TO THE EDITOR
Bladder cancer (BLCA) is a common malignancy of the urinary tract, with an estimated 613000 new cases and 220000 deaths worldwide in 2022[1]. Despite advances in diagnostic and therapeutic techniques, high recurrence rates and lack of targeted therapeutic options remain major clinical challenges[1]. The identification of novel oncogenic drivers and the elucidation of their molecular mechanisms are critical to addressing these challenges. While the role of HOXC6, a member of the HOX transcription factor family, in embryonic development and certain malignancies (e.g. prostate and pancreatic cancer) has been recognised[2,3], its specific function and regulatory pathways in BLCA have not been systematically investigated. In this commentary, we focus on the pioneering work of Lu et al[4], who recently demonstrated that HOXC6 drives BLCA progression through a novel regulatory axis involving the circadian regulator TIMELESS. This finding not only sheds light on the unexplored oncogenic role of HOXC6 in BLCA, but also establishes a mechanistic link between circadian regulation and tumour progression. Figure 1 illustrates the proposed HOXC6-TIMELESS axis in bladder cancer, highlighting the interactions and downstream effects that underpin its role in tumorigenesis. By identifying the HOXC6-TIMELESS axis as a potential driver of BLCA, this study introduces a unique molecular perspective and provides a basis for the development of precision therapies and biomarker strategies targeting this axis. We highlight this significant development, place it in the wider context of BLCA research and fill an important gap in the current literature.
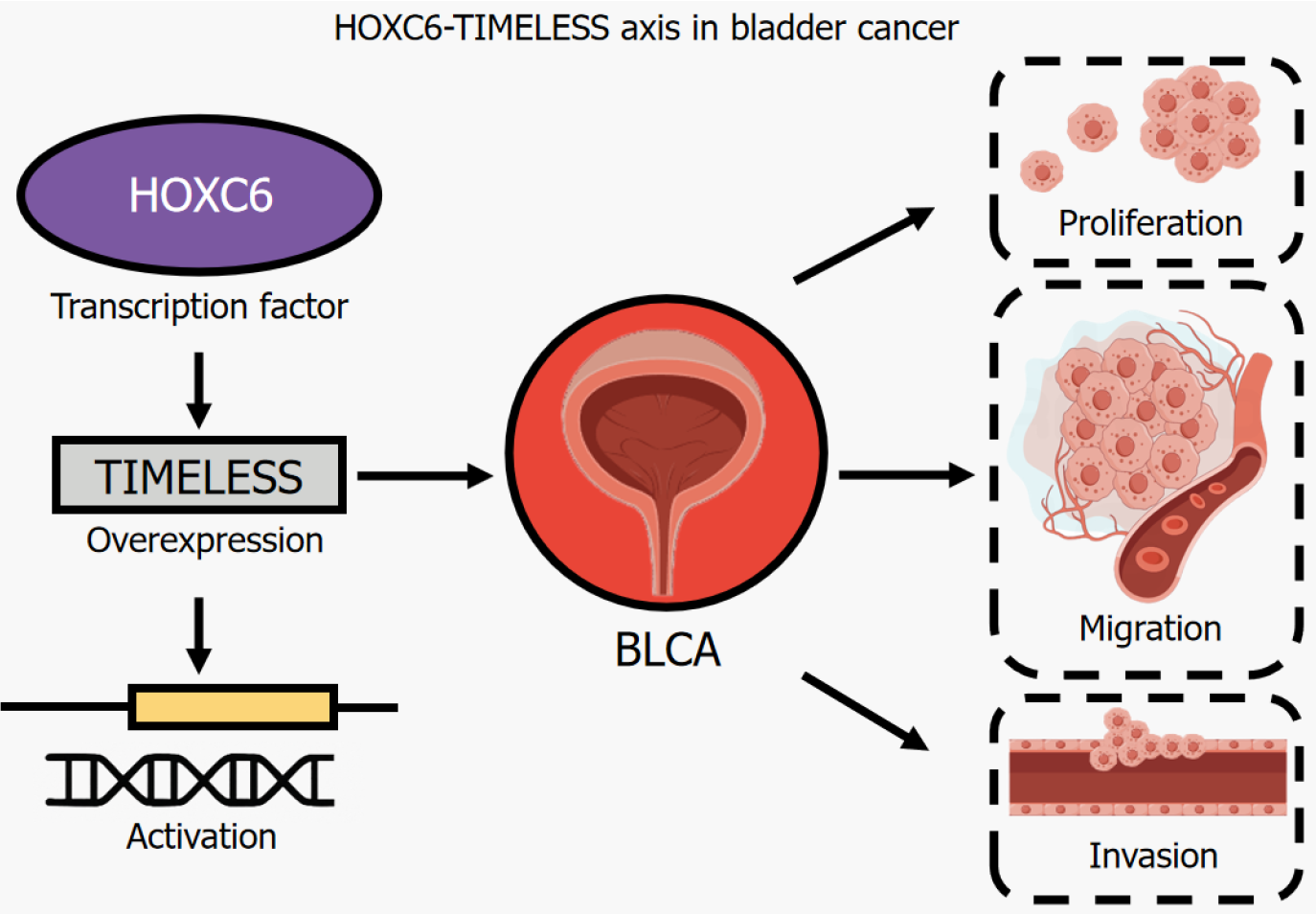
Figure 1 HOXC6-TIMELESS axis in bladder cancer.
