Published online Sep 24, 2025. doi: 10.5306/wjco.v16.i9.109182
Revised: May 25, 2025
Accepted: August 8, 2025
Published online: September 24, 2025
Processing time: 144 Days and 22.3 Hours
Hereditary factors are more prevalent in early-onset colorectal cancers (EOCRC) etiology. Lynch syndrome (LS) is the most common hereditary colorectal cancer (CRC) syndrome that results from mutations in DNA mismatch repair (MMR) genes. This phenomenon is defined as microsatellite instability (MSI). Immunohistochemistry (IHC) is a widely used, practical, and cost-effective method for the screening of MSI. However, using IHC alone may be insufficient to identify patients with MSI and LS.
To determine the clinicopathological features in EOCRC, IHC performance, and the frequency of genetic testing for EOCRC patients.
A retrospective review was conducted on patients with CRC aged ≤ 50 years who underwent surgery at our center between January 2014 and July 2021. MMR proteins were screened using IHC. Of the 131 patients included, IHC was per
Thirty patients with MSI were designated as group 1, whereas 100 with MSS were defined as group 2. The mean age in group 1 was the lowest (median age: 42 vs 46, P < 0.05). Group 1 exhibited a higher frequency of tumors in the right colon and a lower frequency in the rectum. Lymph node involvement and distant metastases were less common in group 1, and in group 2, tumors were generally diagnosed at a more advanced stage. Genetic testing was performed in 53 patients (40%), with a definitive LS diagnosis established in 13/17 patients (76.4%) in group 1 and 1/36 (2.7%) patients in group 2, resulting in a total of 14 patients (26.4%) with confirmed LS.
MSI tumors show a better prognosis. IHC is very effective for screening MSI, but may not be sufficient alone. Low genetic counseling rates highlight the need for hospital-based surveillance programs.
Core Tip: Lynch syndrome (LS) results from microsatellite instability (MSI). Immunohistochemistry (IHC) is a cost-effective and practical method for MSI screening. This retrospective study evaluated clinicopathological features of MSI and microsatellite-stable (MSS) tumors in early-onset colorectal cancer patients between 2014 and 2021. MSI tumors were linked to younger age, right-sided location, and fewer metastases. Genetic testing confirmed LS in 76.4% of MSI and 2.8% of MSS cases based on IHC. However, 59.5% of patients did not receive genetic counseling. While IHC is useful for MSI detection, it may not be sufficient alone. Low counseling rates emphasize the need for hospital-based surveillance programs.
- Citation: Sür Y, Gür EÖ, Cengiz F, Subaşıoğlu A, Güzeliş İ, Demir S, Sari AA, Haciyanli M, Dilek ON. Lynch syndrome association and clinicopathological features in early-onset colorectal cancers: A single-center retrospective study. World J Clin Oncol 2025; 16(9): 109182
- URL: https://www.wjgnet.com/2218-4333/full/v16/i9/109182.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i9.109182
Globally, colorectal cancer (CRC) is the third most common type of cancer and one of the leading causes of cancer-related mortality[1]. Approximately 10% of CRC cases are attributable to genetic factors; however, in early-onset CRCs (EOCRC) diagnosed in individuals ≤ 50 years of age, this proportion increases to 20%[2]. Lynch syndrome (LS), also known as hereditary nonpolyposis CRC (HNPCC), is the most common hereditary CRC syndrome, accounting for 3%-5% of all cases with CRC[3].
LS is an autosomal dominant inherited cancer syndrome caused by pathogenic germline variants in DNA mismatch repair (MMR) genes. The MMR genes affected include MLH1, MSH2, MSH6, and PMS2. These mutations confer a significant predisposition to cancer. The syndrome is associated with a wide spectrum of malignancies, primarily co
Carcinogenesis is accelerated in patients with LS. While it typically takes 8-10 years for a colorectal adenoma to progress to cancer in the general population, this process may be completed within 2-3 years in individuals with LS. Consequently, patients with LS tend to develop rapidly progressing tumors at a younger age[6].
Early diagnosis and treatment in high-risk individuals can reduce morbidity and mortality. Therefore, identifying and screening high-risk individuals, particularly in conditions like LS that cause early-onset, rapidly progressing tumors, is crucial.
The definitive diagnosis of LS is established through genetic testing, which is costly; thus, it is imperative to accurately identify patients who should undergo these tests. The Amsterdam and Bethesda criteria, based on patients’ medical history, age, and family history, are traditionally used to select candidates for genetic testing; however, these criteria alone are insufficient to identify all LS cases[7].
Immunohistochemistry (IHC) has been established as a cost-effective, practical, and reliable screening modality for LS, with numerous studies demonstrating its high sensitivity in syndrome detection. Current guidelines recommend IHC as a primary tool to identify patients eligible for subsequent genetic testing[7].
The aim of the present study was to evaluate the demographic, pathological, and clinical features of patients with CRC ≤ 50 years of age who underwent surgery at our center to assess microsatellite instability (MSI) status via IHC and to compare the clinicopathological characteristics of MSI and microsatellite-stable (MSS) tumors.
As a secondary objective, we analyzed the features of patients who underwent multigene testing at our center and were diagnosed with LS, comparing our IHC results with genetic testing outcomes.
