Published online Sep 24, 2025. doi: 10.5306/wjco.v16.i9.109034
Revised: May 26, 2025
Accepted: August 8, 2025
Published online: September 24, 2025
Processing time: 148 Days and 9.9 Hours
Peritoneal metastases (PM) represent the most frequent and lethal form of dissemination in advanced gastric cancer (GC), with limited efficacy of systemic che
To evaluate the role and outcomes of HIPEC in advanced and high-risk GC th
A systematic review of prospective randomized and controlled clinical trials (2010-2024) was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews guidelines. Studies were selected from PubMed, Cochrane, Scopus, and ClinicalTrials.gov. No geographical restrictions were applied in the search process. Eligible studies included patients with advanced GC (T3+, positive peritoneal cytology/PM) receiving HIPEC in either therapeutic or prophylactic settings. Exclusion criteria included retrospective studies, single-arm trials, and those lacking survival outcomes. Risk of bias was assessed using Risk of Bias 2.0 and Risk of Bias in Non-Randomized Studies of Interventions tools; sensitivity and heterogeneity analyses were also conducted.
Thirteen prospective studies (eight therapeutic, five prophylactic) were included. In therapeutic settings, CRS combined with HIPEC yielded a median OS of 11-24.9 months vs 4-6 months with systemic therapy alone. Co
HIPEC may improve survival and reduce recurrence in selected GC patients, particularly those with low peritoneal burden and CC-0 resection. Further standardization and prospective trials are needed.
Core Tip: This systematic review evaluates prospective trials assessing hyperthermic intraperitoneal chemotherapy (HIPEC) in gastric cancer (GC). Therapeutically, HIPEC combined with cytoreductive surgery improves survival in selected patients with peritoneal metastases. Prophylactically, HIPEC reduces peritoneal recurrence after curative gastrectomy in high-risk patients. While promising, outcomes are influenced by heterogeneity in protocols, chemotherapy regimens, and selection criteria. Completeness of cytoreduction remains the strongest predictor of benefit. Future trials should standardize patient selection and HIPEC approaches to clarify its role within multimodal GC treatment strategies.
- Citation: D'Acapito F, Framarini M, Morgagni P, Di Pietrantonio D, Vittimberga G, Zucchini V, Ercolani G. Advancing gastric cancer treatment: A comprehensive review of hyperthermic intraperitoneal chemotherapy’s role and outcomes. World J Clin Oncol 2025; 16(9): 109034
- URL: https://www.wjgnet.com/2218-4333/full/v16/i9/109034.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i9.109034
Gastric cancer (GC) is a biologically heterogeneous for lymphatic, hematogenous, and intra-abdominal spread. As the fourth leading cause of cancer-related mortality worldwide, it continues to pose a significant clinical burden[1]. Peritoneal metastases (PM) occur in up to 40% of GC patients and are associated with poor outcomes, with median overall survival (OS) ranging from 2 months to 9 months[2]. At initial diagnosis, PM is present in approximately 30%[3] of patients, and its incidence at recurrence ranges from 15% to 52%[4]. In a 2022 study, Solaini et al[5] identified cM-positive status, intestinal-type histology, cT4 tumors, and lower third of the stomach were significantly associated with PM and/or positive peritoneal cytology (CY+). Importantly, nearly 60% of GC-related deaths are attributable to PM[6].
Systemic chemotherapy remains the standard of care for patients with advanced GC patients with PM. According to both Japanese and American Joint Committee on Cancer guidelines, the presence of tumor CY+ is classified as metastatic disease and managed accordingly[7,8]. In contrast, the European Society for Medical Oncology (ESMO) guidelines do not provide specific recommendations for staging or treatment in CY+ cases[9].
Despite progress in immunotherapy and targeted treatments, palliative chemotherapy and best supportive care remain the primary strategies for stage IV GC, as recommended by both the National Comprehensive Cancer Network and ESMO[10]. A critical limitation of systemic therapy in this setting is its reduced efficacy against PM due to physiological barriers that impede drug penetration into the peritoneal cavity[11]. Nevertheless, radical surgical resection may be considered in highly selected patients with limited PM.
More than three decades ago, Sugarbaker et al[12] introduced an innovative strategy combining gastrectomy, cytoreductive surgery (CRS), and hyperthermic intraperitoneal chemotherapy (HIPEC) to improve outcomes in patients with PM. HIPEC combines the concept of direct delivery of the chemotherapeutic agent to the peritoneum, enabling the application of higher local doses with low systemic toxicity and the enhancement of its cytotoxic effects using hy
Studies such as CYTO-CHIP have documented 5-year OS of 26.21% and median OS of 18.8 months with 3-year and 5-year disease-free survival (DFS) of 20.4% and 17.05%, respectively[14]. CRS combined with HIPEC (CRS-H) is a te
This systematic review was designed to evaluate whether the encouraging outcomes reported in retrospective studies for both therapeutic and prophylactic HIPEC-particularly in terms of OS and DFS/relapse-free survival (RFS)-have been confirmed by prospective randomized or controlled clinical trials published over the last 15 years.
A secondary aim was to assess whether universally accepted criteria for selecting candidates for HIPEC have emerged in either setting, and to investigate persisting divergences in the design of systemic chemotherapy and HIPEC protocols across studies.
Unlike prior meta-analyses, including the 2021 Cochrane review that concluded evidence was insufficient to support routine HIPEC use in GC, this study focuses exclusively on prospective evidence to address the current gaps in knowledge.
This study aims to systematically review RCTs and controlled trials (CTs) published since 2010 to evaluate the role and clinical impact of HIPEC in the management of advanced GC. We have excluded data on pressurized intraperitoneal aerosol chemotherapy (PIPAC), as it is currently regarded as a palliative measure. According to a recent meta-analysis by Di Giorgio et al[21], most PIPAC studies focus on feasibility and safety, frequently involving patients previously treated with CRS and HIPEC.
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews guidelines. A systematic literature search was performed in PubMed, Cochrane Library, Scopus, and ClinicalTrials.gov between November 2024 and January 2025, without language re
No geographical restrictions were applied in the search process, and studies were included irrespective of their region of origin, allowing for a comprehensive comparison between Asian and Western clinical practices.
Eligible studies included both randomised and non-randomised prospective clinical trials evaluating the treatment outcomes of using HIPEC in neo-adjuvant, adjuvant or prophylactic setting in patients with advanced GC (T ≥ 3 or nodal positive disease) or metastatic disease (CY+ or macroscopic PM) associated or not with CRS. The following MeSH terms and their variations were adapted for each database using advanced search tools: "Stomach Neoplasms/therapy", "Peritoneal Neoplasms/therapy", "Hyperthermic Intraperitoneal Chemotherapy", "Combined Modality Therapy", and "Humans". There were no restrictions on the time interval between GC diagnosis and surgical intervention, allowing for the inclusion of studies assessing both adjuvant and prophylactic HIPEC strategies. Articles without full-text access or published prior to 2010 were excluded, given the substantial improvements in study methodologies resulting from recent advances in diagnosing and treating stage IV GC. For duplicate publications, only the most recent and comprehensive version was considered. The exclusion criteria were: (1) Single-arm studies; (2) Retrospective studies; (3) Case reports; (4) Editorials; (5) Reviews; (6) Studies lacking survival outcomes (OS or DFS/RFS); and (7) Those not reporting HIPEC-specific protocols.
Study quality and risk of bias were assessed using validated tools. For RCTs, the Cochrane Risk of Bias 2.0 tool (ROB 2.0) was used[22]. For non-randomized prospective studies, the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tool was applied[23]. Sensitivity analysis was performed by excluding individual trial based on sample size, population, and effect size, following scenario-based recommendations from the Cochrane Handbook[24]. It was not our intention to conduct a meta-analysis or any other form of comparative statistical evaluation of outcomes in either treatment setting.
A data charting form was independently developed by one reviewer to determine the variables to be extracted. This form was then discussed within the review team and continuously refined through an iterative process. The extracted data included study characteristics (e.g., authors, year of publication, journal), HIPEC protocol, neoadjuvant or adjuvant chemotherapy regimen if administered, OS (mean and at 1 year, 2 years, 3 years, and 5 years), RFS (mean and at 1 year, 2 years, 3 years, and 5 years), risk of bias, and study limitations.
Peritoneal cancer index (PCI) is a clinical integration of both peritoneal implant size and distribution of nodules on the peritoneal surface, and should be used in the decision of making process as the abdomen is completely explored. The lesion size 0-3 (nodule diameter) and the involvement of abdomino-pelvic regions (0-12) are combined[25].
CRS consists of the set of techniques required to complete six different peritonectomy procedures used to resect cancer on the visceral intra-abdominal surface or to remove cancer from the parietal peritoneal surface. One or all six of these procedures may required, depending on the distribution and volume of peritoneal carcinomatosis (PC)[26].
Completeness of cytoreduction (CC-0) score measures the largest residual tumor nodule at the completion of cancer resection. CC-0 defines no residual peritoneal lesions within the operative field, CC-1 refers to persisting nodules < 2.5 cm after CRS, CC-2 indicates nodules between 2.5 cm and 5 cm, and CC-3 refers to nodules > 5 cm or confluent unresectable tumor nodules[25].