BCLA: Bladder cancer.
HOXC6, a member of the HOX gene family, functions as a transcription factor that regulates cell differentiation and tissue development[5]. However, its aberrant expression has been implicated in oncogenic processes such as proliferation, invasion and metastasis in various cancers[2-5]. Lu et al[4] first identified HOXC6 as a potential oncogenic driver in BLCA through transcriptomic and proteomic analyses, revealing its significant overexpression in BLCA tissues compared to normal bladder tissues. This suggests that HOXC6 may contribute to BLCA progression by modulating key molecular pathways involved in cell proliferation, migration and survival. Functionally, HOXC6 knockdown in BLCA cells suppressed proliferation, migration and invasion, highlighting its role in tumour aggressiveness. Mechanistically, HOXC6 knockdown resulted in an accumulation of cells in the G0/G1 phase, suggesting its role in promoting the G1/S transition. This regulatory function may be mediated through interactions with key cell cycle regulators, including cyclin D1, cyclin E, cyclin-dependent kinases and retinoblastoma protein, which collectively facilitate cell cycle progression[6]. Additionally, HOXC6 has been reported to suppress apoptosis by modulating the expression of pro- and anti-apoptotic genes[2,3]. Studies in cervical cancer have shown that HOXC6 downregulates pro-apoptotic factors such as BAX and PUMA while upregulating BCL-2, an anti-apoptotic protein essential for tumour cell survival[3]. A similar mechanism may underlie HOXC6-driven BLCA progression, highlighting the need for further research into its downstream targets that regulate apoptosis.
The tumour microenvironment (TME) plays a critical role in supporting cancer progression and immune evasion[7]. Studies have shown that HOXC6 contributes to the remodelling of the TME by influencing extracellular matrix remodelling, angiogenesis and immune regulation[8-10]. In oral squamous cell carcinoma, HOXC6 is associated with TGF-β signalling, which is a key driver of EMT and tumour invasion[11]. In BLCA, the role of HOXC6 in TME formation remains largely unexplored, but preliminary evidence suggests that upregulation of HOXC6 is associated with increased metastatic potential. In addition, HOXC6 may regulate immune checkpoints and inflammatory mediators within the TME, thereby modulating immune cell infiltration and response to therapy[10]. Given the growing interest in immunotherapy for the treatment of BLCA, elucidating the role of HOXC6 in the mechanism of immune evasion may have important clinical implications.
TIMELESS is a core component of the circadian regulatory system and plays a critical role not only in maintaining biological rhythms, but also in controlling cell cycle progression, DNA replication, genome stability and oxidative stress response-processes essential for cellular homeostasis[12]. However, dysregulated TIMELESS expression has been implicated in several malignancies, where its aberrant upregulation promotes tumour proliferation, apoptosis resistance and metastasis[13]. Despite its recognised oncogenic potential, the mechanisms underlying TIMELESS overexpression in BLCA remain largely unexplored. Lu et al[4] identified a strong positive correlation between HOXC6 and TIMELESS expression in BLCA, suggesting a transcriptional regulatory relationship. HOXC6 binds directly to the promoter region of TIMELESS, activating its transcription and driving its oncogenic functions. This interaction establishes the HOXC6-TIMELESS axis as a key contributor to BLCA progression, with HOXC6 increasing tumour aggressiveness, at least in part, by upregulating TIMELESS. Pathway enrichment analyses further suggest that the HOXC6-TIMELESS axis is involved in key oncogenic pathways, including chemical carcinogenesis and oxidative stress response[14], both of which are known to drive BLCA malignancy. In addition, studies in nasopharyngeal carcinoma have shown that TIMELESS interacts with the Wnt/β-catenin pathway, suggesting that a similar mechanism may underlie its tumor-promoting effects in BLCA[15]. These findings highlight the need for further investigation into the precise molecular functions of TIMELESS in BLCA and its potential as a therapeutic target.
This study highlights the therapeutic potential of targeting the HOXC6-TIMELESS axis in BLCA, despite certain limitations, including reliance on in vitro analyses and bioinformatic predictions that may not fully capture in vivo tumour complexity, and the need for validation in diverse patient cohorts to account for clinical and genetic variability. Despite these limitations, the identification of HOXC6 as an upstream regulator of TIMELESS unveils novel therapeutic opportunities for the development of diagnostic tools and prognostic biomarkers to guide personalised treatment. Therapeutically, HOXC6's role as a transcriptional regulator makes it a viable target for small molecule inhibitors or gene silencing approaches such as siRNA[16], CRISPR-based genome editing or antisense oligonucleotides[17]. Similarly, disruption of the interaction between HOXC6 and TIMELESS or pharmacological inhibition of TIMELESS could sensitise cancer cells to apoptosis and thereby attenuate tumour progression.
To ensure translational relevance, future research should focus on validating these therapeutic strategies in preclinical models and clinical trials, with particular emphasis on safety and efficacy. These strategies could be evaluated both as stand-alone treatments and in combination with existing options such as chemotherapy, immunotherapy and targeted therapies[18]. In addition, investigation of the broader oncogenic networks affected by the HOXC6-TIMELESS axis may uncover additional pathways and targets, broadening the therapeutic landscape for urinary tract malignancies.