INTRODUCTION
Globally, urothelial carcinoma (UC) ranks as the ninth most prevalent malignancy, with more than half a million new diagnoses reported each year[1]. It predominantly affects the bladder, though it may also arise in the renal pelvis, ureters, or urethra. Approximately 11% of patients present with advanced or metastatic UC (mUC) at diagnosis and despite advances in treatment, mUC is associated with poor outcomes, as recurrence and disease progression are frequent[2]. The prognosis remains poor, with a five-year survival rate below 10% for advanced stages of the disease. Historically, the treatment of advanced or mUC has relied on platinum-based chemotherapy regimens, such as gemcitabine-cisplatin (GC) or methotrexate, vinblastine, and cisplatin[3]. These regimens have shown efficacy in terms of progression-free survival (PFS) and overall survival (OS), yet their high toxicity profiles and limited applicability to patients with comorbidities pose significant limitations[4]. Up to half of all patients are deemed ineligible for cisplatin due to factors such as renal impairment, poor performance status, or advanced age. Immune checkpoint inhibitors (ICIs) revolutionized the treatment landscape, offering new hope for patients unable to tolerate chemotherapy. Agents targeting programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1), such as atezolizumab and pembrolizumab, have demonstrated meaningful survival benefits[5]. However, most patients eventually progress, necessitating additional therapeutic options. The emergence of antibody-drug conjugates (ADCs) represents a breakthrough in mUC treatment. ADCs are designed to deliver cytotoxic agents directly to tumor cells, minimizing off-target effects while enhancing efficacy[6]. With the accelerated Food and Drug Administration (FDA) approvals of enfortumab vedotin (EV) and sacituzumab govitecan (SG), ADCs have become essential components of the therapeutic armamentarium for mUC[7]. These agents have shown significant activity even in heavily pretreated populations, including those refractory to both chemotherapy and ICIs. Ongoing research continues to refine their use, exploring combinations with other therapies and novel targets.
This review explores the evolving landscape of mUC treatment, focusing on the clinical utility of ADCs. It examines their mechanisms of action, clinical trial outcomes, patient selection considerations, and the promise of emerging agents. With further advancements, ADCs have the potential to transform outcomes for a disease that has long been challenging to manage.
DRUG CLASSES IN THE TREATMENT OF MUC
Platinum-based treatments
Platinum-based chemotherapy has served as the backbone of mUC treatment for decades, with cisplatin and carboplatin being the most used agents. Cisplatin exerts its anticancer effects by forming DNA cross-links, which inhibit DNA replication and transcription, leading to apoptosis. GC and dose-dense methotrexate-vinblastine-doxorubicin-cisplatin (dd-MVAC) are well-established regimens, with dd-MVAC showing slightly better efficacy but higher toxicity[8]. For cisplatin-ineligible patients, carboplatin-based combinations, such as gemcitabine-carboplatin, are alternatives, although they are associated with lower OS rates. Limitations of platinum-based treatments include severe nephrotoxicity, ototoxicity, and peripheral neuropathy, which restrict their use in patients with poor performance status or significant comorbidities.
ICIs
ICIs have transformed the treatment paradigm for mUC, particularly for patients who cannot tolerate platinum-based chemotherapy or have progressed following initial therapy. ICIs such as pembrolizumab, atezolizumab, and nivolumab target the PD-1/PD-L1 axis, restoring the immune system’s ability to detect and eliminate cancer cells[9]. Pembrolizumab demonstrated superior OS compared to chemotherapy in the KEYNOTE-045 trial, establishing it as a standard second-line therapy[10]. Despite their benefits, ICIs are effective in only a subset of patients, with response rates ranging from 15% to 25%[11]. The variability in response underscores the importance of biomarkers like PD-L1 expression to guide patient selection. However, not all patients with high PD-L1 expression respond to ICIs, highlighting the need for combination strategies and novel approaches.
ADCs
ADCs represent a novel and highly targeted approach to mUC treatment, combining the specificity of monoclonal antibodies with the cytotoxic potency of chemotherapy. ADCs are designed to deliver their payload directly to cancer cells, reducing off-target effects and enhancing therapeutic efficacy. They consist of three components: A monoclonal antibody that binds to a tumor-specific antigen, a cytotoxic drug, and a linker that connects the two and ensures stability until the ADC reaches its target[12].
