms through integrative multi-omics, longitudinal cohort designs, and functional validation in preclinical models. A major challenge remains in distinguishing correlation from causation and understanding how specific microbial consortia dynamically influence oncogenic processes over time. Longitudinal studies of the fecal microbiome in at-risk populations could help identify microbial markers of increased cancer susceptibility, potentially leading to new early detection tools. Since the microbiome can also modify the effects of environmental pollutants, identifying microbes capable of neu
| 1. | Liang B, Ding H, Huang L, Luo H, Zhu X. GWAS in cancer: progress and challenges. Mol Genet Genomics. 2020;295:537-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | Sud A, Kinnersley B, Houlston RS. Genome-wide association studies of cancer: current insights and future perspectives. Nat Rev Cancer. 2017;17:692-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 282] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 3. | Visscher PM, Wray NR, Zhang Q, Sklar P, McCarthy MI, Brown MA, Yang J. 10 Years of GWAS Discovery: Biology, Function, and Translation. Am J Hum Genet. 2017;101:5-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2025] [Cited by in RCA: 2296] [Article Influence: 255.1] [Reference Citation Analysis (0)] |
| 4. | Byrne L, Toland AE. Polygenic Risk Scores in Prostate Cancer Risk Assessment and Screening. Urol Clin North Am. 2021;48:387-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Romualdo Cardoso S, Gillespie A, Haider S, Fletcher O. Functional annotation of breast cancer risk loci: current progress and future directions. Br J Cancer. 2022;126:981-993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Brandes N, Linial N, Linial M. Genetic association studies of alterations in protein function expose recessive effects on cancer predisposition. Sci Rep. 2021;11:14901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Byun J, Han Y, Li Y, Xia J, Long E, Choi J, Xiao X, Zhu M, Zhou W, Sun R, Bossé Y, Song Z, Schwartz A, Lusk C, Rafnar T, Stefansson K, Zhang T, Zhao W, Pettit RW, Liu Y, Li X, Zhou H, Walsh KM, Gorlov I, Gorlova O, Zhu D, Rosenberg SM, Pinney S, Bailey-Wilson JE, Mandal D, de Andrade M, Gaba C, Willey JC, You M, Anderson M, Wiencke JK, Albanes D, Lam S, Tardon A, Chen C, Goodman G, Bojeson S, Brenner H, Landi MT, Chanock SJ, Johansson M, Muley T, Risch A, Wichmann HE, Bickeböller H, Christiani DC, Rennert G, Arnold S, Field JK, Shete S, Le Marchand L, Melander O, Brunnstrom H, Liu G, Andrew AS, Kiemeney LA, Shen H, Zienolddiny S, Grankvist K, Johansson M, Caporaso N, Cox A, Hong YC, Yuan JM, Lazarus P, Schabath MB, Aldrich MC, Patel A, Lan Q, Rothman N, Taylor F, Kachuri L, Witte JS, Sakoda LC, Spitz M, Brennan P, Lin X, McKay J, Hung RJ, Amos CI. Cross-ancestry genome-wide meta-analysis of 61,047 cases and 947,237 controls identifies new susceptibility loci contributing to lung cancer. Nat Genet. 2022;54:1167-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 8. | Fanfani V, Citi L, Harris AL, Pezzella F, Stracquadanio G. The Landscape of the Heritable Cancer Genome. Cancer Res. 2021;81:2588-2599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Rashkin SR, Graff RE, Kachuri L, Thai KK, Alexeeff SE, Blatchins MA, Cavazos TB, Corley DA, Emami NC, Hoffman JD, Jorgenson E, Kushi LH, Meyers TJ, Van Den Eeden SK, Ziv E, Habel LA, Hoffmann TJ, Sakoda LC, Witte JS. Pan-cancer study detects genetic risk variants and shared genetic basis in two large cohorts. Nat Commun. 2020;11:4423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 194] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 10. | Rotunno M, Barajas R, Clyne M, Hoover E, Simonds NI, Lam TK, Mechanic LE, Goldstein AM, Gillanders EM. A Systematic Literature Review of Whole Exome and Genome Sequencing Population Studies of Genetic Susceptibility to Cancer. Cancer Epidemiol Biomarkers Prev. 2020;29:1519-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Sampson JN, Wheeler WA, Yeager M, Panagiotou O, Wang Z, Berndt SI, Lan Q, Abnet CC, Amundadottir LT, Figueroa JD, Landi MT, Mirabello L, Savage SA, Taylor PR, De Vivo I, McGlynn KA, Purdue MP, Rajaraman P, Adami HO, Ahlbom A, Albanes D, Amary MF, An SJ, Andersson U, Andriole G Jr, Andrulis IL, Angelucci E, Ansell SM, Arici C, Armstrong BK, Arslan AA, Austin MA, Baris D, Barkauskas DA, Bassig BA, Becker N, Benavente Y, Benhamou S, Berg C, Van Den Berg D, Bernstein L, Bertrand KA, Birmann BM, Black A, Boeing H, Boffetta P, Boutron-Ruault MC, Bracci PM, Brinton L, Brooks-Wilson AR, Bueno-de-Mesquita HB, Burdett L, Buring J, Butler MA, Cai Q, Cancel-Tassin G, Canzian F, Carrato A, Carreon T, Carta A, Chan JK, Chang ET, Chang GC, Chang IS, Chang J, Chang-Claude J, Chen CJ, Chen CY, Chen C, Chen CH, Chen C, Chen H, Chen K, Chen KY, Chen KC, Chen Y, Chen YH, Chen YS, Chen YM, Chien LH, Chirlaque MD, Choi JE, Choi YY, Chow WH, Chung CC, Clavel J, Clavel-Chapelon F, Cocco P, Colt JS, Comperat E, Conde L, Connors JM, Conti D, Cortessis VK, Cotterchio M, Cozen W, Crouch S, Crous-Bou M, Cussenot O, Davis FG, Ding T, Diver WR, Dorronsoro M, Dossus L, Duell EJ, Ennas