A total of 131 patients diagnosed with CRC aged ≤ 50 years who underwent surgery at our center between January 2014 and July 2021 were retrospectively evaluated. Sixty-five patients without prior IHC analysis were identified. After obtaining approval from the Hospital Ethics Committee (No. 446, Date: 21.10.2021), IHC was performed retrospectively on the pathology specimens of these 65 patients. Totally, IHC data were obtained for 130 patients. Patients were classified into two groups based on the nuclear staining of MLH1, MSH2, MSH6, and PMS2 proteins. Those with negative nuclear staining were considered to have microsatellite instability (MSI; group 1), whereas those with positive staining were classified as microsatellite stable (MSS; group 2). Subsequently, the two groups were compared with respect to de
In addition, Next-Generation Sequencing (NGS) results of patients referred to the genetics clinic were evaluated. The performance of IHC was assessed by comparing IHC findings with NGS results. Clinical data of patients with a definitive diagnosis of LS were also analyzed.
Patients who underwent surgery at external centers and those aged > 50 years of age were excluded from the study.
For IHC staining, a fully automated staining device (Ventana BenchMark XT) was used with a biotin-free, HRP multimer-based system incorporating a hydrogen peroxide substrate and 3,3′-diaminobenzidine tetrahydrochloride chromogen (ultraView™ Universal diaminobenzidine tetrahydrochloride Detection Kit). The IHC analysis employed Ventana anti-MSH2 (G219-1129), anti-MSH6 (SP93), anti-PMS2 (A16-4), and anti-MLH1 (M1) primary antibodies. Normal colonic mucosa and intratumoral lymphocytes served as positive internal controls for all four antibodies, whereas skeletal muscle was used as the negative control. During evaluation, the presence of nuclear staining for each antibody was regarded as positive, whereas the absence of nuclear staining in tumor cells was interpreted as loss of the corresponding MMR gene protein.
In the genetics clinic, all exonic regions and exon-intron junctions of the following genes were sequenced using NGS: (1) PIK3CA; (2) GREM1; (3) CDKN2A; (4) MSH6; (5) MRE11A; (6) STK11; (7) GEN1; (8) RAD51D; (9) SMARCA4; (10) BRIP1; (11) TP53; (12) POLE; (13) BRCA1; (14) PRSS1; (15) SDHB; (16) SDHC; (17) PMS2; (18) FAM175A; (19) SMAD4; (20) GALNT12; (21) BRCA2; (22) CDK4; (23) PTEN; (24) BLM; (25) RAD51B; (26) RET; (27) ATM; (28) CHEK2; (29) CTNNA1; (30) PALB2; (31) BARD1; (32) AXIN2; (33) RINT1; (34) XRCC2; (35) MET; (36) NTHL1; (37) AIP; (38) NBN; (39) RAD50; (40) ATR; (41) BMPR1A; (42) BUB1B; (43) VHL; (44) SDHD; (45) MLH1; (46) PTCH1; (47) RAD51C; (48) EPCAM; (49) MSH2; (50) BAP1; (51) CDH1; (52) HOXB13; (53) MEN1; (54) GPC3; (55) FANCC; (56) POLD1; (57) MUTYH; (58) APC; (59) FLCN; (60) PALLD; and (61) PMS1.
This study primarily included sample isolation, library preparation, sequencing, and bioinformatics analysis stages. During the sample isolation step, genomic DNA was isolated from peripheral blood samples using the EZ1 DSP DNA Blood Kit (Qiagen). The concentration of the extracted DNA was optimized using the Qubit™ dsDNA High Sensitivity Assay Kit (Thermo Fisher Scientific) and measured with the Qubit™ 3 Fluorometer.
Following DNA quantification and normalization, the library preparation step was performed using the QIAseq Custom DNA Human Hereditary Cancer Panel (Qiagen, Cat No. 333525). In this stage, DNA samples were first enzymatically fragmented. Unique molecular barcodes were attached to each DNA fragment to ensure accurate variant detection and high sensitivity. Target-specific regions of the genes of interest were then amplified, and non-specific fragments were removed. After a final amplification step, the library preparation was completed.
Library quantification was performed using the QIAseq Quant Assay Kit (Qiagen). All libraries were pooled to create a megapool and prepared for sequencing. Sequencing was performed using the MiSeq System (Illumina) according to the manufacturer’s recommendations.
Following sequencing, FASTQ files were processed using Qiagen Clinical Insight-Analyse Universal software. During this step, adaptor sequences were trimmed, low-quality reads were filtered out, and only high-quality reads were retained. For variant annotation, mutation detection, and clinical interpretation, the Qiagen Clinical Insight-Interpret software was used.