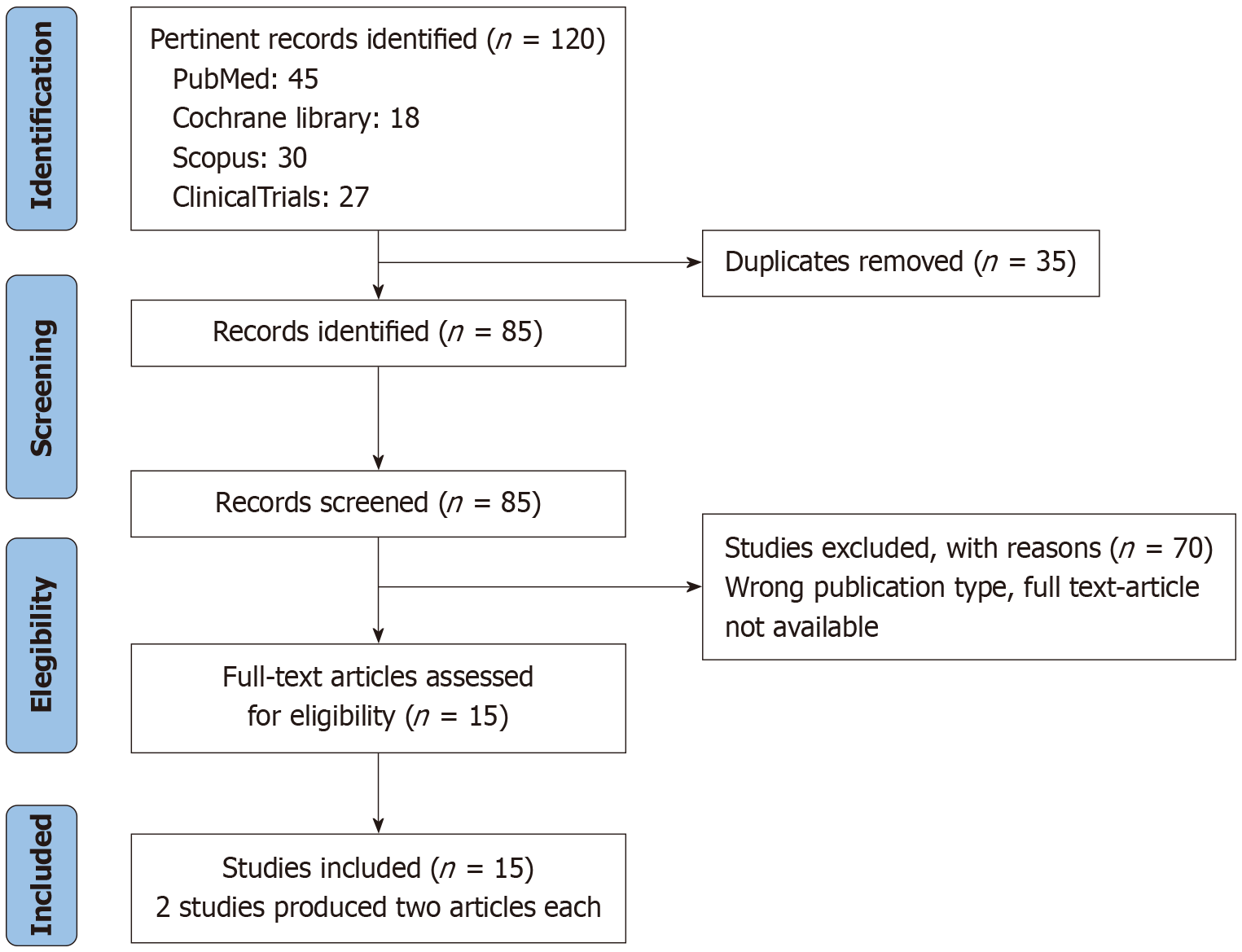
A total of 85 studies were identified after duplicate removal. Initial screening based on titles and abstracts was conducted by six reviewers, leading to the selection of 15 articles for full-text assessment. The 15 articles were derived from 13 studies, as 2 studies produced two articles each. Inclusion was determined based on predefined selection criteria, and any disagreements were resolved through discussion until a consensus was reached (Figure 1).
RCTs and CTs have investigated the use of HIPEC in two main settings. The former examines the therapeutic role of HIPEC in stage IV GC, defined by either CY+ or macroscopic PC. In this context, we analyzed three RCTs[27-29] and six CTs[30-35].
The latter explores the prophylactic use of HIPEC in stage III GC. Here, we reviewed five RCTs[36-40]. Notably, the included studies have explored alternative HIPEC approaches beyond the standard single intraoperative procedure following cytoreduction. HIPEC has also been administered using laparoscopic and bedside approaches and delivered in multiple rounds in both the adjuvant (after CRS) and neoadjuvant (before CRS) settings.
Eight prospective studies have investigated the therapeutic role of HIPEC in patients with GC and PM. However, these studies exhibit substantial heterogeneity in terms of inclusion criteria, disease burden (Tables 1 and 2), systemic chemotherapy protocols, and HIPEC treatment regimens (Tables 3 and 4)[27,30,31,34,35].
| Year | Ref./trial | Arms | Patients cohort | Oncologic exclusion criteria | CY+ | PCI | Ascites | Liver mets | Lung mets | Syncronous/metachronous (PM/CY+) |
| 2011 | Yang et al[27] | Arm 1: CRS-A (n = 34). Arm 2: CRS-H (n = 34) | 68 | Any lung metastasis, liver metastasis, prominent retroperitoneal lymph node metastasis | ND | CRS-H arm: 2-36 (median: 15). CRS-A arm: 3-23 (median: 15) | ND | No | No | Synchronous [PM (n = 51)], metachronous [PM (n = 17)] |
| 2014 | Rudloff/GYMSSA | Arm 1: FOLFOXIRI alone arm-S. Cht (n = 8). Arm 2: GYMS arm— CRS-H + S. Cht (n = 9) | 17 | Disease sites other than either peritoneum, lung or liver. Evidence of extensive para-aortic/retro-pancreatic lymph node metastases. Significant ascites | Yes | GYMS arm: PCI ≤ 20, n = 8/9 (89%); PCI = 21, n = 1/9 (11%) | No | Yes | Yes | Synchronous [PM (n = 14); CY+ (n = 1)], metachronous [PM (n = 2)] |
| 2023 | Rau/GASTRIPEC-I | Arm 1: CRS-A (n = 53). Arm 2: CRS-H (n = 52) | 105 | Further distant metastases except Krukenberg tumors. Pretreated with chemotherapy/radiotherapy | No | PCI ≥ 7 (44%) | Yes (40%) | No | No | Synchronous [PM (n = 105)] |
| Year | Ref./trial | Controlled trial phase | Patient cohort | Positive peritoneal cytology | PCI | Median PCI | Ascites | N stage | Syncronous metastasis only |
| 2020 | Blum Murphy et al[31] | 1 | 27 | Yes | < 8 (52%), 8-14 (15%), 15-21 (11%), > 21 (22%) | 6 | Admitted (41%) | ND | Yes |
| 2020 | Yu et al[30] | 2 | 38 | Yes | ≤ 20 (100%) | 8.8 | Admitted | 72.2% ypN+ (convertion to surgery group) | Yes |
| 2020 | PERISCOPE 1 | 1 and 2 | 25 | Yes | < 7 (100%) | 2 | ND | 68% ypN+ | Yes1 |
| 2021 | Badgwell et al[35] | 2 | 20 | Yes | 0 (40%), 1-2 (35%), > 3 (25%) | ND | ND | 70% ypN+ | Yes |
| 2023 | Green et al[34] | 2 | 41 | Yes | ≤ 10 (100%) | 2 | Excluded if > 500 cc | 68% ypN+ | Yes |
| Year | Ref./trial | Hyperthermic intraperitoneal chemotherapy protocol | Completeness of cytoreduction score (CC 0-1) | Neodjuvant Cht | Neoadjuvant treatment regimen | Adjuvant Cht | Adjuvant treatment regimen | Median OS (month) | 1 year OS | 2 years OS | 3 years OS | Median relapse-free survival (month) |
| 2011 | Yang et al[27] | Cisplatin 120 mg + mitomycin C 30 mg for 60-90 minutes | CRS-A: 58.8% (20 of 34), CRS-H: 58.8% (20 of 34) | No | No | Yes | ND | CRS-A: 6.5 months vs CRS-H: 11.0 months | CRS-A: 29.4% vs CRS-H: 41.2% | CRS-A: 5.9% vs CRS-H: 14.7% | CRS-A: 0% vs CRS-H: 5.9% | ND |
| 2014 | Rudloff/GYMSSA | Oxaliplatin 460 mg/m2 for 30 minutes. Prior to perfusion: 5-fluorouracil 400 mg/m2 IV and leucovorin 20 mg/m2 IV | CRS-H: 88.8% (8 of 9) | Yes. GYMS arm (n = 6/9), SA arm (n = 7/8) | ND | Yes | FOLFOXIRI | SA arm: 4.3 months vs GYMS arm: 11.3 months | SA arm: 0% vs GYMS arm: 44% | SA arm: 0% vs GYMS arm: 22% | SA arm: 0% vs GYMS arm: 22% | ND |
| 2023 | Rau/GASTRIPEC-I | Mitomycin C 15 mg/m2 + cisplatin 75 mg/m2 for 60 minutes | CRS-A: 41.5% (22 of 53), CRS-H: 53.8% (28 of 52) | Yes (all) | HER-2+: Cisplatin, capecitabine, trastuzumab; HER-2-: Epirubicin, oxaliplatin, capecitabine | Yes | HER-2+: Cisplatin, capecitabine, trastuzumab; HER-2-: Epirubicin, oxaliplatin, capecitabine or fluorouracil, leucovorin, oxaliplatin, and docetaxel | CRS-A: 14.9 months vs CRS-H: 14.9 months | CRS-A: 60.5% vs CRS-H: 58.2% | CRS-A: 15.4% vs CRS-H: 25.5% | CRS-A: 0% vs CRS-H: 13.6% | CRS-A: 3.5 months vs CRS-H: 7.1 months |
| Year | Ref./trail | Patient cohort | S. Cht protocol | S. Cht cycles | N-HIPEC | Laparoscopic N-HIPEC | N-HIPEC protocol | N-HIPEC cycles | Convertion to surgery | T-HIPEC protocol | NIPEC | Adjuvant HIPEC bed site | OS media | 1 year OS | 2 years OS | 3 years OS | Relapse-free survival media (month) |
| 2020 | Blum Murphy et al[31] | 27 | 5-FU/OX (n = 24%-89%), 5-FU/OX/Pacli (n = 3%-11%) | 9 (range 4-24) | Yes | 27 patients | Mitomycin C (30 mg) + cisplatin (200 mg). Paclitaxel (dosing was started at 20 mg/m2 and sequentially increased up to 60 mg/m2) | 1 | CRS-H (6/27), CRS-A (1/27) 25.9% | ND | No | No | ND | 73.9% | 58.1% | ND | ND |
| 2020 | Yu et al[30] | 38 | Pacli + S1 (Transtuzumab added in HER-2+) | 4 | Yes | No | Paclitaxel (75 mg/m2) for 60 minutes | 2 | 18 (47.4%) | Paclitaxel (75 mg/m2) for 60 minutes | No | Yes (twice within 72 hours after CRS) | Conversion therapy group 21.1 months | 63.2% | 47.40% | ND | ND |
| 2020 | Koemans/PERISCOPE 1 | 25 | Epirubicin + cisplatin + capecitabin (n = 10), docetaxel + oxaliplatin + capecitabin (n = 7), capecitabin + oxaliplatin (n = 3), 5-FU/OX (n = 2), capecitabin + oxaliplatin (n = 3), Transtuzumab added in HER-2+ | 3 | No | No | No | No | No | Oxaliplatin (460 mg/sm) for 30 minutes | Docetaxel for 90 minutes in a dose-level escalation scheme (0, 50, 75 mg/m2) | No | 15 months | ND | ND | ND | 12 months |