MG, Erickson RL, Feychting M, Flanagan AM, Foretova L, Fraumeni JF Jr, Freedman ND, Beane Freeman LE, Fuchs C, Gago-Dominguez M, Gallinger S, Gao YT, Gapstur SM, Garcia-Closas M, García-Closas R, Gascoyne RD, Gastier-Foster J, Gaudet MM, Gaziano JM, Giffen C, Giles GG, Giovannucci E, Glimelius B, Goggins M, Gokgoz N, Goldstein AM, Gorlick R, Gross M, Grubb R 3rd, Gu J, Guan P, Gunter M, Guo H, Habermann TM, Haiman CA, Halai D, Hallmans G, Hassan M, Hattinger C, He Q, He X, Helzlsouer K, Henderson B, Henriksson R, Hjalgrim H, Hoffman-Bolton J, Hohensee C, Holford TR, Holly EA, Hong YC, Hoover RN, Horn-Ross PL, Hosain GM, Hosgood HD 3rd, Hsiao CF, Hu N, Hu W, Hu Z, Huang MS, Huerta JM, Hung JY, Hutchinson A, Inskip PD, Jackson RD, Jacobs EJ, Jenab M, Jeon HS, Ji BT, Jin G, Jin L, Johansen C, Johnson A, Jung YJ, Kaaks R, Kamineni A, Kane E, Kang CH, Karagas MR, Kelly RS, Khaw KT, Kim C, Kim HN, Kim JH, Kim JS, Kim YH, Kim YT, Kim YC, Kitahara CM, Klein AP, Klein RJ, Kogevinas M, Kohno T, Kolonel LN, Kooperberg C, Kricker A, Krogh V, Kunitoh H, Kurtz RC, Kweon SS, LaCroix A, Lawrence C, Lecanda F, Lee VH, Li D, Li H, Li J, Li YJ, Li Y, Liao LM, Liebow M, Lightfoot T, Lim WY, Lin CC, Lin D, Lindstrom S, Linet MS, Link BK, Liu C, Liu J, Liu L, Ljungberg B, Lloreta J, Di Lollo S, Lu D, Lund E, Malats N, Mannisto S, Le Marchand L, Marina N, Masala G, Mastrangelo G, Matsuo K, Maynadie M, McKay J, McKean-Cowdin R, Melbye M, Melin BS, Michaud DS, Mitsudomi T, Monnereau A, Montalvan R, Moore LE, Mortensen LM, Nieters A, North KE, Novak AJ, Oberg AL, Offit K, Oh IJ, Olson SH, Palli D, Pao W, Park IK, Park JY, Park KH, Patiño-Garcia A, Pavanello S, Peeters PH, Perng RP, Peters U, Petersen GM, Picci P, Pike MC, Porru S, Prescott J, Prokunina-Olsson L, Qian B, Qiao YL, Rais M, Riboli E, Riby J, Risch HA, Rizzato C, Rodabough R, Roman E, Roupret M, Ruder AM, Sanjose Sd, Scelo G, Schned A, Schumacher F, Schwartz K, Schwenn M, Scotlandi K, Seow A, Serra C, Serra M, Sesso HD, Setiawan VW, Severi G, Severson RK, Shanafelt TD, Shen H, Shen W, Shin MH, Shiraishi K, Shu XO, Siddiq A, Sierrasesúmaga L, Sihoe AD, Skibola CF, Smith A, Smith MT, Southey MC, Spinelli JJ, Staines A, Stampfer M, Stern MC, Stevens VL, Stolzenberg-Solomon RS, Su J, Su WC, Sund M, Sung JS, Sung SW, Tan W, Tang W, Tardón A, Thomas D, Thompson CA, Tinker LF, Tirabosco R, Tjønneland A, Travis RC, Trichopoulos D, Tsai FY, Tsai YH, Tucker M, Turner J, Vajdic CM, Vermeulen RC, Villano DJ, Vineis P, Virtamo J, Visvanathan K, Wactawski-Wende J, Wang C, Wang CL, Wang JC, Wang J, Wei F, Weiderpass E, Weiner GJ, Weinstein S, Wentzensen N, White E, Witzig TE, Wolpin BM, Wong MP, Wu C, Wu G, Wu J, Wu T, Wu W, Wu X, Wu YL, Wunder JS, Xiang YB, Xu J, Xu P, Yang PC, Yang TY, Ye Y, Yin Z, Yokota J, Yoon HI, Yu CJ, Yu H, Yu K, Yuan JM, Zelenetz A, Zeleniuch-Jacquotte A, Zhang XC, Zhang Y, Zhao X, Zhao Z, Zheng H, Zheng T, Zheng W, Zhou B, Zhu M, Zucca M, Boca SM, Cerhan JR, Ferri GM, Hartge P, Hsiung CA, Magnani C, Miligi L, Morton LM, Smedby KE, Teras LR, Vijai J, Wang SS, Brennan P, Caporaso NE, Hunter DJ, Kraft P, Rothman N, Silverman DT, Slager SL, Chanock SJ, Chatterjee N. Analysis of Heritability and Shared Heritability Based on Genome-Wide Association Studies for Thirteen Cancer Types. J Natl Cancer Inst. 2015;107:djv279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 12. | Young AI. Solving the missing heritability problem. PLoS Genet. 2019;15:e1008222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 156] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 13. | Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM. Finding the missing heritability of complex diseases. Nature. 2009;461:747-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5794] [Cited by in RCA: 5919] [Article Influence: 348.2] [Reference Citation Analysis (1)] |
| 14. | Balmain A. Cancer as a complex genetic trait: tumor susceptibility in humans and mouse models. Cell. 2002;108:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Balmain A, Gray J, Ponder B. The genetics and genomics of cancer. Nat Genet. 2003;33 Suppl:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 374] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 16. | Ewart-Toland A, Balmain A. The genetics of cancer susceptibility: from mouse to man. Toxicol Pathol. 2004;32 Suppl 1:26-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Hodgson SV, Foulkes WD, Maher ER, Turnbull C. Inherited Susceptibility to Cancer: Past, Present and Future. Ann Hum Genet. 2025;89:354-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Juang JJ, Lu TP, Su MW, Lin CW, Yang JH, Chu HW, Chen CH, Hsiao YW, Lee CY, Chiang LM, Yu QY, Hsiao CK, Chen CJ, Wu PE, Pai CH, Chuang EY, Shen CY. Rare variants discovery by extensive whole-genome sequencing of the Han Chinese population in Taiwan: Applications to cardiovascular medicine. J Adv Res. 2021;30:147-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Weiner DJ, Nadig A, Jagadeesh KA, Dey KK, Neale BM, Robinson EB, Karczewski KJ, O'Connor LJ. Polygenic architecture of rare coding variation across 394,783 exomes. Nature. 