Data were analyzed using IBM Statistical Package for the Social Sciences Statistics version 26.0 (IBM Corp., Armonk, NY, United States). Descriptive statistics were reported as frequencies (n) and percentages for categorical variables and as median with interquartile range (IQR) for continuous variables, following assessment of normality assumptions. The Shapiro-Wilk test was applied to assess the distribution of continuous variables. Comparisons of non-normally dis
The study included 131 patients, comprising 70 men (53.4%) and 61 women (46.6%). Patients were aged between 26 years and 50 years, with a mean age of 42.7 years and a median age of 44 years (IQR: 10 years). During postoperative follow-up, two patients experienced early mortality within the first 30 days due to sepsis resulting from delayed perforation. The median follow-up period was 45 months (IQR: 44 months) A total of 33 patients died during follow-up. Synchronous CRC was detected in four patients. Pathological examination reported tumor differentiation in 130 patients, and po
| Descriptive and clinical features | |
| Age group, median (IQR) | 44 (10) |
| 21-30 | 5 (3.8) |
| 31-40 | 43 (32.8) |
| 41-50 | 83 (63.4) |
| Sex | |
| Male | 70 (53.4) |
| Female | 61 (46.6) |
| Survey (month), median (IQR) | 44.5 (44.7) |
| Tumor diameter (cm), median (IQR) | 5 (2.5) |
| Multiple colorectal tumor | |
| Negative | 127 (96.9) |
| Positive | 4 (3.1) |
| Right colon and transvers colon | |
| Negative | 81 (60.0) |
| Positive | 54 (40.0) |
| Left colon and sigmoid colon | |
| Negative | 90 (66.6) |
| Positive | 45 (33.3) |
| Rectosigmoid colon and rectum | |
| Negative | 99 (73.3) |
| Positive | 36 (26.6) |
| Differentiation degree | |
| Well and moderately | 106 (81.5) |
| Poor | 24 (18.5) |
| Adenocarcinoma component in the tumor | |
| Negative | 6 (4.6) |
| Positive | 125 (95.4) |
| Musinous component in the tumor | |
| Negative | 92 (70.2) |
| Positive | 39 (29.8) |
| Signet-ring cell carcinoma component in the tumor | |
| Negative | 122 (93.1) |
| Positive | 9 (6.9) |
| Lymphovascular invasion | |
| Negative | 73 (55.7) |
| Positive | 58 (44.3) |
| Perineural invasion | |
| Negative | 90 (68.7) |
| Positive | 41 (31.3) |
| Recurrens1 | |
| Negative | 103 (78.6) |
| Positive | 28 (21.4) |
| 2nd tumor | |
| Negative | 120 (91.6) |
| Positive | 11 (8.4) |
| Family history | |
| Negative | 44 (52.4) |
| Positive | 40 (47.6) |
| LN invasion | |
| Negative | 73 (56.5) |
| Positive | 58 (43.5) |
| T stage | |
| T1 | 1 (0.8) |
| T2 | 9 (6.9) |
| T3 | 81 (61.8) |
| T4a ve T4b | 40 (30.5) |
| N stage | |
| N0 | 64 (48.9) |
| N1a, N1b ve N1c | 40 (30.5) |
| N2a ve N2b | 27 (20.6) |
| Metastasis | |
| Negative | 99 (75.6) |
| Positive | 32 (24.4) |
| Stage | |
| 1 | 8 (6.1) |
| 2 | 48 (37.4) |
| 3 | 43 (32.8) |
| 4 | 32 (23.7) |
| Number of positive LNs, median (IQR) | 0 (3) |
| Number of LNs removed, median (IQR) | 20.5 (17) |
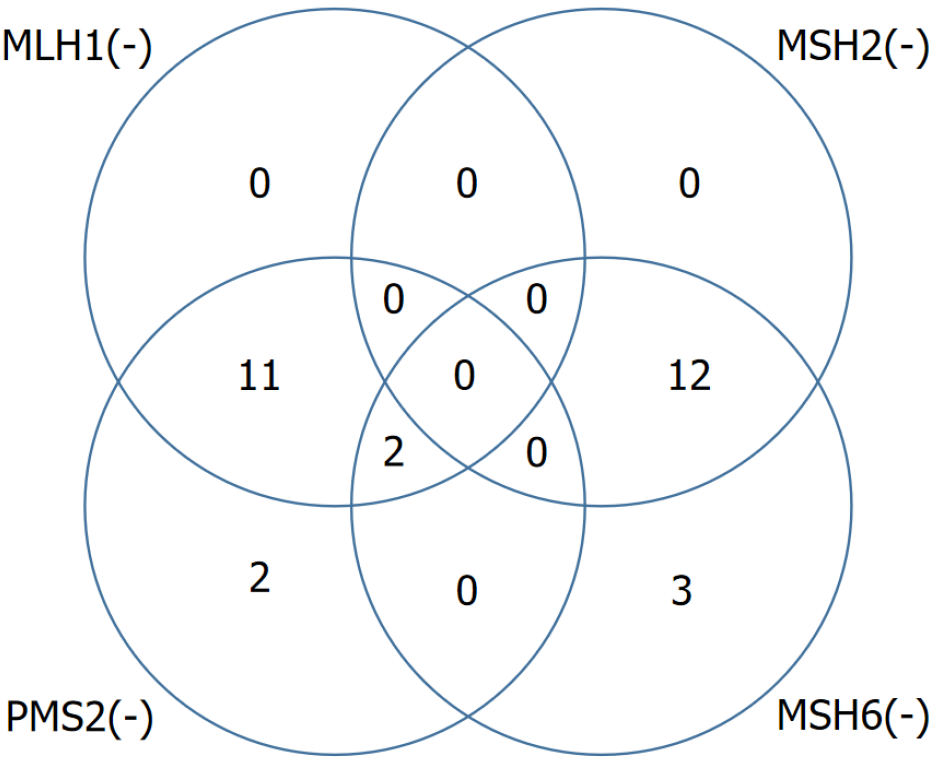
IHC staining data were available for 130 out of 131 patients whose tumor characteristics were evaluated. According to the IHC results, isolated loss of PMS2 expression was observed in 2 patients (1.5%), isolated loss of MSH6 in 3 patients (2.3%), concurrent loss of MLH1 and PMS2 in 11 patients (8.5%), concurrent loss of MSH2 and MSH6 in 12 patients (9.2%), and combined loss of MLH1, PMS2, and MSH6 in 2 patients (1.5%). Overall, 30 patients (22.9%) demonstrated loss of expression in at least one MMR protein (Figure 1).