Nectin-4
EV combines an anti-Nectin-4 monoclonal antibody with monomethyl auristatin E (MMAE), a potent microtubule-disrupting agent, forming an antibody-drug conjugate[13]. UC cells exhibit elevated levels of Nectin-4, a protein involved in cell-cell adhesion[13]. Once EV binds to Nectin-4, the resulting ADC-antigen complex is internalized by the tumor cells, leading to the release of MMAE by intracellular proteases. This process disrupts the microtubule network, causing cell cycle arrest and apoptosis. Preclinical studies suggest that EV's antitumor effects also include a bystander killing effect on adjacent cells—the phenomenon where cells that are not directly targeted by a therapeutic agent, such as a drug or radiation, are still affected or killed as a result of the action on neighboring cells—this suggests that EV may be effective across tumors with differing levels of Nectin-4 expression and could engage an immunogenic response that enhances the activity of anti-PD-1 therapies[14]. Clinical trials indicate that EV is a promising new treatment option for mUC, with a manageable safety profile that could replace chemotherapy. Patients with mild liver or kidney impairment generally tolerate the regimen well, with dose adjustments seldom being necessary.
Based on the EV-301 phase 3 trial results, the recommended dosing schedule for EV is 1.25 mg/kg on days 1, 8, and 15 of a 28-day regimen in patients with relapsed or refractory mUC following prior chemotherapy and immunotherapy[15]. With a median follow-up duration approaching 24 months, EV demonstrated improved survival while maintaining a toxicity profile similar to that of chemotherapy, with a median OS (mOS) of 12.91 months vs 8.94 months [hazard ratio (HR) = 0.704] and median PFS (mPFS) of 5.55 months vs 3.71 months (HR = 0.632). Moreover, interim results from the ongoing phase 3 EV-302 trial indicate that the combination of EV and pembrolizumab (administered at 200 mg on day 1 of each 21-day cycle) significantly improves survival outcomes—nearly doubling OS—in comparison to chemotherapy among patients with metastatic or previously untreated locally advanced UC[16]. The combination of EV and pembrolizumab received accelerated FDA approval in December 2023 for first-line treatment of patients with mUC[17].
In the pre-surgical context, encouraging outcomes were reported in the EV-103 phase 1b/2 study. In Cohort L, patients with muscle-invasive bladder cancer (MIBC) who were ineligible for cisplatin received perioperative EV administered at 1.25 mg/kg on days 1 and 8 within each 21-day treatment cycle. The therapy included three neoadjuvant cycles, followed by six adjuvant cycles beginning eight weeks after surgery[18]. A pathological complete response (pCR) was observed in 34% of patients, and 42% achieved pathological downstaging (pDS). Likewise, in Cohort H, EV was administered over three preoperative cycles to cisplatin-ineligible patients with MIBC prior to radical cystectomy[19]. At the 24-month follow-up, the treatment demonstrated encouraging results, with a pCR rate of 36.4% [95% confidence interval (CI): 17.2-59.3], pDS observed in 50.0% (95%CI: 28.2-71.8) of patients, and an event-free survival rate of 62.0% (95%CI: 38.2-78.9)[20]. These early results have prompted the initiation of phase III trials to more thoroughly evaluate the antitumor potential of EV in MIBC.
Trophoblast cell surface antigen 2
The ADC, SG, comprises the hRS7 IgG1κ antibody bound to the cytotoxic agent SN-38 through a hydrolyzable CL2A linker. The hRS7 antibody specifically binds to trophoblast cell surface antigen 2 (Trop-2), a transmembrane glycoprotein that plays a role in regulating cell proliferation, migration, and survival in both stem cells and cancer cells[21]. Numerous investigations have shown elevated levels of Trop-2 in different cancers, including upper tract UC, making it an attractive therapeutic target due to its limited presence in healthy tissues. SN-38, the active metabolite of camptothecin, exerts its cytotoxic effects by inhibiting topoisomerase I, thereby inducing DNA damage and triggering apoptosis in cancer cells[22].