2023;614:492-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 118] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 20. | Eichler EE, Flint J, Gibson G, Kong A, Leal SM, Moore JH, Nadeau JH. Missing heritability and strategies for finding the underlying causes of complex disease. Nat Rev Genet. 2010;11:446-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1389] [Cited by in RCA: 1225] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 21. | Trerotola M, Relli V, Simeone P, Alberti S. Epigenetic inheritance and the missing heritability. Hum Genomics. 2015;9:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 22. | Mackay TF. Epistasis and quantitative traits: using model organisms to study gene-gene interactions. Nat Rev Genet. 2014;15:22-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 555] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 23. | Mbemi A, Khanna S, Njiki S, Yedjou CG, Tchounwou PB. Impact of Gene-Environment Interactions on Cancer Development. Int J Environ Res Public Health. 2020;17:8089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 24. | Kaprio J. Twins and the mystery of missing heritability: the contribution of gene-environment interactions. J Intern Med. 2012;272:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Awany D, Chimusa ER. Heritability jointly explained by host genotype and microbiome: will improve traits prediction? Brief Bioinform. 2021;22:bbaa175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Sandoval-Motta S, Aldana M, Martínez-Romero E, Frank A. The Human Microbiome and the Missing Heritability Problem. Front Genet. 2017;8:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Bhatt AP, Redinbo MR, Bultman SJ. The role of the microbiome in cancer development and therapy. CA Cancer J Clin. 2017;67:326-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 454] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 28. | Huybrechts I, Zouiouich S, Loobuyck A, Vandenbulcke Z, Vogtmann E, Pisanu S, Iguacel I, Scalbert A, Indave I, Smelov V, Gunter MJ, Michels N. The Human Microbiome in Relation to Cancer Risk: A Systematic Review of Epidemiologic Studies. Cancer Epidemiol Biomarkers Prev. 2020;29:1856-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 29. | Lee MH. Harness the functions of gut microbiome in tumorigenesis for cancer treatment. Cancer Commun (Lond). 2021;41:937-967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 30. | Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science. 2021;371:eabc4552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 868] [Article Influence: 173.6] [Reference Citation Analysis (0)] |
| 31. | Xavier JB, Young VB, Skufca J, Ginty F, Testerman T, Pearson AT, Macklin P, Mitchell A, Shmulevich I, Xie L, Caporaso JG, Crandall KA, Simone NL, Godoy-Vitorino F, Griffin TJ, Whiteson KL, Gustafson HH, Slade DJ, Schmidt TM, Walther-Antonio MRS, Korem T, Webb-Robertson BM, Styczynski MP, Johnson WE, Jobin C, Ridlon JM, Koh AY, Yu M, Kelly L, Wargo JA. The Cancer Microbiome: Distinguishing Direct and Indirect Effects Requires a Systemic View. Trends Cancer. 2020;6:192-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (16)] |
| 32. | Cullin N, Azevedo Antunes C, Straussman R, Stein-Thoeringer CK, Elinav E. Microbiome and cancer. Cancer Cell. 2021;39:1317-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 380] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 33. | Bubier JA, Chesler EJ, Weinstock GM. Host genetic control of gut microbiome composition. Mamm Genome. 2021;32:263-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 34. | Davenport ER. Elucidating the role of the host genome in shaping microbiome composition. Gut Microbes. 2016;7:178-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Yeoh YK, Chen Z, Hui M, Wong MCS, Ho WCS, Chin ML, Ng SC, Chan FKL, Chan PKS. Impact of inter- and intra-individual variation, sample storage and sampling fraction on human stool microbial community profiles. PeerJ. 2019;7:e6172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Zhu A, Sunagawa S, Mende DR, Bork P. Inter-individual differences in the gene content of human gut bacterial species. Genome Biol. 2015;16:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 37. | Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med. 2018;24:392-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 991] [Cited by in RCA: 1675] [Article Influence: 239.3] [Reference Citation Analysis (0)] |
| 38. | Scott AJ, Alexander JL, Merrifield CA, Cunningham D, Jobin C, Brown R, Alverdy J, O'Keefe SJ, Gaskins HR, Teare J, Yu J, Hughes DJ, Verstraelen H, Burton J, O'Toole PW, Rosenberg DW, Marchesi JR, Kinross JM. International Cancer Microbiome Consortium consensus statement on the role of the human microbiome in carcinogenesis. Gut. 2019;68:1624-1632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 207] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 39. | Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev. 2012;70 Suppl 1:S38-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 761] [Article Influence: 54.4] [Reference Citation Analysis (1)] |
