Published online Jul 24, 2025. doi: 10.5306/wjco.v16.i7.108666
Revised: May 22, 2025
Accepted: June 10, 2025
Published online: July 24, 2025
Processing time: 92 Days and 18.9 Hours
The prevalence of colorectal cancer (CRC) in younger people is increasing. Despite advances in precision medicine, the challenges of drug resistance and high costs persist. Nitidine chloride (NC) has pharmacological potential, and kinesin family member 20A (KIF20A) is overexpressed in various tumors; however, their interaction in CRC remains unexplored.
To investigate the KIF20A expression characteristics in CRC cells and determine whether it is a potential target gene for NC in inhibiting CRC treatment.
Single-cell RNA sequencing (scRNA-seq), spatial transcriptomics, and mRNA expression profiling were used to analyze KIF20A expression in CRC cells. Immunohistochemical staining was used to verify KIF20A expression in 416 clinical samples (208 CRC tissue samples and 208 noncancerous control tissue samples). Clustered regularly interspaced short palindromic repeats (CRISPR) technology was used to evaluate the impact of knocking out KIF20A on CRC cell growth. Molecular docking was applied to analyze NC–KIF20A binding. Finally, RNA sequencing and functional enrichment analysis were performed to explore the mechanism of action of NC in CRC cells.
Treating HCT116 cells with NC was found to significantly downregulate KIF20A (P < 0.05), and the molecular docking analysis revealed high-affinity binding between NC and KIF20A (binding energy = -9.6 kcal/mol). The scRNA-seq, spatial transcriptomics, and mRNA expression profiling results confirmed the significantly high expression of KIF20A in CRC tissues (standardized mean difference = 1.33, 95% confidence interval: 0.885-1.77, summary receiver operating characteristic curve area = 0.94). The immunohistochemical analysis of the clinical samples showed high KIF20A expression in the CRC tissues (P < 0.05), with significant correlation between the level of expression and gender, tumor size, and tumor grade (P < 0.05). Knocking out KIF20A significantly inhibited the growth of various CRC cell lines (CRISPR score < -0.3). The functional enrichment analysis indicated that NC may inhibit CRC by disrupting several biological processes, such as mitotic nuclear division, chromosome segregation, and microtubule binding.
Our results indicate that NC binds to KIF20A with high affinity and downregulates its expression in CRC cells, leading to reduced proliferation. Hence, NC has promise as a therapeutic agent in the treatment of CRC, and targeting KIF20A also has potential as a therapeutic strategy. Further KIF20A knockout studies are needed to confirm the binding specificity and mechanistic roles of NC in CRC.
Core Tip: In this study, we identified kinesin family member 20A (KIF20A) as a potential target for nitidine chloride (NC) in colorectal cancer (CRC) cells. Our multi-omics analysis revealed that KIF20A expression was elevated in CRC tissues, particularly in epithelial and proliferative regions, which correlated with clinical parameters. NC treatment significantly downregulated KIF20A in CRC cells, with molecular docking confirming direct binding, and knocking out KIF20A inhibited CRC cell growth. The pathway analysis suggests that NC suppresses CRC by disrupting mitosis, chromosome segregation, and microtubule dynamics by targeting KIF20A.
- Citation: Wu KJ, Zeng DT, He RQ, Li DM, Yao JL, Liu LM, Huang WJ, Qin DY, Li YF, He H, Li SD, Wen JY, Meng L, Shi JR, Chen G, Li H. Nitidine chloride may mediate its antitumor effects by targeting kinesin family member 20A in colorectal cancer cells. World J Clin Oncol 2025; 16(7): 108666
- URL: https://www.wjgnet.com/2218-4333/full/v16/i7/108666.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i7.108666
Colorectal cancer (CRC) is a malignant tumor with a high incidence and high mortality rate worldwide, making it a serious threat to human health. In addition, the prevalence of CRC in younger people has increased in recent years[1,2]. Fortunately, advances in molecular biology have led to the development of precision medicine and new pathways for CRC treatment, although challenges remain, such as drug resistance, insufficient treatment sensitivity, and high treatment costs[3,4]. Thus, there is an urgent need for efficient anti-CRC drugs with low toxicity and high clinical value.
Traditional Chinese medicine (TCM) has been used for thousands of years to treat tumors, and it is playing an increasingly important role in modern comprehensive cancer therapy due to its unique advantages, which include holistic regulation, multi-target intervention, and fewer side effects[5,6]. In addition, research conducted on the antitumor effects of TCM compounds is progressively employing evidence-based scientific research principles and models[7]. As a result, active components of TCM compounds are being isolated, molecular targets are being identified, and mechanisms of action are being elucidated, all of which provide valuable information for the development of antitumor strategies and lay a solid foundation for the modernization and internationalization of TCM[8,9].
Nitidine chloride (NC) is a benzo[c]phenanthridine alkaloid extracted from the Miao medicinal plant Zanthoxylum nitidum that exhibits various pharmacological activities[10]. Early research focused on the antibacterial, anti-inflammatory, and antimalarial effects of NC; however, in recent years, its antitumor potential has gradually gained attention[11]. Studies have shown that NC has significant inhibitory effects on various tumor cells, including lung cancer and liver cancer cells[12,13]. NC exerts these antitumor effects via multiple mechanisms; for example, by inducing apoptosis, inhibiting cell proliferation, arresting the cell cycle, and suppressing both cell migration and invasion[14]. However, research on NC’s antitumor effects and molecular mechanisms in the context of CRC remains insufficient, and further exploration is thus required.
Kinesin family member 20A (KIF20A) is a key member of the kinesin superfamily, which is involved in core biological processes, such as microtubule protein transport and cell division[15]. The abnormally high expression of KIF20A in various tumors has attracted widespread attention[16-18]. It has been found to accelerate tumor progression by promoting tumor cell proliferation, migration, and invasion and inhibiting apoptosis[16,19]. A growing body of evidence suggests that KIF20A is a highly promising therapeutic target in tumor treatment[20,21].