| 2021 | Badgwell et al[35] | 20 | 5-FU+ OX (n = 17/20), Triplet therapy (n = 3/20) | < 8 (20%), 8-10 (75%), > 10 (5%) | Yes | 20 patients | Mitomycin C (30 mg) + cisplatin (200 mg) for 60 minutes | 2 (n = 5/20), 1 (n = 15/20) | No | Mitomycin C (30 mg) + cisplatin (200 mg) for 60 minutes | No | No | 22.1 months | 90% | 50% | 28% | ND |
| 2023 | Green et al[34] | 41 | Fluoruracil, leucovorin, oxaliplatin (56%), fluorouracil, leucovorin, oxaliplatin, and docetaxel (24%), radiotherapy (17%) | ND | Yes | 23 patients | Cisplatin (90 mg/mq) + mitomycin C (10 mg/mq or 30 mg/mq) for 60 minutes | 1 or 2 | No | Cisplatin (90 mg/mq) + mitomycin C (10 mg/mq) for 60 minutes | No | No | 24.9 months | ND | ND | 25% | 7.4 months |
Inclusion criteria varied considerably. For example, the GYMSSA trial enrolled patients with extra-PM, including hepatic and pulmonary lesions, whereas the GASTRIPEC-I trial restricted inclusion to patients with synchronous exclusive PM, low disease burden, following neoadjuvant chemotherapy[28,29]. Notably, two studies (Yu et al[30] and Blum Murphy et al[31]) included patients initially considered unresectable who subsequently underwent neoadjuvant intraperitoneal therapy with the goal of conversion to surgery. Notably, conversion rates ranged from 25.9% (Blum Murphy et al[31]) to 47.4% (Liu et al[39]), underscoring the potential of intraperitoneal therapy not only as a therapeutic modality but also as a tool for surgical patient selection.
Most trials did not impose an upper limit on the PCI, resulting in significant variability in baseline tumor burden. Systemic chemotherapy protocols differ widely, incorporating regimens such as FOLFOXIRI, paclitaxel, and various platinum-based combinations, therapies administered at different phases of treatment. Similarly, HIPEC protocols varied in intraperitoneal agent selection (e.g., Mitomycin C, cisplatin, oxaliplatin, paclitaxel), dosing, and timing. At least one study (PERISCOPE-I) combined CRS and HIPEC with normothermic intraperitoneal chemotherapy (NIPEC) using docetaxel, while others employed laparoscopic HIPEC as a neoadjuvant intervention[32,33].
This lack of standardization complicates the interpretation of oncologic endpoints such as OS and DFS, both of which are closely influenced by initial disease burden and the CC-0. Among patients undergoing CRS-H in RCTs, CC-0/1 was achieved in 67.3% of cases.
Improved survival was strongly associated with CC-0 and receipt of intraperitoneal chemotherapy, with median OS ranging from 11 months to 24.9 months in CRS-H cohorts, compared to 4-6 months in patients treated with surgery alone or systemic therapy only. Similarly, DFS outcomes were significantly improved in HIPEC arms, as demonstrated in GASTRIPEC-I (7.1 months vs 3.5 months; P = 0.0472) and PERISCOPE-I (median DFS: 12 months)[29,33].
In RCTs the heterogeneity assessment revealed moderate clinical variability, particularly in patient selection criteria and systemic chemotherapy regimens[27-29]. A qualitative assessment of heterogeneity was performed across the six included CT, focusing on study design, patient selection, intervention protocols, and reported outcomes. All studies were prospective, single-arm cohort. However, significant clinical and methodological heterogeneity was identified. Patient populations varied in terms of inclusion criteria: Some studies included only patients with CY+ and minimal carcinomatosis (e.g., PCI ≤ 6), while others enrolled patients with more extensive peritoneal disease (PCI up to 20). Differences were also noted in systemic treatment regimens preceding HIPEC, ranging from S-1 plus paclitaxel (Asian studies) to triplet chemotherapy combinations in Western cohorts. In summary, the heterogeneity in patient selection, HIPEC protocols, and systemic treatments across studies[30] necessitates cautious interpretation of pooled results[31-35].
Sensitivity analyses, in RCTs, confirmed that the observed OS benefit of HIPEC was primarily driven by the Yang et al[27] Phase III trial (hazard ratio = 0.46, 95%CI: 0.24-0.89). When the GYMSSA trial was excluded due to its limited statistical power, conclusions remained unchanged. Conversely, removing the Wuhan trial from the analysis resulted in the disappearance of the OS benefit, underscoring its pivotal role. In contrast, DFS and progression-free survival (PFS) improvements were consistently observed across studies, notably in the GASTRIPEC-I trial[27-29].
The sensitivity analysis in CTs showed that sequential exclusion of studies with moderate to serious risk of confounding or missing data did not significantly alter the qualitative outcomes regarding OS and DFS. The removal of early-phase feasibility data (MD Anderson Phase I) resulted in a slight upward shift in reported median OS (from 15-25 months to 20-24 months). Excluding PERISCOPE-I extended analysis did not impact the overall conclusions. Overall, the observed survival benefit of HIPEC in GC with peritoneal dissemination remained consistent across sensitivity scenarios[30], though selection bias remains a critical limitation[31-35].
The risk of bias assessment in RCTs using the ROB 2.0 tool identified two trials (Wuhan Phase III and GASTRIPEC-I) as low risk across all domains. The GYMSSA trial presented some concerns related to the randomization process and selective reporting. Overall, the RCTs included were methodologically sound, supporting a moderate level of confidence in their findings[27-29].
The risk of bias across the included non-randomized prospective cohort studies was systematically assessed using the ROBINS-I tool. All six studies demonstrated a serious overall risk of bias. Specifically, participant selection was deemed at serious risk in all cases, as HIPEC was offered to highly selected patients based on clinical eligibility and surgical resectability, limiting external validity. Missing data was generally well managed, with low risk in most studies, except for a single study reporting moderate concerns due to incomplete follow-up data. Measurement of outcomes[30], particularly OS and DFS, showed moderate risk of bias in all studies, largely attributable to variability in follow-up duration and potential detection bias in recurrence assessment[31-35].