The phase II open-label TROPHY-U-01 study was conducted to investigate the therapeutic activity of SG in mUC. Cohort 1 included patients who had previously received platinum-based chemotherapy and ICIs but progressed. Following a median follow-up of 9.1 months, 27% of patients demonstrated an objective response, while 77% experienced a decrease in measurable disease[23]. The median duration of response (mDOR) was 7.2 (95%CI: 4.7-8.6) months, with mPFS and mOS of 5.4 (95%CI: 3.5-7.2) months and 10.9 (95%CI: 9.0-13.8) months, respectively. The aim of Cohort 3 was to evaluate the efficacy of SG in combination with pembrolizumab for patients with mUC who experienced disease progression after platinum-based chemotherapy[24]. With a median follow-up of 5.8 months, the objective response rate (ORR) was 34% (95%CI: 20.1-50.6), including one CR and 13 partial responses (PR). The mDOR was 2.0 (95%CI: 1.3-2.8) months[23]. Additionally, the 6-month PFS rate was 47%. Ongoing evaluations in Cohorts 4 and 5 focus on the use of SG plus cisplatin, administered either alone or with avelumab, as an initial treatment approach, with subsequent avelumab maintenance in patients with mUC who are eligible for cisplatin[25]. In light of these encouraging results, the FDA granted accelerated approval to SG for the treatment of patients with locally advanced or metastatic UC who had previously been treated with platinum-based chemotherapy and a PD-1 or PD-L1 inhibitor[26].
These findings are being further evaluated in the phase III confirmatory trial TROPiCS-04, which enrolled patients with histologically confirmed UC presenting with metastatic or locally advanced disease following progression on platinum-based chemotherapy and PD-(L)1 inhibitors[27]. Unfortunately, SG did not show a significant improvement in OS vs physician’s choice (paclitaxel, docetaxel, or vinflunine) in the confirmatory study, leading to the withdrawal of approval by the FDA.
Human epidermal growth factor 2
Human epidermal growth factor 2 (HER2) positivity in mUC shows a wide prevalence range—from 7% to 80%—with higher expression levels typically associated with more advanced tumor stage and grade[28]. This suggests a link between HER2 expression and tumor progression, as well as poor prognosis. The positive outcomes observed in breast and gastric cancers have encouraged researchers to explore HER2-targeted ADCs in UC as well[29]. T-DM1 was the ADC approved for the treatment of solid tumors. It consists of the monoclonal antibody trastuzumab, which targets HER2, linked to the cytotoxic agent DM1 through a non-cleavable thioether linker. Trastuzumab is a humanized antibody that specifically targets HER2, while DM1 interferes with microtubule formation by binding to tubulin. Trastuzumab deruxtecan (T-DXd) is another ADC that pairs trastuzumab with a topoisomerase I inhibitor through a cleavable tetrapeptide[30].
The open-label, multi-cohort, multicenter phase II DESTINY-Pantumor-02 trial, demonstrated benefits in ORR, PFS, DOR, and OS from T-DXd in 267 pre-treated patients with various HER2-expressing cancers, including 41 with mUC. The ORR was 39%, and this rate rose to 56.3% in tumors exhibiting high HER2 expression (IHC 3+). Among mUC patients, 1 (2.4%) achieved a CR, 15 (36.6%) had a PR, and 16 (39%) demonstrated stable disease, while 7 (17.1%) experienced disease progression. The mPFS was 7 (95%CI: 4.2-9.7) months, and the mOS was 12.8 (95%CI: 11.2-15.1) months. Around 85% of patients reported treatment-related adverse events (TRAEs) of any grade, with the most common being nausea (55.1%), anemia (27.7%), diarrhea (25.8%), vomiting (24.7%), and fatigue (24.7%). About 41% had grade 3 or higher TRAEs, with interstitial lung disease (ILD) occurring in 10% of cases, resulting in three fatalities[31].
DESTINY-Pantumor-01 is an open-label phase II basket study evaluating the efficacy of T-DXd in patients with unresectable or metastatic solid tumors driven by HER2 mutations. The trial enrolled 102 patients who had progressed after prior treatments, yielding an ORR of 29.4% (95%CI: 20.8%-39.3%). Approximately half of the participants experienced grade 3 or higher TRAEs, with anemia and neutropenia being the most common (16% and 8%, respectively). ILD or pneumonitis, generally of grade 1-2, occurred in 11 patients[32].