Given that KIF20A is highly expressed in tumors and has cancer-promoting characteristics and that NC exhibits unique multi-target antitumor activity, in this study, we aimed to systematically explore whether NC exerts anti-CRC effects by targeting KIF20A. We anticipate that the findings of this study will provide a theoretical basis and experimental support for the development of novel anti-CRC drugs.
The gene protein (ID: 8F18 | pdb_00008f18) and small molecule (ID: CID_4501) three-dimensional structures were obtained from the PubChem and RCSB databases. According to the molecular docking model used in this study[22], the Vina score needed to be < -4 kcal/mol. Hence, the exclusion criteria were as follows: Incomplete gene set or molecular structure data and/or a Vina score > -4 kcal/mol.
NC (96% purity; Chengdu Herbpurify Co. Ltd., China) was dissolved in dimethyl sulfoxide (DMSO; Solarbio, China) and diluted with culture medium to the desired experimental concentrations. The concentrations were based on the 48-hour half-maximal inhibitory concentration (IC50) value determined in a comprehensive dose–response analysis, in which proliferation of HCT116 cells positively correlated with NC concentration and treatment time. The IC50 of NC in HCT116 cells treated for 48 hours was 5.81 ± 0.11 μmol/L (negative control: Cells treated with DMSO).
HCT116 cells (National Collection of Authenticated Cell Cultures and Procell Life Science & Technology Co. Ltd.) were cultured in RPMI 1640 medium (1×; Thermo Fisher, China). The medium was supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin (100 ×; both from Solarbio, China). The cells were maintained at 37 °C with 5% CO2 in a dedicated CO2 incubator under strictly controlled conditions.
HCT116 cells were divided into two groups: The control group (cells treated with 0.1% DMSO) and the NC group (cells treated with 6 μmol/L NC). Each group consisted of three accessory wells. HCT116 cells in the logarithmic growth phase were collected, the concentration of the cell suspension was adjusted, and the cells were seeded in 6-well plates. When the cell fusion rate reached about 60%, the original medium was replaced with medium containing NC or DMSO, and the cells were then incubated in 5% CO2 at 37 °C for 48 hours. Cell samples were sent to HAIYI ERATM for whole genome sequencing.
For the gene expression analysis, total RNA was extracted from cells from the NC and control groups using the AxyPrepTM Multisource Total RNA Miniprep Kit (Axygen, China). The mRNA sequencing library was constructed via mRNA purification, fragmentation, reverse transcription, adapter ligation, and polymerase chain reaction amplification. Sequencing was performed using the Illumina platform.
The mRNA levels of the annotated protein-coding genes were quantified, and the gene expression correlations within/between the groups were assessed as transcripts per million. Significantly downregulated genes (log2-fold change < -1, adjusted P < 0.05) were identified as negatively regulated differentially expressed genes in response to NC treatment.
For the single-cell RNA (scRNA) sequencing (scRNA-seq) analysis of KIF20A, we used the CRC-related GSM8657093 scRNA dataset (made public on February 12, 2025), which is accessible through the Gene Expression Omnibus (GEO) database. First, the Seurat package[23] was used to preprocess and filter the data. Genes that were expressed in < 3 cells and cells with < 50 expressed genes were removed. Next, during the quality control process, cells expressing > 500 genes and a mitochondrial content < 25% were retained. After the data were normalized, a principal component analysis dimensionality reduction was conducted, with the top 20 principal components selected for the clustering analysis. Subsequently, the uniform manifold approximation and projection algorithm was used to perform the secondary dimensionality reduction.
The CRC-related GSM8594568 scRNA dataset (made public on March 17, 2025; accessible through the GEO database) was used in the spatial transcriptome analysis. First, the Seurat package was used to process the data. The data were normalized using the SCTransform method to ensure dataset standardization, spatial data use was specified, and the detailed output was turned off to simplify the computation. Next, the SpatialDimPlot function was used to generate spatial dimension plots and visualize the spatial distribution of the cells in the samples. The cell spatial location and KIF20A expression patterns are presented together.