Five RCTs have evaluated the prophylactic use of HIPEC in patients with locally advanced GC (Tables 5 and 6)[36-40]. Inclusion criteria were generally consistent, with the exception of the Fan et al[38] study, which randomized patients based solely on clinical TNM (cTNM) staging, without subsequent adjustment based on pathological staging. This approach led to the inclusion of a relatively low proportion of high-risk patients (pT4: 32%) and a notable percentage of low-risk individuals (pT1: 8%)[38].
| Year | Ref. | State | Arms | Patient cohort | HIPEC sample | Oncological admission criteria (TNM stage) | Bedsite HIPEC | HIPEC protocol | Neoadjuvant CHT | Neoadjuvant treatment regimen | Adjuvant Cht | Adjuvant treatment regimen |
| 2014 | Cui et al[36] | China | Arm 1: Surgery alone (n = 48). Arm 2: Neoadjuvant Cht + surgery (n = 48). Arm 3: Surgery + postoperative HIPEC (n = 48). Arm 4: Neoadjuvant Cht + surgery + postoperative HIPEC (n = 48) | 192 | Arm 3 + 4: n = 98 | IIIA-IIIC + no Neoadjuvant Cht before enrollement | Yes | Day 1 and 4: 60 mg/m2 cisplatin, 90 minutes. Day 2 and 3: 0.75 g fluorouracil, 90 minutes +1000 cc of perfusate left in place for 23 hours at the end of each cycle | Yes | Paclitaxel, cisplatin, tegafur | Yes, if tumor progression occurred during neodjuvant treatment | Epirubicin, cisplatin, fluorouracil |
| 2019 | Reutovich et al[37] | Belarus | Arm 1: Gastrectomy + HIPEC (n = 76). Arm 2: Gastrectomy only (n = 78) | 154 | 76 | IIB-IIIC + Borrmann type III-IV | No | Cisplatin 50 mg/m2 + doxorubicin 50 mg/m2, 60 minutes closed abdomen | No | _ | No | Not declared |
| 2021 | Fan et al[38] | China | Arm 1: S.Cht (n = 17). Arm 2: T-HIPEC + adjuvant S.Cht (n = 33) | 50 | 33 | IIIA-B | No | Cisplatin (50 mg/L), 30 minutes | No | _ | Yes | S1 + oxaliplatin |
| 2022 | Liu et al[39] | China | 4 weeks after D2 gastrectomy: Arm 1: Bedside HIPEC + oral S1 treatment; arm 2: IV cisplatin + oral S1 treatment | 114 | 57 | III A-B | Yes | Day 1 and 3: Cisplatin (30 mg/m2) + oral S1, (40-60 mg for 14 days) | No | _ | Yes | 6-8 cycles of IV cisplatin (60 mg/m2) + oral S1 (40-60 mg, 2/day) |
| 2023 | Yu et al[40] | China | Arm 1: HIPEC + Cht. Arm 2: Systemic chemotherapy alone | 134 | 67 | IIIA and B + no Neoadjuvant Cht before enrollement | Yes | Cisplatin (40 mg/m2), 60 minutes, 2 cycles within 72 hours after surgery | No | _ | Yes | 6-8 cycles of S1 + oxaliplatin |
| Year | Ref. | State | Arms | Median OS | 1 year OS | 3 years OS | Median RFS | RFS 2 years | RFS 3 years | Peritoneal recurrence |
| 2014 | Cui et al[36] | China | Arm 1: Surgery alone (n = 48). Arm 2: Neoadjuvant Cht + surgery (n = 48). Arm 3: Surgery + postoperative HIPEC (n = 48). Arm 4: Neoadjuvant Cht + surgery + postoperative HIPEC (n = 48) | Arm 1: 27 months. Arm 2: 33 months. Arm 3: 32 months. Arm 4: 36 months | Arm 1: 79.2%. Arm 2: 87.5%. Arm 3: 85.4%. Arm 4: 93.7% | Arm 1: 35.4%. Arm 2: 62.5%. Arm 3: 58.3%. Arm 4: 75.0% | Arm 1: 26 months. Arm 2: 28 months. Arm 3: 31 months. Arm 4: 33 months | Arm 1: 66.7%. Arm 2: 77.1%. Arm 3: 83.3%. Arm 4: 87.5% | ND | ND |
| 2019 | Reutovich et al[37] | Belarus | Arm 1: Gastrectomy + HIPEC (n = 76). Arm 2: Gastrectomy only (n = 78) | ND | ND | ND | Arm 1: 28 months vs arm 2: 13 months | ND | Arm 1: 47% vs arm 2: 27% | 12.8% vs 27.6% |
| 2021 | Fan et al[38] | China | Arm 1: S. Cht (n = 17). Arm 2: Traditional-HIPEC + adjuvant S. Cht (n = 33) | ND | ND | Arm 1: 100% vs arm 2: 87.9% | ND | ND | Arm 1: 88.2% vs arm 2: 84.8% | ND |
| 2022 | Liu et al[39] | China | 4 weeks after D2 gastrectomy: Arm 1: Bedside HIPEC + oral S-1 treatment; arm 2: IV cisplatin + oral S-1 treatment | Arm 1: 42 months vs arm 2: 31 months | ND | ND | Arm 1: 29 months vs arm 2: 15 months | Arm 1: 50.4% vs arm 2: 25.5% | ND | 0% vs 7% |
| 2023 | Yu et al[40] | China | Arm 1: HIPEC + Cht. Arm 2: Systemic chemotherapy alone | 51.4 months | ND | Arm 1: 73.9% vs arm 2: 77.6% | ND | ND | Arm 1: 73.8% vs arm 2: 61.2% | 20.9% vs 40.3% |
All trials included at least two comparative arms: CRS alone (CRS-A) vs CRS-H. The Cui et al’s study[36] expanded upon this design by introducing additional experimental groups to evaluate the effect of neoadjuvant chemotherapy in enhancing the efficacy of both CRS-A and CRS-H approaches.
All HIPEC protocols employed intraperitoneal cisplatin (30-60 mg/m²). In addition, Reutovich et al[37] incorporated doxorubicin, and Cui et al[36] added 5-fluorouracil (5-FU). Two trials utilized single-session traditional HIPEC[37,38], whereas studies by Cui et al[36], Liu et al[39], and Yu et al[40] explored repeated postoperative HIPEC administration[37,38]. The Cui et al’s study[36] delivered four postoperative HIPEC cycles, while Yu et al[40] administered two, both within the first five postoperative days. In contrast, Liu et al[39] integrated HIPEC into the adjuvant phase, administering two cycles prior to each round of systemic chemotherapy for a total of 6-8 treatments.
Systemic adjuvant chemotherapy was included in three of the five trials. While Fan et al[38] and Yu et al[40] used a combination of S-1 and oxaliplatin, Liu et al[39] employed S-1 monotherapy.
The Fan et al’s trial[38], likely influenced by the aforementioned selection bias, failed to demonstrate a significant benefit of HIPEC in terms of DFS or OS. In contrast, the trials by Cui et al[36], Reutovich et al[37], Liu et al[39], and Yu et al[40] all reported a significant protective effect of HIPEC against the development of PM. Furthermore, both Cui et al[36] and Yu et al[40] observed an improvement in OS among patients receiving prophylactic HIPEC.
Although the included RCTs share similar endpoints, moderate heterogeneity exists across the studies in terms of patient selection (T4-only vs mixed T3-T4 populations), HIPEC protocols (intraoperative vs bedside, single-agent vs combination chemotherapy), and systemic therapy regimens. Despite these differences, all trials employed DFS as a primary outcome, and the direction of effect consistently favored HIPEC—except in one neutral study. The overall methodological and clinical heterogeneity is considered moderate but acceptable, justifying the synthesis of findings under a fixed-effects model in the absence of statistical heterogeneity[36-40].
Sensitivity analyses were performed to assess the robustness of the observed benefit of prophylactic HIPEC in patients with locally advanced GC. A leave-one-out approach demonstrated that the overall direction of effect—favoring HIPEC for improved DFS—was consistent across studies[36-40]. However, the magnitude of benefit was influenced by two key trials (Reutovich et al[37] and Yu et al[40]), which reported the most pronounced improvements in DFS and reduction in peritoneal recurrence. Subgroup analyses based on HIPEC modality (intraoperative vs bedside), chemotherapeutic regimen (cisplatin alone vs combination therapy), and pathological staging (T4-only vs T3-T4) suggested that intraoperative cisplatin-based HIPEC in serosa-invasive tumors may offer the most favorable outcomes.
Publication bias was evaluated qualitatively given the limited number of eligible trials (n = 5), which precluded a reliable funnel plot-based analysis. However, no evidence of small-study effects or selective reporting was observed in the included studies. All trials reported negative as well as positive outcomes, and two studies did not demonstrate statistically significant improvements in OS, reducing the likelihood of bias due to selective publication. Still, the relatively small sample sizes and geographic concentration of trials in East Asia underscore the need for additional large-scale, multicenter studies to confirm these findings and mitigate potential bias in the existing literature[36-40].