Disitamab vedotin (DV) is an ADC composed of the humanized anti-HER2 antibody hertuzumab, conjugated to the cytotoxic agent MMAE via a cleavable linker. Hertuzumab exhibits greater selectivity for HER2 compared to trastuzumab and has demonstrated enhanced antibody-dependent cell-mediated cytotoxicity[33]. The phase II trials RC48-C005 and RC48-C009 evaluated the efficacy and safety of DV in a cohort of 107 Chinese patients with HER2-positive mUC who had experienced disease progression following first-line chemotherapy. The combined analysis revealed an ORR of 50.5% (95%CI: 40.6%-60.3%). Additionally, 31.8% of patients achieved stable disease, 48.6% had a PR, and only 1.9% experienced a CR. Higher HER2 expression (IHC 3+ or IHC 2+ with FISH positivity) correlated with a higher ORR (62.2%), while lower expression (IHC 2+ with FISH negativity) resulted in a lower ORR (39.6%). The mOS was 14.2 (95%CI: 9.7-18.8) months, and the mPFS was 5.8 (95%CI: 4.2-7.2) months. The most common TRAEs included peripheral sensory neuropathy (68.2%), leukopenia (50.5%), neutropenia (42.1%), and elevated aspartate aminotransferase levels (42.1%). Grade 3 TRAEs occurred in 54.2% of patients, with peripheral sensory neuropathy (18.7%) and neutropenia (12.1%) being the most frequently reported. No grade 4 or 5 TRAEs were observed[34].
Disiitamab vedotin is also under evaluation in the ongoing RC48-G001 phase II trial trial, either as monotherapy or in combination with pembrolizumab, in patients with HER2-positive mUC. The study stratifies participants into three cohorts according to HER2 expression status and treatment history. Cohorts A and B include patients who have previously received one or two lines of systemic therapy, and both receive DV as a monotherapy. Cohort A is composed of individuals with HER2-positive tumors (defined as IHC 2+ or 3+ and FISH-positive), whereas Cohort B includes those with low HER2 expression (IHC 1+ or IHC 2+ and FISH-negative). Cohort C consists of treatment-naïve patients, regardless of HER2 expression status, who are treated with either DV alone or in combination with pembrolizumab[35].
Separately, the RC48-C011 trial is a phase II, open-label, single-arm study investigating DV in patients with HER2-negative mUC. While updated outcomes are still pending, early findings in 19 enrolled patients—none of whom had HER2 expression—showed an ORR of 26.3% (95%CI: 9.1%-51.2%), a mOS of 16.4 (95%CI: 7.1-21.7) months, and a mPFS of 5.6 (95%CI: 3.9-6.8) months. Most of the reported TRAEs were grade 1 or 2 in severity, reflecting a safety profile consistent with findings from the RC48-C005 and RC48-C009 trials[36].
Adverse events and toxicity management of ADCs in UC
While ADCs offer a favorable toxicity profile compared to conventional chemotherapy, TRAEs remain common and may require dose modifications or treatment discontinuation. Peripheral neuropathy, particularly with EV, is frequently observed (33%-53%) and is likely related to its MMAE component; although often low-grade, it can lead to treatment cessation. Dermatologic toxicities are also prevalent due to Nectin-4 expression in skin tissues, ranging from mild rashes to rare but severe conditions like Stevens-Johnson syndrome and toxic epidermal necrolysis. Hematologic toxicities, including anemia and neutropenia, are more commonly reported with SG, linked to its SN-38 payload, with ≥ G3 neutropenia occurring in up to 36% of patients. Other frequent TRAEs include fatigue, alopecia, nausea, diarrhea, and hyperglycemia. Management involves early recognition, patient education, symptom-directed interventions, and supportive care measures such as growth factor support or endocrinology referral. As ADCs like EV move into earlier disease settings and combination regimens, ongoing efforts aim to optimize dosing schedules and mitigate toxicity while preserving efficacy.
Resistance mechanisms to ADC
The structural complexity of ADCs compared to traditional small molecules presents multiple potential avenues for resistance to develop. ADCs—particularly those with non-cleavable linkers—generally follow a multi-step process involving antigen binding, internalization, and intracellular processing to release their cytotoxic payload. Resistance can arise at any of these steps. Although pinpointing specific intracellular resistance mechanisms remains challenging in preclinical models, several hypotheses have been proposed based on resistant cell line studies. These include impaired cellular uptake due to physical barriers such as thickened basement membranes, and altered internal trafficking, as seen in studies of T-DM1 where disrupted endosomal transport played a role. Additionally, shifts in internalization pathways—from clathrin-mediated to caveolin-dependent endocytosis—have been observed in resistant cell lines, potentially reducing ADC efficacy due to less efficient uptake and processing.