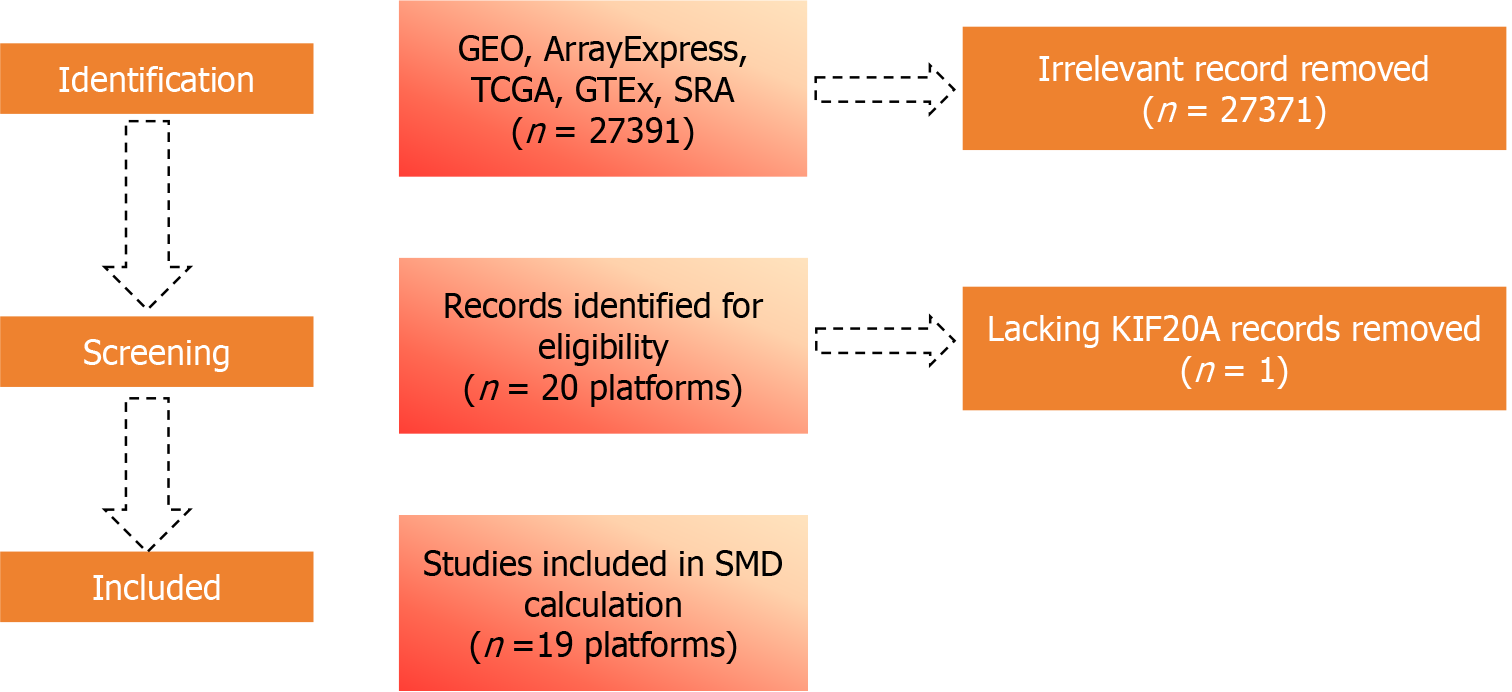
To analyze and compare the expression of KIF20A mRNA in CRC and non-CRC tissue samples, expression data were obtained from the GEO, International Cancer Genome Consortium, Genotype-Tissue Expression Project, Sequence Read Archive, The Cancer Genome Atlas, PubMed, and ArrayExpress databases (Figure 1). The following keywords were used to search the databases: “colorectal cancer”, “colorectal carcinoma”, and “CRC”. The following inclusion criteria were applied: (1) Only datasets containing human primary CRC tissue samples were considered; (2) The experimental group was required to contain CRC diagnostic tissue samples, with normal colorectal tissue samples serving as the control group; and (3) Both the experimental and control groups were required to contain ≥ 3 samples. Datasets with unreported KIF20A expression or metastatic/recurrent CRC tissue samples were excluded.
Datasets from the same GEO platform were merged into a comprehensive matrix. Log2 (x + 1) transformation was used to normalize and log-transform the KIF20A mRNA expression levels. Any batch effects were corrected using the limma and sva R packages. The meta package (version 4.18-2) was used to calculate the standardized mean difference (SMD) of the CRC genes to evaluate the differences between the CRC and non-CRC samples, thereby revealing the potential pathogenic role that KIF20A plays in the occurrence of CRC. The following criteria were used to screen for genes highly expressed in the CRC tissue samples: (1) The gene appeared in ≥ 3 independent studies, (2) The SMD was > 0; and (3) The 95% confidence interval (95%CI) did not include 0. To identify the KIF20A-related co-expressed genes, Spearman’s correlation analysis was conducted based on the following criteria: The gene was co-expressed with KIF20A in ≥ 10 studies, the Spearman’s r was > 0.30, and P was < 0.05. The ClusterProfiler package was used to functionally annotate the intersecting genes, while Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway analyses were performed to explore involvement in biological processes and signaling pathways.
We collected 416 tissue samples from Yulin Red Cross Hospital (208 CRC tissue samples and 208 noncancerous tissue samples). Immunohistochemical staining was performed using an anti-KIF20A antibody (Sc-374508, Invitrogen™, www.scbt.com; 1:1000). After staining, the tissue microarrays were incubated again, stained, dehydrated, and sealed at room temperature. The staining intensity was graded as 0 (no staining, blue), 1 (weak staining, light yellow), 2 (moderate staining, yellow-brown), or 3 (strong staining, dark brown). The proportion of positive cells was scored as 0 (< 5%), 1 (5-25%), 2 (26-50%), 3 (51-75%), or 4 (> 75%). The final immunohistochemical score was calculated by multiplying the intensity score by the percentage score (range: 0-12). Two pathologists independently assessed the samples and determined the immunohistochemical scores. The Wilcoxon rank-sum test was used to compare the relevant clinical baseline data of the CRC tissue and adjacent noncancerous tissue samples. All patients provided informed consent, and the study was approved by the Yulin Red Cross Hospital Ethics Committee (number: Z20210442).