The median age of the 30 patients in the MSI group (group 1) was 42 years (IQR: 7 years), whereas that of the 100 patients in the MSS group (group 2) was 46 years (IQR: 10 years). CRC occurred at a significantly younger age in the MSI group (P = 0.035). The proportion of men was 70% in group 1 compared to 48% in group 2, indicating that MSI was significantly more common among males (P = 0.034). The MSI group had a median follow-up period of 52 months (IQR: 41 months) with a mortality rate of 6.7%, whereas the MSS group had a median follow-up period of 38 months (IQR: 45 months) with a mortality rate of 31%. Survival was significantly longer in the MSI group (P = 0.020) (Table 2).
| Descriptive and clinical features | Microsatellite instabil | Microsatellite stabile | P value |
| Age group, median (IQR) | 42 (7) | 46 (10) | 0.0351 |
| 21-30 | 0 (0) | 5 (5) | |
| 31-40 | 14 (46.7) | 29 (29) | |
| 41-50 | 16 (53.3) | 66 (66) | |
| Sex | |||
| Male | 21 (70) | 48 (48) | 0.0342 |
| Female | 9 (30) | 52 (52) | |
| Survey (month), median (IQR) | 52 (42.5) | 38 (45) | 0.0201 |
| Right colon and transvers colon | |||
| Negative | 10 (31.2) | 71 (69.6) | < 0.0012 |
| Positive | 22 (68.7) | 31 (30.3) | |
| Rectosigmoid colon and rectum | |||
| Negative | 29 (90.6) | 69 (67.6) | 0.0112 |
| Positive | 3 (9.3) | 33 (29.4) | |
| Musinous component in the tumor | |||
| Negative | 15 (50) | 77 (77) | 0.0042 |
| Positive | 15 (50) | 23 (23) | |
| LN metastasis | |||
| Negative | 22 (73.3) | 51 (51) | 0.0312 |
| Positive | 8 (26.7) | 49 (49) | |
| Number of positive LNs, median (IQR) | 0 (1) | 0 (3) | 0.0311 |
| T stage | - | ||
| T1 | 0 (0) | 1 (1) | |
| T2 | 2 (6.7) | 7 (7) | |
| T3 | 21 (70) | 60 (60) | |
| T4a ve T4b | 7 (23.3) | 32 (32) | |
| N stage | - | ||
| N0 | 21 (70) | 42 (42) | |
| N1a, N1b ve N1c | 6 (20) | 34 (34) | |
| N2a ve N2b | 3 (10) | 24 (24) | |
| Metastasis | |||
| Negative | 28 (93.3) | 70 (70) | 0.0092 |
| Positive | 2 (6.7) | 30 (30) | |
| Stage | 0.0122 | ||
| 1 | 2 (6.7) | 6 (6) | |
| 2 | 18 (60) | 30 (30) | |
| 3 | 8 (26.7) | 35 (35) | |
| 4 | 2 (6.7) | 29 (29) | |
| 2nd tumor | |||
| Negative | 23 (76.7) | 96 (96) | 0.0033 |
| Positive | 7 (23.3) | 4 (4) | |
| Next-Generation Sequencing status | < 0.0013 | ||
| Mutasyon positive | 13 (76.5) | 1 (2.7) | |
| Mutasyon negative | 4 (23.5) | 35 (97.3) |
There were no significant differences between the two groups regarding the number of tumors, tumor size, or the presence of synchronous CRC, with both groups having two patients with synchronous CRC. The incidence of tumors in the right and transverse colon was significantly higher in group 1 (68.7%) than in group 2 (30.3%) (P < 0.001), whereas the incidence of tumors located in the rectosigmoid region and rectum was significantly higher in group 2 (29.4%) than in group 1 (9.3%) (P = 0.011) (Table 2).
Poorly differentiated tumors were more frequently observed in group 1 (30%) than in group 2 (15.2%); however, this difference did not reach statistical significance (P = 0.067). The presence of a mucinous component in tumors was significantly more common in group 1 (50%) than in group 2 (23%) (P = 0.004). There was no significant difference between the groups regarding the number of LNs removed during surgery (P = 0.207); however, LN metastasis was significantly less common in group 1 (26.7%) than in group 2 (49%) (P = 0.031) (Table 2).
Moreover, distant organ metastasis was observed significantly less frequently in group 1 (6.7%) than in group 2 (30%) (P = 0.009). When cancer stage at the time of surgery was compared, stage II disease was more common in group 1, whereas stage IV disease was more prevalent in group 2 (P = 0.012). No statistically significant differences were observed between the groups in terms of PNI, LVI, and recurrence rates. Extracolonic cancer was significantly more common in the MSI group (23.3%) than in the MSS group (4%) (P = 0.003). Family history did not differ significantly between the groups (Table 2).