Although retrospective studies have suggested a survival benefit from HIPEC in GC, these findings have not been consistently confirmed by RCTs or CTs[14,41]. This discrepancy likely stems from selection biases inherent in re
Patients with A-CY+ status often exhibit survival outcomes similar to those with macroscopic PM. In a nationwide study by van der Sluis et al[42], the median OS was 12.0 months in CY+ patients compared to 6.7 months in those with visible PM. The CYTO-CHIP trial, which included 54% of patients with only positive cytology, reported a median OS of 18.8 months in the CRS-H group vs 12.1 months in CRS-A group[14].
Some retrospective studies have included patients with serosal invasion-acknowledging its high risk for peritoneal dissemination-under the concept of “ongoing peritoneal carcinomatosis”[43]. However, this creates ambiguity regarding whether such patients should be managed within therapeutic or prophylactic settings. Notably, the prospective studies evaluated in this review, related to the treatment setting, maintain this grouping of A-Cy+ patients with those with macroscopic PM
The extent of peritoneal disease, as measured by PCI, is a critical prognostic factor. Retrospective analyses consistently identify PCI < 7 as the threshold for achieving CC-0 and potential long-term survival. Chia et al[44] reported a 5-year OS of 18% and an 11% cure rate limited to patients with PCI < 7 and CC-0[44]. Similarly, a study by Manzanedo et al[45] from the GECOP registry identified PCI < 7 as the only independent predictor of OS. According to the 2024 Cinquini Multisociety Guidelines, CRS-H only for patients with synchronous PM, a laparoscopic PCI ≤ 6, and response to first-line chemotherapy[46]. The German Registry (2020), which included 235 patients, demonstrated declining median OS with increasing PCI: (1) 18 months for PCI 0-6; (2) 12 months for PCI 7-15; and (3) 5 months for PCI 16-39[41].
There is significant heterogeneity in PCI thresholds among RCTs. Yang et al[27] included 41.1% of patients with PCI ≥ 20; GYMSSA accepted 11% with PCI ≥ 21 and allowed limited liver/Lung metastases[28]; GASTRIPEC-1 enrolled 17.3% with PCI > 13[29]. In CTs, thresholds also varied: (1) Blum Murphy et al[31]: 48% PCI ≥ 8; (2) Badgwell et al[35]: Mean PCI 2, max 13; (3) Yu et al[30]: Cut-off PCI < 21; and (4) Periscope-I and NIH paper, PCI < 10[33,34]. Such inconsistencies may result in the inclusion of patients already beyond curative intent, undermining accurate assessment of HIPEC’s therapeutic role.
Ascites is another variable inconsistently reported-excluded in GYMSSA, unmentioned in Yang et al[27], yet present in 40% of GASTRIPEC-1 patients[28,29]. Tumor histology is also often overlooked. Signet ring cell (SRC) GC, representing 3.4%-32.5% of cases, is highly aggressive, yet its outcomes with CRS + HIPEC remain unclear[47]. Advances in the molecular understanding of the disease have enabled the identification of actionable alterations and therapeutic targets, paving the way for the integration of new agents into the treatment landscape of this complex malignancy. Human epidermal growth factor receptor 2 (HER-2) was the first biomarker to allow the incorporation of an effective targeted therapy-trastuzumab-in combination with chemotherapy in the first-line setting for selected patients[48]. The rarity of HER-2 positivity in peritoneal disease (2%-3% vs 13%-22% in GC overall) further complicates treatment selection[49]. Subsequently, programmed death ligand-1 expression and microsatellite instability-high/mismatch repair deficiency were recognized as predictive biomarkers for the efficacy of anti-programmed cell death protein 1 monoclonal antibodies when combined with chemotherapy as first-line treatment for metastatic GC. More recently, CLDN18.2 has emerged as a noteworthy biomarker in GC[50]. Zolbetuximab, a chimeric immunoglobulin G1 monoclonal antibody targeting CLDN18.2, has shown clinical benefit when combined with oxaliplatin-based chemotherapy in patients with CLDN18.2-positive metastatic GC, as demonstrated by two large phase III trials[51]. The advent of those novel therapeutic strategy (Anti-HER-2 agents, immune checkpoint inhibitors, and anti-claudin 18.2 monoclonal antibodies) underscores the need for molecular profiling in defining the therapeutic pathway of patients with advanced GC.
Lymph node involvement, a well-established negative prognostic factor remains underexplored. Green et al[34] identified the number of positive lymph nodes as the sole independent OS predictor, correlating with higher recurrence and poorer outcomes. While RCTs on intraperitoneal chemotherapy (e.g., Yonemura et al[52], Ikeguchi et al[53]) suggest potential benefit in node-positive patients, retrospective data (e.g., Rau et al[54]) favor node-negative cases, suggesting nodal status as a surrogate marker of tumor biology.
GYMSSA remains the only reviewed study to include patients with lung and liver metastases. However, it imposed a numeric limit on liver lesions without providing justification[28]. Conversely, the 2019 GIRCG study deemed bilobar liver metastases inoperable, associating multiple metastatic sites with a poor prognosis[55].
Multimodal approaches are increasingly adopted, incorporating both preoperative systemic and intraperitoneal chemotherapy. Notable strategies include neoadjuvant intraperitoneal and systemic chemotherapy, as seen in the work of Yonemura et al[56], and perioperative intravenous regimens like Fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT), extensively studied by Al-Batran et al[57], which yield pathological response rates of 43%-60%. Among reviewed studies, particularly those from Asia, systemic chemotherapy is predominantly adjuvant[1]. In contrast, Western protocols favor perioperative chemotherapy (96.8% in Europe, 88.7% in the United States according to the GAST RODATA registry) and the most frequently used regimen was FLOT, applied in both preoperative (47.3%) and pos
HIPEC protocols lack standardization, varying in chemotherapeutic agents (cisplatin, mitomycin, paclitaxel, oxa
Gronau et al[60] reviewed 42 studies, noting significant heterogeneity in drug combinations: HIPEC drug regimens preferred CDDP and MMC as a duplet regimen, which was most frequently used, although the dosages varied greatly. In this context, the different outcome results are difficult to weigh. Van der Speeten et al[61] compared body surface area-based vs concentration-based dosing, highlighting efficacy-toxicity trade-offs. Periscope-1 explored the combination of HIPEC and NIPEC. The PHOENIX-GC trial showed that combining intraperitoneal and intravenous paclitaxel with S-1 yielded a superior 3-year survival rate compared to IV cisplatin and S-1 alone (21.9% vs 6%)[62].
The limited drug residence time in HIPEC (60-90 minutes) restricts penetration into subperitoneal lymphatics. Alternative strategies, such as repeated HIPEC sessions and chemotherapy gels, aim to extend peritoneal drug exposure[63]. Yan et al[64] demonstrated improved 3-year survival (78% vs 53%, P = 0.041) with four HIPEC sessions. Solaini et al’s study[55] suggests HIPEC could facilitate conversion surgery, a strategy yielding OS of 37-56 months in previously unresectable stage III/IV patients. Repeated HIPEC has spurred interest in minimally invasive approaches, including laparoscopic and bedside techniques-the latter primarily reported in Chinese studies. Yonemura et al[65] emphasized the value of postoperative intraperitoneal chemotherapy in eradicating micrometastases, possibly outperforming systemic therapy alone.
Despite these advances, logistical challenges persist. Standardizing protocols across centers is difficult, and systemic chemotherapy often depends on local oncologist discretion. Recruitment remains a barrier, as evidenced by the premature termination of GASTRIPEC due to slow accrual. The Chicago Working Group has advocated using national registry data to mitigate such limitations[66].
Optimal cytoreduction (CC-0) remains the most important prognostic factor. RCTs confirm the safety of CRS + HIPEC, but OS benefits are largely confined to patients with CC-0. In the CYTO-CHIP study (2019), CC-0 patients had significantly better OS than CC-1[14]. This finding was corroborated by Marano et al[67] (2021, SICO Oncoteam), who reported OS of 40.7 months in CC-0 patients vs 10.7 months in CC-0 ≥ 1. Beeharry et al[68] also demonstrated that CRS-H significantly reduced peritoneal recurrence compared to CRS-A (23% vs 3%, P < 0.05).
However, when comparing recent HIPEC trials to retrospective series, no substantial improvement in OS or DFS has been observed with traditional HIPEC. This may be due to limited sample sizes-across three RCTs, only 95 patients were enrolled in CRS-H arms, and just 71 completed treatment. Of these, 78.8% achieved CC-0 or CC-1.
In the GASTRIPEC-I trial, the median OS was identical in both study arms (14.9 months for CRS plus HIPEC and CRS-A). However, a notable divergence was observed in the 3-year OS rates 13.6% in the CRS-H group vs 0% in the CRS-alone group[29]. This apparent discrepancy may be attributed to two key factors. First, the observed survival benefit at later time points likely reflects a tailing effect driven by a small number of long-term survivors in the HIPEC group. The authors of the trial specifically acknowledged that both OS and PFS outcomes were influenced by a limited number of patients with extended survival, a phenomenon that is particularly relevant in oncologic studies where a minority of patients may derive durable benefit. Second, the trial was underpowered due to premature closure following slow recruitment, with only 105 of the planned 180 patients enrolled. This limited sample size reduces the statistical power to detect significant differences in long-term outcomes, especially when event numbers are low at the tail of the survival curve. Therefore, the survival tail seen in the CRS-H group should be interpreted cautiously but may signal a clinically meaningful benefit for a select subgroup of patients, such as those who achieved CC (CC-R0). These findings underscore the importance of considering both median survival and long-term survival rates when evaluating the efficacy of HIPEC in the management of PM from GC.