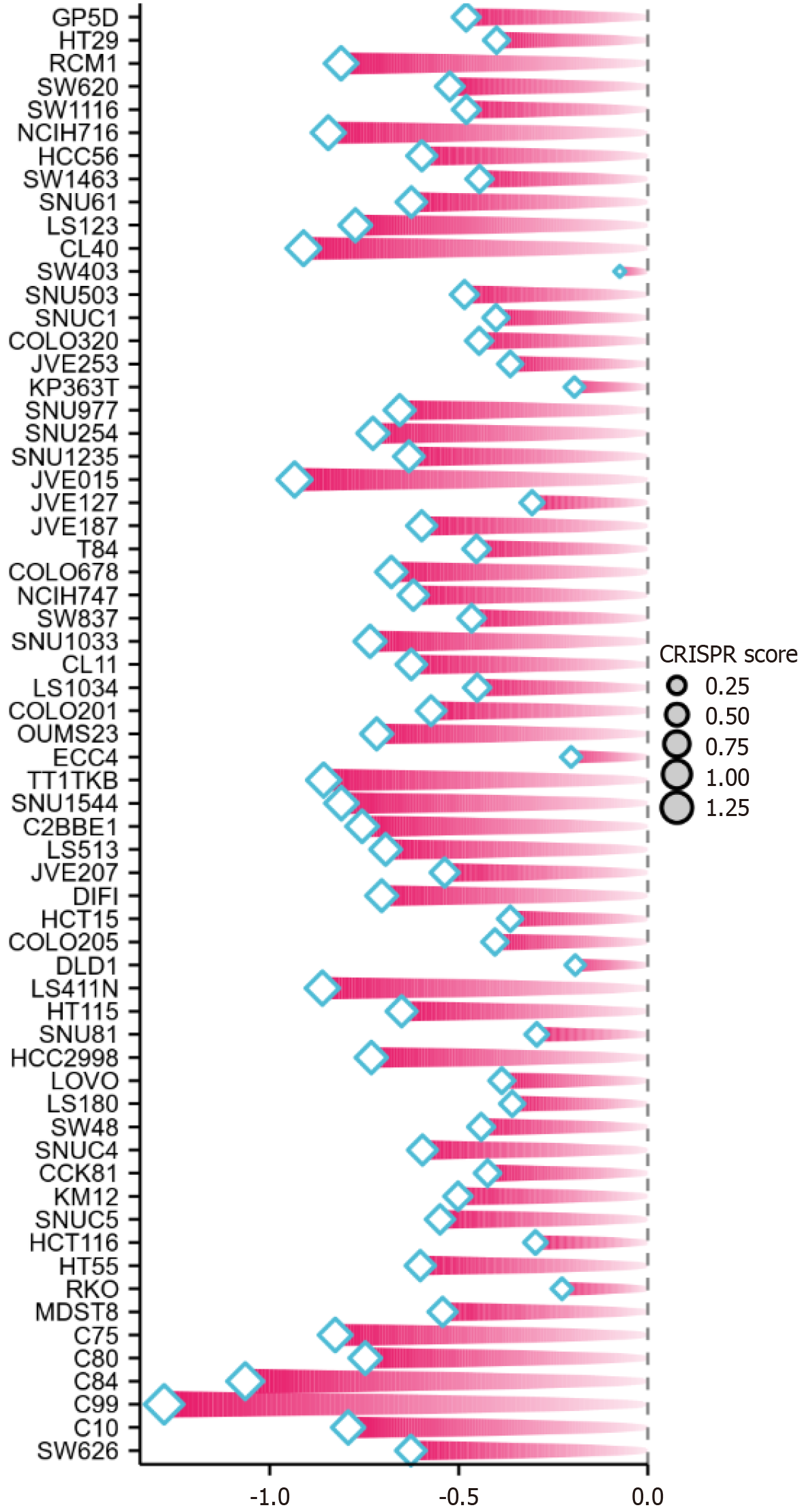
To study the role that KIF20A plays in CRC cells, we used clustered regularly interspaced short palindromic repeats (CRISPR) knockout screening technology. The CERES algorithm was used to calculate dependency scores and hence determine the criticality of KIF20A in various CRC cell lines. Negative dependency scores indicated that knocking out KIF20A impedes cell growth and that KIF20A is necessary for cell proliferation. Conversely, positive dependency scores implied that knocking out KIF20A promotes growth and that KIF20A has potential inhibitory effects[24].
We used the Wilcoxon rank-sum test to assess differences in KIF20A expression. When P was < 0.05, the result was considered statistically significant. A fixed-effects model was used to calculate the SMD when heterogeneity was low (I² < 50%); otherwise, a random-effects model was used. The pROC package was used to plot receiver operating characteristic curves, and STATA 18.0 software was used to generate sROC curves. Area under the curve (AUC) values were used to determine KIF20A expression, with larger AUC values indicating greater expression. Begg’s test was used to evaluate publication bias. A P value > 0.05 indicated no publication bias.
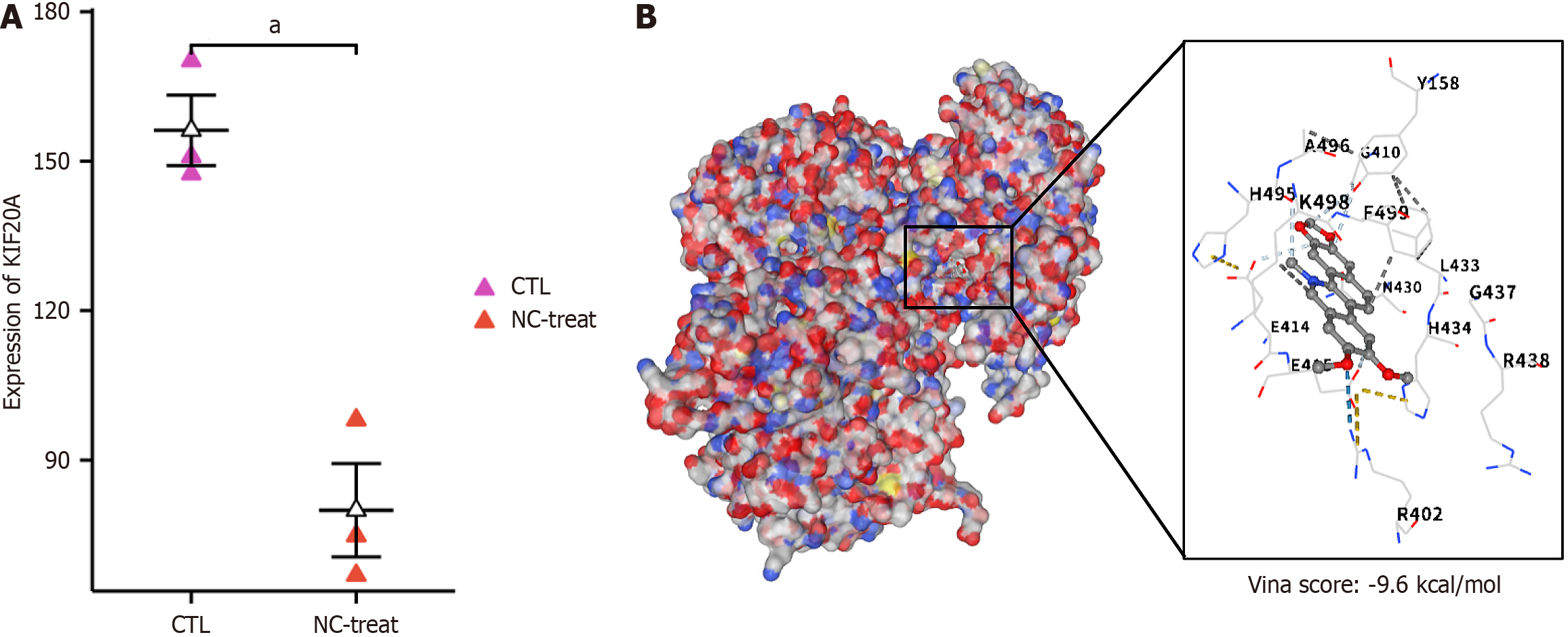
HCT116 cells treated with NC exhibited significant downregulation of KIF20A mRNA (Figure 2A, P < 0.05). The molecular docking analysis showed that the binding energy between NC and KIF20A reached -9.6 kcal/mol (Figure 2B), indicating stable, high-affinity binding between NC and KIF20A.