Among the 53 patients who underwent NGS analysis following referral to the genetics clinic, MLH1 mutations in the absence of a BRAF mutation were detected in 2 patients, MSH2 mutations in 7 patients, MSH6 mutations in 2 patients, and a PMS2 mutation in 1 patient. Additionally, one patient exhibited MSH2 and MLH1 mutations, and another patient had MLH1 and PMS2 mutations. Totally, 14/53 patients (26.4%) who underwent NGS analysis received a definitive diagnosis of LS. Detailed information on patients with LS is presented in Table 3.
| Cases | Sex | Age | Location | Immunohistochemistry-mismatch repair | Next-Generation Sequencing | Ex-on | Mutation | Tumor | Family history |
| 1 | Female | 36 | 1 | MSH2/MSH6 | MSH2 | 13 | c.2074G > A p.P316 L | CRC | Multiple CRC and gastric cancer |
| 2 | Male | 43 | 1 | MLH1/PMS2 | MLH1 | 15 | c.1676T > G p.L559R | CRC | Multiple CRC |
| 3 | Female | 38 | 1 | MSH2/MSH6 | MSH2 | 12 | c.1861C > T p.Arg621Ter | CRC, EC | CRC and EC |
| 4 | Male | 46 | 1 ve 1 | MSH2/MSH6 | MSH2 | 12 | c.1799C > T p.A600V | Synchron CRC, urinary tract ca | Father with CRC, mother with EC |
| 5 | Male | 46 | 1 | 0 | MSH6 | 4 | c.2194C > T p.R732* | CRC | Father with CRC, Grandma and 3 aunt with breast cancer |
| 6 | Male | 42 | 1 | MSH2/MSH6 | MSH2 | 1 | c.70C > T p.Gln24Ter | CRC | 0 |
| 7 | Female | 45 | 2 | MSH2/MSH6 | MSH2, MLH1 | 12 1 | c.1861C > T p.Arg621Terc49A > C/p.Asn17His | CRC | Brother, sister and her doughter with CRC |
| 8 | Male | 39 | 1 | PMS2 | PMS2 | 3 | c.182delA p.Y61fs*15 | CRC | Uncle with CRC |
| 9 | Male | 40 | 2 | MLH1/PMS2 | MLH1 | Splice site | c.1990-1G > C | CRC | Father and brother with CRC sister with renal cancer |
| 10 | Male | 40 | 1 | MLH1/PMS2 | MLH1, PMS2 | İntron 17 exon 8 | c.1990-1G > C c1609G > A/p.Glu537 Lys | CRC | Father and brother with CRC sister with renal cancer |
| 11 | Male | 34 | 2 | MSH2/MSH6 | MSH2 | 5 | c.942G > A p.Q314Q | CRC | Father and uncle with CRC |
| 12 | Male | 44 | 2 | MSH2/MSH6 | MSH2 | 12 | C.1927G > T p.E643* | CRC | Father and uncle with gastric cancer |
| 13 | Male | 43 | 1 | MSH2/MSH6 | MSH6 | 4 | c.660A > C p.E220D | CRC, small intestine cancer | Father, uncle and aunt with CRC |
| 14 | Male | 37 | 1 | MSH2/MSH6 | MSH2 | 2 | c.352dupT, p.Y118fs*5 | CRC | 0 |
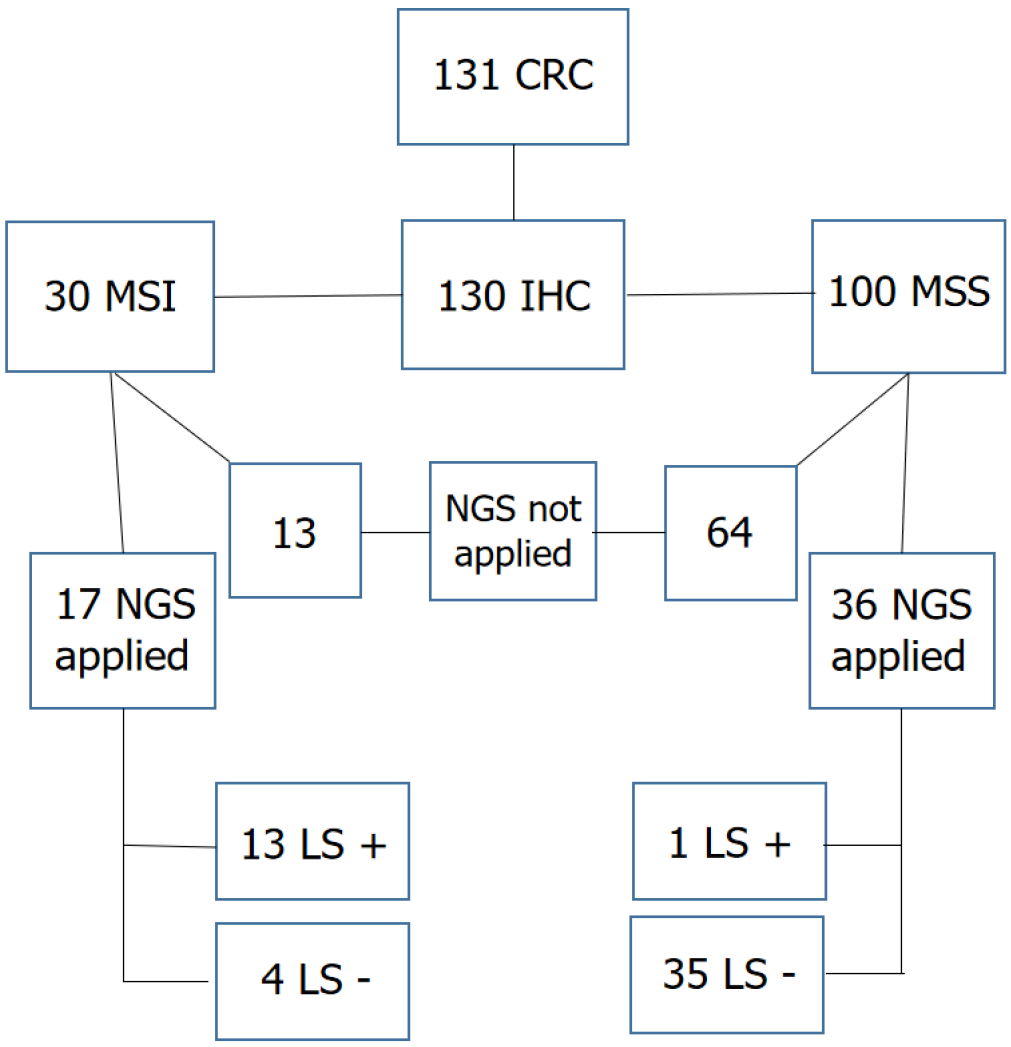
Genetic testing was performed in 17 of the 30 patients in group 1, with 13 receiving a definitive LS diagnosis. The sensitivity of IHC for the diagnosis of LS was 76.4% in the present study. In group 2, NGS analysis was performed in 36 of the 100 patients, and one patient received a definitive diagnosis of LS. The specificity of IHC was 97.2% (Figure 2).