Notably, non-traditional applications of HIPEC, particularly in neoadjuvant settings, appear more promising, with conversion surgery yielding substantial survival benefits. Currently, two Western trials (PERISCOPE II and NCT 04107077) are investigating the conventional application of HIPEC in GC[69]. To date, none of these studies have in
The peritoneal cavity remains the most common site of tumor recurrence following radical gastrectomy with D2 Lymphadenectomy in patients with locally advanced GC. The current standard perioperative approach involves systemic chemotherapy; however, peritoneal relapse still occurs in approximately 40% of cases despite appropriate treatment[70].
Administering chemotherapy directly into the peritoneal cavity exploits the blood-peritoneal barrier, which limits systemic absorption and allows for higher local drug concentrations with reduced systemic toxicity[11]. Building on this locoregional strategy, prophylactic HIPEC after curative gastrectomy has been proposed as a strategy to prevent peritoneal recurrence in patients without evidence of PM.
A retrospective study reported the presence of cancer cells in the peritoneal lavage fluid of 29.7% of T3 and 34.8% of T4 patients after radical surgery. Furthermore, PM developed in 59.4% of these patients within five years, compared to 21.3% who experienced hematogenous spread[71]. A 2022 meta-analysis by Zhang et al[72] confirmed that patients who received prophylactic HIPEC had significantly higher 3-year OS rates compared to those treated with surgery alone, without a corresponding increase in morbidity.
Despite these encouraging findings, prophylactic HIPEC has not yet been adopted as a standard adjuvant therapy in Western clinical practice. Most of the supporting evidence comes from Asian studies, and the reliance on cTNM staging for patient selection in the absence of PM presents a significant limitation[1].
Nevertheless, the available data consistently support the role of prophylactic HIPEC in improving locoregional disease control. With the exception of Fan et al’s study[38]-which included a small proportion of T1 (8%) and a relatively lower proportion of T4 (32%) patients-all studies demonstrated a clear benefit in PFS. Moreover, Yu et al’s study[40] was the first to establish lymph node positivity as an independent predictor of disease progression, further reinforcing the need for precise patient selection.
Cui et al’s study[36] further supports that, even in a prophylactic setting, combining neoadjuvant systemic chemotherapy with HIPEC improves both DFS and OS, supporting the integration of multimodal treatment strategies in managing high-risk patients.
Currently, four Western trials (GASTRICHIP, PREVENT, CHIMERA, and GOETH) and three Chinese trials (NCT 02356276, NCT02960061, and NCT02381847) are ongoing to investigate the prophylactic use of HIPEC in GC[73,74].
The integration of HIPEC into the multidisciplinary management of advanced GC remains an area of active investigation, supported by promising-but heterogeneous-clinical evidence. While select studies have demonstrated survival benefits, these are most apparent in patients undergoing CC-0, with low peritoneal disease burden and favorable biological response to systemic chemotherapy.
The growing interest in neoadjuvant and conversion strategies, including minimally invasive approaches such as bedside or laparoscopic HIPEC, has shown potential in downstaging disease and enabling curative-intent surgery in patients initially deemed unresectable. However, at present, this therapeutic strategy may be best regarded as a means of prolonging survival, rather than achieving cure[75].
Further randomized trials will be necessary to assess the impact of emerging targeted therapies-both systemic and intraperitoneal-on clinical outcomes in this patient population.
Prophylactic HIPEC appears to be effective in reducing the incidence of peritoneal recurrence, particularly when used in conjunction with systemic chemotherapy. Nevertheless, significant challenges remain, including the lack of standardized treatment protocols, heterogeneity in systemic therapy regimens, and variability in patient selection criteria. It is desirable that international societies focused on peritoneal disease, particularly PSOGI-the peritoneal surface oncology group international society and ISSPP-international society for study of pleura and peritoneum, take an active role in promoting the standardization of treatment protocols.
Ultimately, the reviewed literature highlights the critical importance of rigorous patient selection in optimizing outcomes. Despite the variability across studies, the addition of HIPEC-whether for therapeutic or prophylactic purposes-has been associated with improved outcomes, particularly in terms of DFS. Continued research and harmonization of treatment protocols are essential to fully define HIPEC’s role within the broader therapeutic landscape of GC.
We are hopeful that the results of ongoing studies will contribute significantly to the advancement of knowledge about this multimodal approach in both therapeutic and prophylactic settings.
We thank Prof. Cucchetti A for suggestions and support in performing the qualitative analyses of the reviewed studies.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68648] [Article Influence: 13729.6] [Reference Citation Analysis (201)] |
| 2. | Rijken A, Lurvink RJ, Luyer MDP, Nieuwenhuijzen GAP, van Erning FN, van Sandick JW, de Hingh IHJT. The Burden of Peritoneal Metastases from Gastric Cancer: A Systematic Review on the Incidence, Risk Factors and Survival. J Clin Med. 2021;10:4882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 3. | Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, Lemmens VE, de Hingh IH. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134:622-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 450] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 4. | Seyfried F, von Rahden BH, Miras AD, Gasser M, Maeder U, Kunzmann V, Germer CT, Pelz J, Kerscher AG. Incidence, time course and independent risk factors for metachronous peritoneal carcinomatosis of gastric origin--a longitudinal experience from a prospectively collected database of 1108 patients. BMC Cancer. 2015;15:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Solaini L, Bencivenga M, D'ignazio A, Milone M, Marino E, De Pascale S, Rosa F, Sacco M, Fumagalli Romario U, Graziosi L, De Palma G, Marrelli D, Morgagni P, Ercolani G. Which gastric cancer patients could benefit from staging laparoscopy? A GIRCG multicenter cohort study. Eur J Surg Oncol. 2022;48:1778-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Yonemura Y, Endou Y, Shinbo M, Sasaki T, Hirano M, Mizumoto A, Matsuda T, Takao N, Ichinose M, Mizuno M, Miura M, Ikeda M, Ikeda S, Nakajima G, Yonemura J, Yuuba T, Masuda S, Kimura H, Matsuki N. Safety and efficacy of bidirectional chemotherapy for treatment of patients with peritoneal dissemination from gastric cancer: Selection for cytoreductive surgery. J Surg Oncol. 2009;100:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 842] [Article Influence: 280.7] [Reference Citation Analysis (2)] |
| 8. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4699] [Article Influence: 522.1] [Reference Citation Analysis (4)] |
| 9. | Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 912] [Article Influence: 228.0] [Reference Citation Analysis (0)] |
| 10. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 1168] [Article Influence: 292.0] [Reference Citation Analysis (0)] |
| 11. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1119] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 12. | Sugarbaker PH, Cunliffe WJ, Belliveau J, de Bruijn EA, Graves T, Mullins RE, Schlag P. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin Oncol. 1989;16:83-97. [PubMed] |
| 13. | Kok HP, Beck M, Löke DR, Helderman RFCPA, van Tienhoven G, Ghadjar P, Wust P, Crezee H. Locoregional peritoneal hyperthermia to enhance the effectiveness of chemotherapy in patients with peritoneal carcinomatosis: a simulation study comparing different locoregional heating systems. Int J Hyperthermia. 2020;37:76-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Bonnot PE, Piessen G, Kepenekian V, Decullier E, Pocard M, Meunier B, Bereder JM, Abboud K, Marchal F, Quenet F, Goere D, Msika S, Arvieux C, Pirro N, Wernert R, Rat P, Gagnière J, Lefevre JH, Courvoisier T, Kianmanesh R, Vaudoyer D, Rivoire M, Meeus P, Passot G, Glehen O; FREGAT and BIG-RENAPE Networks. Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J Clin Oncol. 2019;37:2028-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 279] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 15. | Mussa A, Sandrucci S, Zanon C. Intraoperative chemohyperthermia for advanced gastric cancer: a new procedure with closed abdomen and previously constructed anastomosis. Tumori. 2001;87:S18-S20. [PubMed] |
| 16. | Yang XJ, Li Y, Yonemura Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat gastric cancer with ascites and/or peritoneal carcinomatosis: Results from a Chinese center. J Surg Oncol. 2010;101:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Newton AD, Bartlett EK, Karakousis GC. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a review of factors contributing to morbidity and mortality. J Gastrointest Oncol. 2016;7:99-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 18. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26160] [Article Influence: 1189.1] [Reference Citation Analysis (2)] |