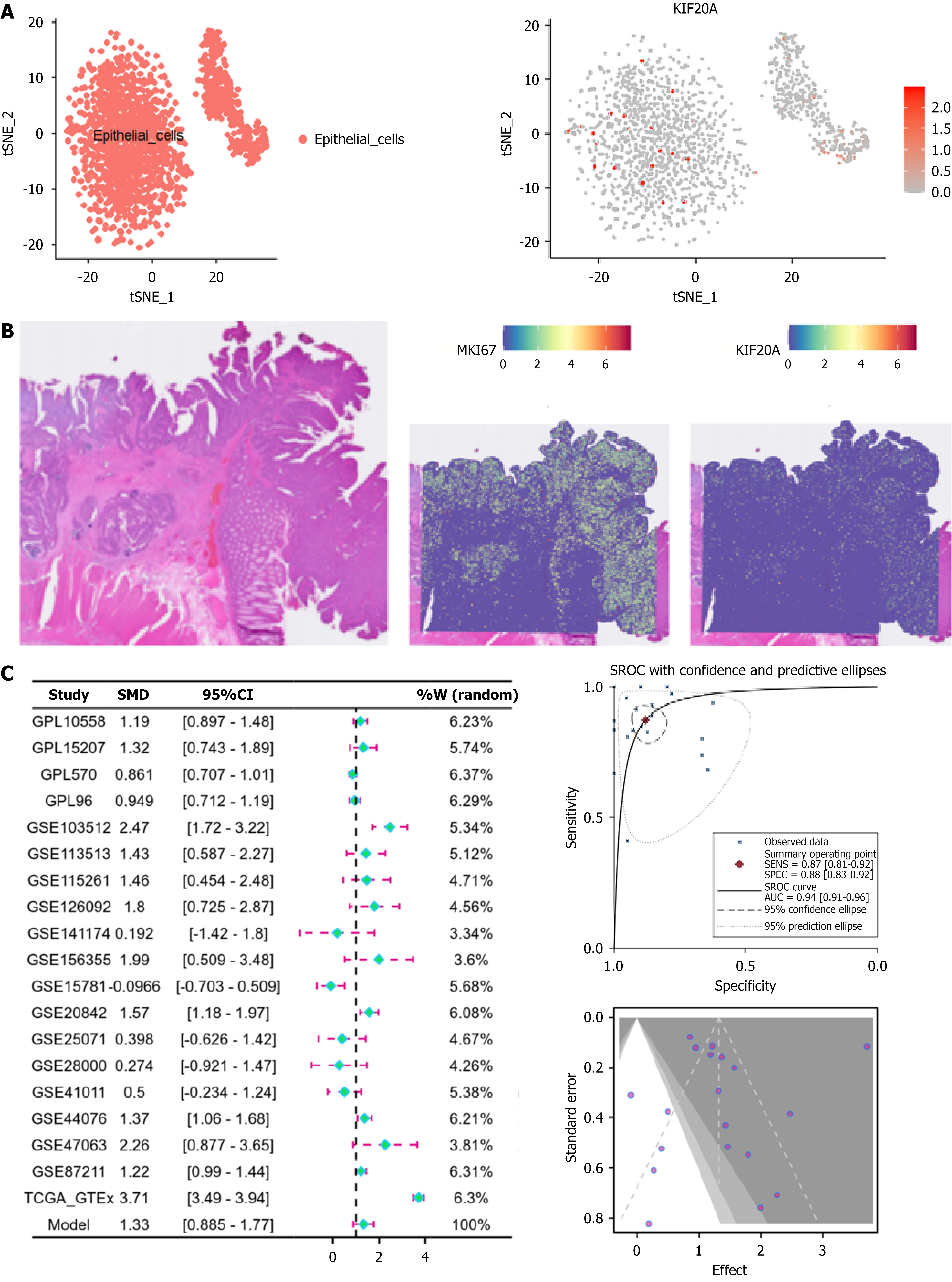
The scRNA-seq, spatial transcriptomics, and mRNA-level analyses showed that KIF20A was highly expressed in epithelial CRC cells [SMD = 1.33, 95%CI: 0.885-1.77], sROC AUC = 0.94; Figure 3A) and in tissues with high KI67 expression (Figure 3B). The Begg’s test result indicated no publication bias (P > 0.05), and the funnel plot showed no heterogeneity (Figure 3C). As shown in Figures 4 and 5, the high levels of KIF20A expression observed in the CRC tissues were verified through immunohistochemical staining of the clinical samples (P < 0.05). Additionally, KIF20A expression significantly correlated with gender, tumor size, and tumor grades I-III in the clinical baseline data (P < 0.05; Figure 5).