Notably, 78 of the 131 patients (59.5%) included in the study did not receive genetic counseling. Additionally, familial adenomatous polyposis was diagnosed in 2 patients based on the genetic tests performed.
Globally, the incidence of CRC in individuals ≤ 50 years of age is steadily increasing[8,9]. These early-onset cases are considered more aggressive. Consequently, establishing early diagnosis and prevention strategies is of paramount importance[10]. Therefore, the present study focused on CRCs diagnosed in patients ≤ 50 years of age.
Tumors with MSI may present in two distinct ways: (1) As hereditary, early-onset cases; and (2) As sporadic cases in older adults. In sporadic MSI cases that occur among older adults, mostly due to promoter hypermethylation of the MLH1 gene[7]. Moreover, among the two patients in whom an isolated MLH1 mutation was detected via NGS, the BRAF gene mutation was absent. This finding is consistent with our CRC patient population of < 50 years of age, considering that sporadic MSI CRC cases generally occur in older adults.
Distinct clinicopathological features exist between tumors with MSI and MSS. Mucinous carcinoma and signet ring cell carcinoma types are more frequent in tumors with MSI[10,11]. In the present study, consistent with the literature, the rate of mucinous component presence was significantly higher in MSI cases; however, no significant difference was observed regarding the occurrence of signet ring cell tumors.
Moreover, MSI tumors are more commonly located in the proximal colon[12]. In the present study, while the overall incidence of proximal colon tumors was 40%, the rate was 68.7% in MSI cases. Although a proximal location might delay the onset of symptoms and diagnosis, survival was longer in the MSI group.
Tumors with MSI tend to have a more favorable prognosis than MSS[13]. Zumstein et al[12] demonstrated that, owing to fewer adverse histological features, MSI tumors were frequently diagnosed at an earlier stage. Although the rates of LVI and PNI were lower in the MSI group in the present study, albeit the difference was not statistically significant, the rate of LN metastasis was significantly lower. The literature suggests that metastasis is rare in cases with MSI and CRC[14]. However, the impact of MSI on overall survival in metastatic CRC remains unclear[14]. In the present study, the incidence of distant organ metastasis was significantly lower in the MSI group. Therefore, it is evident that MSI tumors are diagnosed at a significantly earlier stage than MSS tumors. Given their favorable prognostic features and the strong possibility of early-stage diagnosis, it is imperative that patients with these tumors be enrolled in appropriate screening and surveillance programs for early detection.
Although the underlying causes of multiple primary cancers remain largely unclear, MSI can lead to the development of multiple primary malignancies[15]. In the present study, when the incidence of noncolorectal cancers was compared, it was found that they were significantly more common in the MSI group. This highlights the importance of MSI determination and surveillance.
In the general population, the prevalence of LS is approximately 3%-5%. However, this increases to 10% in individuals < 50 years of age and up to 23% in those < 35 years of age[13]. In our cohort, the prevalence was 26.4%, with 14 out of 53 patients exhibiting this finding. Identifying LS in CRC patients enables more rigorous follow-up programs, facilitating the early detection of synchronous or metachronous tumors and other extracolonic malignancies[4]. Moreover, a diagnosis of LS in an index patient can lead to the early diagnosis of LS-associated CRC and other malignancies in at-risk relatives[16]. Conversely, excluding LS may obviate the need for close follow-up in these individuals[17].
The definitive diagnosis of LS is established by genetic testing. However, the criteria for selecting patients for these costly tests remain unclear. Hampel et al[18] reported that among 23 patients with confirmed LS mutations, 5 did not meet the Amsterdam or Bethesda criteria. In the present study, more than half (52%) of the patients for whom family history data were available had a negative family history. Notably, among the 14 patients diagnosed with LS via genetic testing, two had no family history of cancer and did not meet the Amsterdam or Bethesda criteria. Consequently, several studies in the literature strongly advocate for universal LS screening in all patients with CRC, irrespective of clinicopathological features or family history[12,19]. Therefore, we believe that a positive family history is not a prerequisite for LS screening in a CRC patient. However, once LS is diagnosed, all family members should be enrolled in an LS screening program.