PATIENT SELECTION
Selecting the appropriate frontline therapy for mUC is essential for achieving optimal outcomes. This decision requires assessing clinical, molecular, and patient-specific factors. Most mUC patients are elderly, with many over 70 years old, often presenting with comorbidities that may limit treatment options. Cisplatin-based chemotherapy is a standard approach but is unsuitable for many patients due to renal impairment or poor performance status. Cisplatin eligibility generally requires a creatinine clearance (CrCl) of 60 mL/minutes or higher, whereas patients with CrCl below this threshold are typically treated with carboplatin-based regimens or ICIs[40]. Although criteria for cisplatin use are clearly established, guidelines for carboplatin eligibility are less consistent, resulting in variation across clinical practices. Patients with poor performance status (ECOG ≥ 3), severe renal impairment (CrCl < 30 mL/minute), peripheral neuropathy (grade > 3), or advanced heart failure are generally considered ineligible for platinum-based chemotherapy[41]. However, factors such as age, uncontrolled diabetes, or inadequate bone marrow reserve can further influence treatment decisions. Molecular biomarkers, particularly PD-L1 expression, play an increasing role in treatment selection. Patients with high PD-L1 expression who are ineligible for cisplatin may benefit from ICIs like pembrolizumab or atezolizumab[42]. However, PD-L1 status alone should not determine therapy but be part of a broader clinical assessment. Additionally, histological subtypes, such as micropapillary, sarcomatoid, or neuroendocrine variants, may require tailored approaches, including neoadjuvant chemotherapy or clinical trials[43]. Emerging therapies, such as EV and lurbinectedin, show promise for certain subtypes, though response rates vary. Patient preferences and quality of life are crucial in therapy selection. Discussing treatment risks, benefits, and impact on daily life ensures alignment with patient goals[44]. Additionally, clinical trial eligibility can offer access to innovative treatments. Genomic profiling, particularly FGFR3 mutations, is increasingly influencing therapy choices, though targeted treatments are currently reserved for second-line use[45]. Ongoing research may expand their role in frontline therapy, further personalizing mUC management.
PROMISING NEW ADCS
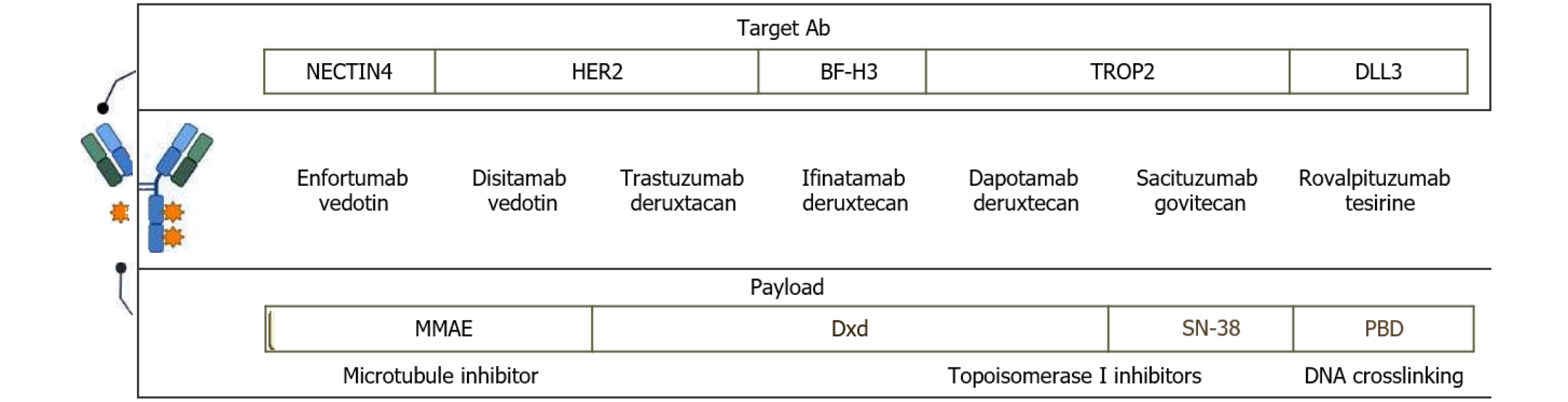
Emerging ADCs are rapidly expanding the therapeutic landscape for mUC, targeting novel antigens and leveraging innovative technologies. Advancements in ADCs involve modifying their target, linker, or payload (Figure 1). Datopotamab deruxtecan (Dato-DXd) is a TROP2-targeting ADCs that employs the Dxd cytotoxic payload, and showed a 19.2% response rate in mUC during the phase 1 TROPION-PanTumor01 trial[46]. Similarly, ifinatamab deruxtecan, targeting B7-H3 (highly expressed in bladder cancer), is under evaluation in a phase 1 trial[47]. DV, an ADC targeting HER2 and carrying an MMAE payload, demonstrated a 51% response rate in HER2-positive tumors based on pooled data from phase II studies. A phase 3 study is currently comparing DV plus pembrolizumab to chemotherapy in untreated HER-2-positive mUC[48]. Resistance mechanisms to ADCs remain unclear, particularly regarding sequential use of ADCs with identical payloads or targets. For instance, it is uncertain whether DV maintains efficacy after first-line EV plus pembrolizumab. However, findings from HER-2-positive breast cancer suggest that similar ADCs may still be effective when used sequentially. New approaches, such as bicycle agents, offer potential solutions. These agents have short half-lives and release their payloads within