Knocking out KIF20A was found to significantly inhibit the growth of cells from multiple CRC cell lines, including the C99, HCT116, and C84 cell lines (CRISPR score < -0.3; Figure 6).
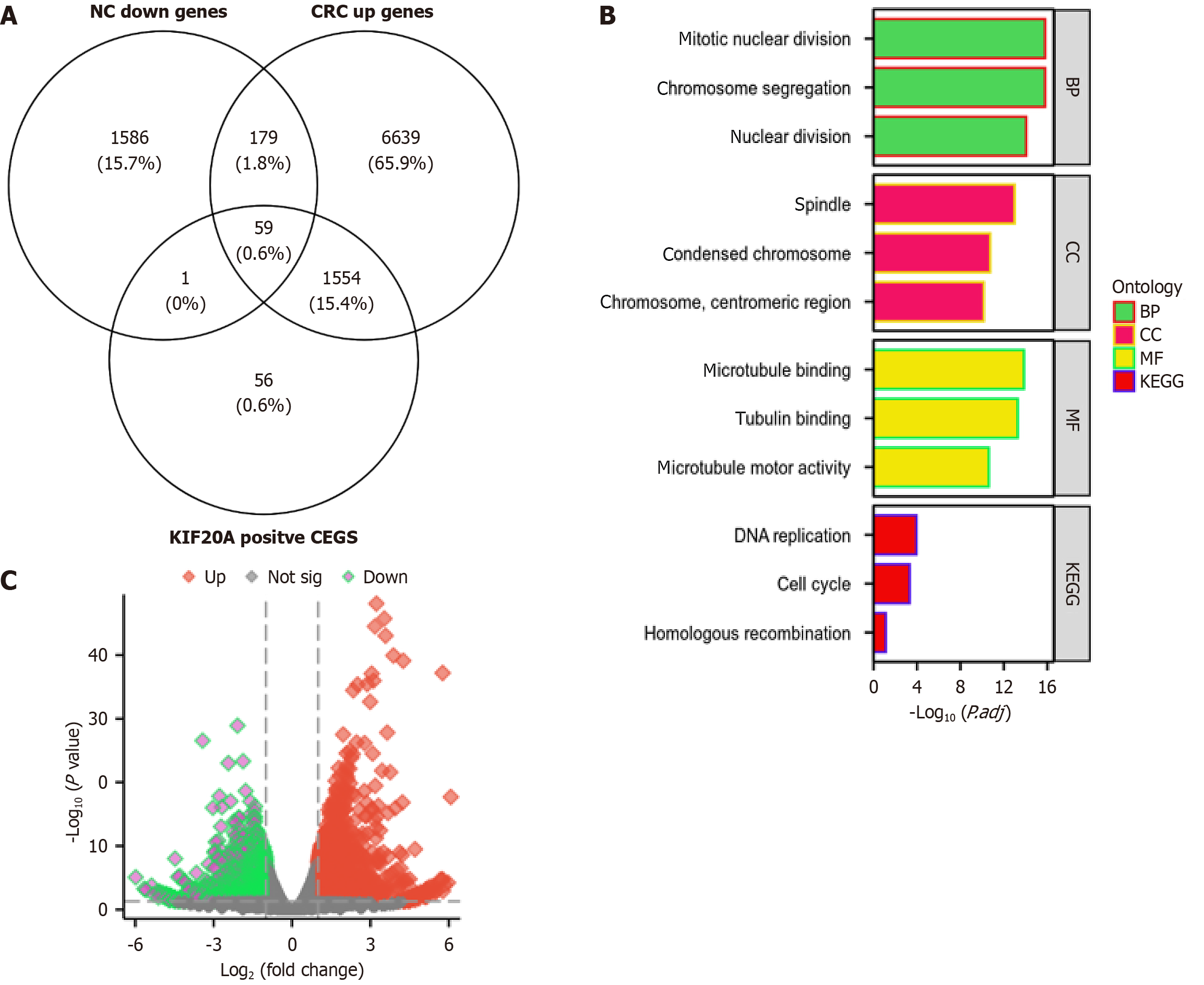
We performed an enrichment analysis using intersecting genes that were highly expressed in CRC cells, that positively correlated with KIF20A expression, and that were downregulated in CRC cells after NC treatment (Figure 7A and B). These genes were significantly enriched in several functional processes, including mitotic nuclear division, chromosome segregation, nuclear division, spindle, chromosome condensation, microtubule binding, tubulin binding, microtubule motor activity, DNA replication, cell cycle, and homologous recombination (Figure 7C).
Using a multi-omics approach, we have shown that KIF20A exhibits significantly high expression in CRC tissues. The immunohistochemical staining results verified that KIF20A expression was higher in CRC tissues compared to adjacent tissues. Our results also show that KIF20A expression significantly correlates with clinical characteristics, such as patient gender and tumor size. These findings echo those of previous studies and provide further evidence that KIF20A plays a role in CRC development[16-18].
When we assessed the effect of NC on KIF20A levels in HCT116 cells to examine its potential as an antitumor agent, we found that KIF20A was significantly downregulated. In addition, the molecular docking analysis revealed that NC likely binds to KIF20A with high affinity, which strongly suggests that NC may exert its anti-CRC activity by specifically targeting KIF20A. This finding provides a new theoretical basis for NC’s antitumor activity and opens new pathways for developing anti-CRC strategies that focus on inhibiting KIF20A.