Since 2014, the National Comprehensive Cancer Network has recommended universal MSI testing for colorectal carcinomas[20]. IHC, which is widely used in histopathological evaluations, is widely utilized in the screening of LS cases, too. Several studies have indicated that IHC is nearly equivalent to genetic testing in determining MSI status[20,21]. In the present study, LS was detected in 13/17 patients with MSI using IHC (with 76.4% sensitivity), whereas it was detected in only one patient among the 36 patients with MSS using IHC (with 97.2% specificity). These findings suggest that while IHC is highly effective in identifying patients who should undergo genetic testing for LS, it may lack sufficient diagnostic accuracy when used as a standalone diagnostic tool. Our study identified a CRC patient with MSS status by IHC but confirmed LS diagnosis through NGS (MSH6 mutation), who had a positive family history of CRC. Based on these observations, when combining both IHC-detected MSI and family history as screening criteria for LS, the specificity reached 100% in our cohort.
Hechtman et al[22] reported that approximately 6% of patients identified as having a high risk of MSI by genetic testing exhibited intact MMR protein expression on IHC staining. They attributed this finding to missense mutations, which result in non-functional but antigenically preserved MMR proteins. Similarly, in our series, among 14 patients diagnosed with LS through NGS, 1 patient (7.1%) showed intact MMR protein expression on IHC. This finding emphasizes that patients with clinical suspicion of LS should undergo genetic testing even if IHC results appear intact.
In the study by Keshinro et al[23], which included all age groups in the general population, IHC staining analysis revealed concurrent loss of MLH1 and PMS2 in 79% of tumors, concurrent loss of MSH2 and MSH6 in 10%, isolated loss of MSH6 in 3%, and isolated loss of PMS2 in 2%. In contrast, in our study focusing on patients with MMR-deficient tumors based on IHC staining, concurrent loss of MLH1 and PMS2 was observed in 36.6%, concurrent loss of MSH2 and MSH6 in 40%, isolated loss of MSH6 in 9.9%, and isolated loss of PMS2 in 6.6%. The frequency of concurrent MSH2 and MSH6 Loss, associated with a high risk of LS, was four times higher, whereas the rate of MLH1 and PMS2 Loss, typically linked to a lower LS risk, was less than half compared to Keshinro et al's findings[23]. These results are consistent with the fact that our study included patients under 50 years of age, supporting the known higher prevalence of LS in EOCRC.
The literature has also noted low rates of genetic testing in EOCRC cases[12,24]. Jones et al[24] found that, among 98 EOCRC patients, only 34 underwent routine genetic testing. In the present study, only 53/131 EOCRC patients received routine genetic counseling. This finding highlights the need for improved referral practices for genetic testing.
It should be noted that the present study is limited by its retrospective, single-center design and relatively small sample size. In the future, we would be interested in collaborating on a multicenter study with researchers working on similar topics in our country to strengthen the generalizability and statistical power of the findings.
Although IHC effectively identifies patients who should undergo genetic testing for LS, it may be insufficient as an individual parameter. Therefore, clinicians must be well acquainted with the clinical features of LS-associated tumors, recognize the limitations of the Amsterdam and Bethesda criteria, and ensure that suspected patients are promptly referred for genetic testing. Particularly in cases of EOCRC, an underlying genetic pathology should be considered. Notably, early diagnosis can facilitate the detection of associated conditions and the enrollment of at-risk relatives in surveillance programs. Given that a large proportion of our patients (59.5%) did not receive genetic counseling and were lost to follow-up, it is imperative to establish dedicated cancer follow-up units in hospitals. These patients should receive appropriate genetic counseling and be included in relevant surveillance programs.
Izmir Katip Celebi University Faculty of Medicine, Izmir Ataturk Training and Research Hospital, kindly supported this work.