| 40. | Grice EA, Segre JA. The human microbiome: our second genome. Annu Rev Genomics Hum Genet. 2012;13:151-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 453] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 41. | Sepich-Poore GD, Guccione C, Laplane L, Pradeu T, Curtius K, Knight R. Cancer's second genome: Microbial cancer diagnostics and redefining clonal evolution as a multispecies process: Humans and their tumors are not aseptic, and the multispecies nature of cancer modulates clinical care and clonal evolution: Humans and their tumors are not aseptic, and the multispecies nature of cancer modulates clinical care and clonal evolution. Bioessays. 2022;44:e2100252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4573] [Cited by in RCA: 3850] [Article Influence: 202.6] [Reference Citation Analysis (0)] |
| 43. | Ahn J, Hayes RB. Environmental Influences on the Human Microbiome and Implications for Noncommunicable Disease. Annu Rev Public Health. 2021;42:277-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 44. | Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7:e7502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 478] [Article Influence: 68.3] [Reference Citation Analysis (4)] |
| 45. | Kurilshikov A, Medina-Gomez C, Bacigalupe R, Radjabzadeh D, Wang J, Demirkan A, Le Roy CI, Raygoza Garay JA, Finnicum CT, Liu X, Zhernakova DV, Bonder MJ, Hansen TH, Frost F, Rühlemann MC, Turpin W, Moon JY, Kim HN, Lüll K, Barkan E, Shah SA, Fornage M, Szopinska-Tokov J, Wallen ZD, Borisevich D, Agreus L, Andreasson A, Bang C, Bedrani L, Bell JT, Bisgaard H, Boehnke M, Boomsma DI, Burk RD, Claringbould A, Croitoru K, Davies GE, van Duijn CM, Duijts L, Falony G, Fu J, van der Graaf A, Hansen T, Homuth G, Hughes DA, Ijzerman RG, Jackson MA, Jaddoe VWV, Joossens M, Jørgensen T, Keszthelyi D, Knight R, Laakso M, Laudes M, Launer LJ, Lieb W, Lusis AJ, Masclee AAM, Moll HA, Mujagic Z, Qibin Q, Rothschild D, Shin H, Sørensen SJ, Steves CJ, Thorsen J, Timpson NJ, Tito RY, Vieira-Silva S, Völker U, Völzke H, Võsa U, Wade KH, Walter S, Watanabe K, Weiss S, Weiss FU, Weissbrod O, Westra HJ, Willemsen G, Payami H, Jonkers DMAE, Arias Vasquez A, de Geus EJC, Meyer KA, Stokholm J, Segal E, Org E, Wijmenga C, Kim HL, Kaplan RC, Spector TD, Uitterlinden AG, Rivadeneira F, Franke A, Lerch MM, Franke L, Sanna S, D'Amato M, Pedersen O, Paterson AD, Kraaij R, Raes J, Zhernakova A. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet. 2021;53:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 1227] [Article Influence: 245.4] [Reference Citation Analysis (0)] |
| 46. | McLaughlin-Drubin ME, Munger K. Viruses associated with human cancer. Biochim Biophys Acta. 2008;1782:127-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 255] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 47. | Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609-e616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1068] [Cited by in RCA: 1057] [Article Influence: 105.7] [Reference Citation Analysis (0)] |
| 48. | Ryu G, Kim H, Koh A. Approaching precision medicine by tailoring the microbiota. Mamm Genome. 2021;32:206-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 49. | Hughes DA, Bacigalupe R, Wang J, Rühlemann MC, Tito RY, Falony G, Joossens M, Vieira-Silva S, Henckaerts L, Rymenans L, Verspecht C, Ring S, Franke A, Wade KH, Timpson NJ, Raes J. Genome-wide associations of human gut microbiome variation and implications for causal inference analyses. Nat Microbiol. 2020;5:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 50. | Li D, Zhong C, Yang M, He L, Chang H, Zhu N, Celniker SE, Threadgill DW, Snijders AM, Mao JH, Yuan Y. Genetic and microbial determinants of azoxymethane-induced colorectal tumor susceptibility in Collaborative Cross mice and their implication in human cancer. Gut Microbes. 2024;16:2341647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 51. | Lopera-Maya EA, Kurilshikov A, van der Graaf A, Hu S, Andreu-Sánchez S, Chen L, Vila AV, Gacesa R, Sinha T, Collij V, Klaassen MAY, Bolte LA, Gois MFB, Neerincx PBT, Swertz MA; LifeLines Cohort Study, Harmsen HJM, Wijmenga C, Fu J, Weersma RK, Zhernakova A, Sanna S. Effect of host genetics on the gut microbiome in 7,738 participants of the Dutch Microbiome Project. Nat Genet. 2022;54:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 332] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 52. | Nagarajan A, Scoggin K, Gupta J, Threadgill DW, Andrews-Polymenis HL. Using the collaborative cross to identify the role of host genetics in defining the murine gut microbiome. Microbiome. 2023;11:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 53. | Qin Y, Havulinna AS, Liu Y, Jousilahti P, Ritchie SC, Tokolyi A, Sanders JG, Valsta L, Brożyńska M, Zhu Q, Tripathi A, Vázquez-Baeza Y, Loomba R, Cheng S, Jain M, Niiranen T, Lahti L, Knight R, Salomaa V, Inouye M, Méric G. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat Genet. 2022;54:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 380] [Article Influence: 95.0] [Reference Citation Analysis (1)] |