| 19. | Glehen O, Gilly FN, Arvieux C, Cotte E, Boutitie F, Mansvelt B, Bereder JM, Lorimier G, Quenet F, Elias D; Association Française de Chirurgie. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17:2370-2377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 350] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 20. | Gill RS, Al-Adra DP, Nagendran J, Campbell S, Shi X, Haase E, Schiller D. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: a systematic review of survival, mortality, and morbidity. J Surg Oncol. 2011;104:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 21. | Di Giorgio A, Macrì A, Ferracci F, Robella M, Visaloco M, De Manzoni G, Sammartino P, Sommariva A, Biacchi D, Roviello F, Pastorino R, Pires Marafon D, Rotolo S, Casella F, Vaira M. 10 Years of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): A Systematic Review and Meta-Analysis. Cancers (Basel). 2023;15:1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 22. | Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. United States: Wiley, 2019: 205-228. |
| 23. | Sterne JA, Hernán MA, McAleenan A, Reeves BC, Higgins JP. Assessing risk of bias in a non-randomized study. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. United States: Wiley, 2019: 621-641. |
| 24. | Higgins JP, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. United States: Wiley, 2019: 143-176. |
| 25. | Sugarbaker PH. A curative approach to peritoneal carcinomatosis from colorectal cancer. Semin Oncol. 2005;32:S68-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 1212] [Article Influence: 39.1] [Reference Citation Analysis (33)] |
| 27. | Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, Zhou YF, Xiong B, Yonemura Y, Li Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 508] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 28. | Rudloff U, Langan RC, Mullinax JE, Beane JD, Steinberg SM, Beresnev T, Webb CC, Walker M, Toomey MA, Schrump D, Pandalai P, Stojadinovic A, Avital I. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol. 2014;110:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 29. | Rau B, Lang H, Koenigsrainer A, Gockel I, Rau HG, Seeliger H, Lerchenmueller C, Reim D, Wahba R, Angele M, Heeg S, Keck T, Weimann A, Topp S, Piso P, Brandl A, Schuele S, Jo P, Pratschke J, Wegel S, Rehders A, Moosmann N, Gaedcke J, Heinemann V, Trips E, Loeffler M, Schlag PM, Thuss-Patience P. Effect of Hyperthermic Intraperitoneal Chemotherapy on Cytoreductive Surgery in Gastric Cancer With Synchronous Peritoneal Metastases: The Phase III GASTRIPEC-I Trial. J Clin Oncol. 2024;42:146-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 85] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 30. | Yu P, Ye Z, Dai G, Zhang Y, Huang L, Du Y, Cheng X. Neoadjuvant systemic and hyperthermic intraperitoneal chemotherapy combined with cytoreductive surgery for gastric cancer patients with limited peritoneal metastasis: a prospective cohort study. BMC Cancer. 2020;20:1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Blum Murphy M, Ikoma N, Wang X, Estrella J, Roy-Chowdhuri S, Das P, Minsky BD, Song S, Mansfield P, Ajani J, Badgwell B. Phase I Trial of Hyperthermic Intraperitoneal Chemoperfusion (HIPEC) with Cisplatin, Mitomycin, and Paclitaxel in Patients with Gastric Adenocarcinoma and Associated Carcinomatosis or Positive Cytology. Ann Surg Oncol. 2020;27:2806-2811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | van der Kaaij RT, Wassenaar ECE, Koemans WJ, Sikorska K, Grootscholten C, Los M, Huitema A, Schellens JHM, Veenhof AAFA, Hartemink KJ, Aalbers AGJ, van Ramshorst B, Boerma D, Boot H, van Sandick JW. Treatment of PERItoneal disease in Stomach Cancer with cytOreductive surgery and hyperthermic intraPEritoneal chemotherapy: PERISCOPE I initial results. Br J Surg. 2020;107:1520-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Koemans WJ, van der Kaaij RT, Wassenaar ECE, Boerma D, Boot H, Sikorska K, Los M, Grootscholten C, Hartemink KJ, Veenhof AAFA, Kodach L, Snaebjornsson P, van Sandick JW. Tumor characteristics and clinical outcome of peritoneal metastasis of gastric origin treated with a hyperthermic intraperitoneal chemotherapy procedure in the PERISCOPE I trial. J Surg Oncol. 2021;123:904-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Green BL, Blumenthaler AN, Gamble LA, McDonald JD, Robinson K, Connolly M, Epstein M, Hernandez JM, Blakely AM, Badgwell BD, Davis JL. Cytoreduction and HIPEC for Gastric Carcinomatosis: Multi-institutional Analysis of Two Phase II Clinical Trials. Ann Surg Oncol. 2023;30:1852-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Badgwell B, Ikoma N, Murphy MB, Wang X, Estrella J, Roy-Chowdhuri S, Das P, Minsky BD, Lano E, Song S, Mansfield P, Ajani J. A Phase II Trial of Cytoreduction, Gastrectomy, and Hyperthermic Intraperitoneal Perfusion with Chemotherapy for Patients with Gastric Cancer and Carcinomatosis or Positive Cytology. Ann Surg Oncol. 2021;28:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 36. | Cui HB, Ge HE, Bai XY, Zhang W, Zhang YY, Wang J, Li X, Xing LP, Guo SH, Wang ZY. Effect of neoadjuvant chemotherapy combined with hyperthermic intraperitoneal perfusion chemotherapy on advanced gastric cancer. Exp Ther Med. 2014;7:1083-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Reutovich MY, Krasko OV, Sukonko OG. Hyperthermic intraperitoneal chemotherapy in serosa-invasive gastric cancer patients. Eur J Surg Oncol. 2019;45:2405-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Fan B, Bu Z, Zhang J, Zong X, Ji X, Fu T, Jia Z, Zhang Y, Wu X. Phase II trial of prophylactic hyperthermic intraperitoneal chemotherapy in patients with locally advanced gastric cancer after curative surgery. BMC Cancer. 2021;21:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 39. | Liu L, Sun L, Zhang N, Liao CG, Su H, Min J, Song Y, Yang X, Huang X, Chen D, Chen Y, Zhang HW, Zhang H. A novel method of bedside hyperthermic intraperitoneal chemotherapy as adjuvant therapy for stage-III gastric cancer. Int J Hyperthermia. 2022;39:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Yu P, Huang X, Huang L, Dai G, Xu Q, Fang J, Ye Z, Chai T, Du Y. Hyperthermic intraperitoneal chemotherapy (HIPEC) plus systemic chemotherapy versus systemic chemotherapy alone in locally advanced gastric cancer after D2 radical resection: a randomized-controlled study. J Cancer Res Clin Oncol. 2023;149:11491-11498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Rau B, Brandl A, Piso P, Pelz J, Busch P, Demtröder C, Schüle S, Schlitt HJ, Roitman M, Tepel J, Sulkowski U, Uzunoglu F, Hünerbein M, Hörbelt R, Ströhlein M, Beckert S, Königsrainer I, Königsrainer A; Peritoneum Surface Oncology Group and members of the StuDoQ|Peritoneum Registry of the German Society for General and Visceral Surgery (DGAV). Peritoneal metastasis in gastric cancer: results from the German database. Gastric Cancer. 2020;23:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 42. | Van Der Sluis K, Taylor SN, Kodach LL, van Dieren JM, de Hingh IHJT, Wijnhoven BPL, Verhoeven RHA, Vollebergh MA, van Sandick JW. Tumor-positive peritoneal cytology in patients with gastric cancer is associated with poor outcome: A nationwide study. Eur J Cancer. 2024;199:113541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Yano M, Yasuda T, Fujiwara Y, Takiguchi S, Miyata H, Monden M. Preoperative intraperitoneal chemotherapy for patients with serosa-infiltrating gastric cancer. J Surg Oncol. 2004;88:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Chia CS, You B, Decullier E, Vaudoyer D, Lorimier G, Abboud K, Bereder JM, Arvieux C, Boschetti G, Glehen O; BIG RENAPE Group. Patients with Peritoneal Carcinomatosis from Gastric Cancer Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Is Cure a Possibility? Ann Surg Oncol. 2016;23:1971-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 45. | Manzanedo I, Pereira F, Rihuete Caro C, Pérez-Viejo E, Serrano Á, Gutiérrez Calvo A, Regueira FM, Casado-Adam Á, Cascales-Campos PA, Arteaga X, García-Fadrique A, Gómez Sanz R, López García A, Zozaya G, Arjona Á, Gil Martínez J. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Gastric Cancer with Peritoneal Carcinomatosis: Multicenter Study of Spanish Group of Peritoneal Oncologic Surgery (GECOP). Ann Surg Oncol. 2019;26:2615-2621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 46. | Cinquini M, Kusamura S, Tralongo AC, Fittipaldo VA, Deraco M, Monteforte M. Multi-Society Guideline on Cytoreductive Surgery and HIPEC: Methodology and Overview of the Results. J Surg Oncol. 2024;130:1173-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 47. | Liu X, Cai H, Sheng W, Yu L, Long Z, Shi Y, Wang Y. Clinicopathological Characteristics and Survival Outcomes of Primary Signet Ring Cell Carcinoma in the Stomach: Retrospective Analysis of Single Center Database. PLoS One. 2015;10:e0144420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Mathias-Machado MC, de Jesus VHF, Jácome A, Donadio MD, Aruquipa MPS, Fogacci J, Cunha RG, da Silva LM, Peixoto RD. Claudin 18.2 as a New Biomarker in Gastric Cancer-What Should We Know? Cancers (Basel). 2024;16:679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 49. | Imano M, Satou T, Itoh T, Yasuda A, Kato H, Shinkai M, Peng YF, Tsubaki M, Yasuda T, Imamoto H, Nishida S, Takeyama Y, Okuno K, Shiozaki H. Peritoneal metastatic lesions of gastric cancer exhibit low expression of human epidermal growth factor receptor 2. Target Oncol. 2012;7:213-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 2229] [Article Influence: 445.8] [Reference Citation Analysis (1)] |