| 1. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3360] [Article Influence: 560.0] [Reference Citation Analysis (2)] |
| 2. | Strum WB, Boland CR. Clinical and Genetic Characteristics of Colorectal Cancer in Persons under 50 Years of Age: A Review. Dig Dis Sci. 2019;64:3059-3065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Perrod G, Rahmi G, Cellier C. Colorectal cancer screening in Lynch syndrome: Indication, techniques and future perspectives. Dig Endosc. 2021;33:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Anyla M, Lefevre JH, Creavin B, Colas C, Svrcek M, Lascols O, Debove C, Chafai N, Tiret E, Parc Y. Metachronous colorectal cancer risk in Lynch syndrome patients-should the endoscopic surveillance be more intensive? Int J Colorectal Dis. 2018;33:703-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Maratt JK, Stoffel E. Identification of Lynch Syndrome. Gastrointest Endosc Clin N Am. 2022;32:45-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Rivero-Sánchez L, Gavric A, Herrero J, Remedios D, Alvarez V, Albéniz E, Gordillo J, Puig I, López-Vicente J, Huerta A, López-Cerón M, Salces I, Peñas B, Parejo S, Rodriguez E, Herraiz M, Carretero C, Gimeno-Garcia AZ, Saperas E, Alvarez C, Arnau-Collell C, Ortiz O, Sánchez A, Jung G, Balaguer F, Pellisé M. The "diagnose and leave in" strategy for diminutive rectosigmoid polyps in Lynch syndrome: a post hoc analysis from a randomized controlled trial. Endoscopy. 2022;54:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Eikenboom EL, van der Werf-'t Lam AS, Rodríguez-Girondo M, Van Asperen CJ, Dinjens WNM, Hofstra RMW, Van Leerdam ME, Morreau H, Spaander MCW, Wagner A, Nielsen M. Universal Immunohistochemistry for Lynch Syndrome: A Systematic Review and Meta-analysis of 58,580 Colorectal Carcinomas. Clin Gastroenterol Hepatol. 2022;20:e496-e507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022;7:262-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 515] [Article Influence: 128.8] [Reference Citation Analysis (7)] |
| 9. | Akimoto N, Ugai T, Zhong R, Hamada T, Fujiyoshi K, Giannakis M, Wu K, Cao Y, Ng K, Ogino S. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol. 2021;18:230-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 482] [Article Influence: 96.4] [Reference Citation Analysis (0)] |
| 10. | Burnett-Hartman AN, Lee JK, Demb J, Gupta S. An Update on the Epidemiology, Molecular Characterization, Diagnosis, and Screening Strategies for Early-Onset Colorectal Cancer. Gastroenterology. 2021;160:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (1)] |
| 11. | Luo C, Cen S, Ding G, Wu W. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond). 2019;39:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 212] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 12. | Zumstein V, Vinzens F, Zettl A, Heinimann K, Koeberle D, von Flüe M, Bolli M. Systematic immunohistochemical screening for Lynch syndrome in colorectal cancer: a single centre experience of 486 patients. Swiss Med Wkly. 2016;146:w14315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Stoffel EM, Murphy CC. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology. 2020;158:341-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 431] [Article Influence: 71.8] [Reference Citation Analysis (10)] |
| 14. | Goldstein J, Tran B, Ensor J, Gibbs P, Wong HL, Wong SF, Vilar E, Tie J, Broaddus R, Kopetz S, Desai J, Overman MJ. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H). Ann Oncol. 2014;25:1032-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 15. | Kim HS, Cho NB, Yoo JH, Shin KH, Park JG, Kim YI, Kim WH. Microsatellite instability in double primary cancers of the colorectum and stomach. Mod Pathol. 2001;14:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Stoffel E, Mukherjee B, Raymond VM, Tayob N, Kastrinos F, Sparr J, Wang F, Bandipalliam P, Syngal S, Gruber SB. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621-1627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 270] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 17. | Vasen HF, Abdirahman M, Brohet R, Langers AM, Kleibeuker JH, van Kouwen M, Koornstra JJ, Boot H, Cats A, Dekker E, Sanduleanu S, Poley JW, Hardwick JC, de Vos Tot Nederveen Cappel WH, van der Meulen-de Jong AE, Tan TG, Jacobs MA, Mohamed FL, de Boer SY, van de Meeberg PC, Verhulst ML, Salemans JM, van Bentem N, Westerveld BD, Vecht J, Nagengast FM. One to 2-year surveillance intervals reduce risk of colorectal cancer in families with Lynch syndrome. Gastroenterology. 2010;138:2300-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 18. | Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Nakagawa H, Sotamaa K, Prior TW, Westman J, Panescu J, Fix D, Lockman J, Comeras I, de la Chapelle A. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med. 2005;352:1851-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 1035] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 19. | Moreira L, Balaguer F, Lindor N, de la Chapelle A, Hampel H, Aaltonen LA, Hopper JL, Le Marchand L, Gallinger S, Newcomb PA, Haile R, Thibodeau SN, Gunawardena S, Jenkins MA, Buchanan DD, Potter JD, Baron JA, Ahnen DJ, Moreno V, Andreu M, Ponz de Leon M, Rustgi AK, Castells A; EPICOLON Consortium. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308:1555-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 410] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 20. | Cheah PL, Li J, Looi LM, Koh CC, Lau TP, Chang SW, Teoh KH, Mun KS, Nazarina AR. Screening for microsatellite instability in colorectal carcinoma: Practical utility of immunohistochemistry and PCR with fragment analysis in a diagnostic histopathology setting. Malays J Pathol. 2019;41:91-100. [PubMed] |
| 21. | Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn. 2008;10:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 501] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 22. | Hechtman JF, Rana S, Middha S, Stadler ZK, Latham A, Benayed R, Soslow R, Ladanyi M, Yaeger R, Zehir A, Shia J. Retained mismatch repair protein expression occurs in approximately 6% of microsatellite instability-high cancers and is associated with missense mutations in mismatch repair genes. Mod Pathol. 2020;33:871-879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 23. | Keshinro A, Ganesh K, Vanderbilt C, Firat C, Kim JK, Chen CT, Yaeger R, Segal NH, Gonen M, Shia J, Stadler ZK, Weiser MR. Characteristics of Mismatch Repair-Deficient Colon Cancer in Relation to Mismatch Repair Protein Loss, Hypermethylation Silencing, and Constitutional and Biallelic Somatic Mismatch Repair Gene Pathogenic Variants. Dis Colon Rectum. 2023;66:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Jones HG, Radwan R, Davies M, Evans M, Khot U, Chandrasekaran TV, Williams N, Murray A, Jones W, Harris D, Beynon J. Clinicopathological characteristics of colorectal cancer presenting under the age of 50. Int J Colorectal Dis. 2015;30:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/