To further elucidate how NC mediates its anti-CRC effect at the molecular level, we conducted a systematic analysis of the genes that were highly expressed in CRC cells, that positively correlated with KIF20A expression, and that were downregulated after NC treatment. The results showed that these genes are mainly enriched in key biological processes, including mitosis, chromosome distribution, spindle formation, microtubule binding, and DNA replication. Previous studies have established that KIF20A/MKlp2 is a microtubule motor protein and a member of the kinesin-6 subfamily, and that it plays a key role in the organization of the central spindle and cytokinesis during late mitosis[25].
The results of the dependency analysis, which was performed using the CRISPR algorithm, showed that knocking out KIF20A would likely significantly inhibit the proliferation of cells from multiple CRC cell lines (C99, HCT116, and C84). This finding is consistent with those of previous studies that showed that knocking out KIF20A led to cell cycle arrest and increased apoptosis[26,27].
In pharmacodynamic studies, NC has been shown to inhibit CRC cell proliferation and induce apoptosis in a dose- and time-dependent manner[28]. In-depth mechanistic studies have also revealed that NC treatment results in the significant upregulation of pro-apoptotic molecules (Bax, p53, and cleaved caspases 3 and 9) and inhibition of anti-apoptotic proteins (e.g., Bcl-2)[29]. It has also been shown that NC, which is a structurally unique benzophenanthridine alkaloid, exerts its antitumor effects through multi-target mechanisms, including inhibiting cancer cell growth and interfering with protein synthesis[12,30,31].
In this study, we systematically elucidated the molecular mechanism by which NC inhibits CRC cell proliferation. It acts by downregulating KIF20A. This finding provides insight into KIF20A’s clinical significance in CRC and expands our understanding of NC’s antitumor mechanism.
NC exhibits high-affinity binding to KIF20A and downregulates its expression in CRC cells, which is associated with the suppression of cell proliferation. These findings suggest that KIF20A is a potential target of NC in CRC and that binding to KIF20A mediates the anti-proliferative effect of NC. As a potential specific inhibitor of KIF20A, NC shows promise as a new drug candidate in the CRC treatment field. Given the clinical relevance of KIF20A and its effect on cancer cell viability, targeting KIF20A and its pathways represents a promising therapeutic strategy for CRC management. However, further experiments, such as KIF20A knockout studies, are necessary to confirm the specificity of the NC–KIF20A interaction and to elucidate its mechanistic role in therapeutic outcomes.
We would like to thank “Guangxi Zhuang Autonomous Region Clinical Medicine Research Center for Molecular Pathology and Intelligent Pathology Precision Diagnosis” for providing technical support.
| 1. | Zheng Z, Wang L. Perspective on enhancing CRC risk prediction. Gut. 2025;gutjnl-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Fang H, Dai W, Gu R, Zhang Y, Li J, Luo W, Tong S, Han L, Wang Y, Jiang C, Wang X, Wang R, Cai G. Correction: myCAF-derived Exosomal PWAR6 accelerates CRC liver metastasis via altering glutamine availability and NK cell function in the tumor microenvironment. J Hematol Oncol. 2025;18:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Cherri S, Libertini M, Noventa S, Oneda E, Meriggi F, Zaniboni A. What Is Next for Refractory Colorectal Cancer CRC? Looking Beyond SUNLIGHT, FRESCO2, RECURSE and CORRECT. Int J Mol Sci. 2025;26:2522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 4. | Brockmueller A, Buhrmann C, Moravejolahkami AR, Shakibaei M. Resveratrol and p53: How are they involved in CRC plasticity and apoptosis? J Adv Res. 2024;66:181-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Aljabali AAA, Obeid MA, Bashatwah RM, Qnais E, Gammoh O, Alqudah A, Mishra V, Mishra Y, Khan MA, Parvez S, El-Tanani M, Hatahet T. Phytochemicals in Cancer Therapy: A Structured Review of Mechanisms, Challenges, and Progress in Personalized Treatment. Chem Biodivers. 2025;e202402479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Wang S, Wang H, Zhang Y, Yin G, Zhang X, Zhang F. Epimedium and its chemical constituents in cancer treatment: A comprehensive review of traditional applications, antitumor effects, pharmacokinetics, delivery systems, and toxicology. J Ethnopharmacol. 2025;347:119738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Lin P, Qin Z, Chen X, Zhang X, Lin Y, Wang Q, He L, Guo J, Xu D, He R, Wu H, Yao X, Yao Z. Deciphering the effective components of a TCM formula for atherosclerosis by three-dimensional pattern recognition of exogenous components correlated with endogenous metabolites. J Ethnopharmacol. 2025;346:119647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Jiao Y, He Q, Li X, Chen Y, Tian T, Cao L, Zhang Z. Genome-wide identification of starch metabolism gene families in Potentilla anserina and the expression pattern in response to abiotic stress factors. BMC Plant Biol. 2025;25:201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Gu Z, Yang C, Li M. Impact of the five-pattern personality traits of traditional Chinese medicine on workplace violence and depression among nurses. BMC Nurs. 2025;24:68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Luo FH, Chen ZH, Zeng FF, Yang X, Li JJ, Zhang FX, Shi W. Botany, phytochemistry, pharmacologic activities, traditional applications, pharmacokinetics, quality control and toxicity of Zanthoxyli Radix: An updated review. J Ethnopharmacol. 