| 51. | Shah MA, Shitara K, Ajani JA, Bang YJ, Enzinger P, Ilson D, Lordick F, Van Cutsem E, Gallego Plazas J, Huang J, Shen L, Oh SC, Sunpaweravong P, Soo Hoo HF, Turk HM, Oh M, Park JW, Moran D, Bhattacharya P, Arozullah A, Xu RH. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med. 2023;29:2133-2141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 377] [Article Influence: 125.7] [Reference Citation Analysis (0)] |
| 52. | Yonemura Y, de Aretxabala X, Fujimura T, Fushida S, Katayama K, Bandou E, Sugiyama K, Kawamura T, Kinoshita K, Endou Y, Sasaki T. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepatogastroenterology. 2001;48:1776-1782. [PubMed] |
| 53. | Ikeguchi M, Kondou A, Oka A, Tsujitani S, Maeta M, Kaibara N. Effects of continuous hyperthermic peritoneal perfusion on prognosis of gastric cancer with serosal invasion. Eur J Surg. 1995;161:581-586. [PubMed] |
| 54. | Rau B, Brandl A, Thuss-Patience P, Bergner F, Raue W, Arnold A, Horst D, Pratschke J, Biebl M. The efficacy of treatment options for patients with gastric cancer and peritoneal metastasis. Gastric Cancer. 2019;22:1226-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 55. | Solaini L, Ministrini S, Bencivenga M, D'Ignazio A, Marino E, Cipollari C, Molteni B, Mura G, Marrelli D, Graziosi L, Roviello F, De Manzoni G, Tiberio GAM, Morgagni P. Conversion gastrectomy for stage IV unresectable gastric cancer: a GIRCG retrospective cohort study. Gastric Cancer. 2019;22:1285-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 56. | Yonemura Y, Elnemr A, Endou Y, Hirano M, Mizumoto A, Takao N, Ichinose M, Miura M, Li Y. Multidisciplinary therapy for treatment of patients with peritoneal carcinomatosis from gastric cancer. World J Gastrointest Oncol. 2010;2:85-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 92] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (8)] |
| 57. | Al-Batran SE, Homann N, Pauligk C, Illerhaus G, Martens UM, Stoehlmacher J, Schmalenberg H, Luley KB, Prasnikar N, Egger M, Probst S, Messmann H, Moehler M, Fischbach W, Hartmann JT, Mayer F, Höffkes HG, Koenigsmann M, Arnold D, Kraus TW, Grimm K, Berkhoff S, Post S, Jäger E, Bechstein W, Ronellenfitsch U, Mönig S, Hofheinz RD. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol. 2017;3:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 356] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 58. | Pelc Z, Sędłak K, Endo Y, Van Sandick J, Gisbertz S, Pera M, Baiocchi GL, Morgagni P, Framarini M, Hoelscher A, Moenig S, Kołodziejczyk P, Gockel I, Piessen G, Eveno C, Da Costa PM, Davies A, Baker C, Allum W, Romario UF, Rosati R, Reim D, D'ugo D, Wijnhoven B, De Manzoni G, Kielan W, Schneider P, Badgwell BB, Pawlik TM, Polkowski W, Rawicz-Pruszyński K. Hyperthermic intraperitoneal chemotherapy (HIPEC) for gastric cancer with peritoneal metastasis - Joint analysis of European GASTRODATA and American national cancer database. Am J Surg. 2025;242:116235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 59. | Kusamura S, Dominique E, Baratti D, Younan R, Deraco M. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2008;98:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 60. | Gronau F, Feldbruegge L, Oberwittler F, Gonzalez-Moreno S, Villeneuve L, Eveno C, Glehen O, Kusamura S, Rau B. HIPEC in Peritoneal Metastasis of Gastric Origin: A Systematic Review of Regimens and Techniques. J Clin Med. 2022;11:1456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 61. | Van der Speeten K, Kusamura S, Villeneuve L, Piso P, Verwaal VJ, González-Moreno S, Glehen O. The 2022 PSOGI International Consensus on HIPEC Regimens for Peritoneal Malignancies: HIPEC Technologies. Ann Surg Oncol. 2024;31:7090-7110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Ishigami H, Fujiwara Y, Fukushima R, Nashimoto A, Yabusaki H, Imano M, Imamoto H, Kodera Y, Uenosono Y, Amagai K, Kadowaki S, Miwa H, Yamaguchi H, Yamaguchi T, Miyaji T, Kitayama J. Phase III Trial Comparing Intraperitoneal and Intravenous Paclitaxel Plus S-1 Versus Cisplatin Plus S-1 in Patients With Gastric Cancer With Peritoneal Metastasis: PHOENIX-GC Trial. J Clin Oncol. 2018;36:1922-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 276] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 63. | Huang O, Lu X, Xu X, Shi Y. Fibrin-sealant-delivered cisplatin chemotherapy versus cisplatin hyperthermic intraperitoneal perfusion chemotherapy for locally advanced gastric cancer without peritoneal metastases: a randomized phase-II clinical trial with a 40-month follow-up. Cell Biochem Biophys. 2015;71:1171-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Yan K, Wu K, Yan L, Liang L, Yuan Y. Efficacy of postoperative intraperitoneal hyperthermic perfusion chemotherapy with oxaliplatin + 5-Fluorouracil in the treatment of gastric cancer patients with peritoneal carcinomatosis. J BUON. 2019;24:1587-1594. [PubMed] |
| 65. | Yonemura Y, Prabhu A, Sako S, Ishibashi H, Mizumoto A, Takao N, Ichinose M, Motoi S, Liu Y, Nishihara K, Brandl A, Fushida S. Long Term Survival after Cytoreductive Surgery Combined with Perioperative Chemotherapy in Gastric Cancer Patients with Peritoneal Metastasis. Cancers (Basel). 2020;12:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 66. | Chicago Consensus Working Group. The Chicago Consensus on peritoneal surface malignancies: Management of gastric metastases. Cancer. 2020;126:2541-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 67. | Marano L, Marrelli D, Sammartino P, Biacchi D, Graziosi L, Marino E, Coccolini F, Fugazzola P, Valle M, Federici O, Baratti D, Deraco M, Di Giorgio A, Macrì A, Pasqual EM, Framarini M, Vaira M, Roviello F; Italian Peritoneal Surface Malignancies Oncoteam (S. I.C.O.). Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Synchronous Peritoneal Metastases: Multicenter Study of 'Italian Peritoneal Surface Malignancies Oncoteam-S.I.C.O.'. Ann Surg Oncol. 2021;28:9060-9070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 68. | Beeharry MK, Zhu ZL, Liu WT, Yao XX, Yan M, Zhu ZG. Prophylactic HIPEC with radical D2 gastrectomy improves survival and peritoneal recurrence rates for locally advanced gastric cancer: personal experience from a randomized case control study. BMC Cancer. 2019;19:932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (2)] |
| 69. | Koemans WJ, van der Kaaij RT, Boot H, Buffart T, Veenhof AAFA, Hartemink KJ, Grootscholten C, Snaebjornsson P, Retel VP, van Tinteren H, Vanhoutvin S, van der Noort V, Houwink A, Hahn C, Huitema ADR, Lahaye M, Los M, van den Barselaar P, Imhof O, Aalbers A, van Dam GM, van Etten B, Wijnhoven BPL, Luyer MDP, Boerma D, van Sandick JW. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy versus palliative systemic chemotherapy in stomach cancer patients with peritoneal dissemination, the study protocol of a multicentre randomised controlled trial (PERISCOPE II). BMC Cancer. 2019;19:420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 70. | Martins M, Santos-Sousa H, Araújo F, Nogueiro J, Sousa-Pinto B. Impact of Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy in the Treatment of Gastric Cancer with Peritoneal Carcinomatosis: A Systematic Review and Meta-analysis. Ann Surg Oncol. 2022;29:7528-7537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 71. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K; ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 1979] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 72. | Zhang JF, Lv L, Zhao S, Zhou Q, Jiang CG. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Combined with Surgery: A 12-Year Meta-Analysis of this Promising Treatment Strategy for Advanced Gastric Cancer at Different Stages. Ann Surg Oncol. 2022;29:3170-3186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 73. | Götze TO, Piso P, Lorenzen S, Bankstahl US, Pauligk C, Elshafei M, Amato G, Reim D, Bechstein WO, Königsrainer A, Mönig SP, Rau B, Schwarzbach M, Al-Batran SE. Preventive HIPEC in combination with perioperative FLOT versus FLOT alone for resectable diffuse type gastric and gastroesophageal junction type II/III adenocarcinoma - the phase III "PREVENT"- (FLOT9) trial of the AIO /CAOGI /ACO. BMC Cancer. 2021;21:1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 74. | Noiret B, Piessen G, Eveno C. Update of randomized controlled trials evaluating cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in prevention and therapy of peritoneal metastasis: a systematic review. Pleura Peritoneum. 2022;7:51-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 75. | Sugarbaker PH, Van der Speeten K. Aggressive local treatments may prolong survival but do not have curative intent. J Gastrointest Oncol. 2021;12:S68-S69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/