| 54. | Tabrett A, Horton MW. The influence of host genetics on the microbiome. F1000Res. 2020;9:F1000 Faculty Rev-F1000 Faculty R84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 55. | Dutta D, Lim SH. Bidirectional interaction between intestinal microbiome and cancer: opportunities for therapeutic interventions. Biomark Res. 2020;8:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 56. | Xu F, Fu Y, Sun TY, Jiang Z, Miao Z, Shuai M, Gou W, Ling CW, Yang J, Wang J, Chen YM, Zheng JS. The interplay between host genetics and the gut microbiome reveals common and distinct microbiome features for complex human diseases. Microbiome. 2020;8:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 57. | Mody D, Verma V, Rani V. Modulating host gene expression via gut microbiome-microRNA interplay to treat human diseases. Crit Rev Microbiol. 2021;47:596-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 58. | Nichols RG, Davenport ER. The relationship between the gut microbiome and host gene expression: a review. Hum Genet. 2021;140:747-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 59. | Patrignani P, Tacconelli S, Bruno A. Gut microbiota, host gene expression, and aging. J Clin Gastroenterol. 2014;48 Suppl 1:S28-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Aguilar C, Mano M, Eulalio A. MicroRNAs at the Host-Bacteria Interface: Host Defense or Bacterial Offense. Trends Microbiol. 2019;27:206-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 61. | Krautkramer KA, Rey FE, Denu JM. Chemical signaling between gut microbiota and host chromatin: What is your gut really saying? J Biol Chem. 2017;292:8582-8593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 62. | Nikolaieva N, Sevcikova A, Omelka R, Martiniakova M, Mego M, Ciernikova S. Gut Microbiota-MicroRNA Interactions in Intestinal Homeostasis and Cancer Development. Microorganisms. 2022;11:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 63. | Qin Y, Wade PA. Crosstalk between the microbiome and epigenome: messages from bugs. J Biochem. 2018;163:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 64. | Balskus EP. Colibactin: understanding an elusive gut bacterial genotoxin. Nat Prod Rep. 2015;32:1534-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 65. | Lai YR, Chang YF, Ma J, Chiu CH, Kuo ML, Lai CH. From DNA Damage to Cancer Progression: Potential Effects of Cytolethal Distending Toxin. Front Immunol. 2021;12:760451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 66. | Puschhof J, Sears CL. Microbial metabolites damage DNA. Science. 2022;378:358-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Wilson MR, Jiang Y, Villalta PW, Stornetta A, Boudreau PD, Carrá A, Brennan CA, Chun E, Ngo L, Samson LD, Engelward BP, Garrett WS, Balbo S, Balskus EP. The human gut bacterial genotoxin colibactin alkylates DNA. Science. 2019;363:eaar7785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 465] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 68. | Dougherty MW, Jobin C. Intestinal bacteria and colorectal cancer: etiology and treatment. Gut Microbes. 2023;15:2185028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 69. | Hirayama Y, Sato M, Watanabe K. Advancing the Biosynthetic and Chemical Understanding of the Carcinogenic Risk Factor Colibactin and Its Producers. Biochemistry. 2022;61:2782-2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 70. | Scott N, Whittle E, Jeraldo P, Chia N. A systemic review of the role of enterotoxic Bacteroides fragilis in colorectal cancer. Neoplasia. 2022;29:100797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 71. | Sears CL. Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clin Microbiol Rev. 2009;22:349-369, Table of Contents. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 72. | Cao Y, Oh J, Xue M, Huh WJ, Wang J, Gonzalez-Hernandez JA, Rice TA, Martin AL, Song D, Crawford JM, Herzon SB, Palm NW. Commensal microbiota from patients with inflammatory bowel disease produce genotoxic metabolites. Science. 2022;378:eabm3233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 73. | Environmental Chemicals, the Human Microbiome, and Health Risk: A Research Strategy. Washington (DC): National Academies Press (US); 2017-Dec-29 . [PubMed] |
| 74. | Giambò F, Costa C, Teodoro M, Fenga C. Role-Playing Between Environmental Pollutants and Human Gut Microbiota: A Complex Bidirectional Interaction. Front Med (Lausanne). 2022;9:810397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Gupta N, Yadav VK, Gacem A, Al-Dossari M, Yadav KK, Abd El-Gawaad NS, Ben Khedher N, Choudhary N, Kumar P, Cavalu S. Deleterious Effect of Air Pollution on Human Microbial Community and Bacterial Flora: A Short Review. Int J Environ Res Public Health. 2022;19:15494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 76. | Campana AM, Laue HE, Shen Y, Shrubsole MJ, Baccarelli AA. Assessing the role of the gut microbiome at the interface between environmental chemical exposures and human health: Current knowledge and challenges. Environ Pollut. 2022;315:120380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 77. | Koontz JM, Dancy BCR, Horton CL, Stallings JD, DiVito VT, Lewis JA. The Role of the Human Microbiome in Chemical Toxicity. Int J Toxicol. 