2025;337:118783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Lu Q, Luo S, Shi Z, Yu M, Guo W, Li C. Nitidine chloride, a benzophenanthridine alkaloid from Zanthoxylum nitidum (Roxb.) DC., exerts multiple beneficial properties, especially in tumors and inflammation-related diseases. Front Pharmacol. 2022;13:1046402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 12. | Xiong DD, Chen ZD, Li JD, Deng YL, He RQ, Huang ZG, An SQ, Dang YW, Chen G. Nitidine chloride inhibits the progression of hepatocellular carcinoma by suppressing IGF2BP3 and modulates metabolic pathways in an m(6)A-dependent manner. Mol Med. 2025;31:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 13. | Li JD, He RQ, Dang YW, Huang ZG, Xiong DD, Zhang L, Du XF, Chen G. Unveiling expression patterns, mechanisms, and therapeutic opportunities of transmembrane protein 106C: From pan-cancers to hepatocellular carcinoma. World J Gastrointest Oncol. 2025;17:92437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Chen F, Peng S, Li C, Yang F, Yi Y, Chen X, Xu H, Cheng B, Xu Y, Xie X. Nitidine chloride inhibits mTORC1 signaling through ATF4-mediated Sestrin2 induction and targets IGF2R for lysosomal degradation. Life Sci. 2024;353:122918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Ranaivoson FM, Crozet V, Benoit MPMH, Abdalla Mohammed Khalid A, Kikuti C, Sirkia H, El Marjou A, Miserey-Lenkei S, Asenjo AB, Sosa H, Schmidt CF, Rosenfeld SS, Houdusse A. Nucleotide-free structures of KIF20A illuminate atypical mechanochemistry in this kinesin-6. Open Biol. 2023;13:230122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Yang M, Huang H, Zhang Y, Wang Y, Zhao J, Lee P, Ma Y, Qu S. Identification and validation of KIF20A for predicting prognosis and treatment outcomes in patients with breast cancer. Sci Rep. 2024;14:31543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Zhang R, Li L, Li H, Bai H, Suo Y, Cui J, Wang Y. Ginsenoside 20(S)-Rg3 reduces KIF20A expression and promotes CDC25A proteasomal degradation in epithelial ovarian cancer. J Ginseng Res. 2024;48:40-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 18. | Wang Q, Wu H, Wu Q, Zhong S. Berberine targets KIF20A and CCNE2 to inhibit the progression of nonsmall cell lung cancer via the PI3K/AKT pathway. Drug Dev Res. 2023;84:907-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 19. | Hu X, Chen Y, Ying H, He C, Ren Y, Tian Y, Tan Y. Metabolic-associated fatty liver disease (MAFLD) promotes the progression of hepatocellular carcinoma by enhancing KIF20A expression. Int Immunopharmacol. 2025;154:114589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Zhao X, Xuan F, Li Z, Yin X, Zeng X, Chen J, Fang C. A KIF20A-based thermosensitive hydrogel vaccine effectively potentiates immune checkpoint blockade therapy for hepatocellular carcinoma. NPJ Vaccines. 2025;10:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 21. | Xu J, Cheng W, Wang Y, Zhou Y, Wang Z, Dai Y, Li Y, Chen P, Liu T, Li Y, Li G, Qu W, Chen J. KIF20A activated by transcription factor GATA2 promotes cell growth in hepatitis B virus-related hepatocellular carcinoma. Front Cell Infect Microbiol. 2024;14:1497427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Liu Y, Yang X, Gan J, Chen S, Xiao ZX, Cao Y. CB-Dock2: improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022;50:W159-W164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 901] [Article Influence: 225.3] [Reference Citation Analysis (0)] |
| 23. | Rich JM, Moses L, Einarsson PH, Jackson K, Luebbert L, Booeshaghi AS, Antonsson S, Sullivan DK, Bray N, Melsted P, Pachter L. The impact of package selection and versioning on single-cell RNA-seq analysis. bioRxiv. 2024;2024.04.04.588111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 24. | Ho KH, Huang TW, Liu AJ, Shih CM, Chen KC. Cancer Essential Genes Stratified Lung Adenocarcinoma Patients with Distinct Survival Outcomes and Identified a Subgroup from the Terminal Respiratory Unit Type with Different Proliferative Signatures in Multiple Cohorts. Cancers (Basel). 2021;13:2128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Wu WD, Yu KW, Zhong N, Xiao Y, She ZY. Roles and mechanisms of Kinesin-6 KIF20A in spindle organization during cell division. Eur J Cell Biol. 2019;98:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Geng A, Qiu R, Murai K, Liu J, Wu X, Zhang H, Farhoodi H, Duong N, Jiang M, Yee JK, Tsark W, Lu Q. KIF20A/MKLP2 regulates the division modes of neural progenitor cells during cortical development. Nat Commun. 2018;9:2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Chen S, Zhao L, Liu J, Han P, Jiang W, Liu Y, Hou J, Wang F, Li J. Inhibition of KIF20A enhances the immunotherapeutic effect of hepatocellular carcinoma by enhancing c-Myc ubiquitination. Cancer Lett. 2024;598:217105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Zhai H, Hu S, Liu T, Wang F, Wang X, Wu G, Zhang Y, Sui M, Liu H, Jiang L. Nitidine chloride inhibits proliferation and induces apoptosis in colorectal cancer cells by suppressing the ERK signaling pathway. Mol Med Rep. 2016;13:2536-2542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Ou X, Lu Y, Liao L, Li D, Liu L, Liu H, Xu H. Nitidine chloride induces apoptosis in human hepatocellular carcinoma cells through a pathway involving p53, p21, Bax and Bcl-2. Oncol Rep. 2015;33:1264-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Zhang J, Wu L, Lian C, Lian S, Bao S, Zhang J, Wang P, Ma J, Li Y. Nitidine chloride possesses anticancer property in lung cancer cells through activating Hippo signaling pathway. Cell Death Discov. 2020;6:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Yu F, Tan W, Chen Z, Shen X, Mo X, Mo X, He J, Deng Z, Wang J, Luo Z, Yang J. Nitidine chloride induces caspase 3/GSDME-dependent pyroptosis by inhibting PI3K/Akt pathway in lung cancer. Chin Med. 2022;17:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/