the tumor microenvironment without requiring internalization. BT8009, a bicycle toxin conjugate, demonstrated a 50% response rate in mUC patients during a phase 1/2 trial[49]. The upcoming Duravelo-2 trial will compare BT8009 plus pembrolizumab to chemotherapy in first-line treatment. Lastly, rovalpituzumab tesirine, an ADC targeting DLL3, showed promise in preclinical small-cell bladder cancer models but underperformed in a phase 3 Lung cancer study, exhibiting lower overall survival and increased side effects[50]. Meanwhile, CDCP1 is an emerging ADC target, with a CDCP1-directed ADC showing in vitro breast cancer cell death and high expression in bladder cancer samples[51].
Figure 1 A classification of antibody-drug conjugates based on their target antigens and cytotoxic payloads.
The antibody-drug conjugates (ADCs) are categorized according to their antigen specificity, including anti-NECTIN4, anti-HER2, anti-B7-H3, anti-TROP2, and anti-DLL3. Each ADC is listed alongside its corresponding payload, which is color-coded according to its mechanism of action: Microtubule inhibitors, topoisomerase I inhibitors (Dxd, SN-38), and DNA crosslinking agents. MMAE: Monomethyl auristatin E; Dxd: Deruxtecan; PBD: Pyrrolobenzodiazepine.
CONCLUSION
The future of ADCs lies in addressing challenges such as tumor resistance, antigen heterogeneity, and payload delivery. Strategies to enhance payload potency, improve antigen specificity, and refine linker designs are actively being pursued. The integration of ADCs with other therapeutic modalities, such as ICIs and targeted therapies, is a particularly promising avenue. Combination regimens leveraging the immune-priming effects of ICIs with the cytotoxic precision of ADCs have shown synergistic activity in preclinical models, and ongoing trials aim to validate these findings in clinical settings. Efforts are also focused on identifying predictive biomarkers to better stratify patients and optimize treatment outcomes. Advanced genomic and proteomic profiling may allow for the selection of patients most likely to benefit from specific ADCs, enhancing personalization. Additionally, liquid biopsies to monitor treatment response and detect resistance early during therapy could significantly improve clinical management. The advent of next-generation ADCs with dual payloads, improved bystander effects, and mechanisms to overcome efflux pump-mediated resistance is expected to redefine therapeutic possibilities. These innovations, combined with real-world evidence from expanded clinical use, will shape the future landscape of ADC development. With continued research and technological advancements, ADCs are poised to establish themselves as a cornerstone of mUC management, offering new hope for patients facing this challenging disease.
ADCs represent a paradigm shift in mUC treatment, offering targeted and effective options for refractory populations. EV, SG, and trastuzumab deruxtecan have set the stage for ADCs as standard-of-care therapies. With ongoing innovation and clinical trials, ADCs are poised to further revolutionize the treatment landscape, offering renewed hope to patients with mUC.
Looking ahead, personalized medicine is expected to be a pivotal breakthrough in optimizing the use of ADCs in UC. The integration of liquid biopsy technologies offers a promising avenue for real-time, non-invasive monitoring of treatment response, early detection of resistance mechanisms, and dynamic adjustment of therapeutic strategies. Coupled with genomic and proteomic profiling, these tools could enable more accurate patient selection and individualized treatment plans, ultimately enhancing the efficacy and safety of ADC-based therapies in clinical practice.