2019;38:251-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 78. | Chiu K, Warner G, Nowak RA, Flaws JA, Mei W. The Impact of Environmental Chemicals on the Gut Microbiome. Toxicol Sci. 2020;176:253-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 79. | Jin Y, Wu S, Zeng Z, Fu Z. Effects of environmental pollutants on gut microbiota. Environ Pollut. 2017;222:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 469] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 80. | Krych L, Hansen CH, Hansen AK, van den Berg FW, Nielsen DS. Quantitatively different, yet qualitatively alike: a meta-analysis of the mouse core gut microbiome with a view towards the human gut microbiome. PLoS One. 2013;8:e62578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 81. | Park JC, Im SH. Of men in mice: the development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp Mol Med. 2020;52:1383-1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (71)] |
| 82. | Yang J, Chun J. Taxonomic composition and variation in the gut microbiota of laboratory mice. Mamm Genome. 2021;32:297-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | Hugenholtz F, de Vos WM. Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol Life Sci. 2018;75:149-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 424] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 84. | Collaborative Cross Consortium. The genome architecture of the Collaborative Cross mouse genetic reference population. Genetics. 2012;190:389-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 379] [Cited by in RCA: 379] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 85. | Dorman A, Binenbaum I, Abu-Toamih Atamni HJ, Chatziioannou A, Tomlinson I, Mott R, Iraqi FA. Genetic mapping of novel modifiers for Apc(Min) induced intestinal polyps' development using the genetic architecture power of the collaborative cross mice. BMC Genomics. 2021;22:566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 86. | Lawley KS, Rech RR, Elenwa F, Han G, Perez Gomez AA, Amstalden K, Welsh CJ, Young CR, Threadgill DW, Brinkmeyer-Langford CL. Host genetic diversity drives variable central nervous system lesion distribution in chronic phase of Theiler's Murine Encephalomyelitis Virus (TMEV) infection. PLoS One. 2021;16:e0256370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 87. | Luo YS, Cichocki JA, Hsieh NH, Lewis L, Wright FA, Threadgill DW, Chiu WA, Rusyn I. Using Collaborative Cross Mouse Population to Fill Data Gaps in Risk Assessment: A Case Study of Population-Based Analysis of Toxicokinetics and Kidney Toxicodynamics of Tetrachloroethylene. Environ Health Perspect. 2019;127:67011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 88. | Mao JH, Kim YM, Zhou YX, Hu D, Zhong C, Chang H, Brislawn CJ, Fansler S, Langley S, Wang Y, Peisl BYL, Celniker SE, Threadgill DW, Wilmes P, Orr G, Metz TO, Jansson JK, Snijders AM. Genetic and metabolic links between the murine microbiome and memory. Microbiome. 2020;8:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 89. | Milhem A, Abu Toamih-Atamni HJ, Karkar L, Houri-Haddad Y, Iraqi FA. Studying host genetic background effects on multimorbidity of intestinal cancer development, type 2 diabetes and obesity in response to oral bacterial infection and high-fat diet using the collaborative cross (CC) lines. Animal Model Exp Med. 2021;4:27-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 90. | Mosedale M, Cai Y, Eaddy JS, Kirby PJ, Wolenski FS, Dragan Y, Valdar W. Human-relevant mechanisms and risk factors for TAK-875-Induced liver injury identified via a gene pathway-based approach in Collaborative Cross mice. Toxicology. 2021;461:152902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 91. | Noll KE, Ferris MT, Heise MT. The Collaborative Cross: A Systems Genetics Resource for Studying Host-Pathogen Interactions. Cell Host Microbe. 2019;25:484-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 92. | Zeiss CJ, Gatti DM, Toro-Salazar O, Davis C, Lutz CM, Spinale F, Stearns T, Furtado MB, Churchill GA. Doxorubicin-Induced Cardiotoxicity in Collaborative Cross (CC) Mice Recapitulates Individual Cardiotoxicity in Humans. G3 (Bethesda). 2019;9:2637-2646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 93. | He L, Zhong C, Chang H, Inman JL, Celniker SE, Ioakeim-Ioannidou M, Liu KX, Haas-Kogan D, MacDonald SM, Threadgill DW, Kogan SC, Mao JH, Snijders AM. Genetic architecture of the acute and persistent immune cell response after radiation exposure. Cell Genom. 2023;3:100422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 94. | Yang H, Wang X, Blanco-Gómez A, He L, García-Sancha N, Corchado-Cobos R, Pérez-Baena MJ, Jiménez-Navas A, Wang P, Inman JL, Snijders AM, Threadgill DW, Balmain A, Chang H, Perez-Losada J, Mao JH. A susceptibility gene signature for ERBB2-driven mammary tumour development and metastasis in collaborative cross mice. EBioMedicine. 2024;106:105260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 95. | Bubier JA, Philip VM, Quince C, Campbell J, Zhou Y, Vishnivetskaya T, Duvvuru S, Blair RH, Ndukum J, Donohue KD, Foster CM, Mellert DJ, Weinstock G, Culiat CT, O'Hara BF, Palumbo AV, Podar M, Chesler EJ. A Microbe Associated with Sleep Revealed by a Novel Systems Genetic Analysis of the Microbiome in Collaborative Cross Mice. Genetics. 2020;214:719-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 96. | Lundberg R, Toft MF, August B, Hansen AK, Hansen CH. Antibiotic-treated versus germ-free rodents for microbiota transplantation studies. Gut Microbes. 2016;7:68-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 97. | Martín R, Bermúdez-Humarán LG, Langella P. Gnotobiotic Rodents: An In Vivo Model for the Study of Microbe-Microbe Interactions. Front Microbiol. 2016;7:409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 98. | Bhattarai Y, Kashyap PC. Germ-Free Mice Model for Studying Host-Microbial Interactions. Methods Mol Biol. 2016;1438:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 99. | Darnaud M, De Vadder F, Bogeat P, Boucinha L, Bulteau AL, Bunescu A, Couturier C, Delgado A, Dugua H, Elie C, Mathieu A, Novotná T, Ouattara DA, Planel S, Saliou A, Šrůtková D, Yansouni J, Stecher B, Schwarzer M, Leulier F, Tamellini A. A standardized gnotobiotic mouse model harboring a minimal 15-member mouse gut microbiota recapitulates SOPF/SPF phenotypes. Nat Commun. 2021;12:6686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 100. | Bokoliya SC, Dorsett Y, Panier H, Zhou Y. Procedures for Fecal Microbiota Transplantation in Murine Microbiome Studies. Front Cell Infect Microbiol. 2021;11:711055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 101. | Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci U S A. 2006;103:10011-10016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 501] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 102. | Steimle A, De Sciscio A, Neumann M, Grant ET, Pereira GV, Ohno H, Martens EC, Desai MS. Constructing a gnotobiotic mouse model with a synthetic human gut microbiome to study host-microbe cross talk. STAR Protoc. 2021;2:100607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 103. | Zucoloto AZ, Yu IL, McCoy KD, McDonald B. Generation, maintenance, and monitoring of gnotobiotic mice. STAR Protoc. 2021;2:100536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Al-Asmakh M, Zadjali F. Use of Germ-Free Animal Models in Microbiota-Related Research. J Microbiol Biotechnol. 2015;25:1583-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 105. | Basic M, Bleich A. Gnotobiotics: Past, present and future. Lab Anim. 2019;53:232-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 106. | Gheorghe CE, Ritz NL, Martin JA, Wardill HR, Cryan JF, Clarke G. Investigating causality with fecal microbiota transplantation in rodents: applications, recommendations and pitfalls. Gut Microbes. 2021;13:1941711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 107. | Kubelkova K, Benuchova M, Kozakova H, Sinkora M, Krocova Z, Pejchal J, Macela A. Gnotobiotic mouse model's contribution to understanding host-pathogen interactions. Cell Mol Life Sci. 2016;73:3961-3969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 108. | Lynn MA, Ryan FJ, Tee YC, Lynn DJ. Protocol to colonize gnotobiotic mice in early life and assess the impact on early life immune programming. STAR Protoc. 2022;3:101914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 109. | Schwarzer M, Tlaskalova-Hogenova H, Leulier F, Schabussova I. Editorial: Employing Experimental Gnotobiotic Models to Decipher the Host-Microbiota Cross-Talk in Health and Disease. Front Immunol. 2021;12:729052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 110. | Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9292] [Cited by in RCA: 8342] [Article Influence: 595.9] [Reference Citation Analysis (4)] |
| 111. | McDonald D, Hyde E, Debelius JW, Morton JT, Gonzalez A, Ackermann G, Aksenov AA, Behsaz B, Brennan C, Chen Y, DeRight Goldasich L, Dorrestein PC, Dunn RR, Fahimipour AK, Gaffney J, Gilbert JA, Gogul G, Green JL, Hugenholtz P, Humphrey G, Huttenhower C, Jackson MA, Janssen S, Jeste DV, Jiang L, Kelley ST, Knights D, Kosciolek T, Ladau J, Leach J, Marotz C, Meleshko D, Melnik AV, Metcalf JL, Mohimani H, Montassier E, Navas-Molina J, Nguyen TT, Peddada S, Pevzner P, Pollard KS, Rahnavard G, Robbins-Pianka A, Sangwan N, Shorenstein J, Smarr L, Song SJ, Spector T, Swafford AD, Thackray VG, Thompson LR, Tripathi A, Vázquez-Baeza Y, Vrbanac A, Wischmeyer P, Wolfe E, Zhu Q; American Gut Consortium, Knight R. American Gut: an Open Platform for Citizen Science Microbiome Research. mSystems. 2018;3:e00031-e00018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 608] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 112. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J; MetaHIT Consortium, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9101] [Cited by in RCA: 8091] [Article Influence: 505.7] [Reference Citation Analysis (4)] |
| 113. | Hermida LC, Gertz EM, Ruppin E. Predicting cancer prognosis and drug response from the tumor microbiome. Nat Commun. 2022;13:2896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 114. | Zhou YH, Sun G. Improve the Colorectal Cancer Diagnosis Using Gut Microbiome Data. Front Mol Biosci. 2022;9:921945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 115. | Lee KA, Thomas AM, Bolte LA, Björk JR, de Ruijter LK, Armanini F, Asnicar F, Blanco-Miguez A, Board R, Calbet-Llopart N, Derosa L, Dhomen N, Brooks K, Harland M, Harries M, Leeming ER, Lorigan P, Manghi P, Marais R, Newton-Bishop J, Nezi L, Pinto F, Potrony M, Puig S, Serra-Bellver P, Shaw HM, Tamburini S, Valpione S, Vijay A, Waldron L, Zitvogel L, Zolfo M, de Vries EGE, Nathan P, Fehrmann RSN, Bataille V, Hospers GAP, Spector TD, Weersma RK, Segata N. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat Med. 2022;28:535-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 308] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 116. | Coker OO, Liu C, Wu WKK, Wong SH, Jia W, Sung JJY, Yu J. Altered gut metabolites and microbiota interactions are implicated in colorectal carcinogenesis and can be non-invasive diagnostic biomarkers. Microbiome. 2022;10:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 170] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 117. | Yang X, An H, He Y, Fu G, Jiang Z. Comprehensive analysis of microbiota signature across 32 cancer types. Front Oncol. 2023;13:1127225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 118. | Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11842] [Cited by in RCA: 10973] [Article Influence: 731.5] [Reference Citation Analysis (0)] |
| 119. | Liang H, Jo JH, Zhang Z, MacGibeny MA, Han J, Proctor DM, Taylor ME, Che Y, Juneau P, Apolo AB, McCulloch JA, Davar D, Zarour HM, Dzutsev AK, Brownell I, Trinchieri G, Gulley JL, Kong HH. Predicting cancer immunotherapy response from gut microbiomes using machine learning models. Oncotarget. 2022;13:876-889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 120. | Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261-5267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12842] [Cited by in RCA: 13532] [Article Influence: 712.2] [Reference Citation Analysis (0)] |
| 121. | Werner JJ, Koren O, Hugenholtz P, DeSantis TZ, Walters WA, Caporaso JG, Angenent LT, Knight R, Ley RE. Impact of training sets on classification of high-throughput bacterial 16s rRNA gene surveys. ISME J. 2012;6:94-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 488] [Cited by in RCA: 401] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 122. | Mao AW, Barck H, Young J, Paley A, Mao J-, Chang H. Identification of a novel cancer microbiome signature for predicting prognosis of human breast cancer patients. Clin Transl Oncol. 2022;24:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 123. | Lee TS, Mumford D. Hierarchical Bayesian inference in the visual cortex. J Opt Soc Am A Opt Image Sci Vis. 2003;20:1434-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 910] [Cited by in RCA: 695] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 124. | Lee TS, Mumford D, Romero R, Lamme VA. The role of the primary visual cortex in higher level vision. Vision Res. 1998;38:2429-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 290] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 125. | Fioravanti D, Giarratano Y, Maggio V, Agostinelli C, Chierici M, Jurman G, Furlanello C. Phylogenetic convolutional neural networks in metagenomics. BMC Bioinformatics. 2018;19:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 126. | Lo C, Marculescu R. MetaNN: accurate classification of host phenotypes from metagenomic data using neural networks. BMC Bioinformatics. 2019;20:314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 127. | Mao XY, Perez-Losada J, Abad M, Rodríguez-González M, Rodríguez CA, Mao JH, Chang H. iCEMIGE: Integration of CEll-morphometrics, MIcrobiome, and GEne biomarker signatures for risk stratification in breast cancers. World J Clin Oncol. 2022;13:616-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 128. | Vaswani A, Shazeer N, Parmar N, Uszkoreit J, Jones L, Gomez AN, Kaiser Ł, Polosukhin I: Attention is all you need. In: Luxburg UV, Guyon I, Wallach H, Fergus R, editors. Proceedings of the 31st International Conference on Neural Information Processing Systems; 2017 Dec 4-9; California, United States. New York: Curran Associates Inc., 2017: 6000-6010. |
| 129. | Dosovitskiy A, Beyer L, Kolesnikov A, Weissenborn D, Zhai X, Unterthiner T, Dehghani M, Minderer M, Heigold G, Gelly S, Uszkoreit J, Houlsby N. An Image is Worth 16x16 Words: Transformers for Image Recognition at Scale. 2021 Preprint. Available from: arXiv:2010.11929. [DOI] [Full Text] |
| 130. | Verma P, Berger J. Audio Transformers. 2025 Preprint. Available from: arXiv:2105.00335. [DOI] [Full Text] |
| 131. | Shang J, Tang X, Guo R, Sun Y. Accurate identification of bacteriophages from metagenomic data using Transformer. Brief Bioinform. 2022;23:bbac258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 132. | Wolf T, Debut L, Sanh V, Chaumond J, Delangue C, Moi A, Cistac P, Rault T, Louf R, Funtowicz M, Davison J, Shleifer S, von Platen P, Ma C, Jernite Y, Plu J, Xu C, Le Scao T, Gugger S, Drame M, Lhoest Q, Rush A. Transformers: State-of-the-Art Natural Language Processing. In: Proceedings of the 2020 Conference on Empirical Methods in Natural Language Processing: System Demonstrations. Texas: Association for Computational Linguistics, 2020: 38-45. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/